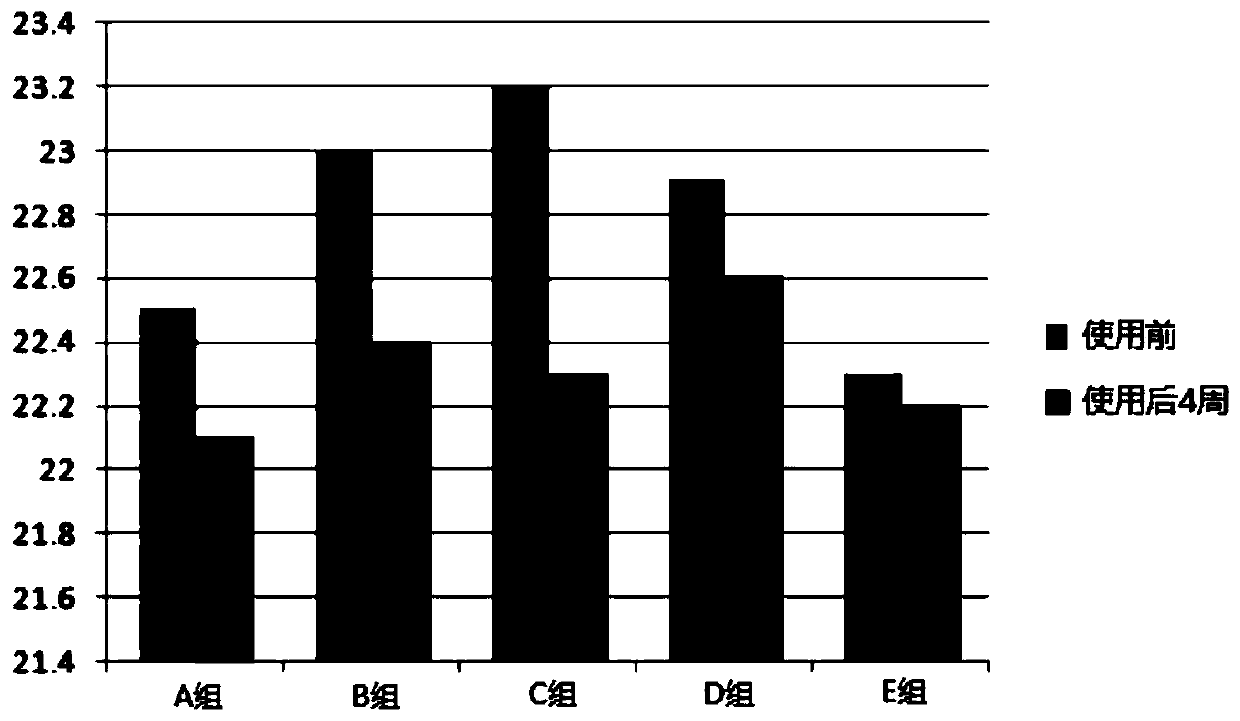
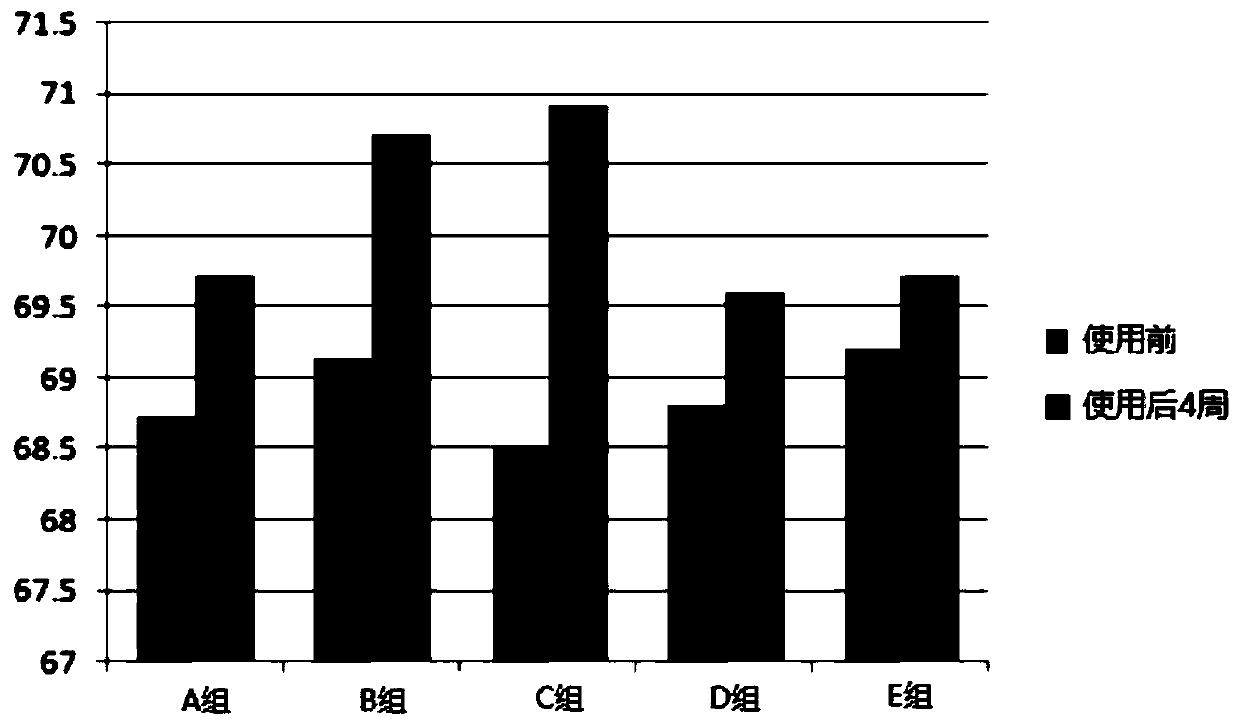
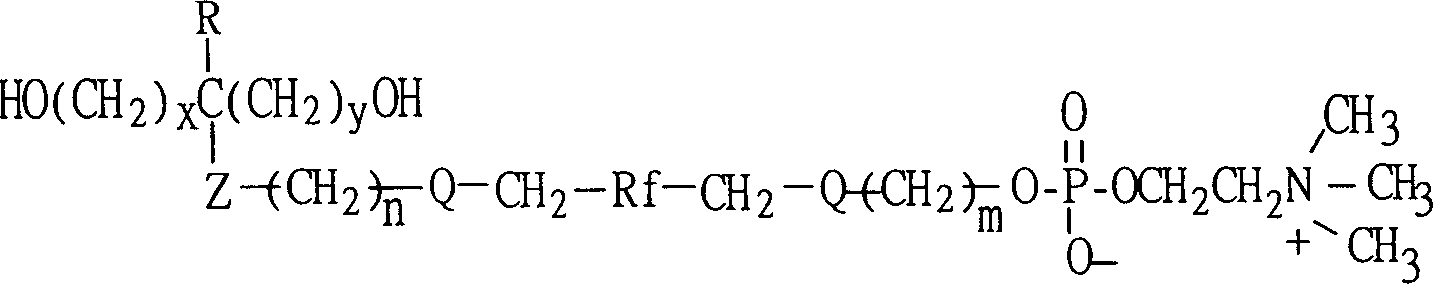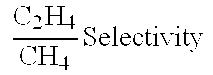Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
242 results about "Phosphatidyl Cholines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphatidylcholine is a ubiquitous, naturally occurring phospholipid molecule. It is the major lipid, or fat, of cell membranes and blood proteins. Also known as PC, phosphatidylcholine serves as the body’s main source of choline, an essential nutrient and precursor to the neurotransmitter, acetylcholine.
Proteinic drug delivery system using membrane mimetics
A mixed liposome pharmaceutical formulation with multilamellar vesicles, comprises a proteinic pharmaceutical agent, water, an alkali metal lauryl sulphate in a concentration of from 1 to 10 wt. / wt. %, at least one membrane-mimetic amphiphile and at least one phospholipid. The membrane-mimetic amphiphile is hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, lauramidopropyl betain, lauramide monoisopropanolamide, sodium cocoamphopropionate, bishydroxypropyl dihydroxypropyl stearammonium chloride, polyoxyethylene dihydroxypropyl stearammonium chloride, dioctadecyldimethylammonium chloride, sulphosuccinates, stearamide DEA, gamma-linoleic acid, borage oil, evening of primrose oil, monoolein, sodium tauro dihydro fusidate, fusidic acid, alkali metal isostearyl lactylates, alkaline earth metal isostearyl lactylates, panthenyl triacetate, cocamidopropyl phosphatidyl PG-diammonium chloride, stearamidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidylcholine, polysiloxy pyrrolidone linoleyl phospholipid, trihydroxy-oxo-cholanylglycine and alkali metal salts thereof, and octylphenoxypolythoxyethanol, polydecanol X-lauryl ether, polydecanol X-oleyl ether, wherein X is from 9 to 20, or combinations thereof. The phospholipid is phospolipid GLA, phosphatidyl serine, phosphatidylethanolamine, inositolphosphatides, dioleoylphosphatidylethanolamine, sphingomyelin, ceramides, cephalin, triolein, lecithin, saturated lecithin and lysolecithin, or a combination thereof. The amount of each membrane mimetic amphiphile and phospholipid is present 1 to 10 wt. / wt. % of the total formulation, and the total concentration of membrane mimetic amphiphiles and phospholipids is less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA
Artificial low-density lipoprotein carriers for transport of substances across the blood-brain barrier
InactiveUS7803400B2Convenience to mergeFacilitate and improve treatmentBiocidePowder deliveryDiseaseLipid formation
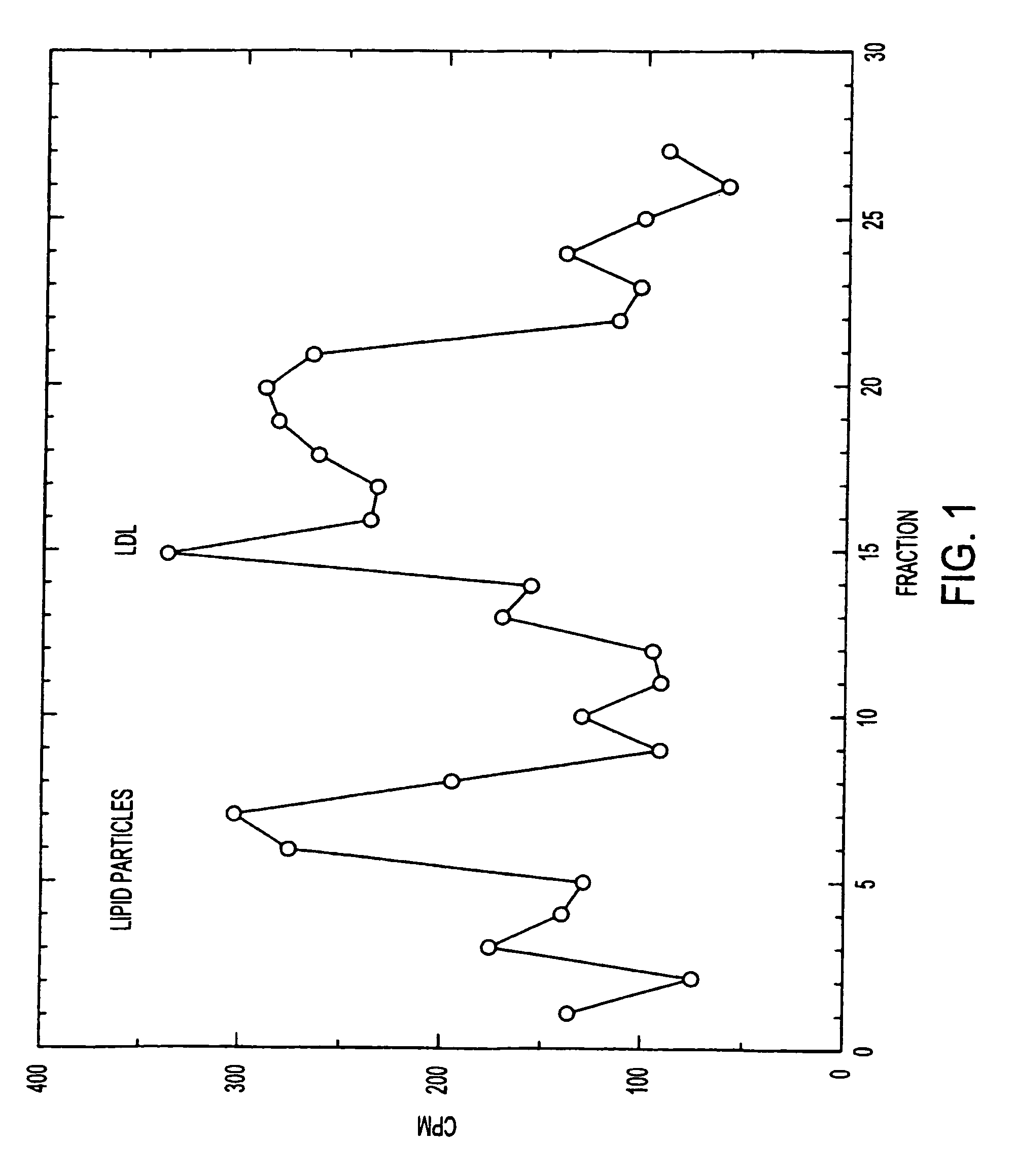
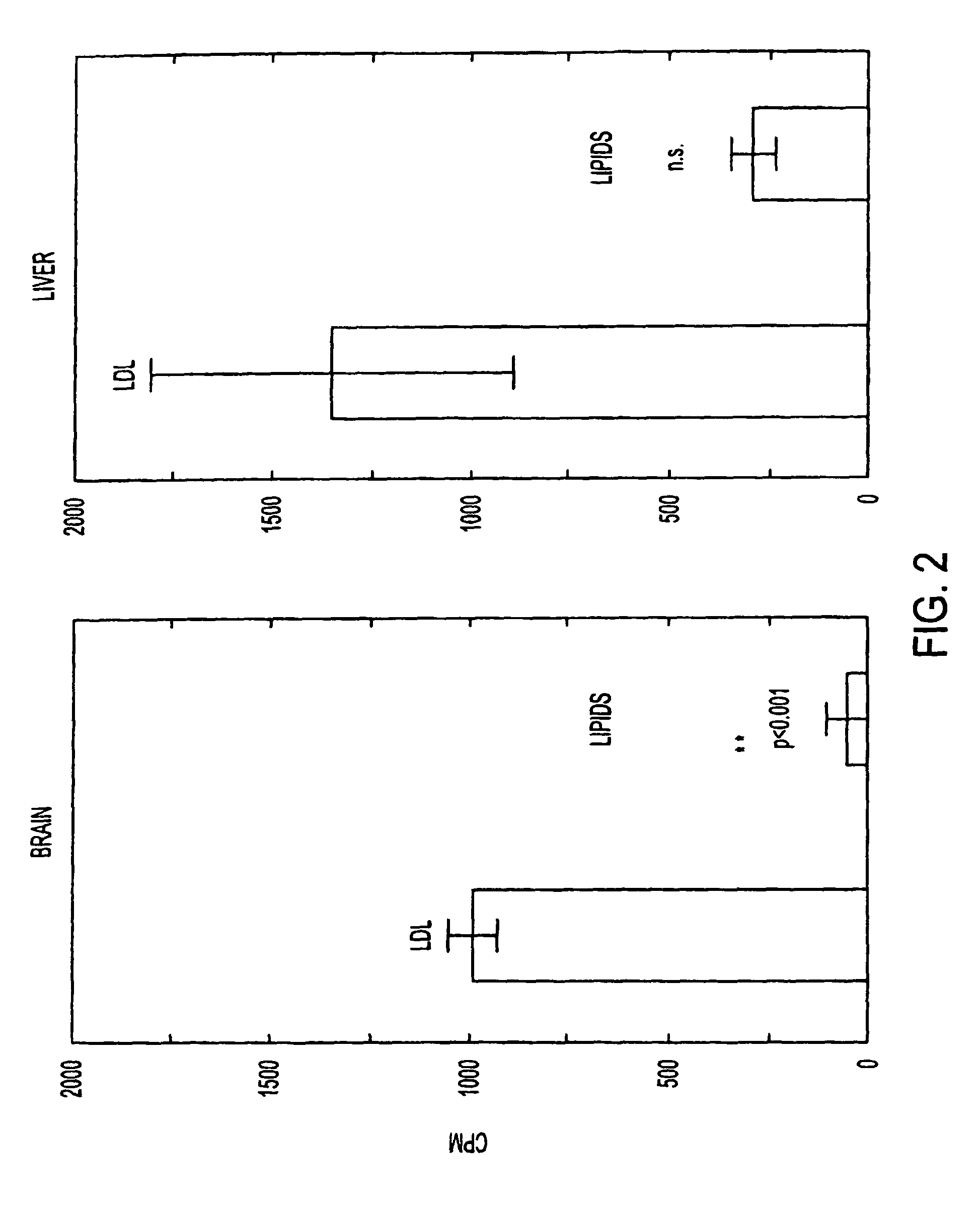
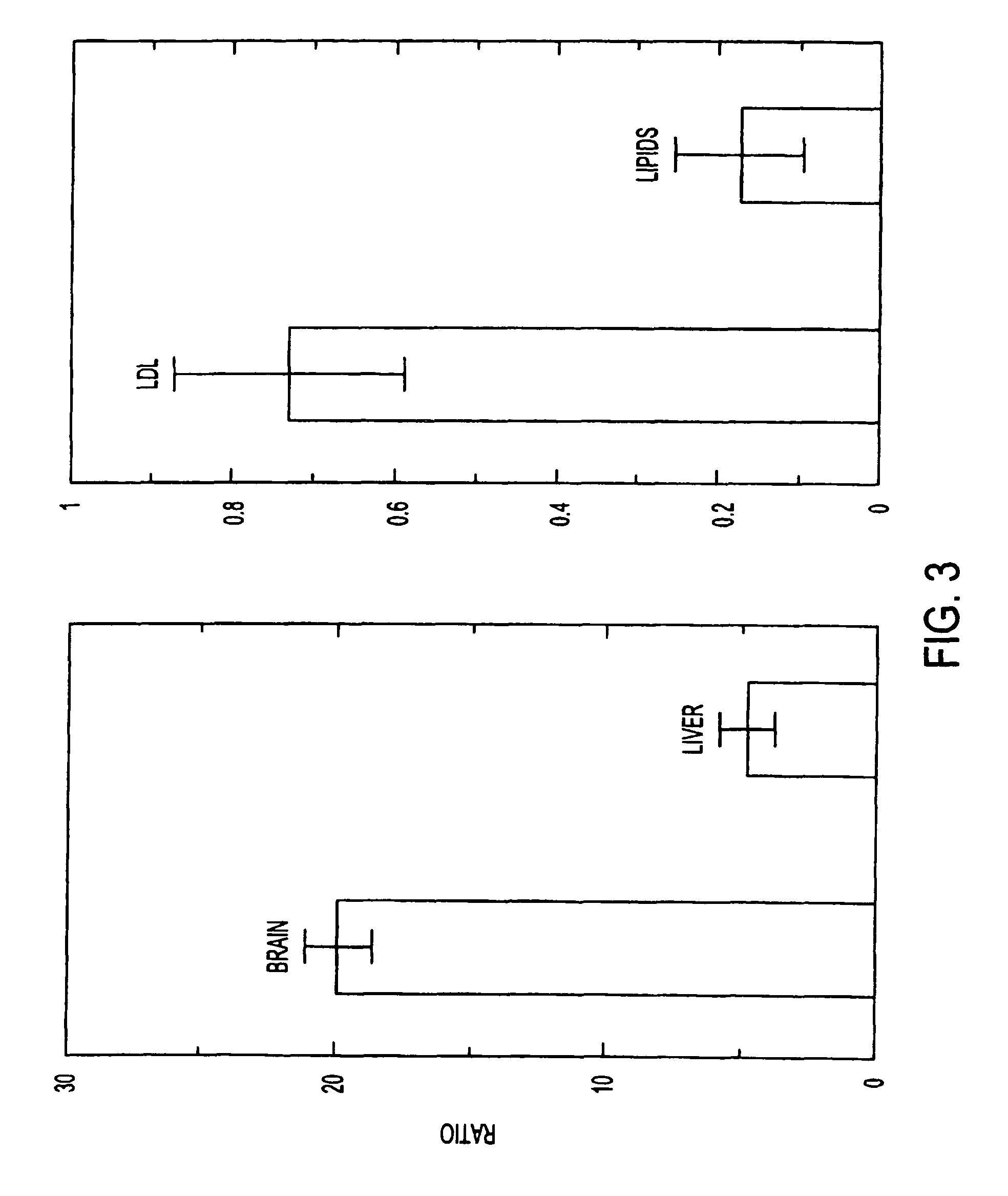
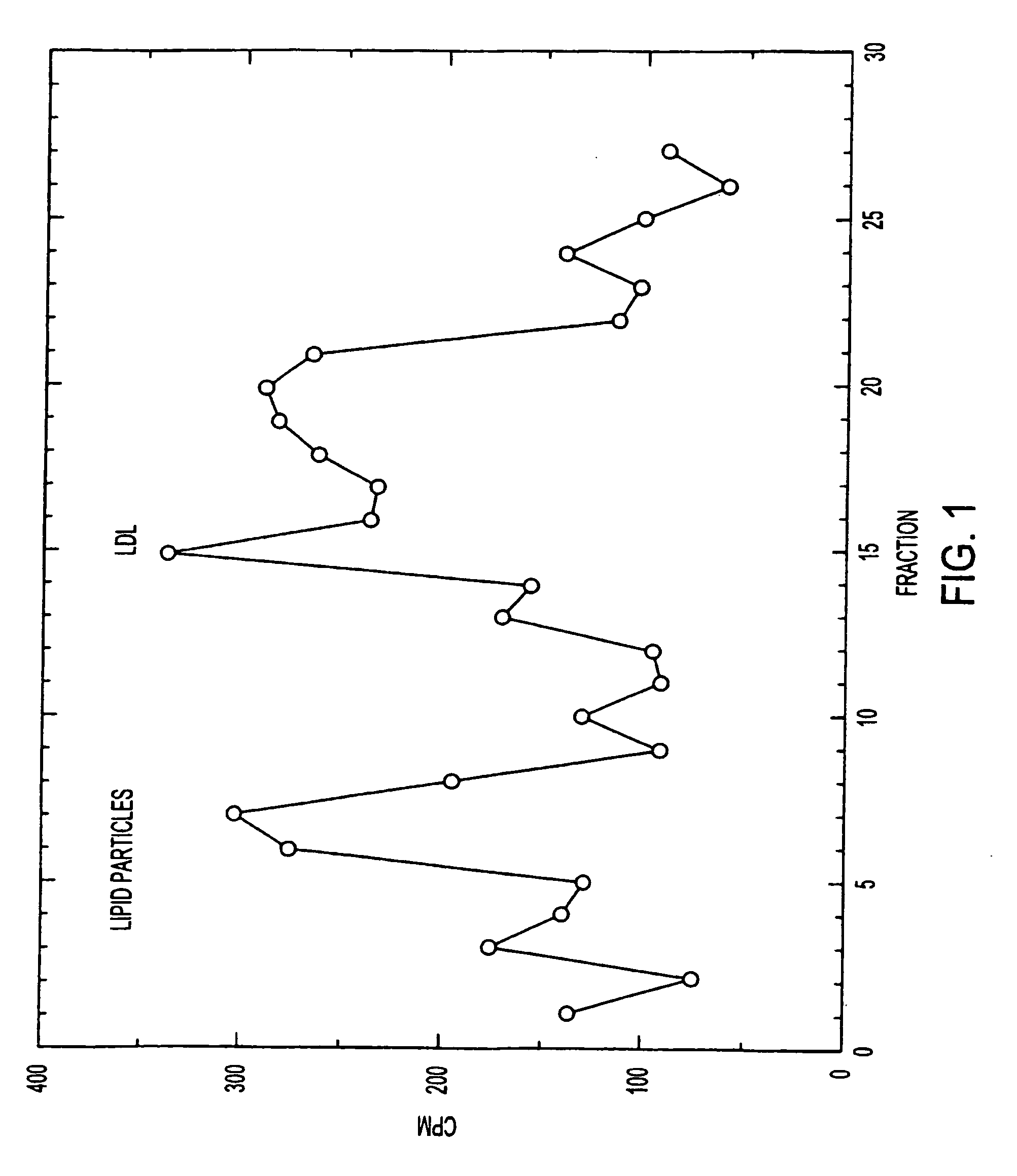
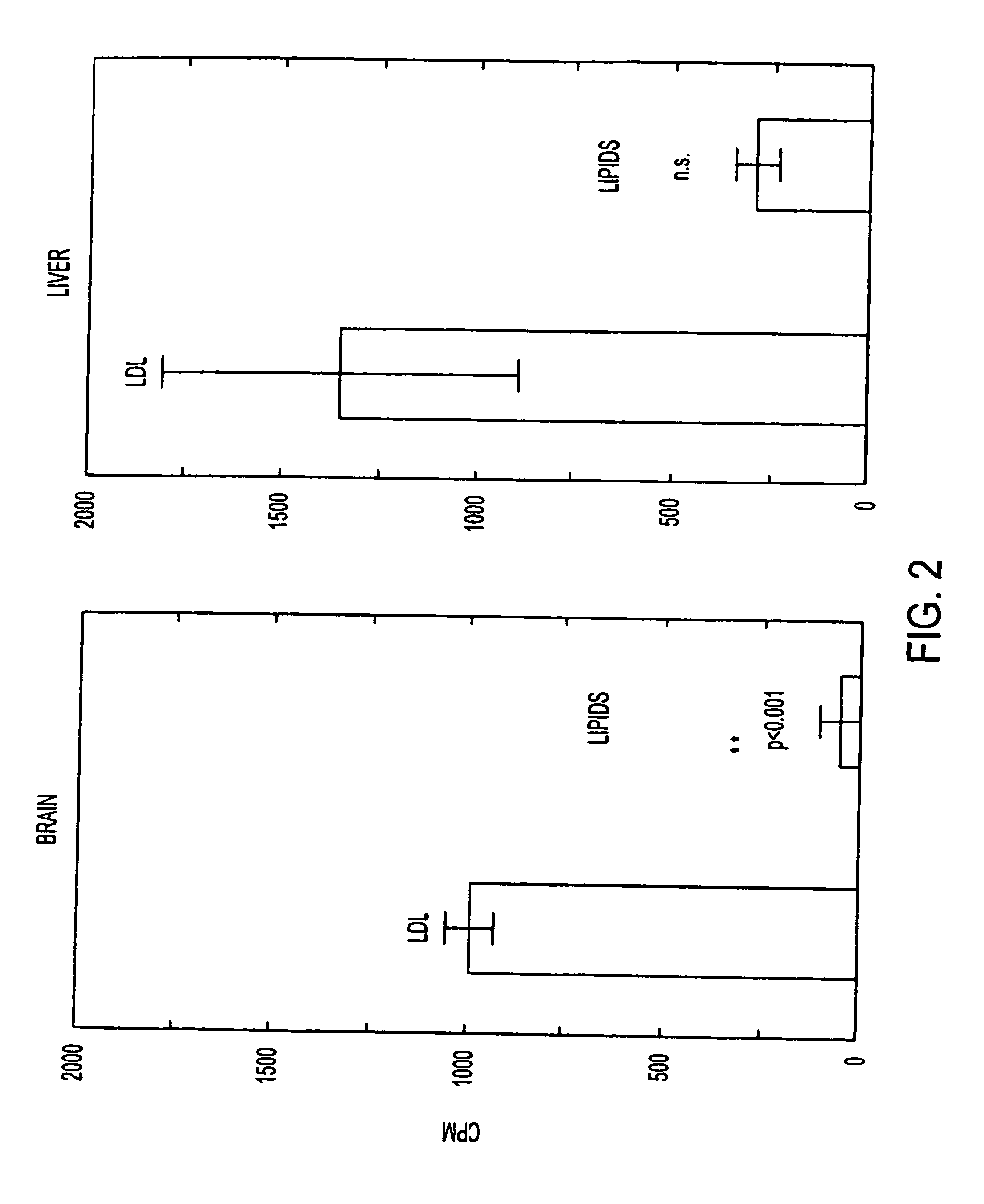
This invention relates to a highly efficient artificial low-density lipoprotein (LDL) carrier system for the targeted delivery therapeutic agents across the blood-brain barrier (BBB). In particular, this invention relates to artificial LDL particles comprised of three lipid elements: phosphatidyl choline, fatty-acyl-cholesterol esters, and at least one apolipoprotein. The present invention further relates to compositions, methods and kits comprising artificial LDL particles for targeting drugs to and across the BBB for the prevention and treatment of brain diseases.
Owner:WEST VIRGINIA UNIVERSITY
Whitening cream and preparation method thereof
ActiveCN110721104ASimple recipeEasy to prepareCosmetic preparationsToilet preparationsGallic acid esterAcetophenone
The invention belongs to the technical field of cosmetics, and particularly relates to whitening cream and a preparation method thereof. The whitening cream comprises the following preparation raw materials of nicotinamide, resveratrol, 3-o-ethyl ascorbic acid, a peony extract, diglucosyl gallic acid, a caviar extract, phosphatidylcholine, ceramide 3, p-hydroxyacetophenone, tocopheryl acetate, sodium hyaluronate, 7-10% of a humectant, an emollient, an emulsifying agent, a thickening agent, a neutralizer, essence and water. The whitening cream disclosed by the invention can take effect in multiple ways of melanin generation inducement, melanin generation process, melanin transfer, melanin digestion and discharge process and the like, and the purpose of quickly whitening skin and dispellingfreckles can be achieved.
Owner:泉后(广州)生物科技研究院有限公司
Method for preparing glycerophosphorylcholine (GPC) by phospholipase-catalyzed hydrolysis
The invention discloses a method for preparing GPC by phospholipase-catalyzed hydrolysis and belongs to the technical field of lipid development and application. The method comprises the following steps: preparing GPC by using powdered phospholipid, alcohol soluble phospholipids, high-purity PC as raw materials and by phospholipase-catalyzed hydrolysis of phosphorylcholine (PC) in a water phase; and decolorizing by using active carbon to obtain GPC aqueous solution, converting into an alcohol phase, purifying by using a cation resin adsorption resolution and anion adsorption process to obtain high-purity GPC aqueous solution, performing rotating evaporation at a low temperature and under reduced pressure to obtain colorless transparent L-alpha-GPC solution, wherein the chemical purity of the obtained GPC is 98.8 percent, the optical purity ee of the obtained GPC is 99 percent, and the melting point (mp) of the obtained GPC is 142 to 143 DEG C (C is equal to 2.6, the H2O content is 14 percent and the pH value is 5.8). The invention provides a new thought and a new preparation method for preparing GPC and provides new application of phospholipase in lipid science.
Owner:JIANGNAN UNIV
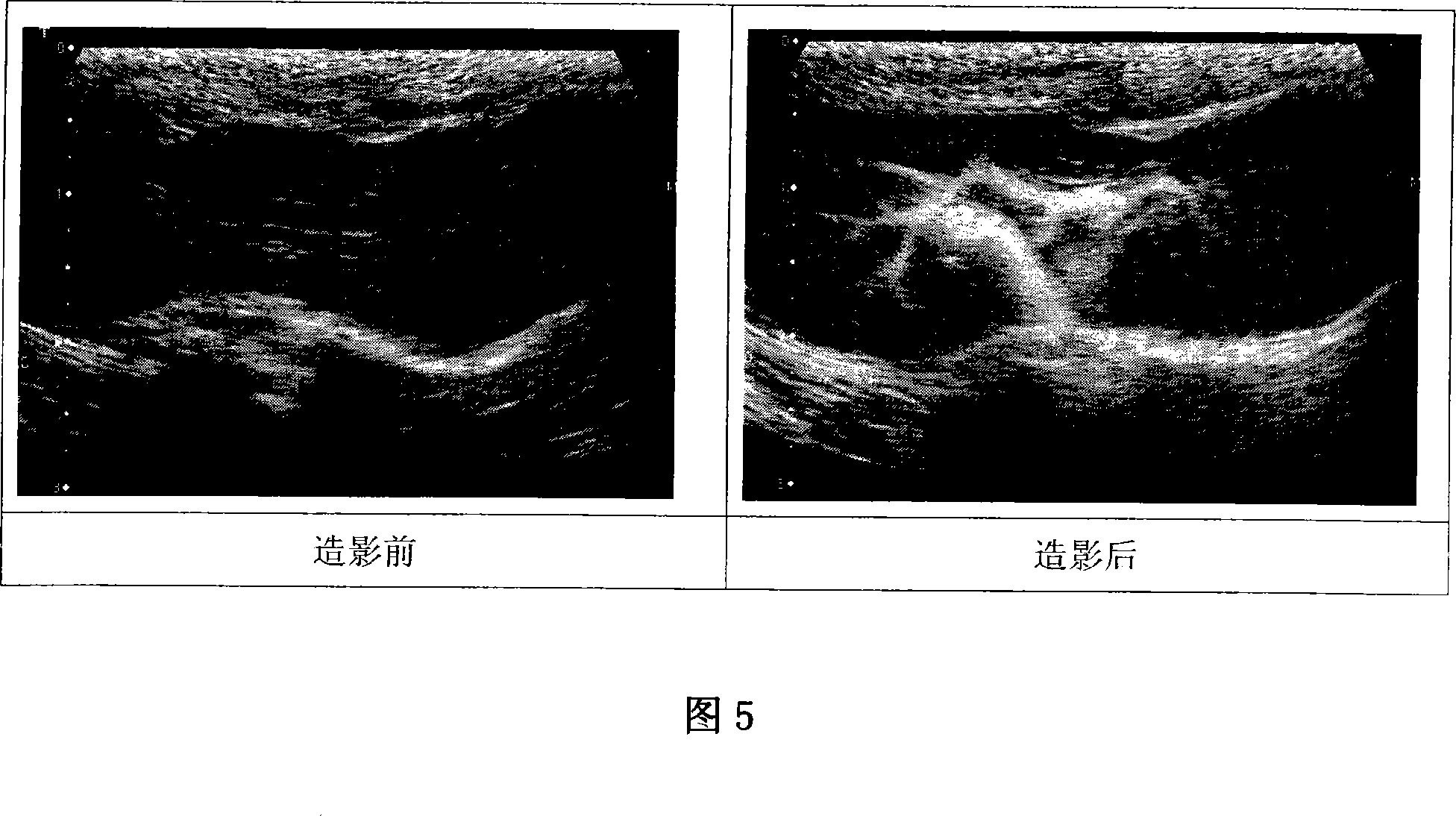
Lipid microvesicle ultrasound angiography powder agent internally containing mixture gas of fluorine carbon/nitrogen gas and production of the same
InactiveCN101130095AEnhanced Ultrasound ImagingEnables mass productionEchographic/ultrasound-imaging preparationsLipid formationDispersity
The invention discloses a hollow lipid microfoam composition with fluorocarbon / nitrogen and preparing method, wherein the microfoam filming material is composed of phosphatide, protective and polymer; the phosphatide is selected from phosphatidyl Choline and phosphatidyl ethanolamine; the protective can be middle and macromolecular hydroxyethyl amidon; the polymer is selected from boluoshamu 188 or medically acceptable surface activator for vein injection; the lipid filming material covers the fluorocarbon liquid to form emulsion particle, which forms hollow lipid microball under high temperature instantaneously through gasifying the liquid fluorocarbon; the fluorocarbon / nitrogen is guided to produce the lipid microball for ultrasonic imaging with effectively reinforcing time over 60 min. The invention has high yield rate and fast manufacturing speed, which improves the microball dispersity and microball density effectively.
Owner:CHONGQING RUNQI PHARMA TECH DEV
Lipid-based dispersions useful for drug delivery
The invention provides lipid-based dispersion comprising comprising, a) phosphatidyl choline; b) an anionic phospholipid; optionally c) up to 1% cholesterol by weight of total lipids; and optionally d) a therapeutic agent; wherein the mean particle size measured by dynamic light scattering is less than 100 nm. The invention also provides pharmaceutical compositions comprising such a dispersion as well as methods of producing a therapeutic effect in a mammal comprising administering an effective amount of such a dispersion.
Owner:GILEAD SCI INC
Compositions for reducing incidence of necrotizing enterocolitis
InactiveCN1200701CDigestive systemPhosphorous compound active ingredientsCholesterolNecrotic enterocolitis
Formulations for gastrointestinal administration containing long-chain polyunsaturated fatty acids (PUFAs), such as essentially cholesterol-free arachidonic acid (AA) and docosaccharide, and methods for their preparation are described Hexaenoic acid (DHA). More specifically, the invention relates to methods of reducing the incidence of necrotizing enterocolitis by administering a composition thereby providing n-6 and n-3 long chain PUFAs, phospholipids and / or choline. Compositions from egg yolk lipids are preferred as they contain n-6 and n-3 long chain PUFAs and mainly in the phosphatidylcholine form. Think this provides synergy. Methods of preparing such a composition with improved sensory properties and stability are also provided.
Owner:ABBOTT LAB INC +1
Nonviral targeted nanoparticle system for gene transfer and drug delivery
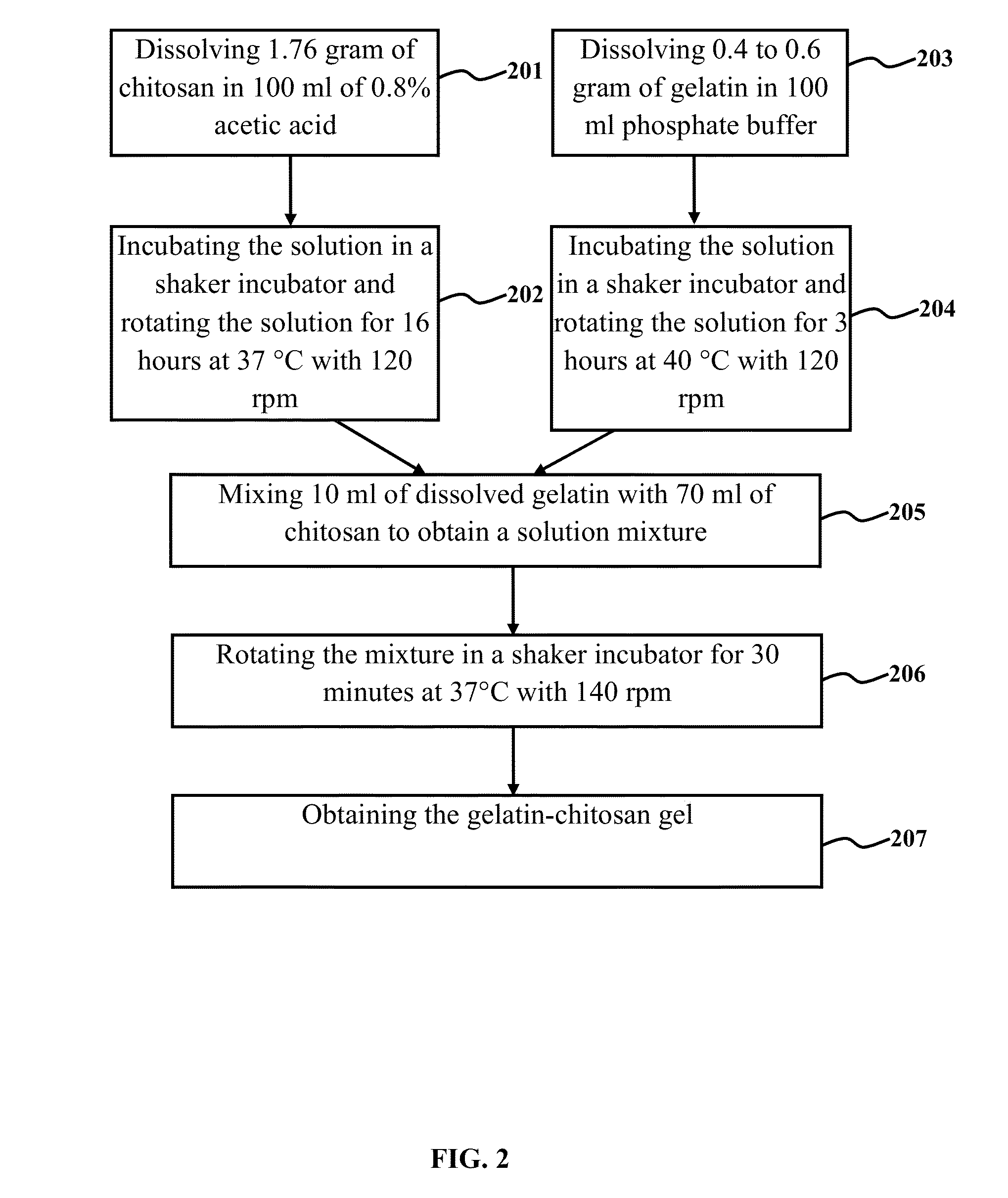
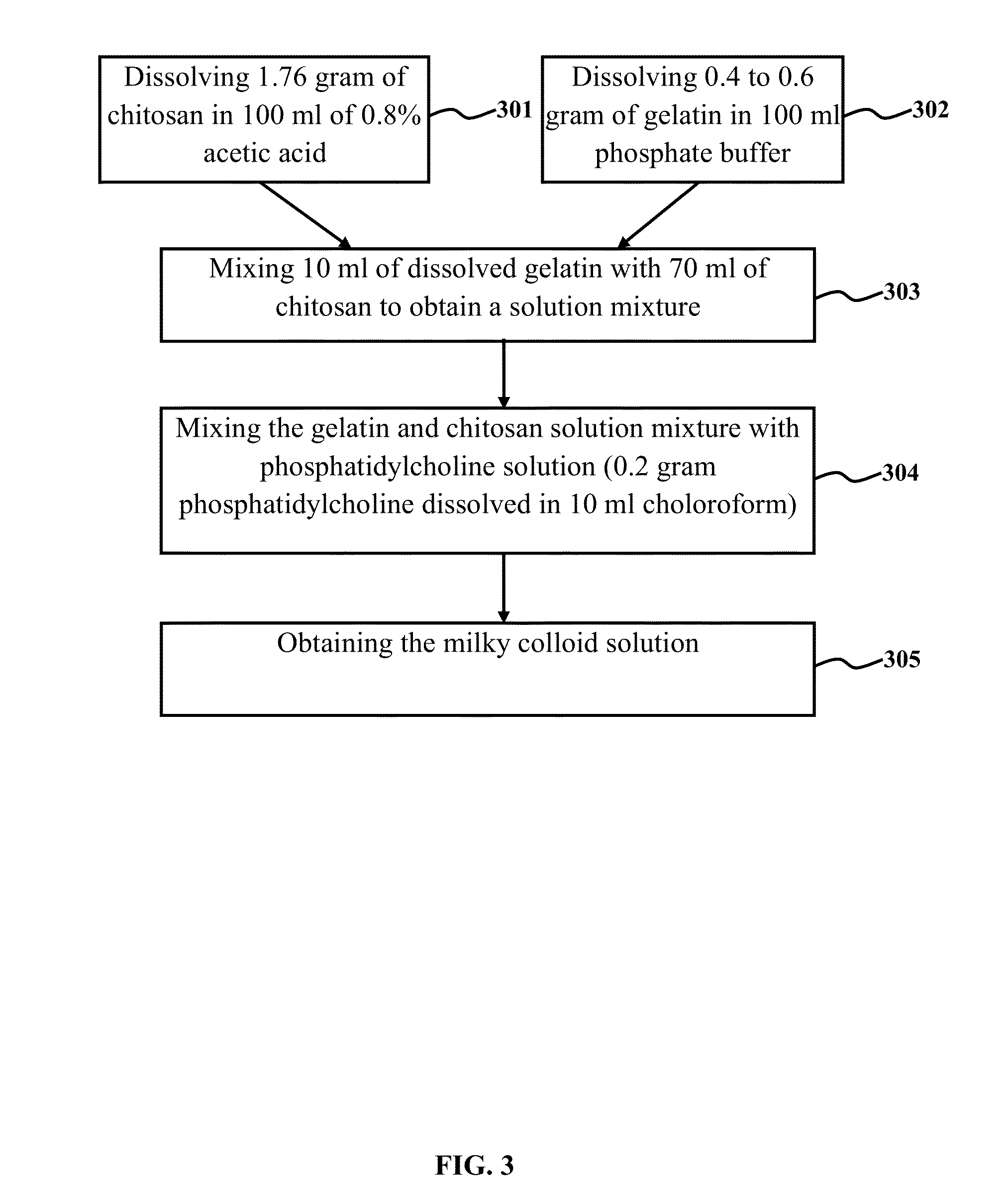
The embodiments herein provide a nanoparticle system for targeted gene delivery or a drug delivery and a method of synthesising the same. The nanoparticle composition for targeted gene transfer and drug delivery comprises a protein, a chitosan and a lipid. A method of synthesizing the nanoparticles involves preparing a gelatine and chitosan gel. A milky colloid solution is prepared with the gelatine, chitosan solution and a phosphatidylcholine. The milky colloid is homogenized for the self assembly of the nanoparticles. The milky colloid is subjected to high speed and high pressure homogenizer. The CHO cells are transfected with nanoparticles and lipofectamine 2000 for comparing the transfection efficiency. The nanoparticles deliver DNA, RNA, ribozyme and nucleotide sequences. The nanoparticles deliver lipophylic and hydrophilic drugs. The transfection efficiency of gene and drug is higher when the target cells are transferred with nanoparticles, compared to the cells transferred with lipofectamine 2000.
Owner:GHANAVI JALALEDIN +1
A method for preparing lysophosphatidylcholine by enzymatic alcoholysis
InactiveCN102277393AImprove conversion rateImprove solubilityFermentationPhosphoric Acid EstersPhosphoric acid
The invention discloses a method for preparing lysophosphatidyl choline by enzymatic alcoholysis, which comprises: adding phosphatidylcholine into low-carbon alcohol solution, uniformly stirring and mixing at a certain temperature, adding lipase, and allowing the lipase to catalyze the alcoholysis of phosphatidylcholine with constant-temperature stirring to obtain the lysophosphatidyl choline. The method has the advantages that: (1) the solubility of phosphatidylcholine, lysophosphatidyl choline serving as the product, and aliphatic ester, glycerophosphorylcholine and very small amount of fatty acid, which serve as byproducts, in the reaction system is improved, and the conversion rate of the lysophosphatidyl choline is high; (2) the aliphatic ester, a small amount of glycerophosphate and a very small amount of fatty acid are generated in an alcoholysis reaction process, but the pH value of the system is not changed, and the influence of the solvent on the activity of the lipase is relieved and the catalytic reaction activity of the lipase is high; (3) the properties of the fatty acid, the aliphatic ester and glycerophosphorylcholine, which are products of side reactions, are very different from those of the lysophosphatidyl choline, so the lysophosphatidyl choline product can be separated and purified very easily; and (4) the alcohol, which is the product of the reaction, can be removed easily.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Breviscapinum long-circulating nanoliposome and its preparation method
InactiveCN1843368ASimple preparation processImprove in vivo and in vitro stabilityOrganic active ingredientsLiposomal deliveryYolkFreeze-drying
The invention discloses a breviscapinum long-circulating nanoliposome and its preparation method, wherein the constituents include breviscapine, phosphatide, polyethylene glycol derived phosphatide (PEG2000-DSPE) and cholestrin by a molar ratio of 55:25-55:3-7:2.2-18.3, the dose type of the medicinal preparation includes injections, freeze-dried powder injections and sprays. The phospholipids can be soya bean lecithin, yolk phosphatidy icholine, hydrogenated soya bean lecithin, hydrogenated yolk lecithin, di-palmityl phosphatidyl choline or DPOG.
Owner:TSINGHUA UNIV
Vitamin E phosphate/phosphatidycholine liposomes to protect from or ameliorate cell damage
This invention relates to protecting cells from damage and stimulating cell repair by administration of vitamin E phosphate encapsulated in phosphatidylcholine liposomes.
Owner:LAMB ROBERT
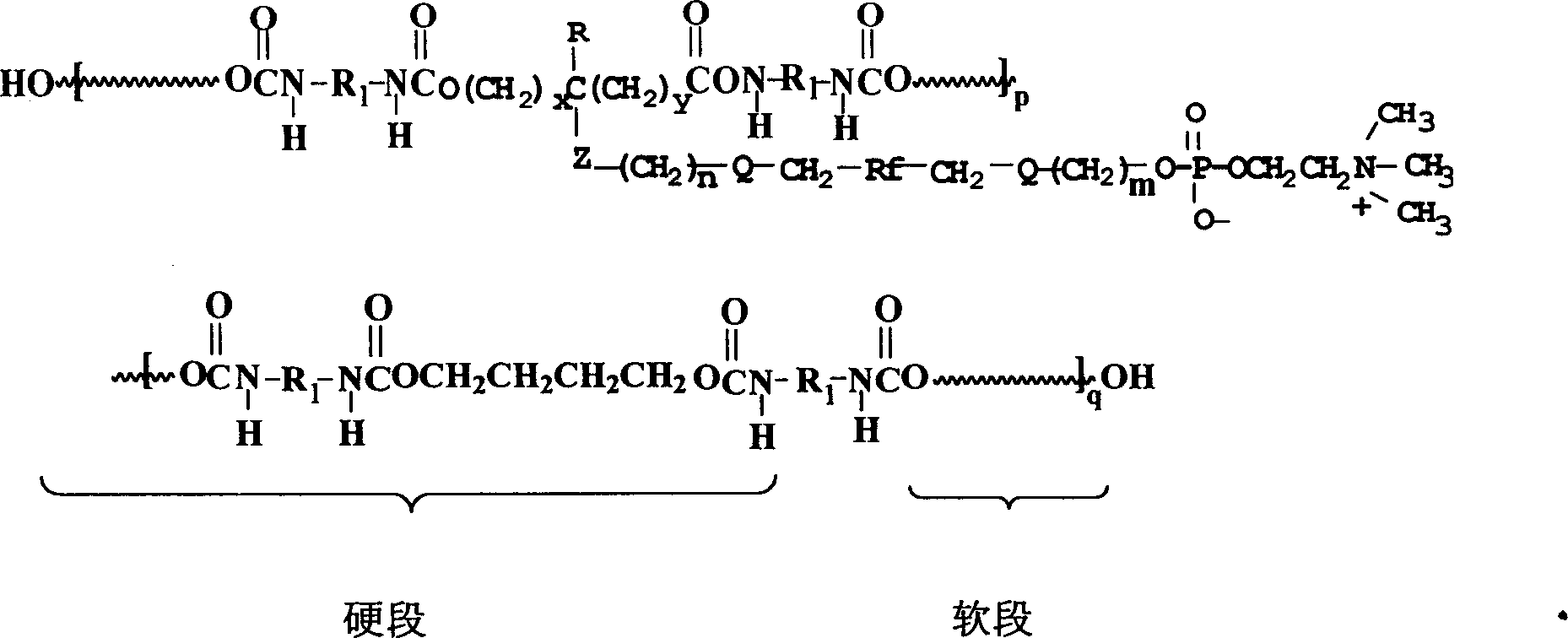
Polyurethane material with side chain possessing fluorophosphatidylcholine and its preparation method
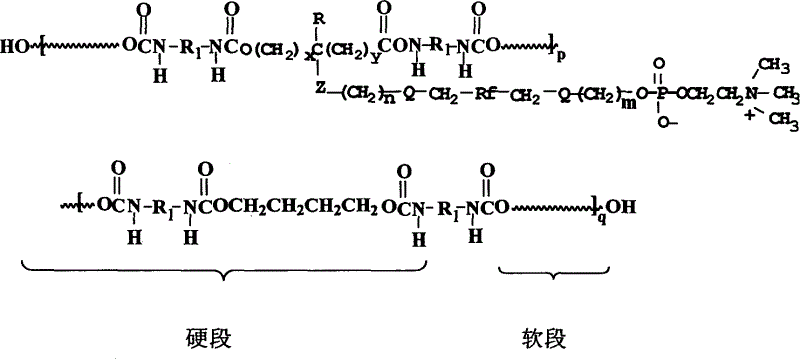
The invention provides a polyurethane material with side chain possessing fluorophosphatidylcholine and its preparation method, which is prepared through alternating copolymerization of a soft segment composed of polyether dihydroxy alcohol and / or polycarbonate diatomic alcohol and a rigid chain segment formed by diisocyanate and chain expanding agent, characterized in that the copolymer comprises the following repetition structural elements, wherein the partial rigid chain segment side chain contains fluorine-containing phosphatidyl choline groups, whose weight average molecular weight is 30000-60000.
Owner:SICHUAN UNIV
Liposome delivery system for treating cartilage diseases and preparation method of liposome delivery system
ActiveCN105078889AReduce volumeKnockout is safe and effectiveOrganic active ingredientsGenetic material ingredientsDiseaseCholesterol
The invention belongs to the technical field of medicine; in order to solve the problem that no efficient and safe cartilage siRNA delivery system is provided for limiting application of RNAi in treating cartilage diseases, the invention provides a cartilage siRNA liposome delivery system and a preparation method of the delivery system. The delivery system consists of lipidosome, a lipidosome modifier and a small nucleic acid drug, wherein the lipidosome is phosphatidylcholine, cholesterol or Dlin-KC2-DMA; the lipidosome modifier is a polyethylene glycol lipid conjugate; and the small nucleic acid drug is Ihh siRNA. According to the invention, a liposome nano based cartilage RNA interference delivery system is modified, and the formed composite has positive charges, is safe and small in volume, and can be distributed in a full-lamellar cartilage tissue. Carrier experiments verify that siRNA can be delivered into cartilage cells to successful knock out corresponding genes inside the cartilage cells, therefore a bottleneck of gene knockout in the cartilage tissue is broken through; and the siRNA, as a target gene, can be used for treating PTOA (post-traumatic osteoarthritis).
Owner:魏垒 +4
Water-based emulsion containing lecithin and DHA and preparation method and application of water-based emulsion
InactiveCN104490773AImprove bioavailabilityCosmetic preparationsOrganic active ingredientsGlycerolConjugated linoleic acid
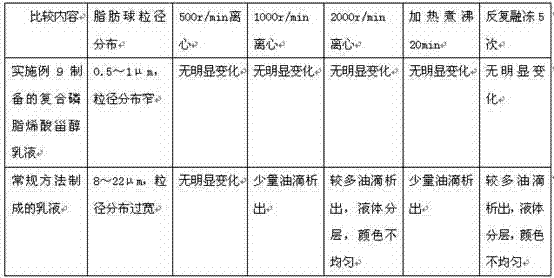
The invention provides an emulsion composition containing lecithin, DHA, other fat-soluble substances and purified water. The content of the purified water in the emulsion composition is more than 70% by weight, the weight ratio of the lecithin to the DHA is 1: (0.005-1), the particle size of fat balls in the emulsion is less than 5 mu m, and the other fat-soluble substances comprise one or more of the following components: arachidonic acid, linolenic acid, alpha-linolenic acid, gamma-linolenic acid, linoleic acid, conjugated linoleic acid, conjugated linoleic acid glyceride, phosphatidylserine, polyene phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl inositol, phosphatidyl glycerol, phytosterol, plant sterol ester, plant stanol ester and medium and long-chain fatty acid. The invention further provides a preparation method of the emulsion composition and application of the emulsion composition in the functional aspects of supplementing phospholipids and unsaturated fatty acids by oral administration, protecting heart and brain blood vessels, reducing blood lipids, reducing blood pressure, treating fatty liver and the like.
Owner:FUZHOU QIANZHENG PHARMA
Artificial low-density lipoprotein carriers for transport of substances across the blood-brain barrier
InactiveUS20080160094A1Convenience to mergeFacilitate and improve treatmentPowder deliveryBiocideDiseaseLipid formation
This invention relates to a highly efficient artificial low-density lipoprotein (LDL) carrier system for the targeted delivery therapeutic agents across the blood-brain barrier (BBB). In particular, this invention relates to artificial LDL particles comprised of three lipid elements: phosphatidyl choline, fatty-acyl-cholesterol esters, and at least one apolipoprotein. The present invention further relates to compositions, methods and kits comprising artificial LDL particles for targeting drugs to and across the BBB for the prevention and treatment of brain diseases.
Owner:WEST VIRGINIA UNIVERSITY
Lipidic Compositions for Induction of Immune Tolerance
InactiveUS20120164189A1Improve the level ofDecrease in ILSSPowder deliverySnake antigen ingredientsL serineTolerability
This invention provides a method for inducing immune tolerance toward an antigen comprising the antigen in lipidic particles or lipidic compositions. The lipidic particles are made up of phosphatidylserine and phosphatidylcholine, or phosphatidylinositol and phosphatidylcholine. The lipidic compositions comprise the antigen and O-phospho-L-serine. Administration of these composition results in inducing immune tolerance to the antigen.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Apparatus and Method for Reducing the Occurrence of Post-Surgical Adhesions
A method for inhibiting formation of adhesions following abdominal surgery which involves application of an anti-static fatty acid ethoxylated amide (Cocamide DEA) in a matrix that is placed in the peritoneal cavity at the conclusion of an abdominal surgery and which releases this anti-adhesive chemical over a predetermined time in a range up to seven days. Tests conducted on laboratory rats established that the method reduced the incidence of adhesions from 100 percent (100%) in a test model to near zero in the majority of treated animals. In an alternative embodiment, andrographalide was delivered via a pump with similar results. In still another embodiment, an effective amount of 50% phosphatidylchorene and propylene glycol was delivered, via a pump, into the abdominal cavity, again with similar results.
Owner:ANHESE
A lyophilized powder injection of polyene phosphatidylcholine and preparation method thereof
The invention relates to a phosphatidyl choline freeze powder injection and relative preparation, wherein said formula is formed by phosphatidyl choline, solubilizer, support agent and other additive components; the phosphatidyl choline content is 10-60%; the solubilizer can colalin, glycocholic acid, cholaic acid, deoxycholic acid, anthrodsoxycholic acid, or taurodeoxycholic acid and relative salt, loxapine and tween; the support agent can be freeze protector. The invention has high stability, which can be stored for 1 year at normal temperature, without allergic and excitation.
Owner:四川思达康药业有限公司
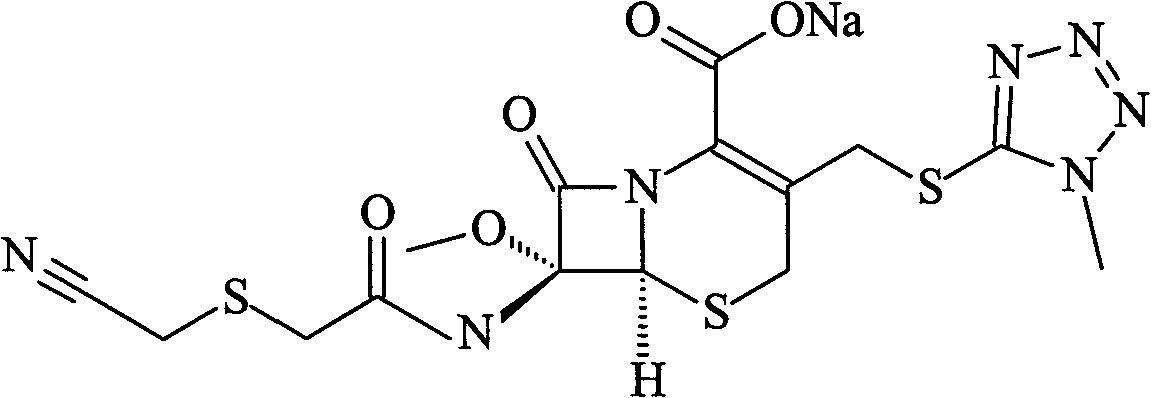
Cefmetazole sodium proliposome preparation
ActiveCN101623264AImprove stabilityImprove therapeutic indexAntibacterial agentsOrganic active ingredientsPolyeneChemistry
The invention provides a cefmetazole sodium proliposome preparation which comprises the following components by weight part: 1 part of cefmetazole sodium, 3-15 parts of liposome carrier and 2-10 parts of proppant, wherein the liposome carrier comprises polyene phosphatidyl choline, cholesterol and oleinic acid according to a weight ratio of (4-20):(1-5):1. The cefmetazole sodium proliposome preparation has good preparation stability and cannot crack because of dewatering, fusion, ice crystal generation, and the like in a freeze-drying process; and after hydrated re-dissolution, the cefmetazole sodium proliposome preparation still can maintain good entrapment rate.
Owner:灵康药业集团股份有限公司
Nutritional composition for preventing and improving fatty liver and preparation method of nutritional composition
ActiveCN104172196AHigh activityImprove liver functionVitamin food ingredientsAcidic food ingredientsNutritional compositionSide effect
The invention discloses a nutritional composition for preventing and improving fatty liver. The nutritional composition comprises hydrolyzed small molecular protein powder, high-F-value oligopeptide, dietary fiber, germ meal, an edible mushroom extract, a medicinal and edible extract, phosphatidylcholine, phytosterol, se-enriched yeast powder, omega-3 fatty acid and vitamins. The nutritional composition is capable of improving the immunity of a human body, improving the liver function, reducing the blood lipid and cholesterol, dispelling effects of alcohol, and inhibiting the absorption of the human body to the cholesterol and the fat. Raw materials used in the nutritional composition are common food raw materials common in the market and new resource food raw materials approved by the Ministry of Health, and are convenient to purchase. Effective ingredients in the nutritional composition are contained in daily foods, and quantified to achieve a treatment effect, and are high in safety and small in side effects. The nutritional composition is simple in preparation process, and is obtained by adopting the steps of mixing the raw materials and then packaging.
Owner:广州金酮医疗科技有限公司
Pharmaceutical compositions for the administration of aptamers
Pharmaceutical compositions comprising an aptamer and an amino acid ester or amide or an aptamer; a divalent metal cation; and a carboxylic acid, a phospholipid, a phosphatidyl choline, or a sphingomyelin. Methods of treating or preventing a condition in an animal comprising administering to the animal the pharmaceutical compositions.
Owner:IDEXX LABORATORIES
Somatostatin receptor agonist formulations
The present invention relates to compositions forming a low viscosity mixture of: a) 20-50 wt.% of at least one diacyl glycerol; b) 20-54 wt.% of at least one phosphatidyl choline (PC); c) 5-15wt.% of at least one biocompatible, organic mono-alcoholic solvent; d) 1 to 20 wt.% polar solvent e) 5 to 150 mg / ml of at least one peptide somatostatin receptor agonist comprising pasireotide; f) optionally at least one antioxidant; wherein the ratio of components a:b is in the range 40:60 to 54:46; wherein the pre-formulation forms, or is capable of forming, at least one liquid crystalline phase structure upon contact with excess aqueous fluid. The invention further relates to methods of treatment comprising administration of such compositions, and to pre-filled administration devices and kits containing the formulations.
Owner:CAMURUS AB
Preparation and application of high-purity lysophosphatidylcholine
ActiveCN103131736AHigh yieldLower reaction costOrganic active ingredientsDigestive systemDrugPhospholipase A2
The invention provides a novel effective method for preparing high-purity lysophosphatidylcholine, and a purpose for applying the high-purity lysophosphatidylcholine for preventing and / or treating metabolic bone diseases. According to the novel preparation method, phosphatidylcholine is converted into lysophosphatidylcholine under the catalysis of phospholipase A2. During the conversion process, a novel phospholipase A2 activity enhancer is added. Therefore, phospholipase A2 dose is reduced, lysophosphatidylcholine yield is improved, and high-purity lysophosphatidylcholine can be prepared. The prepared high-purity lysophosphatidylcholine can be used in medicine compositions used for preventing and / or treating metabolic bone diseases.
Owner:FUBICHENG SHANGHAI PHARMA TECH CO LTD
Novel phosphatidase B and application thereof
The invention belongs to the technical field of genetic engineering of enzymes, and particularly relates to a method for obtaining phosphatidase B mutants with improved enzyme activity through a PCR (sequential error-prone) technology in vitro directed evolution, using the phosphatidase B mutants to prepare glycerophosphatidylcholine and performing oil degumming. The phosphatidase B mutant PLBM is obtained; the enzyme activity is improved by 25 percent through being compared with that of wild phosphatidase B.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Olefin selective membrane comprising an ionic liquid and a complexing agent
InactiveUS20120190905A1Promote high degree of transportMore separatedSemi-permeable membranesGas treatmentTetrafluoroborateCadmium Cation
Owner:DOW GLOBAL TECH LLC
Preparation method of high-purity phosphatidylcholine
ActiveCN103613612ANot easy to form dead adsorptionPromote regenerationPhosphatide foodstuff compositionsPhospholipinAlcohol ethyl
The invention discloses a preparation method of high-purity phosphatidylcholine. The preparation method comprises the following steps: preparing an alcohol-soluble raw phospholipid ethanol solution by taking low-alcohol alcohol-soluble raw phospholipid as a raw material; loading the alcohol-soluble raw phospholipid ethanol solution into a dextrangel SephadexLH-20 chromatographic column; after the alcohol-soluble raw phospholipid ethanol solution is eluted by using high-concentration ethanol; collecting an eluent; removing solvents in the eluent by evaporating, so that high-purity phosphatidylcholine is obtained. Dextrangel SephadexLH-2 used in the invention has less possibility of forming dead adsorption and is easily regenerated and used indiscriminately, and eluted solvents are single and non-toxic; solvents adopted in the preparation method are less in type, and high in indiscriminate use degree in production; the preparation method disclosed by the invention is simple in whole process and short in production cycle, the purity of obtained products is over 85%, and the industrialized production is easily realized.
Owner:WENGYUAN GUANGYE QINGYI FOOD TECH +1
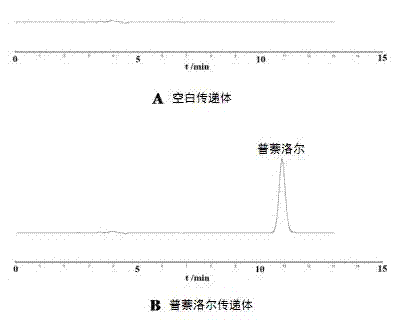
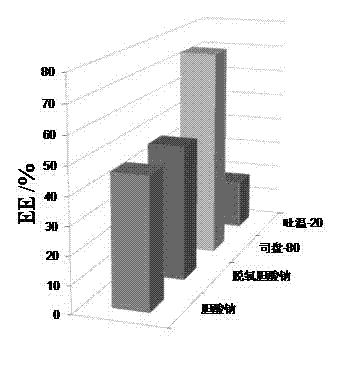
Percutaneous-absorption-promoting propranolol composite phospholipid transfersome, and prepartion method and application thereof
InactiveCN102846546APromote percutaneous absorptionImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsInfantile haemangiomaBlood plasma
The invention provides a composite phospholipid transfersome which promotes propranolol percutaneous absorption, and a preparation method thereof. According to the invention, two phospholipid materials with different phase-change temperatures, which are dipalmitoyl phosphatidyl choline and soybean lecithin, are adopted as a composite phospholipid material. Compared with a transfersome with a single phospholipid material in prior art, the propranolol composite phospholipid transfersome prepared with the phospholipid material provided by the invention has substantially improved encapsulation efficiency, reduced leakage, improved stability in rat plasma, and substantially improved bioavailability after percutaneous administration. The propranolol composite phospholipid transfersome provided by the invention is especially suitable to be used for treating infantile hemangioma. With the transfersome, propranolol percutaneous administration can be realized, and propranolol can directly act upon a hemangioma affected part. The treatment effect is improved, toxic and side effects are reduced, and children medication compliance can be improved. Also, the invention provides a preparation method of the propranolol composite phospholipid transfersome.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for preparing Polyene Phosphatidylcholine injection liquid
InactiveCN1961886AOrganic active ingredientsDigestive systemPolyene phosphatidylcholinePolymer science
The invention relates to a process for preparing polyene phosphatidyl choline injection, which consists of mixing polyene phosphatidyl choline, solubilizing agent and water for injection proportionally, charging right amount of preservative agent and anti-oxidizing agent, and mixing to colloid form dispersible system.
Owner:ZHEJIANG HAILISHENG PHARM CO LTD
Potentiated topical composition
A topical composition for skin care or administration of a pharmacologically active agent in form of a lotion, cream or similar comprises from 5% to 70% by weight of pentane-1,5-diol and a cosmetically or pharmaceutically acceptable carrier, with the proviso that the composition does not comprise polysiloxane, volatile siloxane, phosphatidyl-choline, creatine, carnitine, panthenol, pyruvic acid, monoglyceride of lauric acid, monoglyceride of myristic acid. Also disclosed are corresponding methods of administration, a patch for holding said composition against the skin, and methods of preventing or treating a dry skin condition and of keeping skin in a humid state.
Owner:AMBRIA DERMATOLOGY
Mesotherapy Cream
InactiveUS20080058287A1Promotes breakdown and leachingTo promote metabolismBiocidePhosphorous compound active ingredientsCholic acidBULK ACTIVE INGREDIENT
The present disclosure concerns a cream-based composition and method which reduces fat deposits upon topical application to the skin. The active ingredients of the composition include the active ingredients deoxycholic acid and phosphatidyl choline which are mixed together with a cream composition. The active ingredients may be combined with any suitable pharmaceutical vehicle to provide the fat-reducing composition claimed herein. The composition is topically applied to a skin area over a period of days or months in order to reduce fat deposits that have collected beneath the dermis.
Owner:NATURAL DESIGNS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com