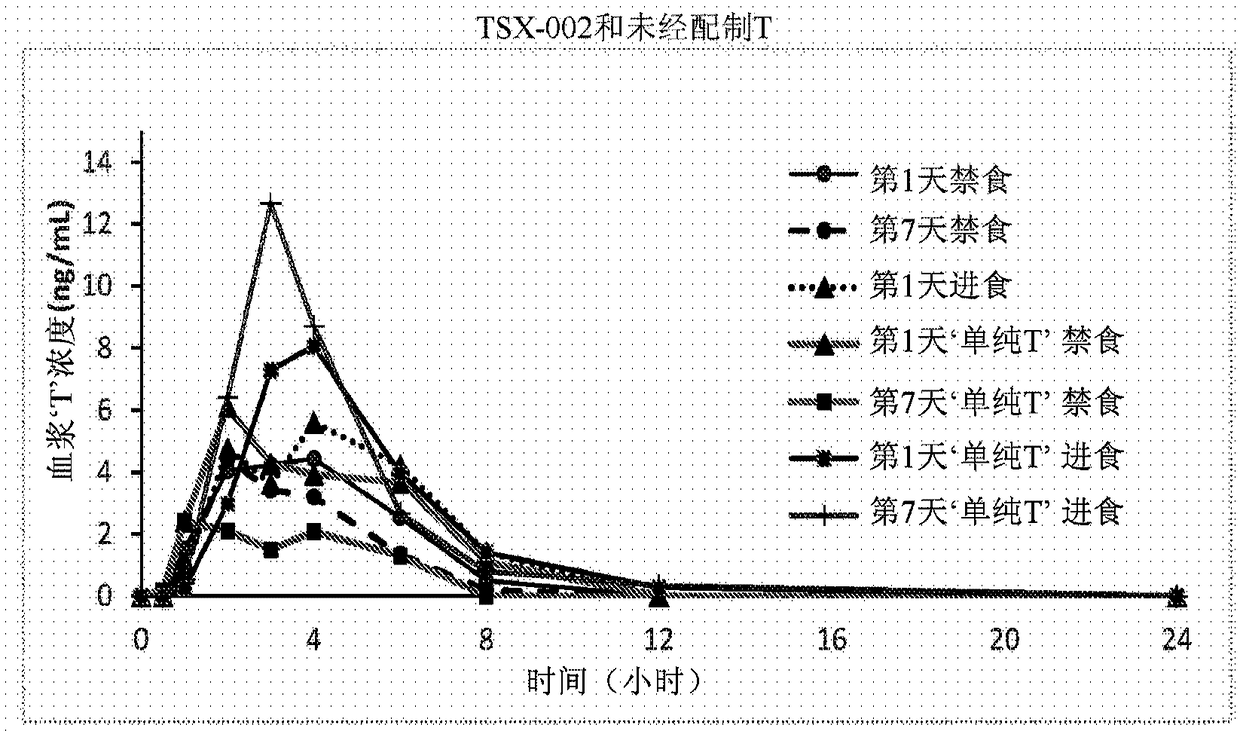
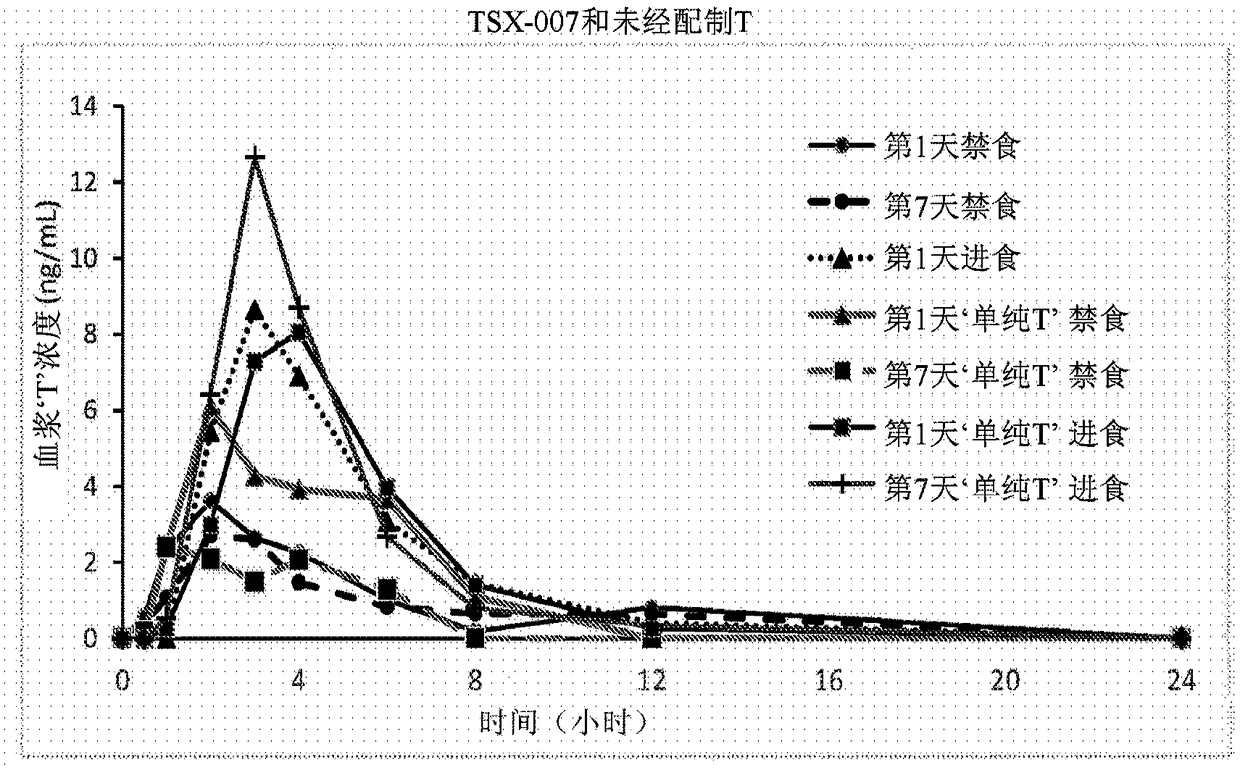
Proliposomal testosterone undecanoate formulations
A proliposome and testosterone undecanoate technology, which can be used in liposome delivery, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as significant performance differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Preparation of Proliposome Powder Dispersion
[0047] The proliposomal powder dispersion of the present invention can be prepared by dissolving TU in a solvent. Heat (eg, 45 to 55° C.) can optionally be applied during dissolution. The solvent is any solvent in which TU dissolves, but is preferably a water-miscible solvent such as ethanol; however, the solvent should generally not contain 10% or more water (vol / vol). Other exemplary solvents include methanol, chloroform, dichloromethane, acetone, isopropanol, and diethyl ether. After TU was dissolved (ie the solution became clear), DSPC was also dissolved in the TU solution until the solution became clear again. The solvent is removed by any suitable technique, such as by evaporation, by placing the solution under vacuum, by spray drying, or by use of a drying gas or the like. The solvent removal process was continued until a dry mass of TU and DSPC dispersion was formed. The average particle size of the resulting ...
Embodiment
[0067] Example 1. Testosterone Undecanoate Uncoated, Lipid Free Control Formulation TU1-044. To prepare TU1-044, 95 mg of testosterone undecanoate (TU) purchased from Pfizer Inc., Kalamazoo, MI was weighed and manually filled to uncoated size 1 Plus capsules.
[0068] Example 2. Enteric-coated testosterone undecanoate, lipid-free control preparation TU1-076. To prepare TU1-076, 95 mg of testosterone undecanoate (TU) purchased from Pfizer Inc., Kalamazoo, MI was weighed and manually filled to uncoated size 1 Plus capsules. The filled capsules were coated with methacrylic acid copolymer NF, type C ( L 30D-55). Plus capsules contain United States Pharmacopeia (USP) grade hydroxypropyl methylcellulose and water.
Embodiment 3
[0069] Example 3. Testosterone undecanoate + DSPC + cholesterol (1.0:0.9:0.1) preparation TU1-040. To prepare TU1-040, TU (3.95 g) was dissolved in 19 mL of EtOH at 45 to 55 °C and mixed until a clear solution formed. Distearoylphosphatidylcholine (DSPC) (3.55 g) and cholesterol (0.395 g) were added to the drug solution, and the mixture continued to mix at 45 to 55° C. until a clear solution formed. Mixing and heating were continued under vacuum until a dried mass formed. Pass the dried material through a No. 60 screen. Fill dried and sieved powder to uncoated size "0" Plus capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com