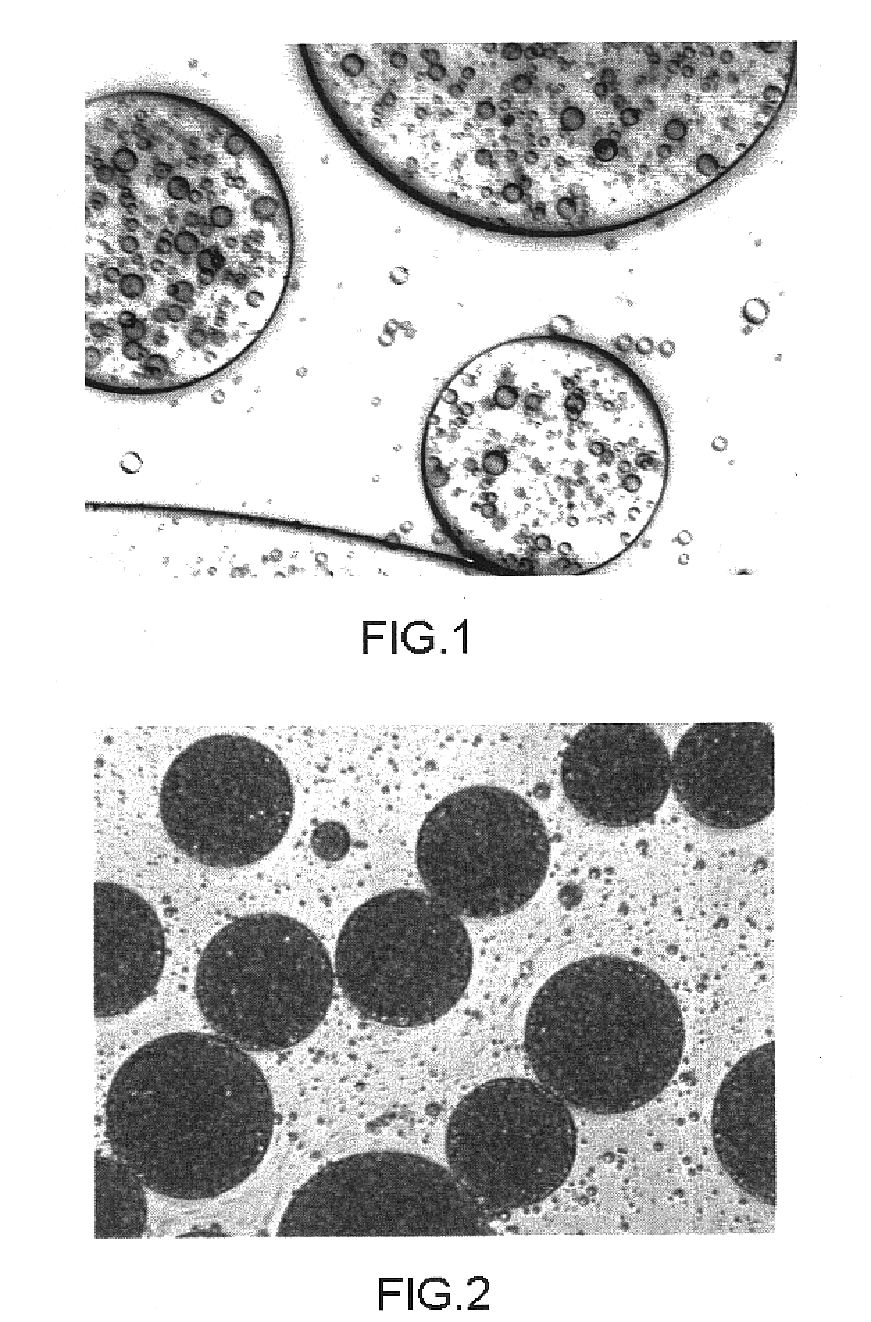
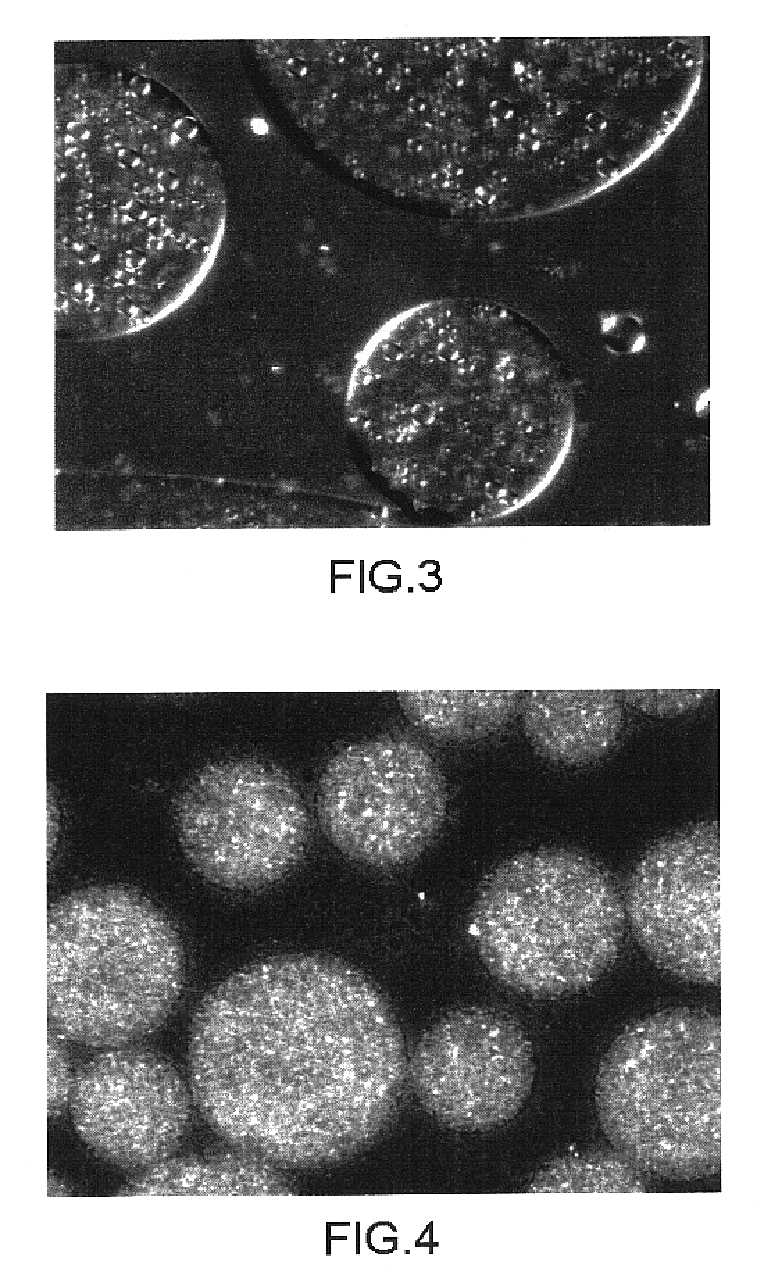
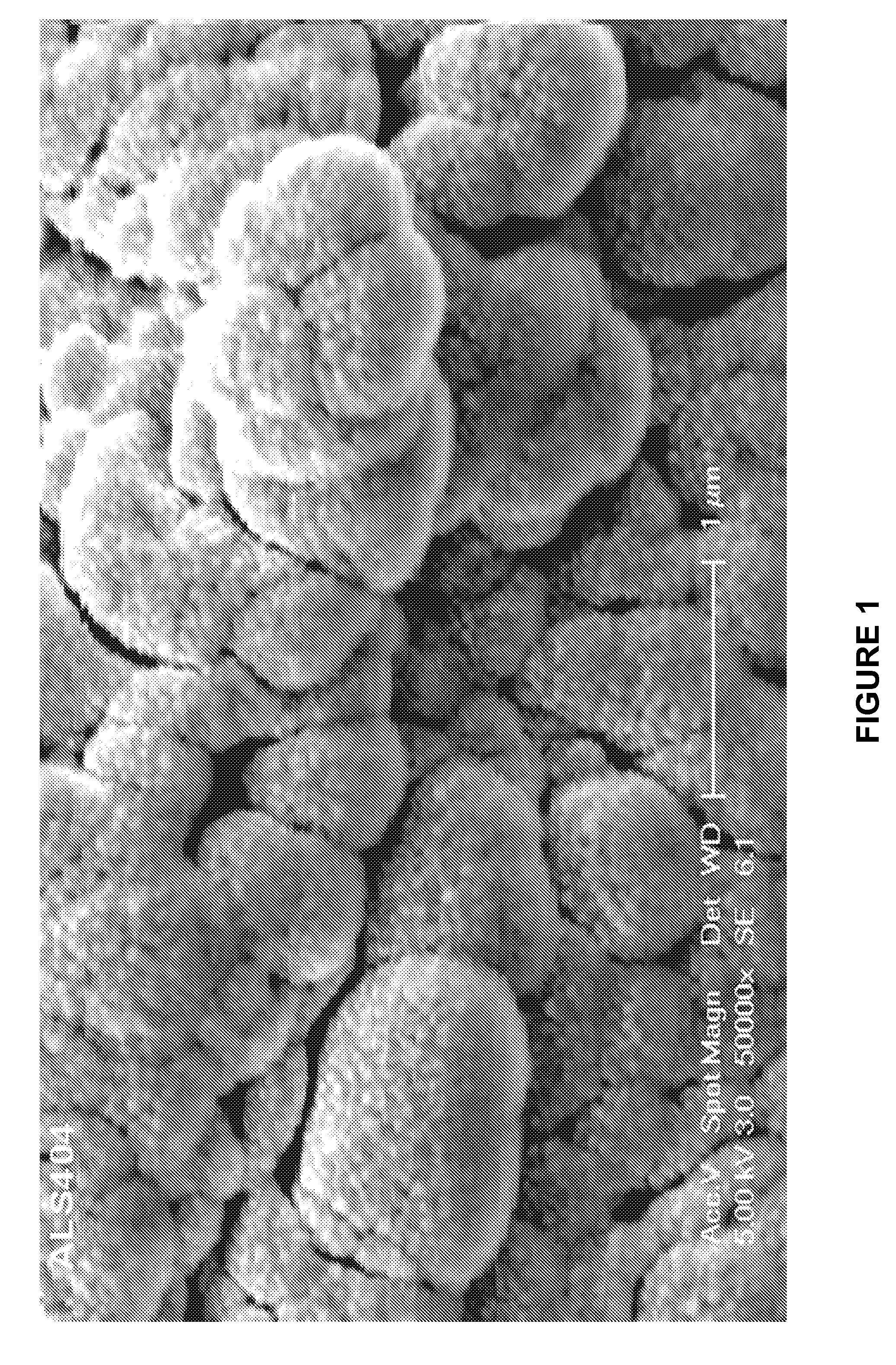
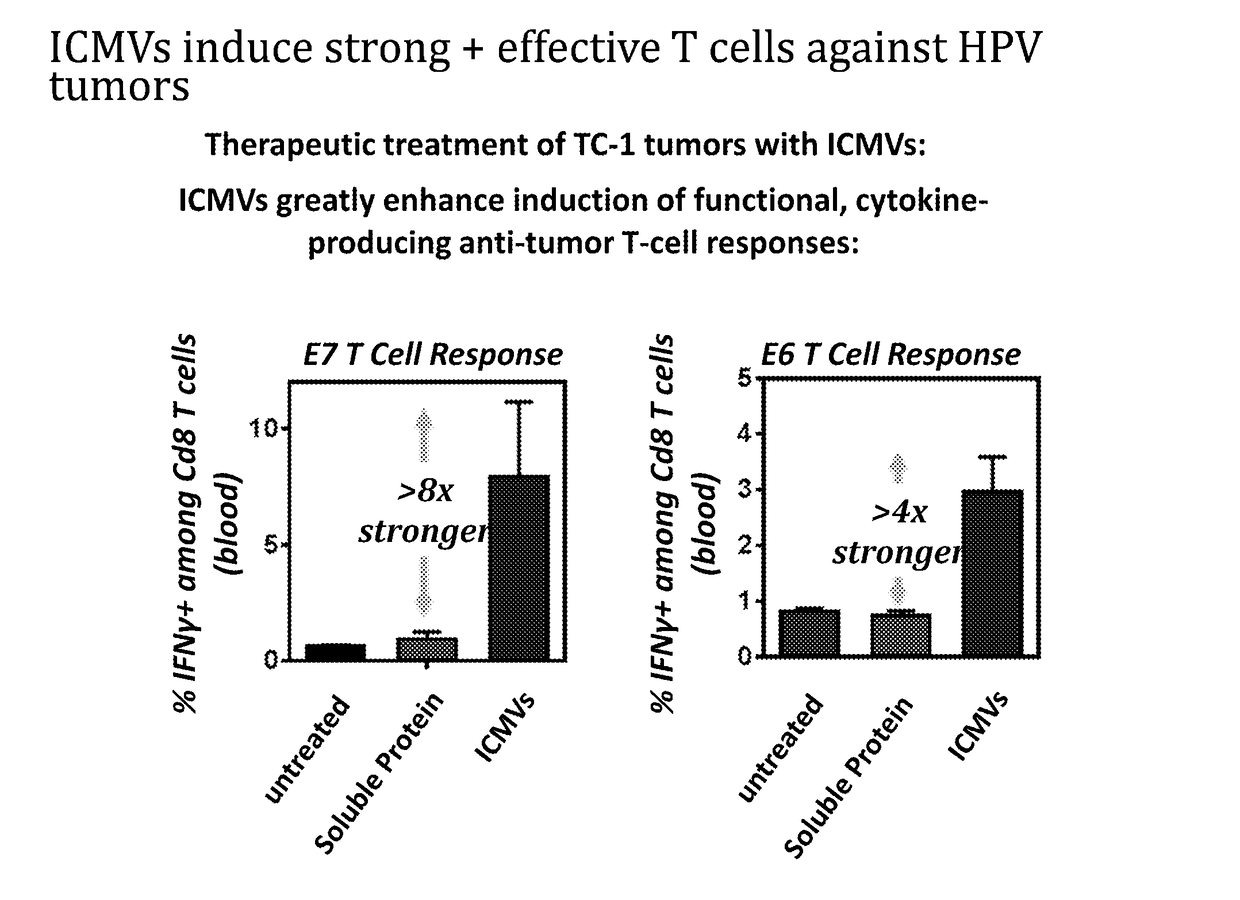
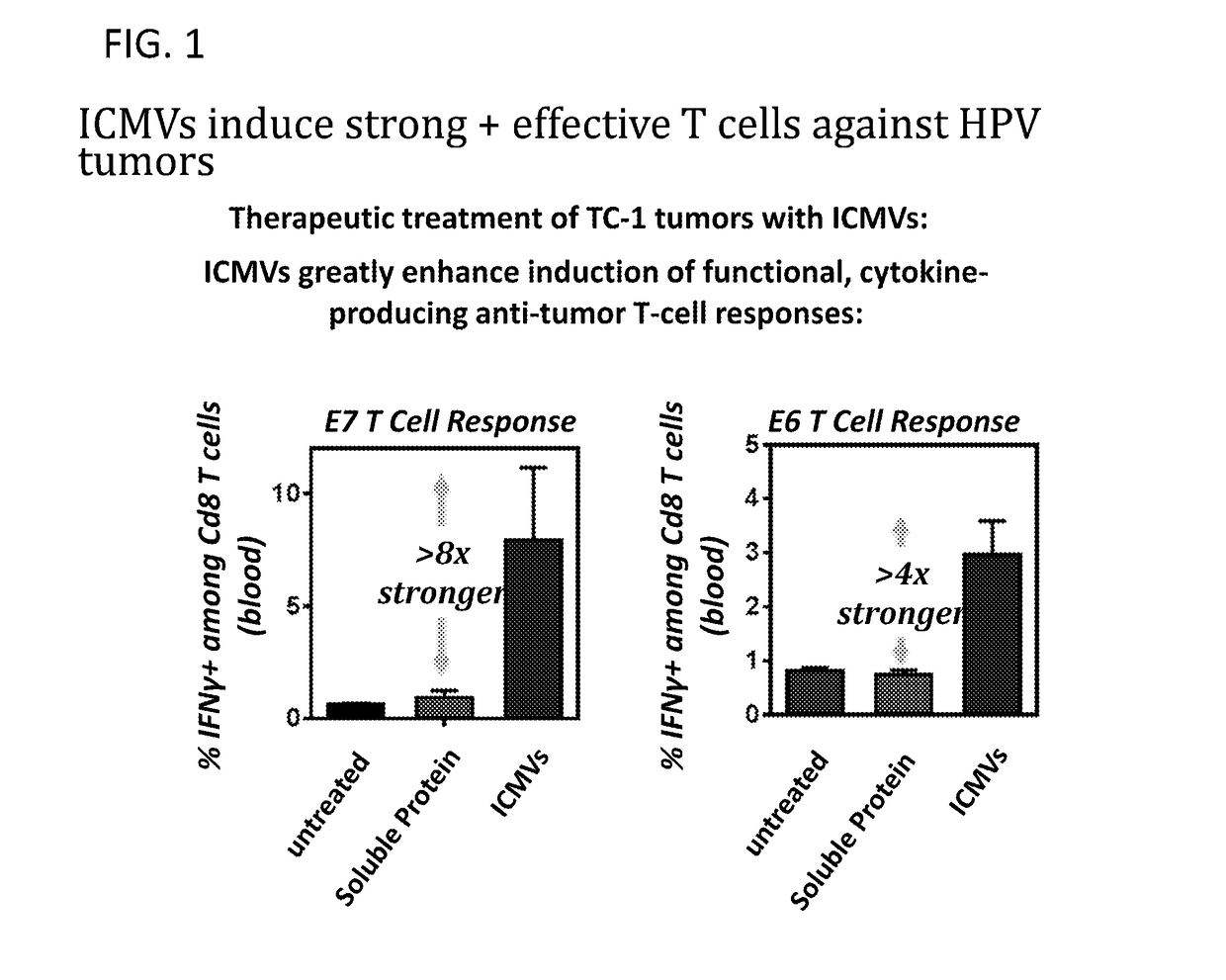
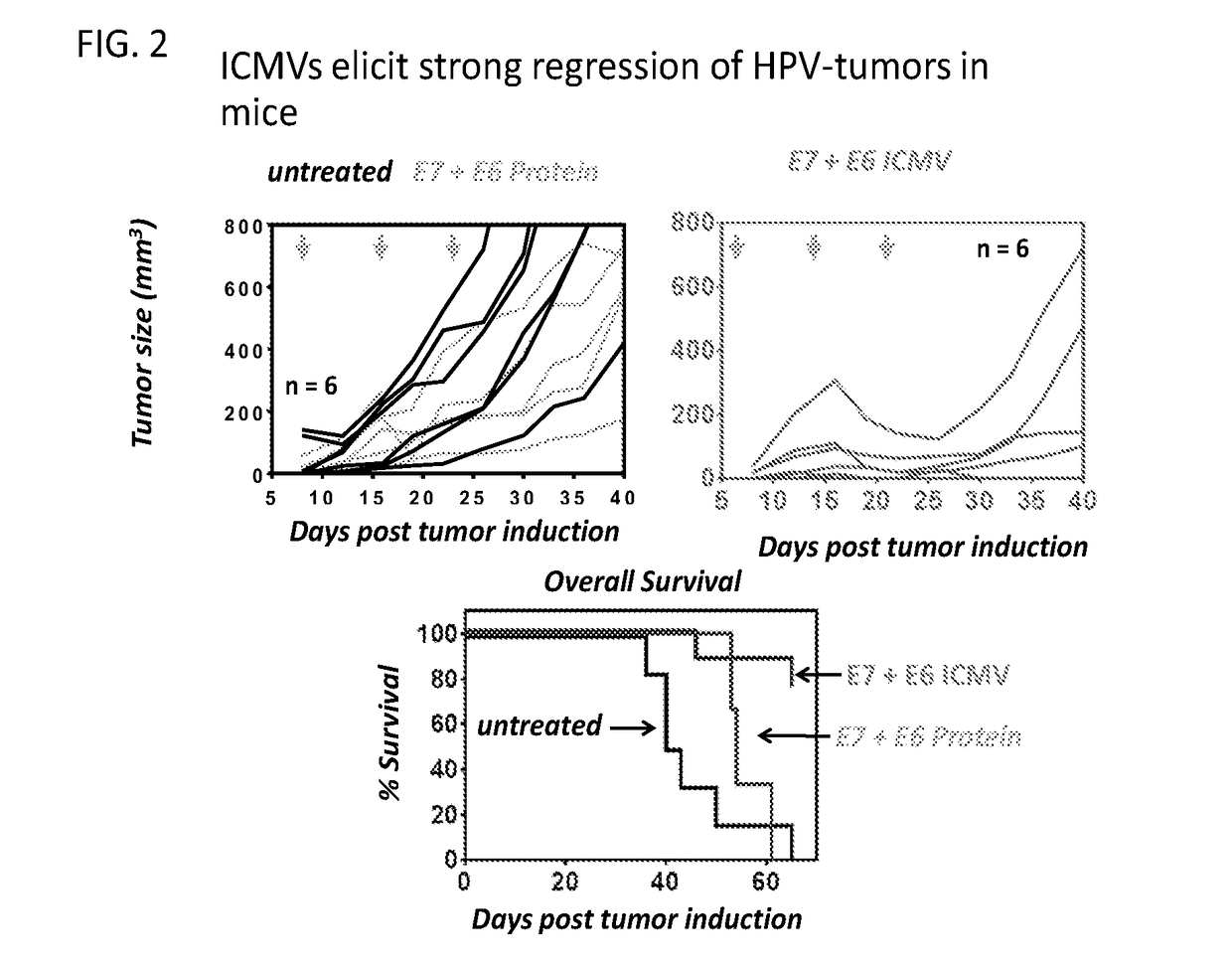
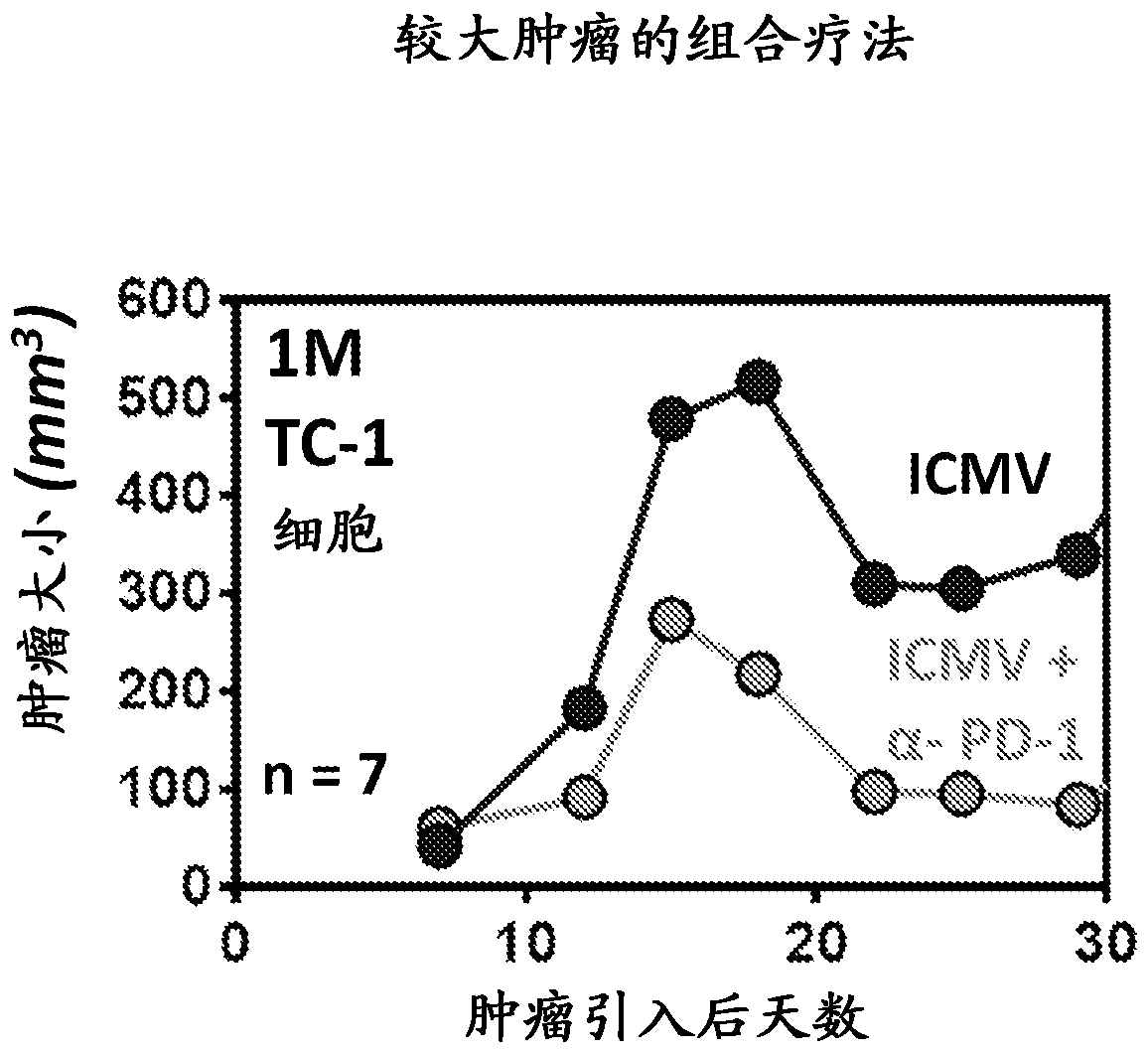
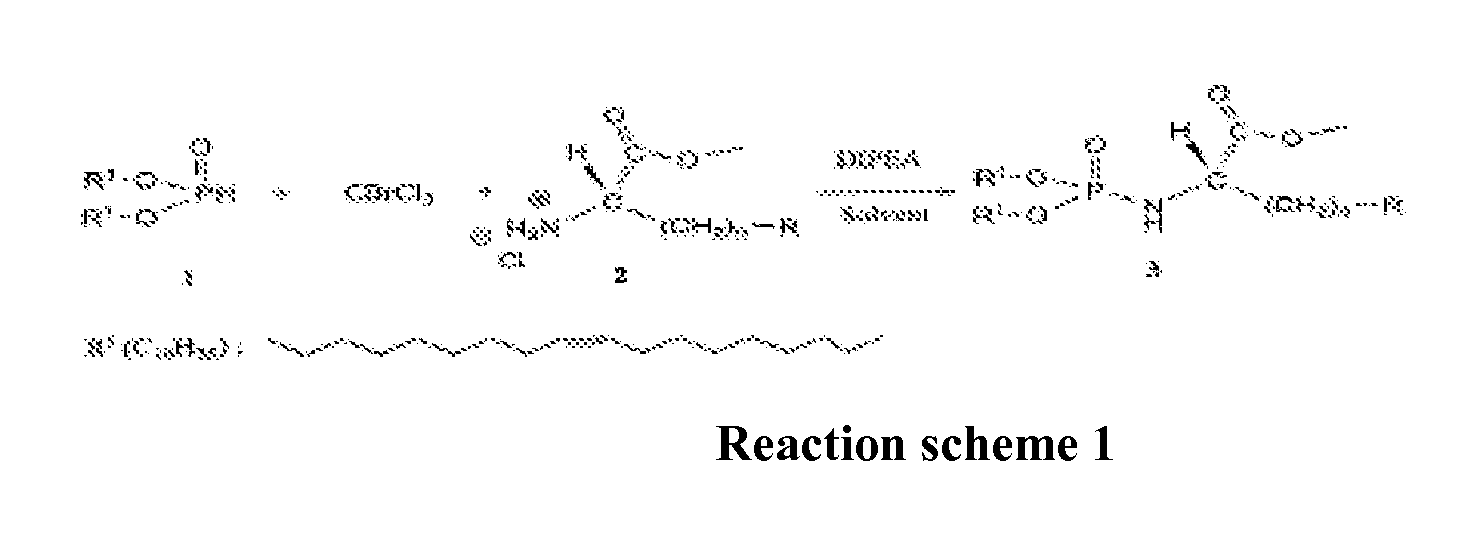
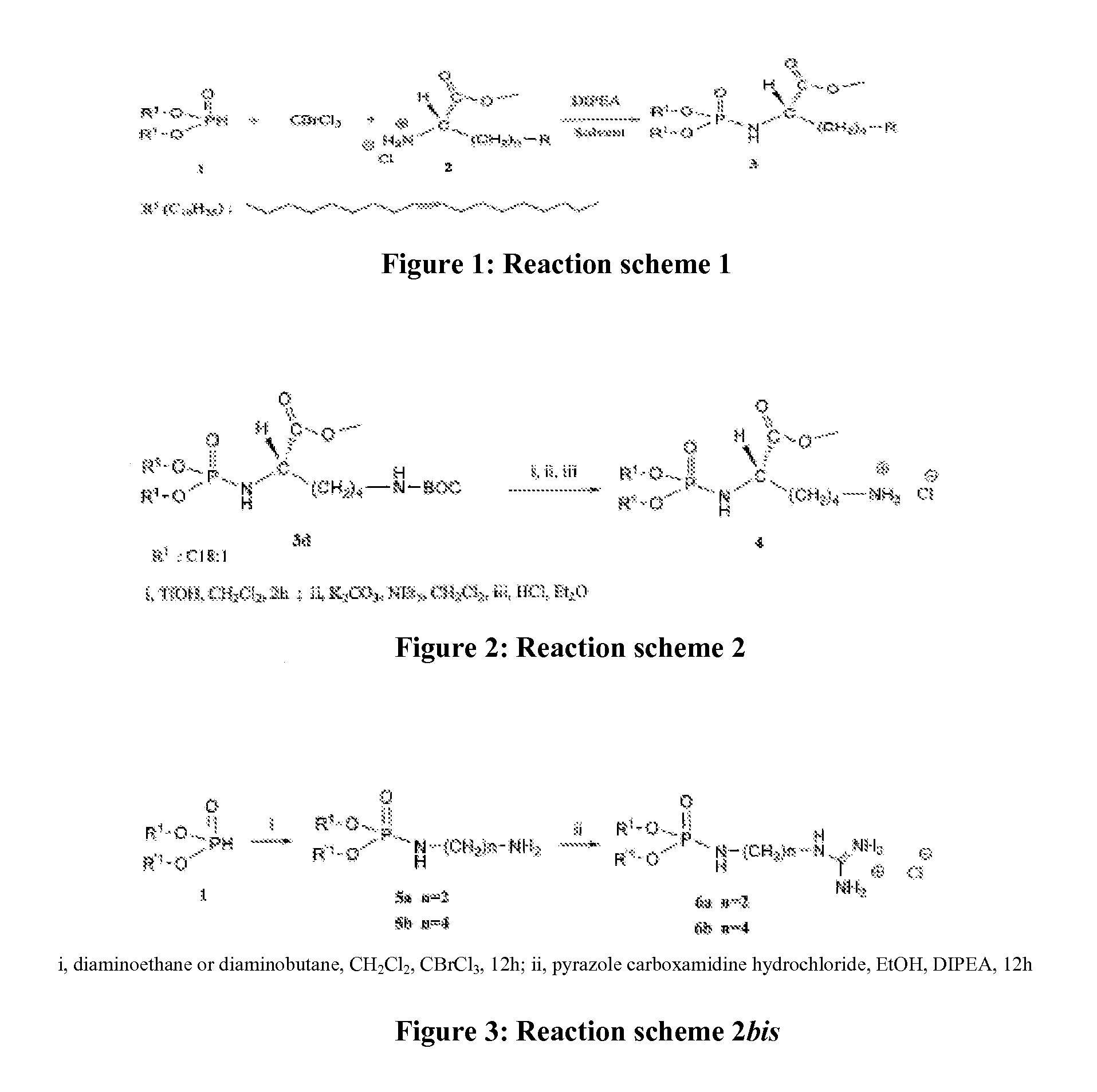
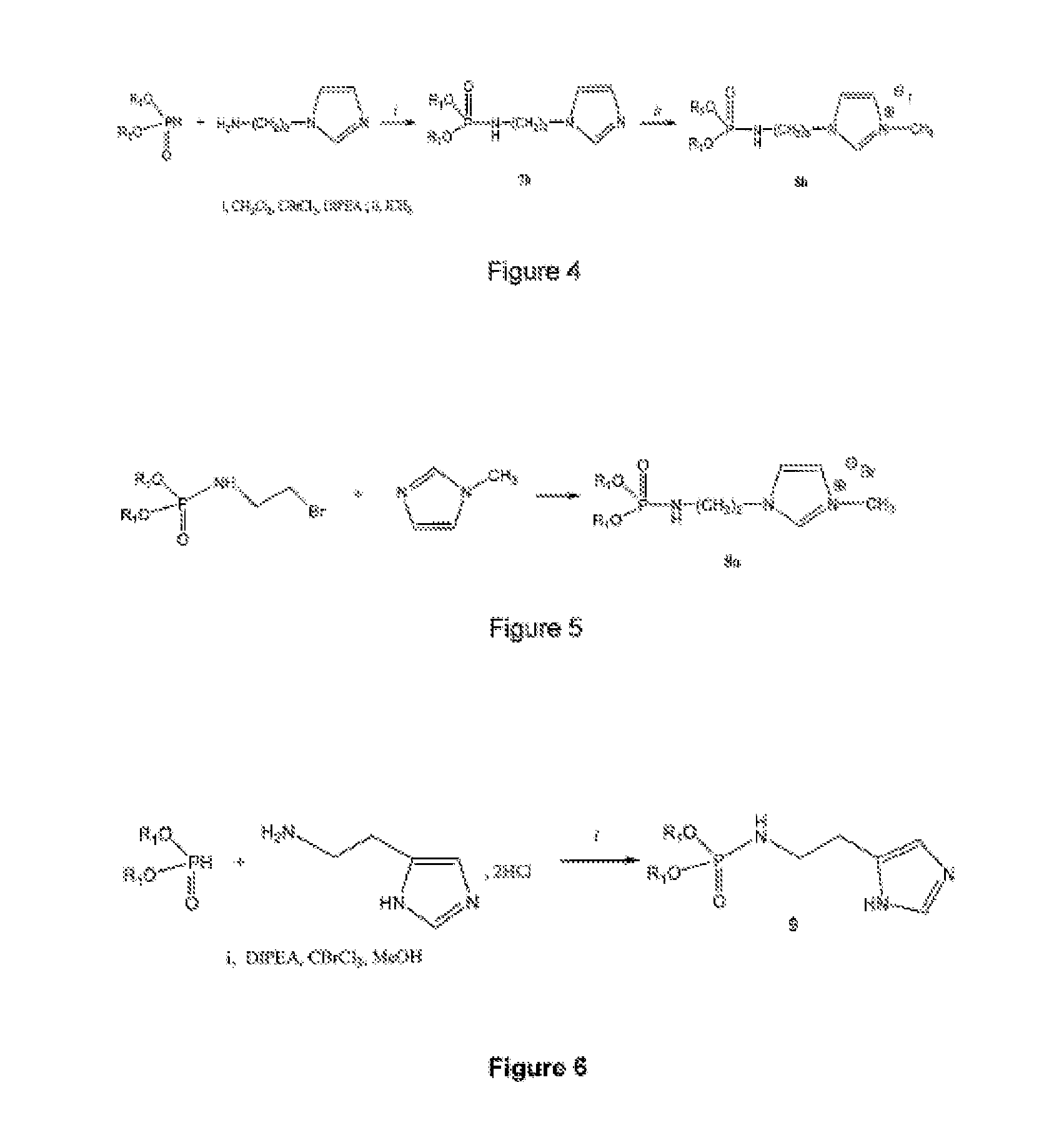
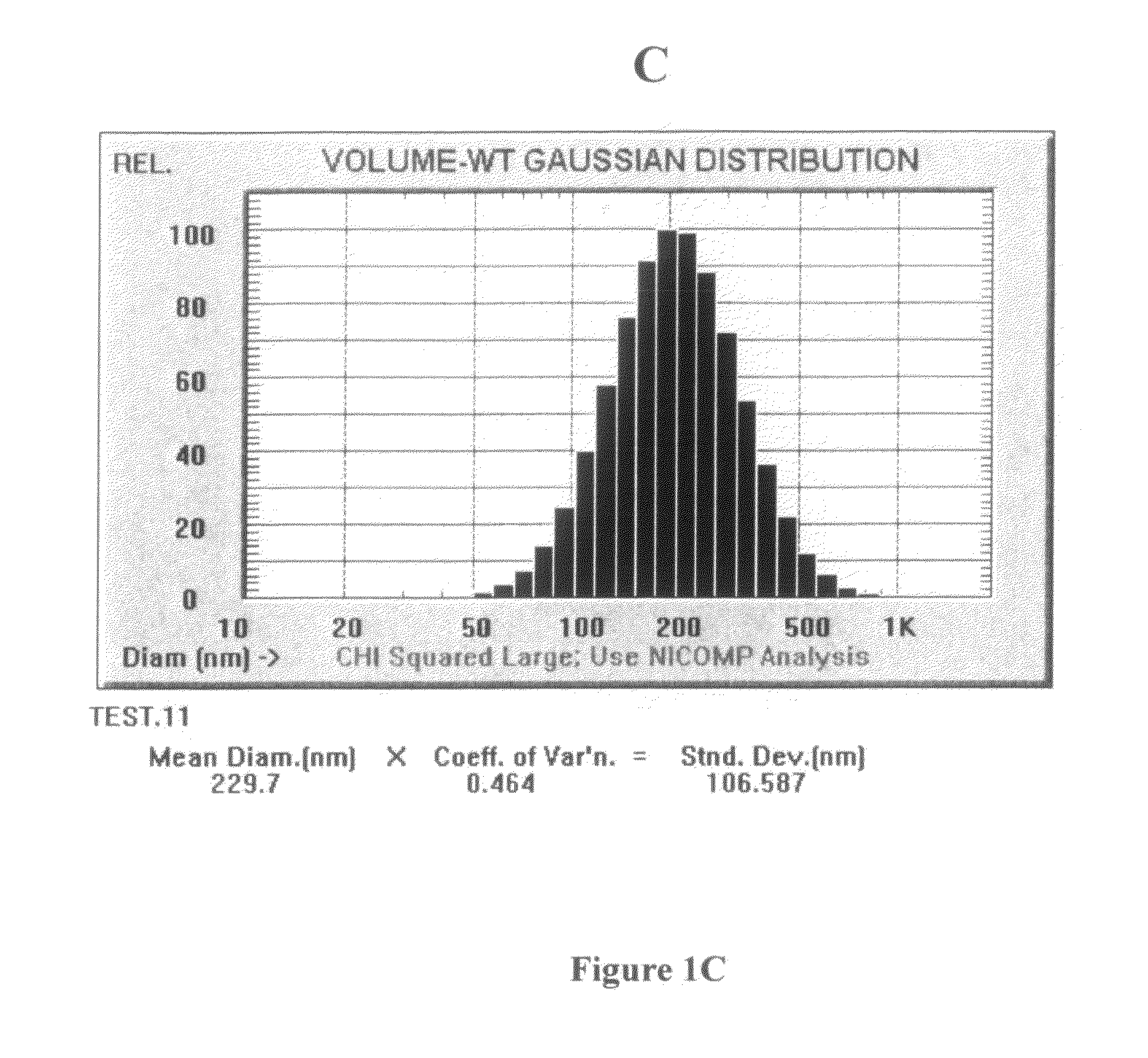
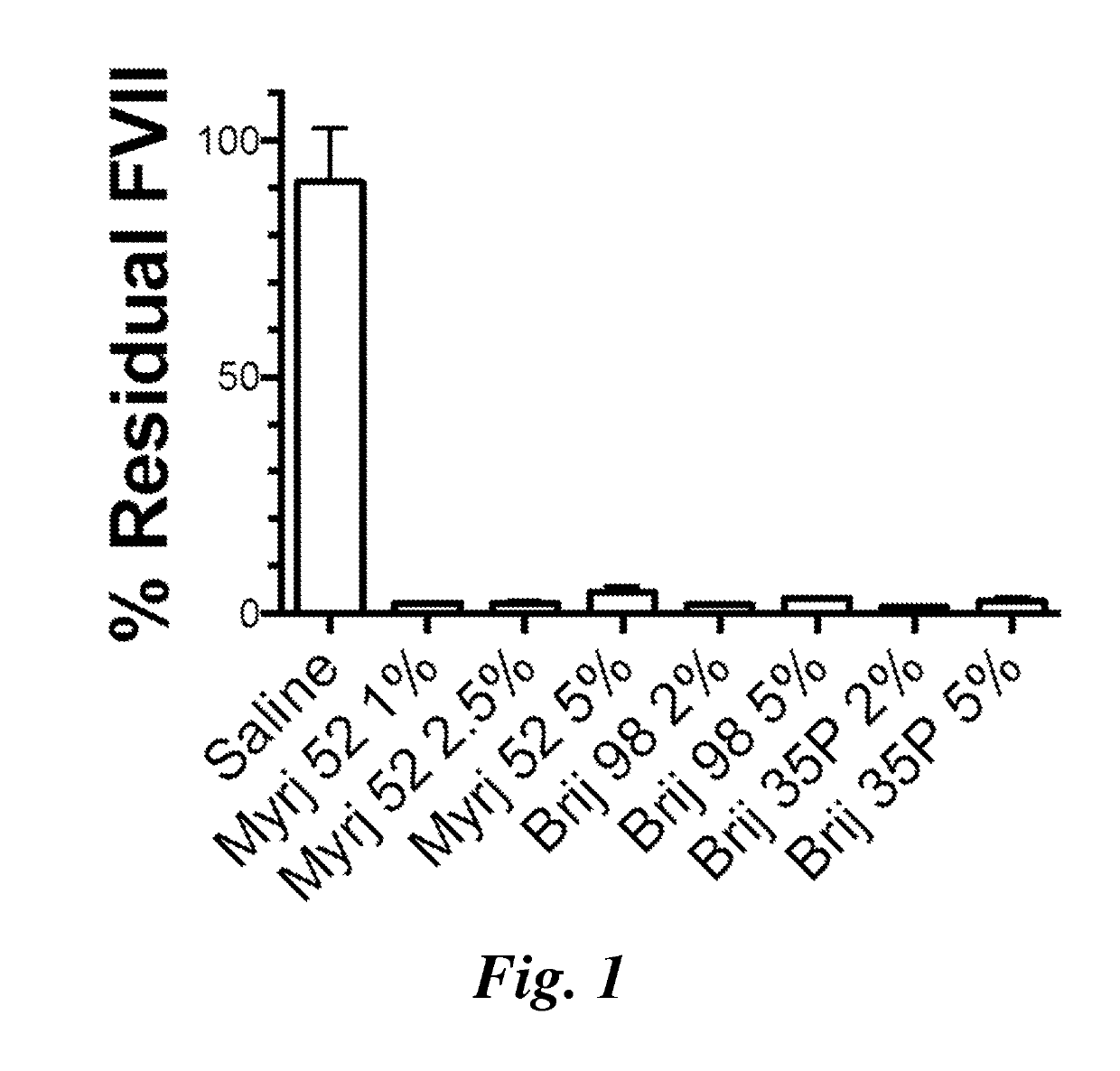
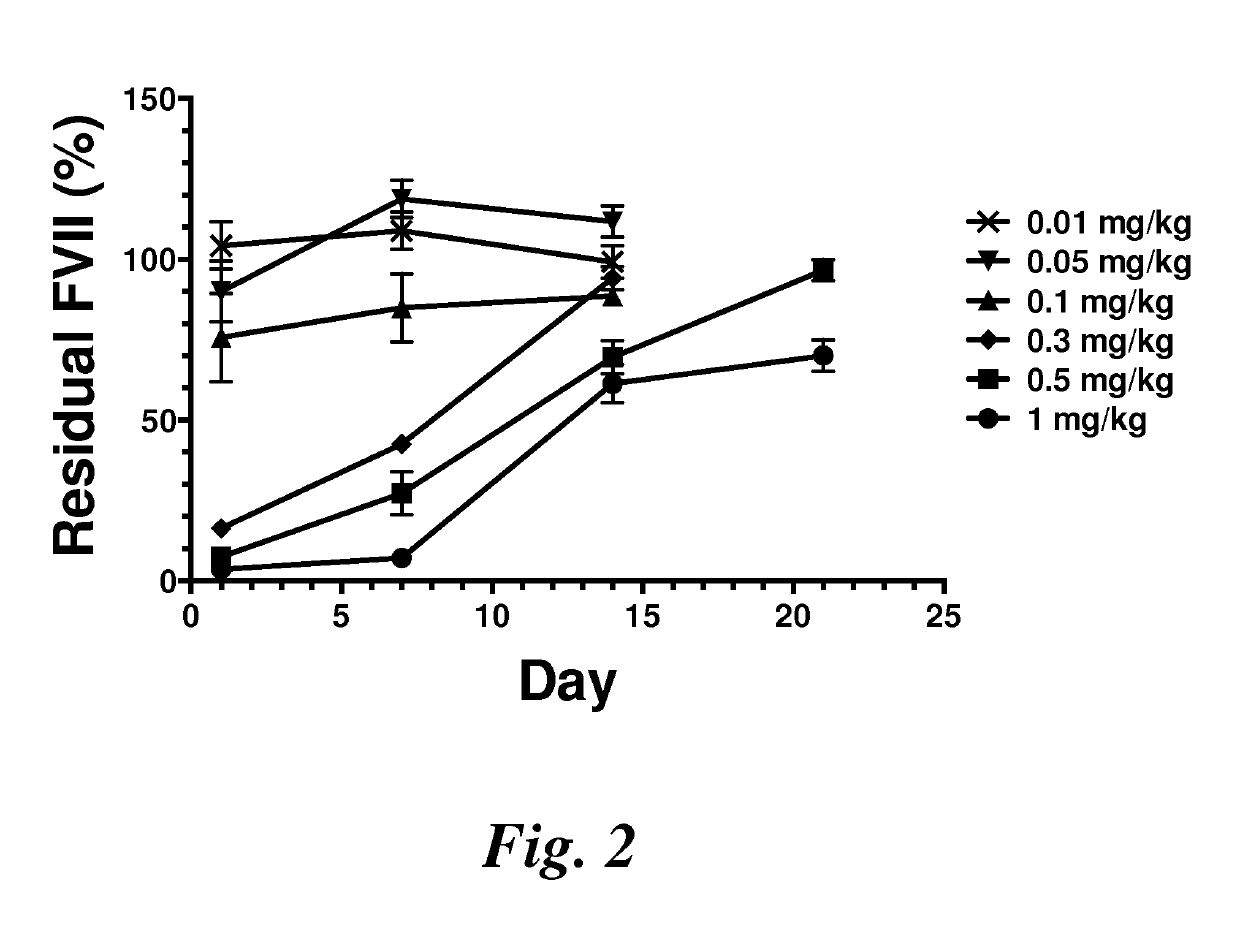
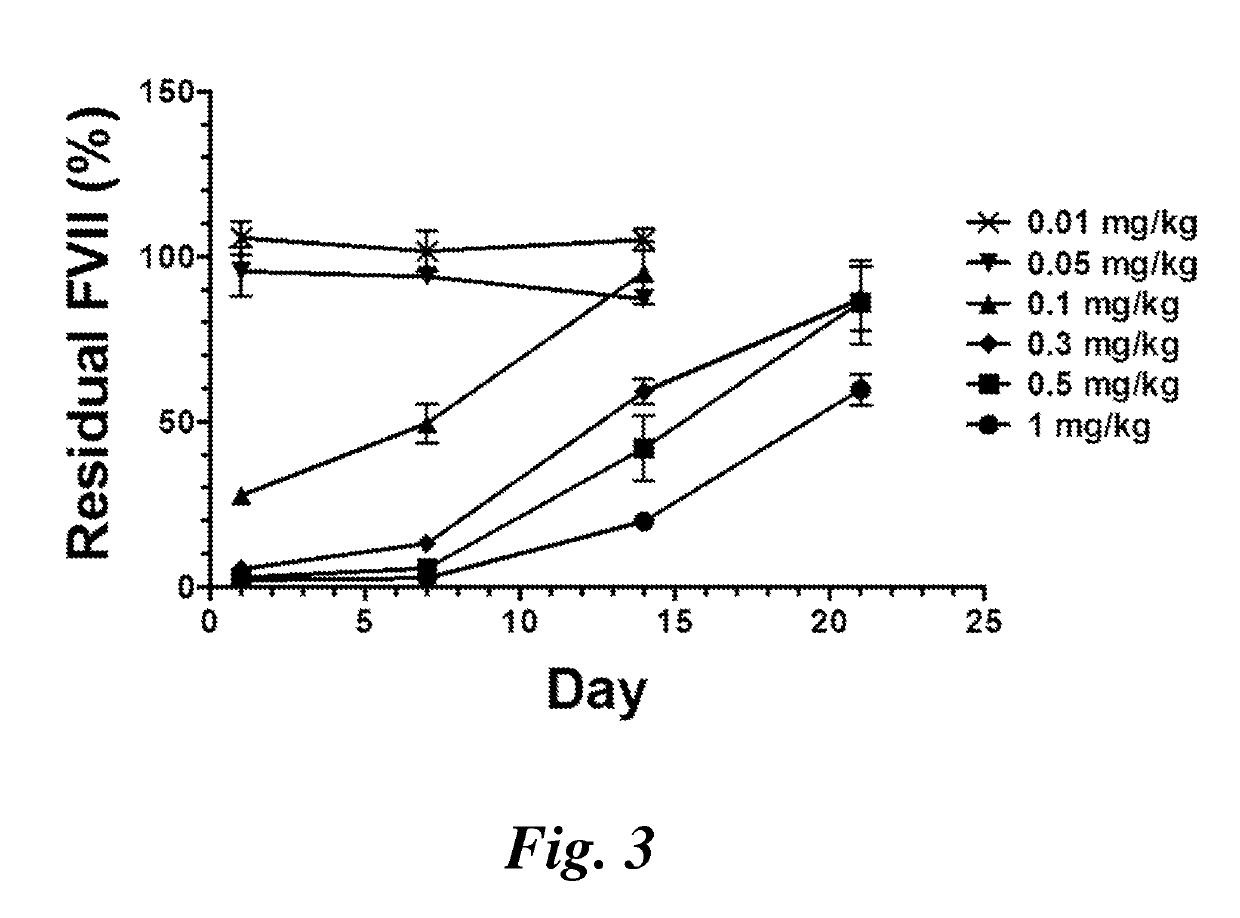
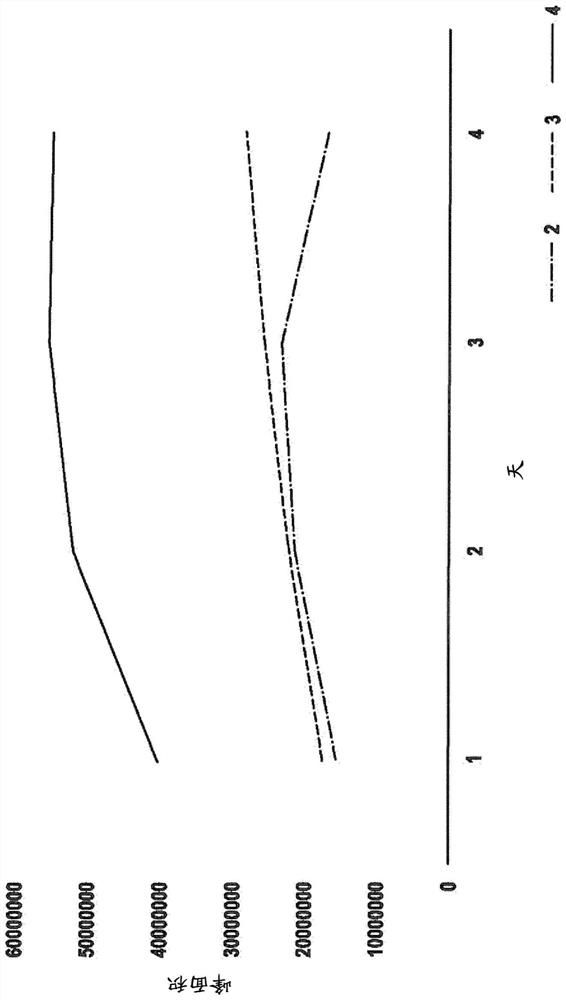
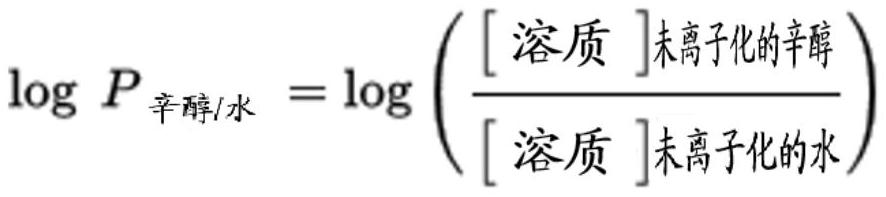
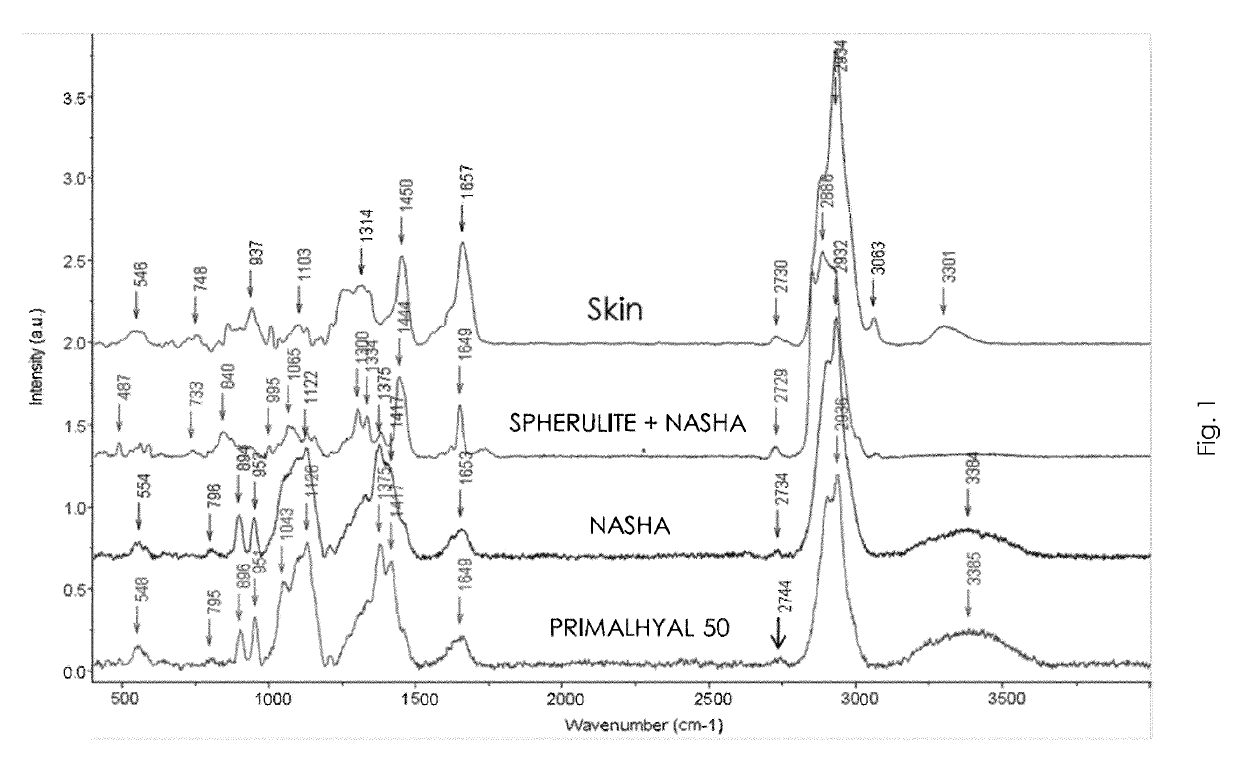
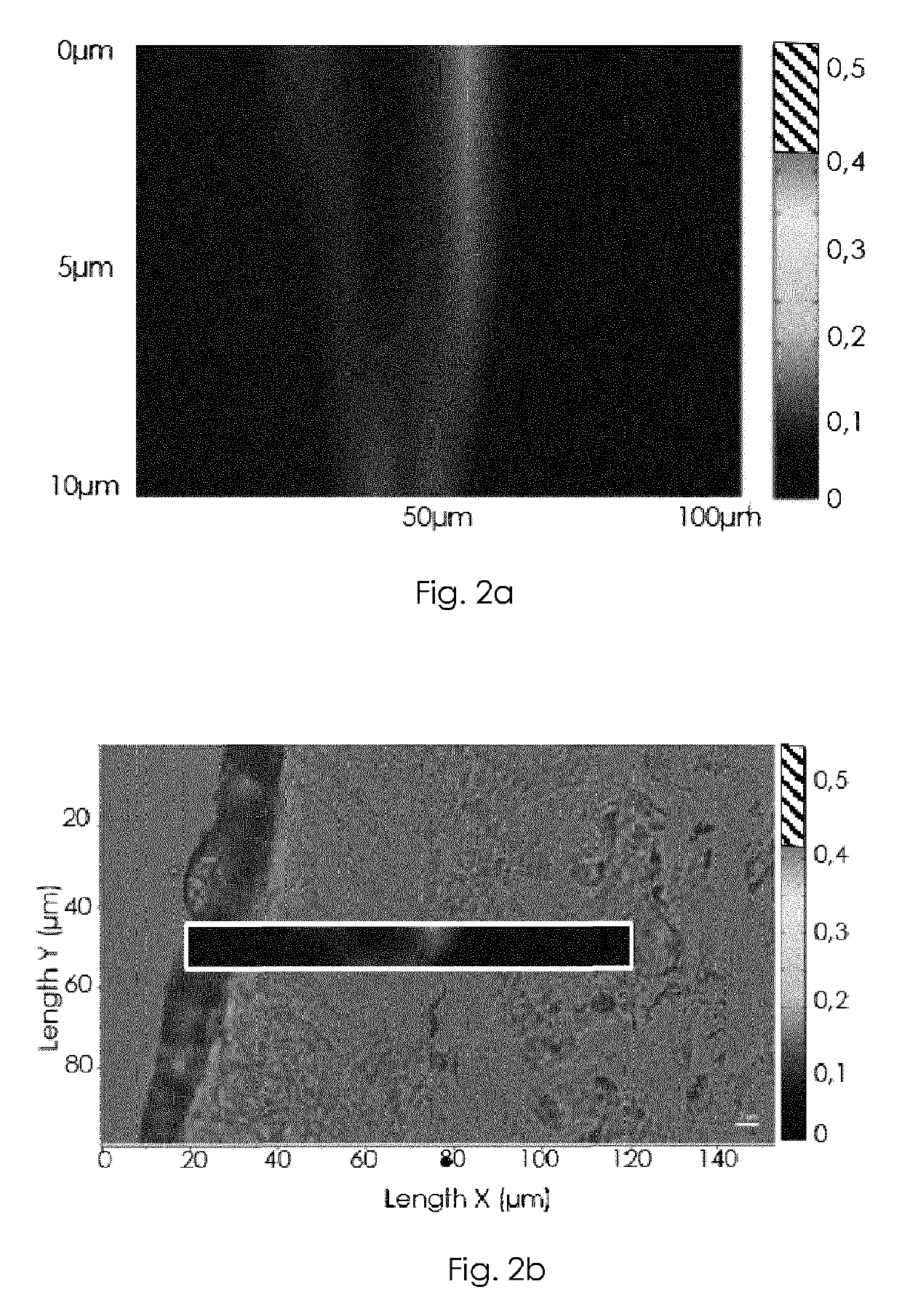
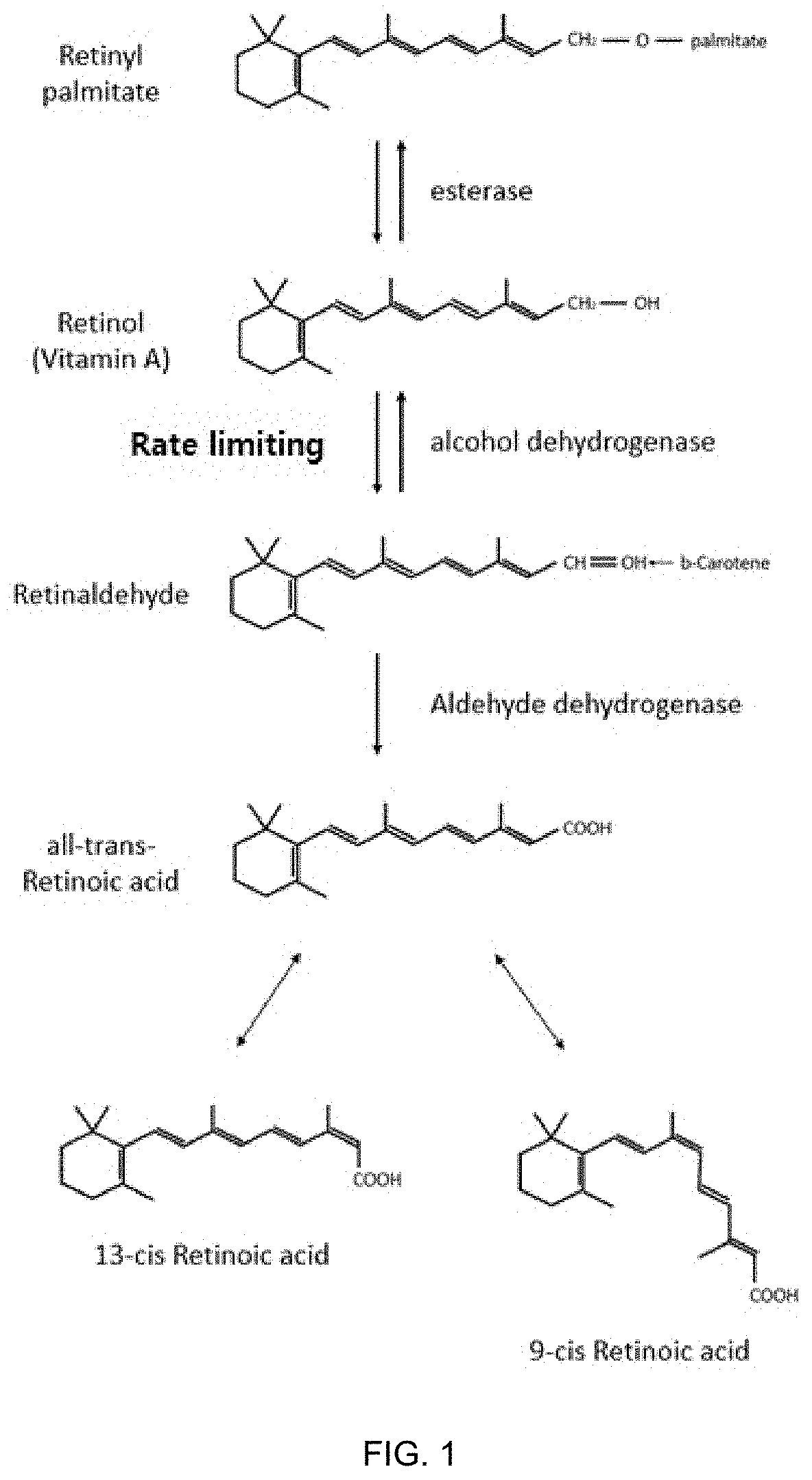
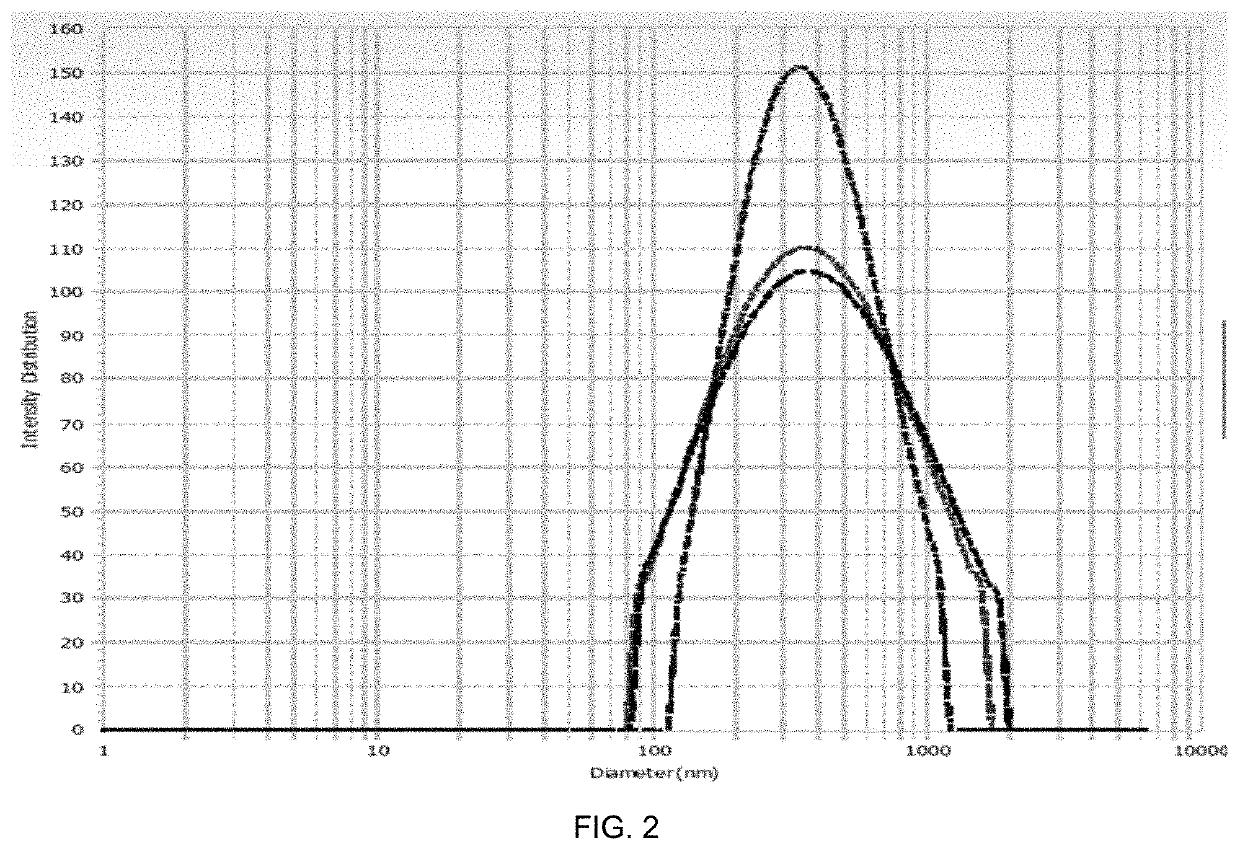
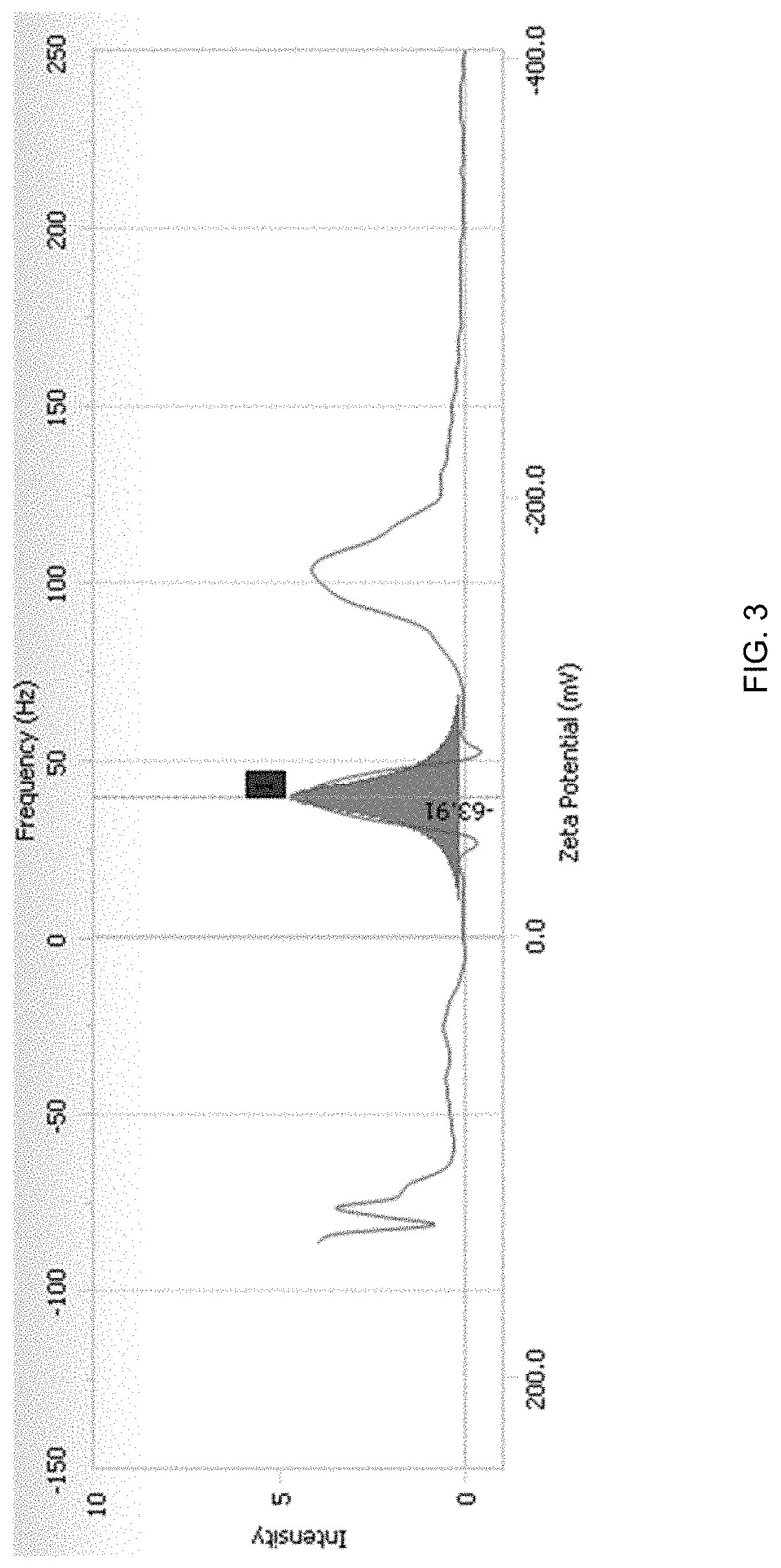
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Multilamellar vesicles" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
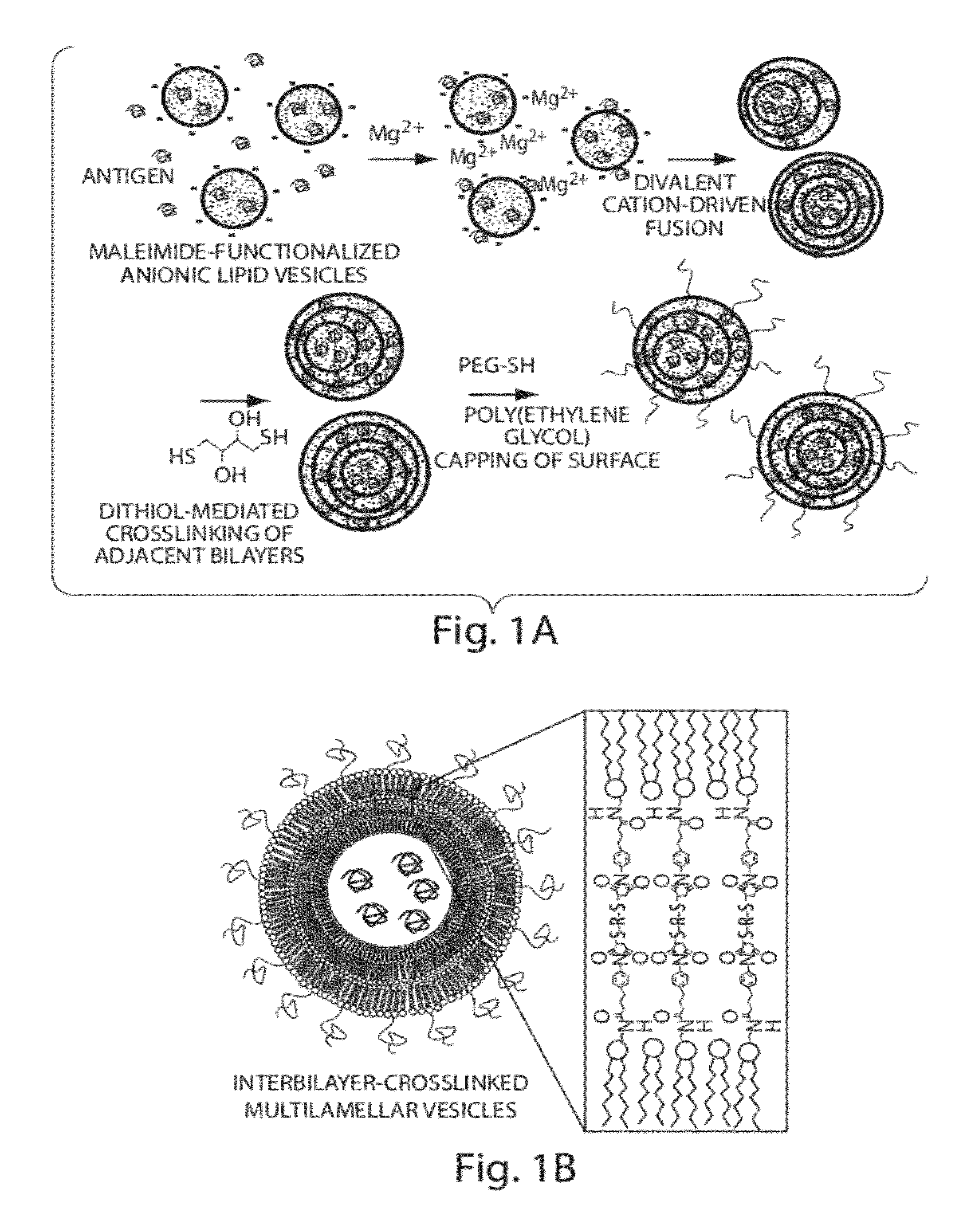
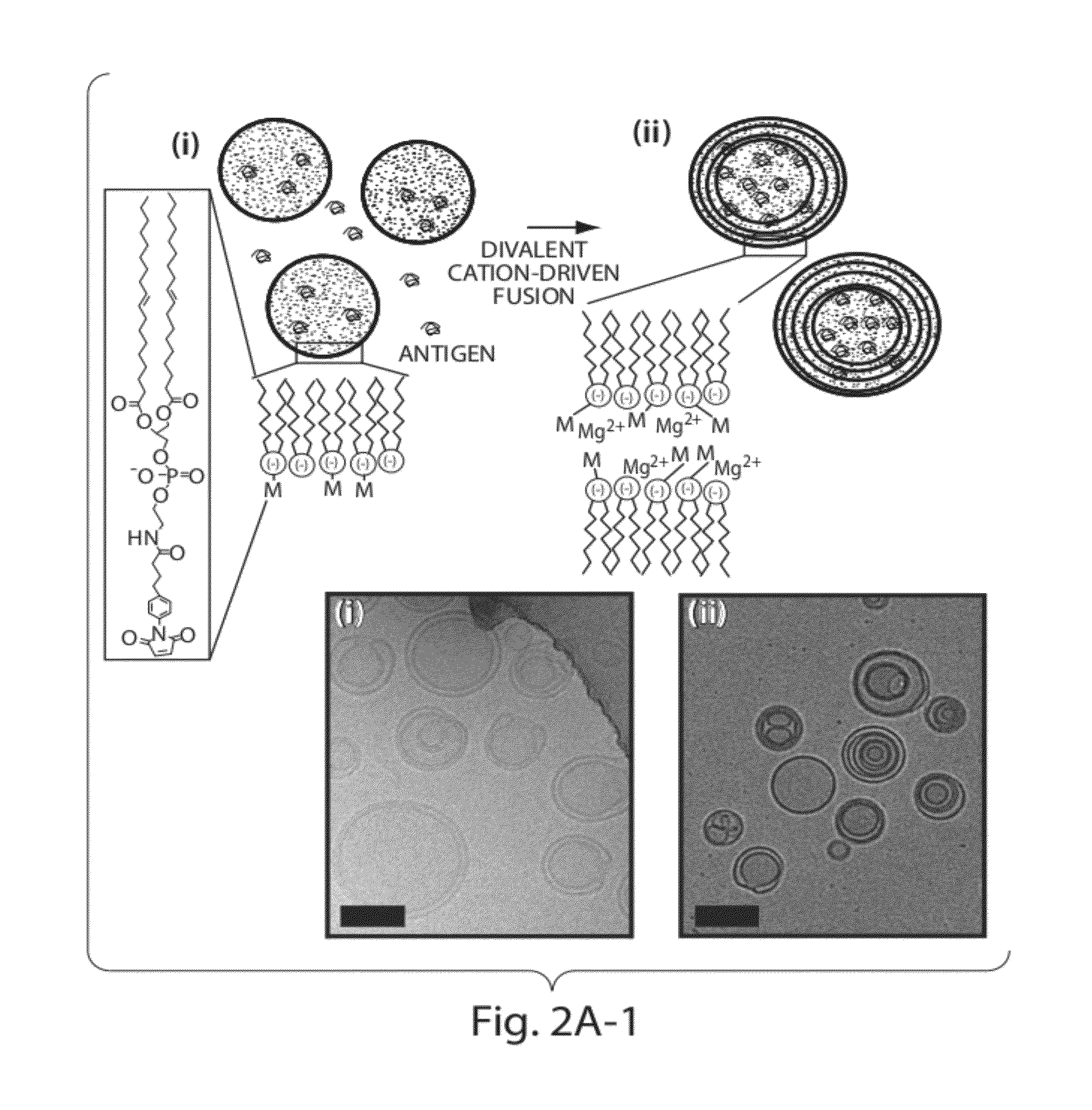
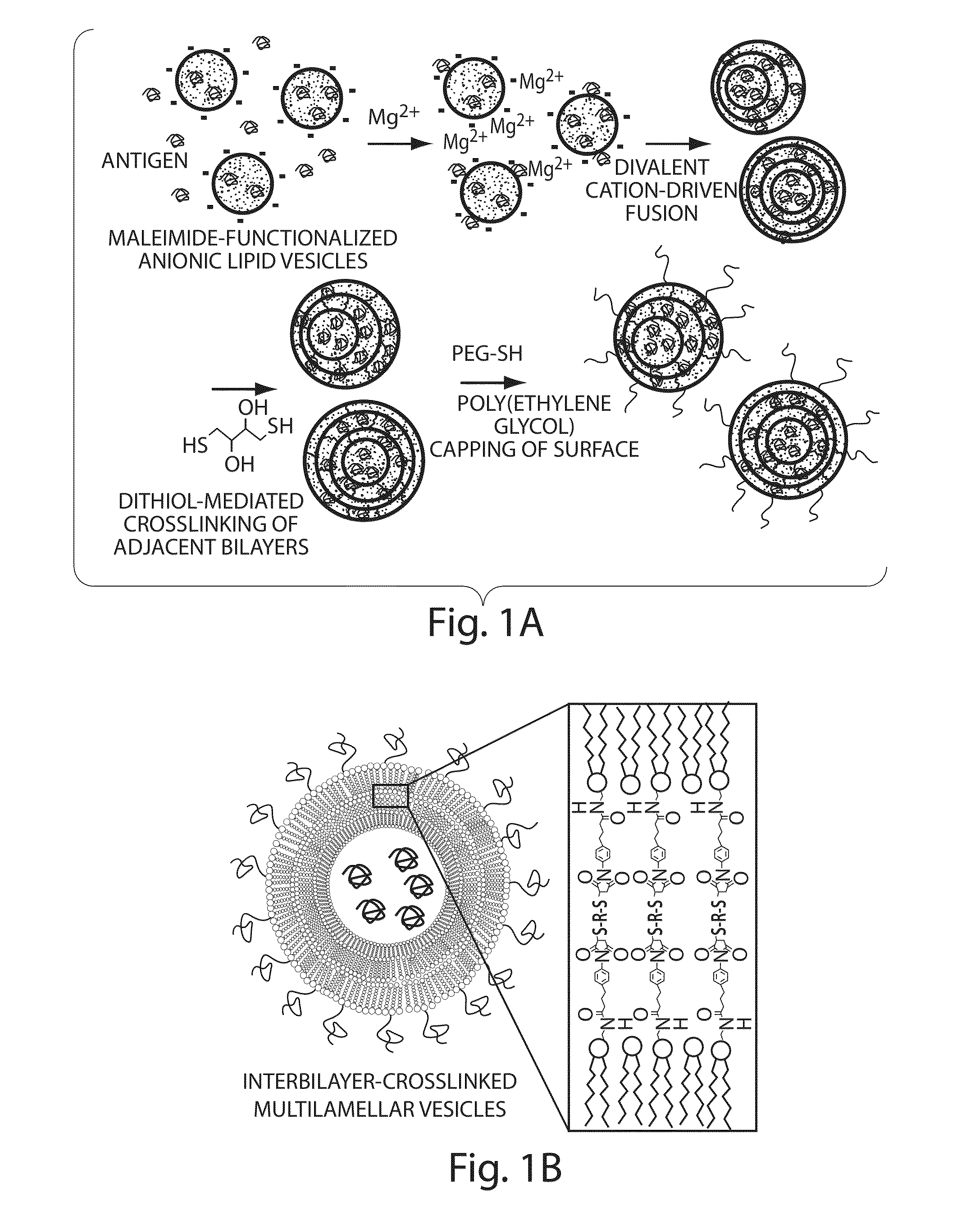
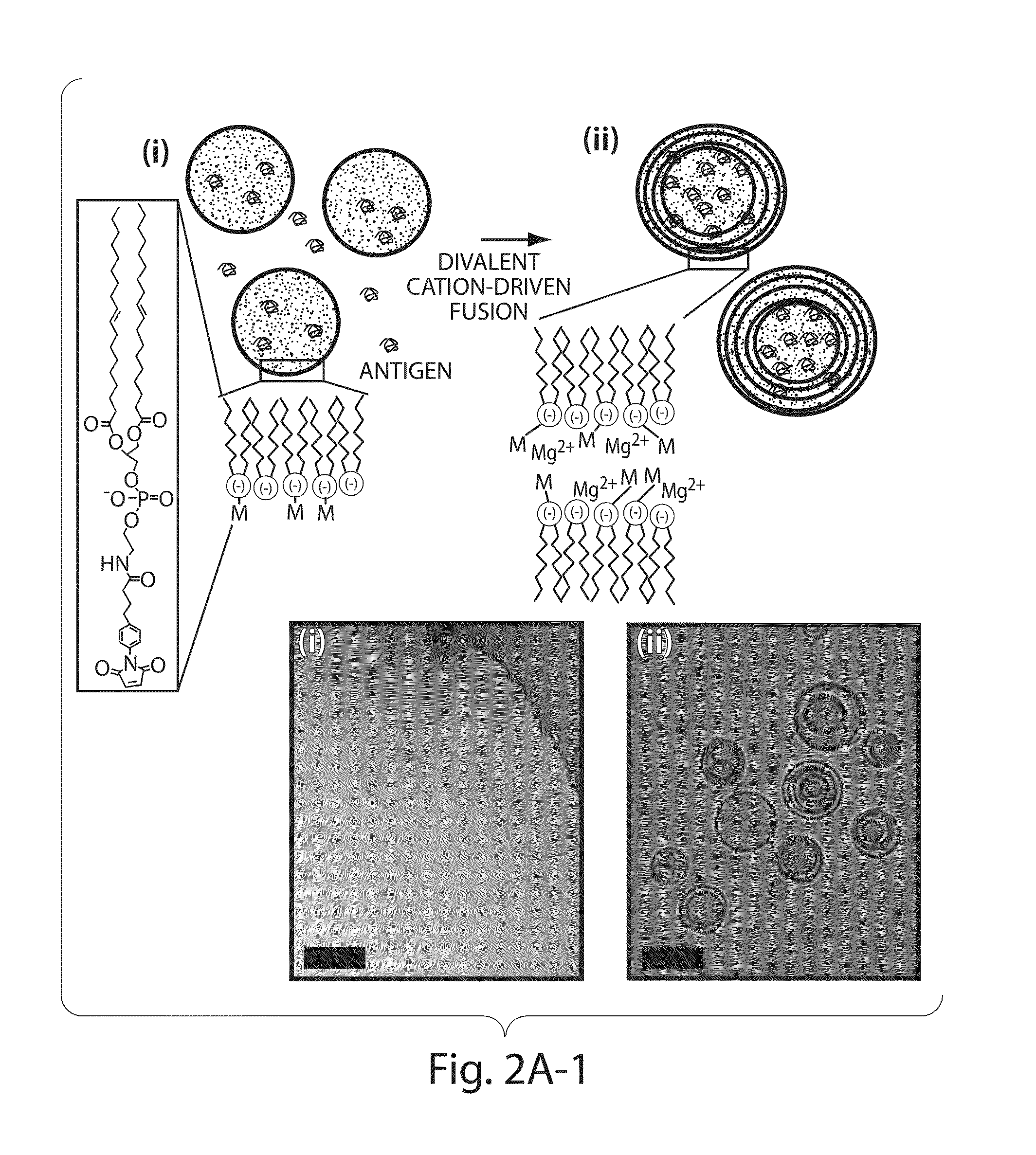
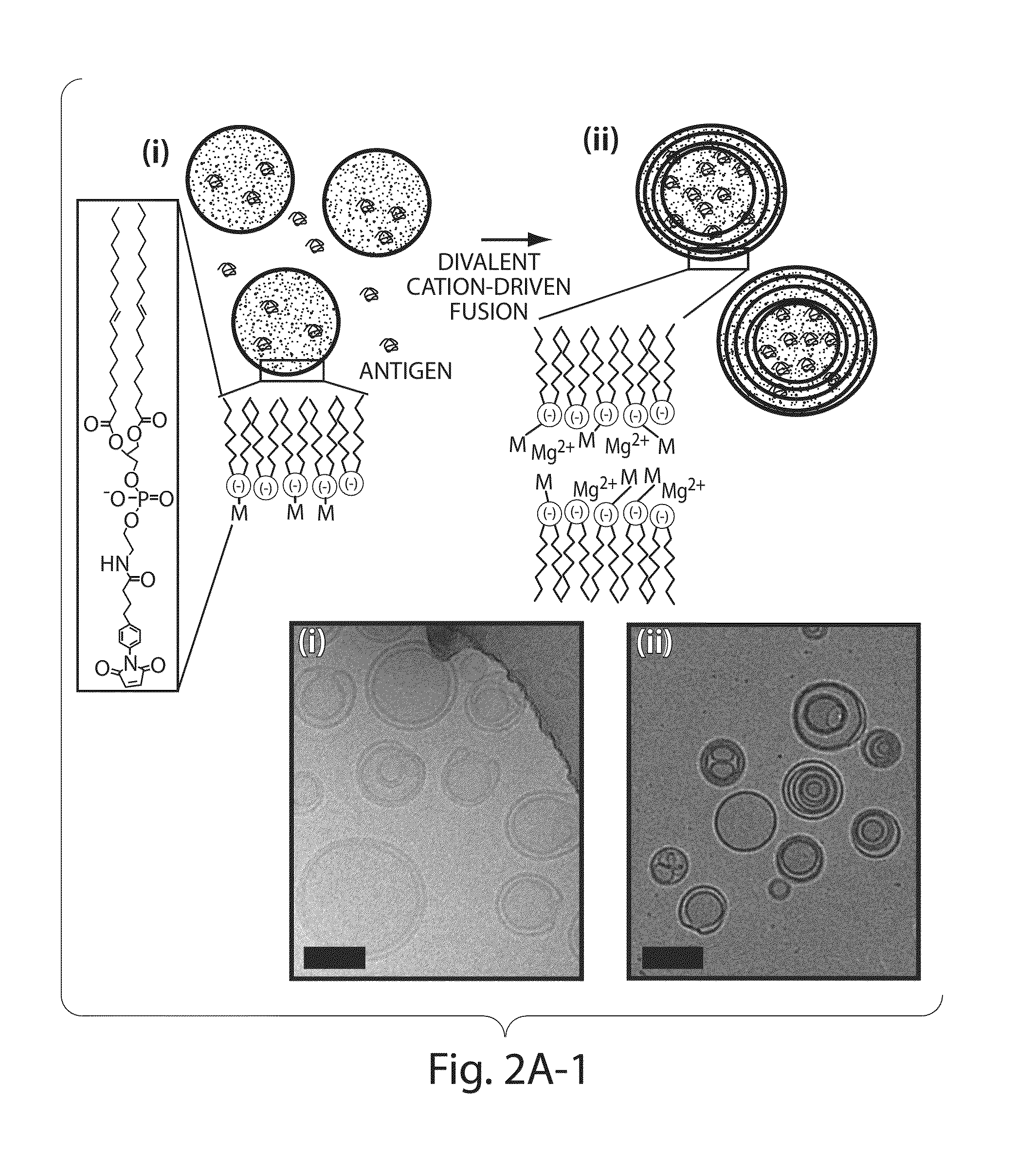
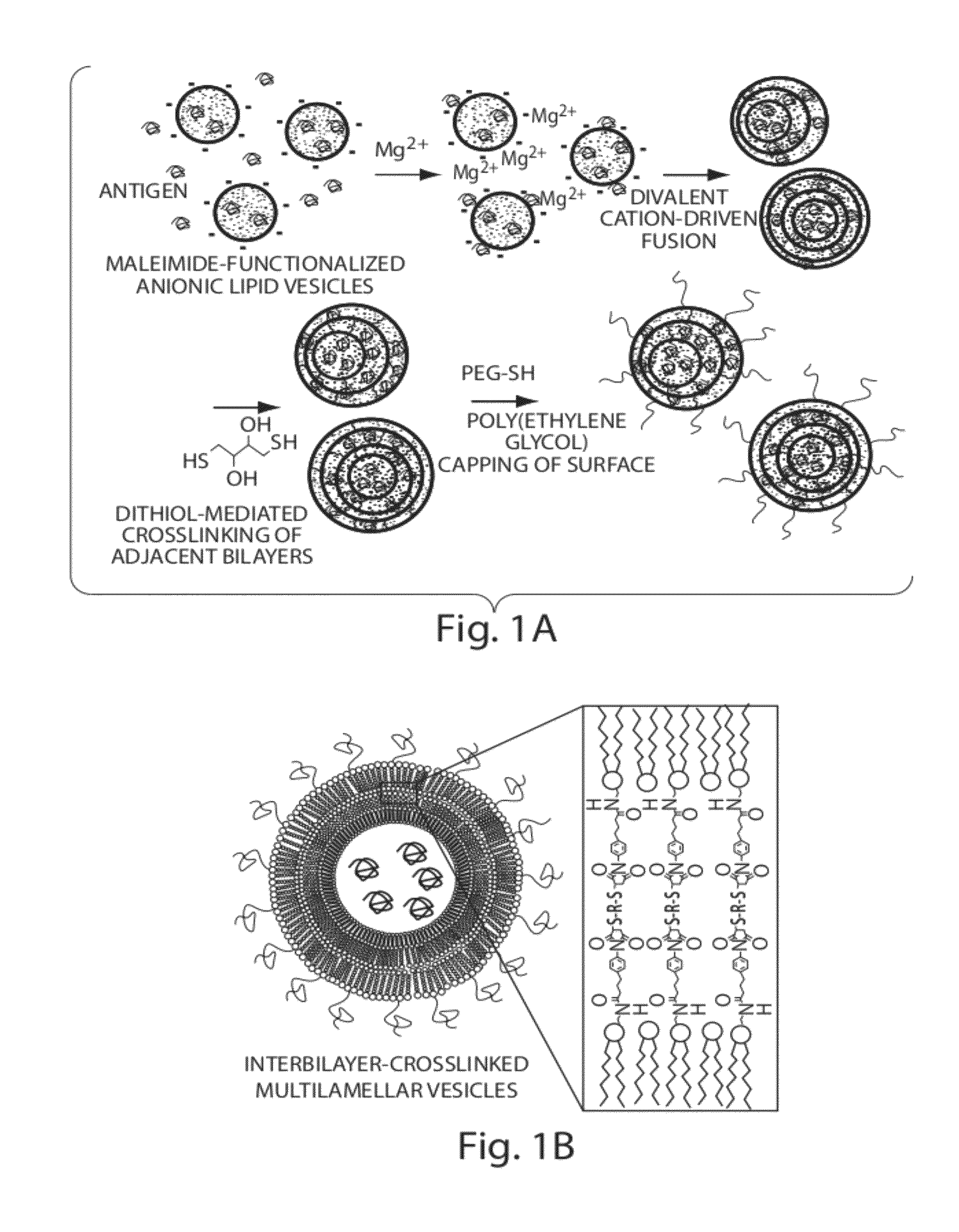
Lipid vesicle compositions and methods of use
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Lipid vesicle compositions and methods of use
ActiveUS20110229529A1Increase load capacityLong release profileOrganic active ingredientsVirusesDiagnostic agentVesicle/vacuole
Owner:MASSACHUSETTS INST OF TECH
Hair treatment compositions
The invention provides a hair treatment composition such as a shampoo or conditioner suitable for topical application to hair for the repair and prevention of damage, comprising (i) cholesterol, and (ii) a hair benefit agent which is a mixture of a basic amino acid and a fatty acid. Preferably the cholesterol and hair benefit agent are compounded into a dispersion of multilamellar vesicles, stabilised with nonionic surfactant.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
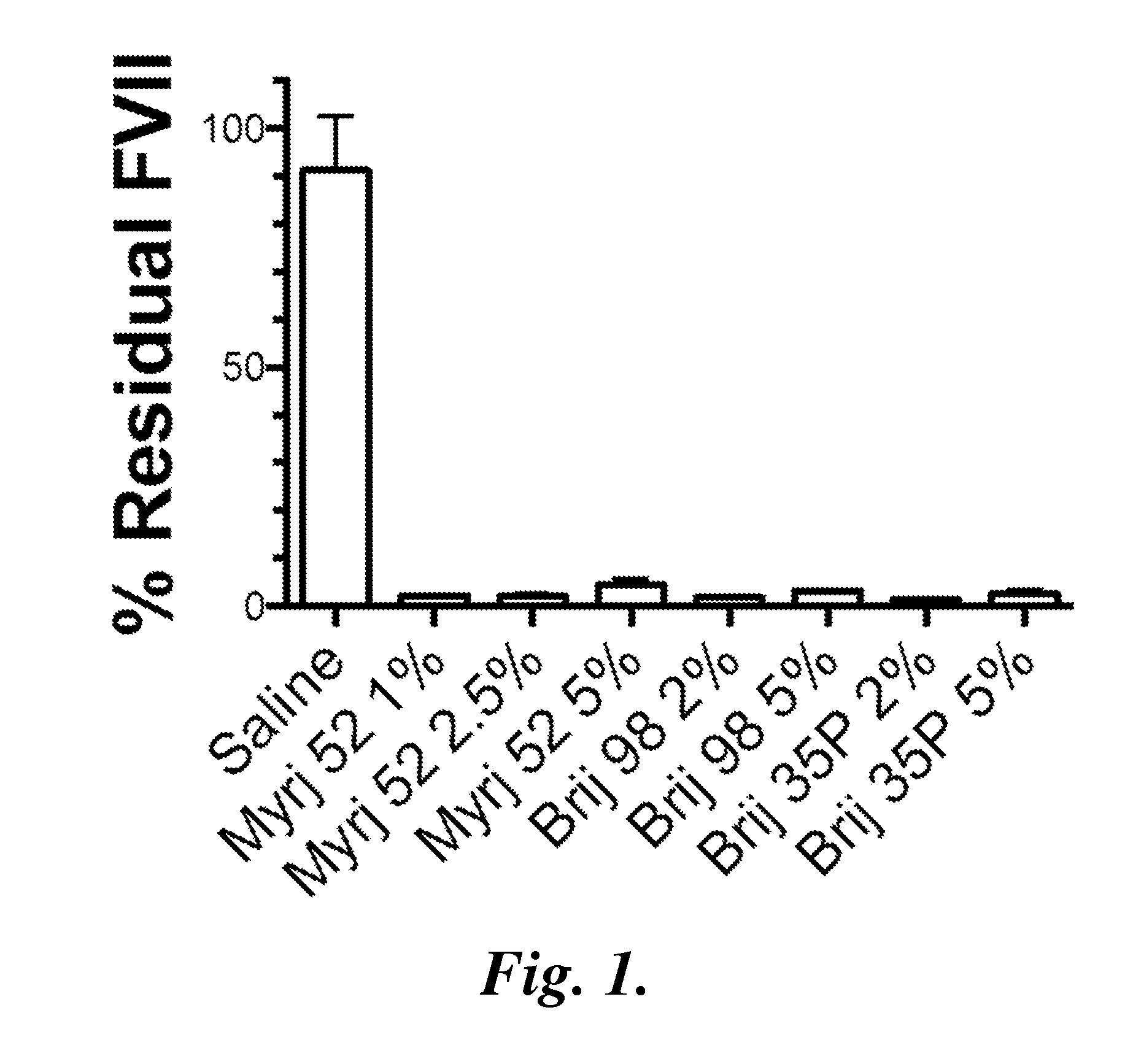
Compositions and methods for dosing liposomes of certain sizes to treat or prevent disease
InactiveUS20040009216A1Improve the level ofLower levelOrganic active ingredientsSenses disorderDiseaseRegimen
Owner:PFIZER INC +1
Shampoo compositions
InactiveUS20020119113A1Easily visualisedEnhancing performance and deliveryCosmetic preparationsHair removalSterolBULK ACTIVE INGREDIENT
A shampoo composition comprising an active ingredient and multilamellar vesicles, characterised in that the multilamellar vesicles consist essentially of anionic surfactant and a sterol.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Lipid vesicle compositions and methods of use
ActiveUS8747869B2Increase load capacityImprove the level ofOrganic active ingredientsFatty acid chemical modificationLipid formationDiagnostic agent
Owner:MASSACHUSETTS INST OF TECH
Lipid nanoparticles for transfection and related methods
ActiveUS20160022580A1Avoid mixingReduce the amount requiredBiocideOrganic active ingredientsNanoparticleAqueous medium
Transfection reagent composition, lipid nanoparticles prepared from the transfection reagent composition, kits that include the transfection reagent composition, and methods for making and using lipid nanoparticles prepared from the transfection reagent composition. Lipids when dispersed in aqueous media readily form liposomes, such as unilamellar vesicles and multilamellar vesicles. Liposomes have been used successfully to encapsulate and deliver a wide range of chemicals including nucleic acids, proteins and. small molecule drugs, to cells.
Owner:THE UNIV OF BRITISH COLUMBIA
Liposomal formulation of irinotecan
InactiveUS20050019387A1Easy to useBiocidePharmaceutical non-active ingredientsMedicineCompound (substance)
The present invention is for novel compositions and methods for treating cancer, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include liposome entrapped irinotecan in which the liposome can contain any of a variety of neutral or charged liposome-forming compounds and cardiolipin. The liposomes of the present invention can be either multilamellar vesicles and unilamellar vesicles, as desired.
Owner:NEOPHARMA INC
Surfactant Mixtures for Improving the Deposition of Active Substances and for Reducing the Skin Irritant Action
InactiveUS20120183591A1Improve efficiencyMore cost-effectivelyCosmetic preparationsBiocideAlcoholPolyol
A cosmetic or pharmaceutical rinse-off formulation contains 0.2% by weight to 20% by weight of a phase A; 0.1% by weight to 50% by weight of an active substance; a detergent; and the remainder being water. The phase A is a mixture of 20% by weight to 90% by weight of a surfactant mixture of isethionates, acyl lactylates and one of alkyl glutamates and alkyl glucosides and 10% by weight to 80% by weight of water, alcohol, polyol or mixtures thereof. The phase A forms multilamellar vesicles. The cosmetic or pharmaceutical rinse-off formulation is a clear product with the multilamellar vesicles of phase A having an average diameter of less than 100 nm.
Owner:IFAC INST FUR ANGEWANDTE COLLOIDTECH
Lipid vesicle compositions and methods of use
ActiveUS9149432B2Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Media in the form of complex dispersions, method for preparing same and uses
The invention concerns novel media in the form of complex dispersions, method for preparing same and their uses. Said media consist of a first medium in the form of droplets wherein is dispersed a phase containing organized double layers of surfactants, preferably in the form of multilamellar vesicles, said droplets being in emulsion in a second medium called continuous phase non-miscible with the first medium. Said media can be used as base for cosmetic compositions. They can contain active principles, in particular for controlling their release and / or protecting and / or controlling their pH. They can also be used to make polymer microspheres containing an active principle. They are also useful as antigen vector.
Owner:CAPSULIS
Liposomal formulation of irinotecan
The present invention is for novel compositions and methods for treating cancer, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include liposome entrapped irinotecan in which the liposome can contain any of a variety of neutral or charged liposome-forming compounds and cardiolipin. The liposomes of the present invention can be either multilamellar vesicles and unilamellar vesicles, as desired.
Owner:NEOPHARMA INC
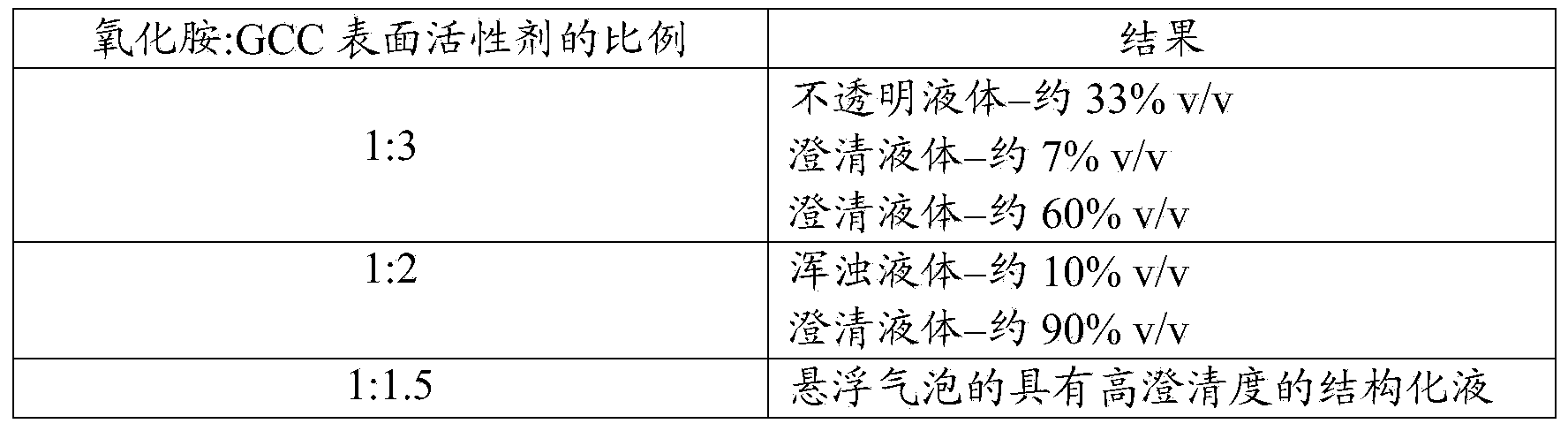
Structured Surfactant Suspending Systems
A structured surfactant system with a very high degree of clarity. The system comprises water and a mixture of at least one surfactant having a HLB (Hydrophilic Lipophilic Balance) value of less than 10, and at least one surfactant having an HLB value of 10 or greater. The structured surfactant system forms multilamellar vesicles and has suspending properties without added electrolytes, carbohydrates, or polymeric thickeners. This makes the structured surfactant system particularly useful in personal care compositions.
Owner:STEPAN COMPANY
Vinorelbine compositions and methods of use
The present invention is for novel compositions and methods for treating cancer, particularly, for treating cancer in mammals and more particularly in humans. The therapeutic compositions of the present invention include liposome entrapped vinorelbine in which the liposome can contain any of a variety of neutral or charged liposome-forming compounds and cardiolipin. The liposomes of the present invention can be either multilamellar vesicles or unilamellar vesicles, as desired.
Owner:NEOPHARMA INC
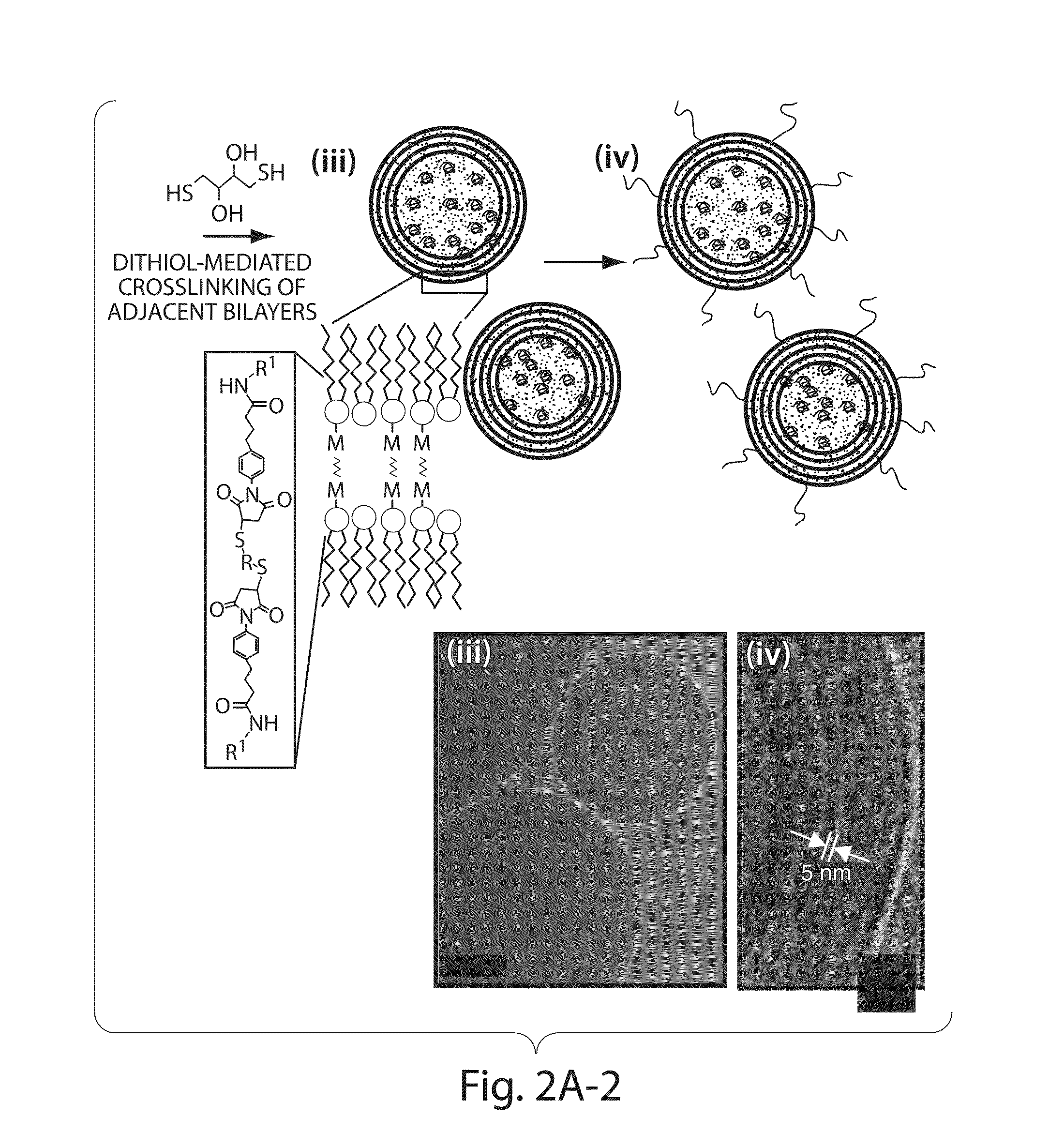
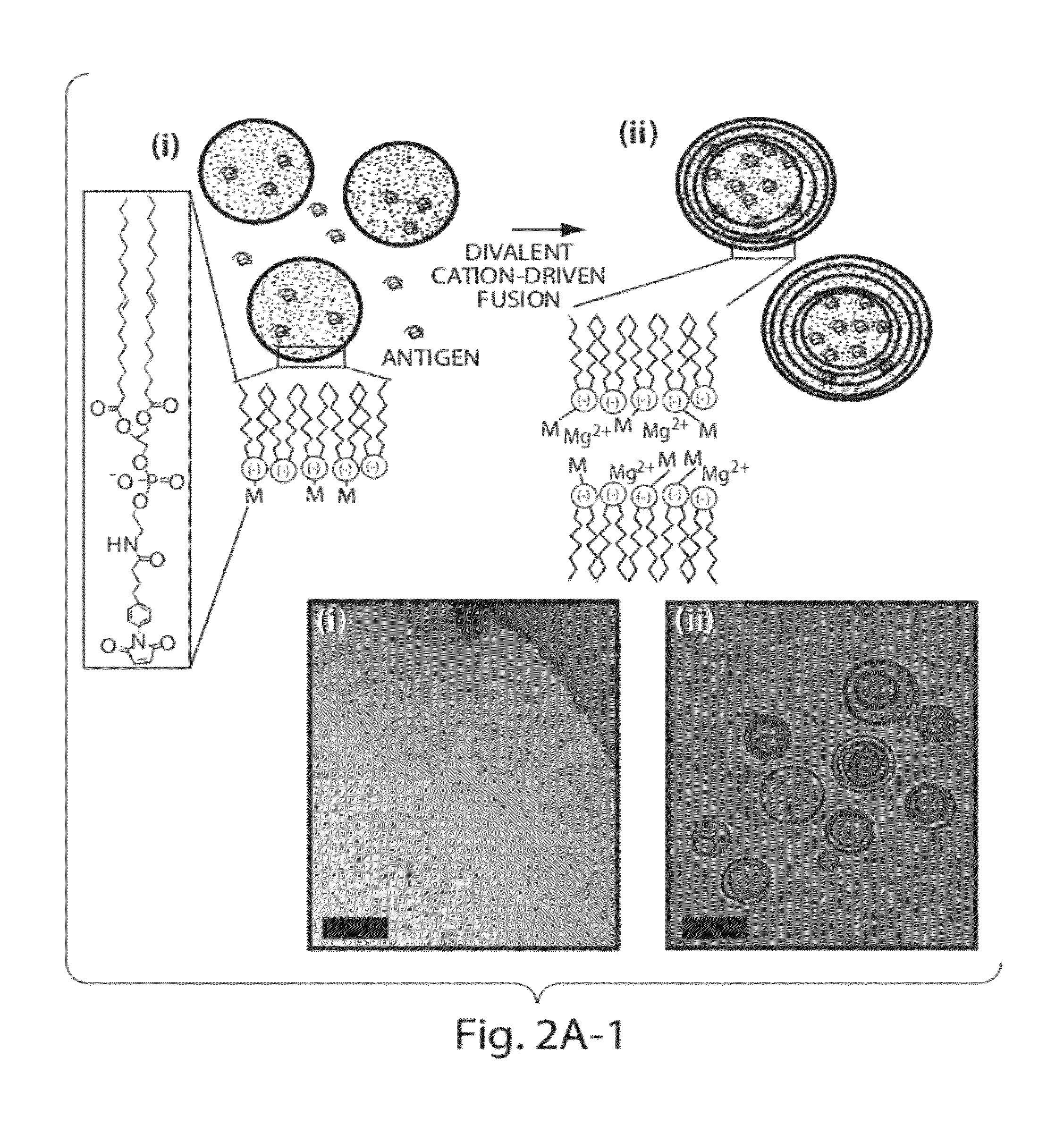
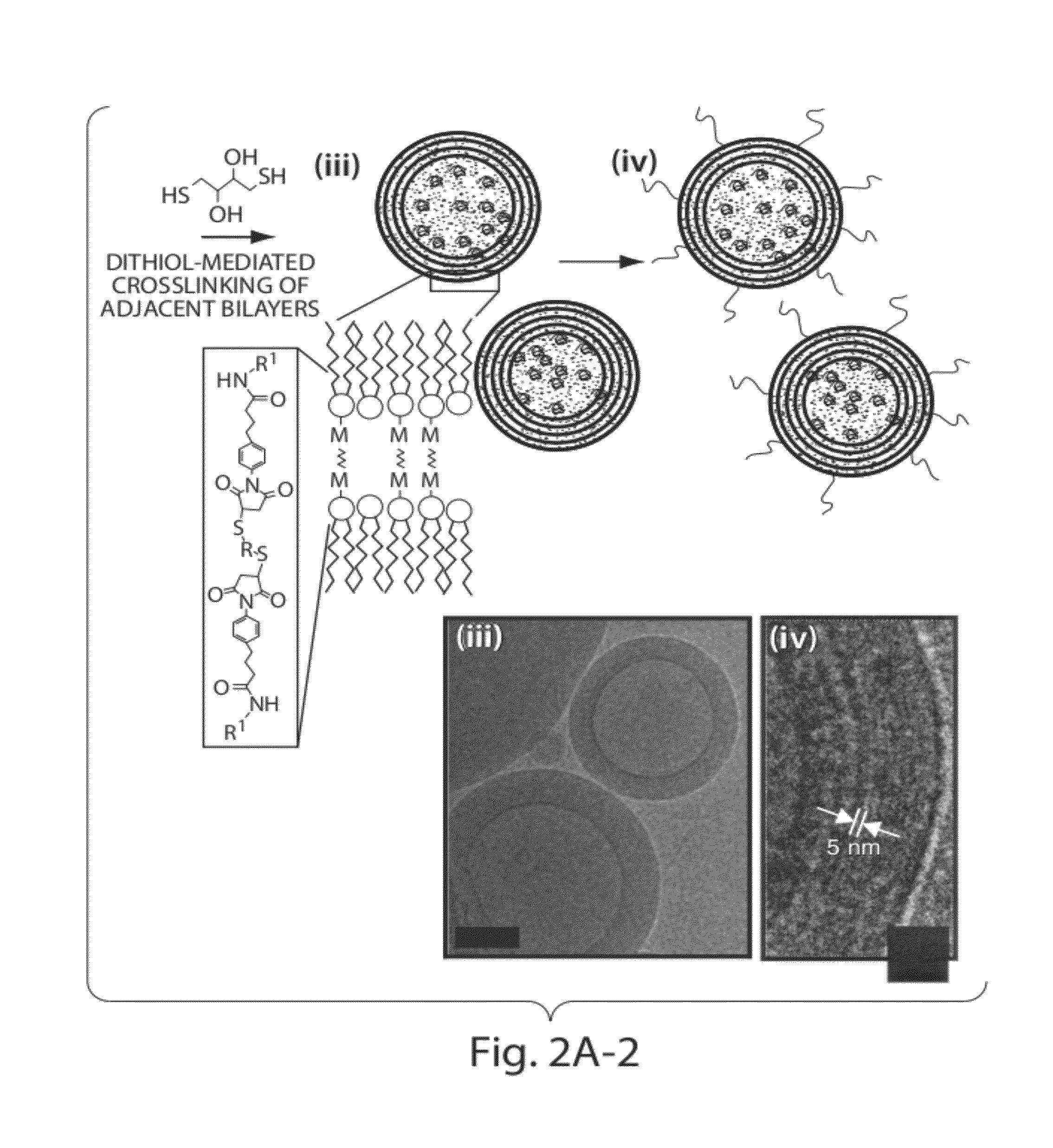
Multilamellar lipid vesicle compositions and methods of use
InactiveUS20170246288A1Improve packaging efficiencyOrganic active ingredientsPeptide/protein ingredientsAntigenVesicle/vacuole
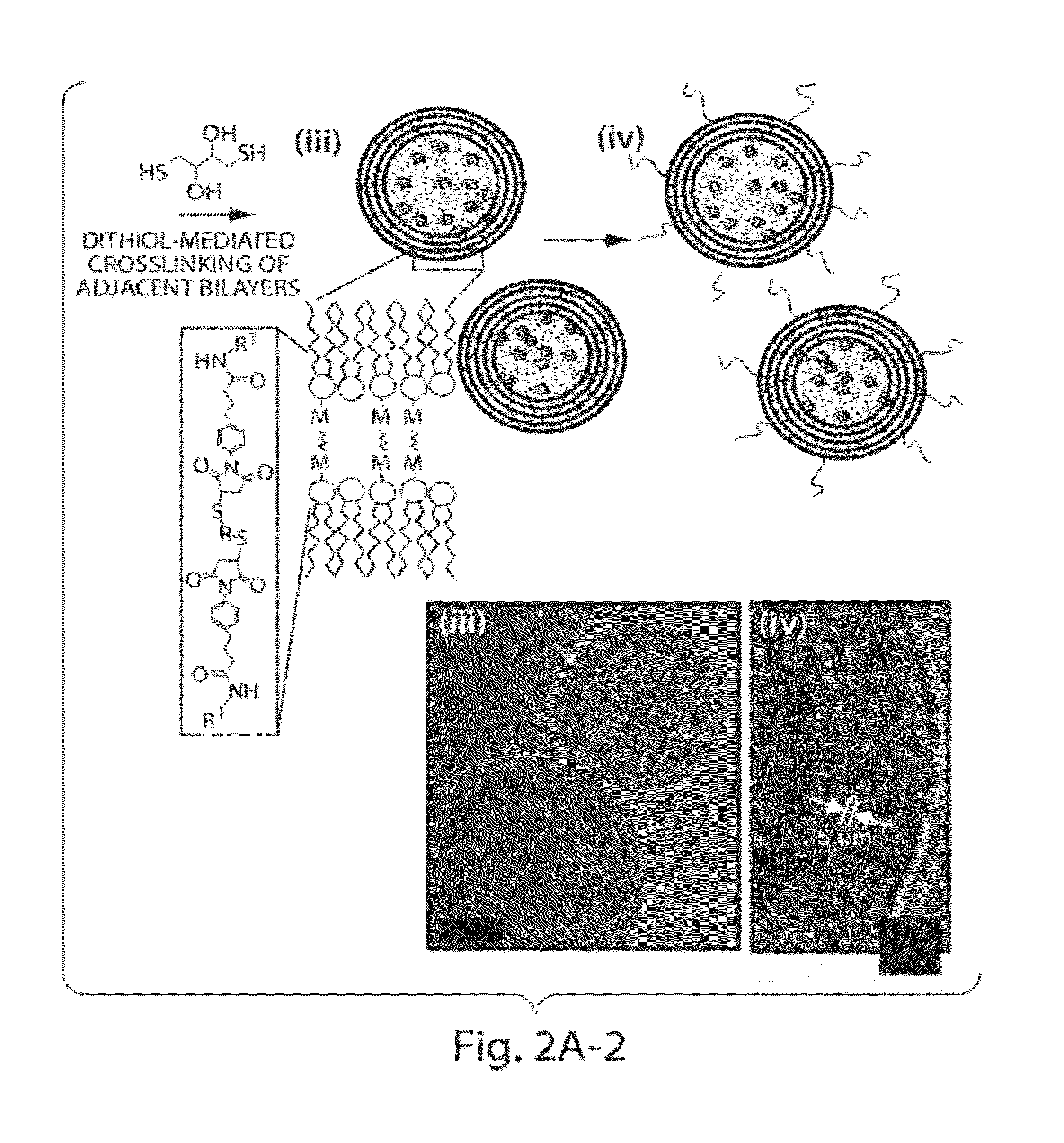
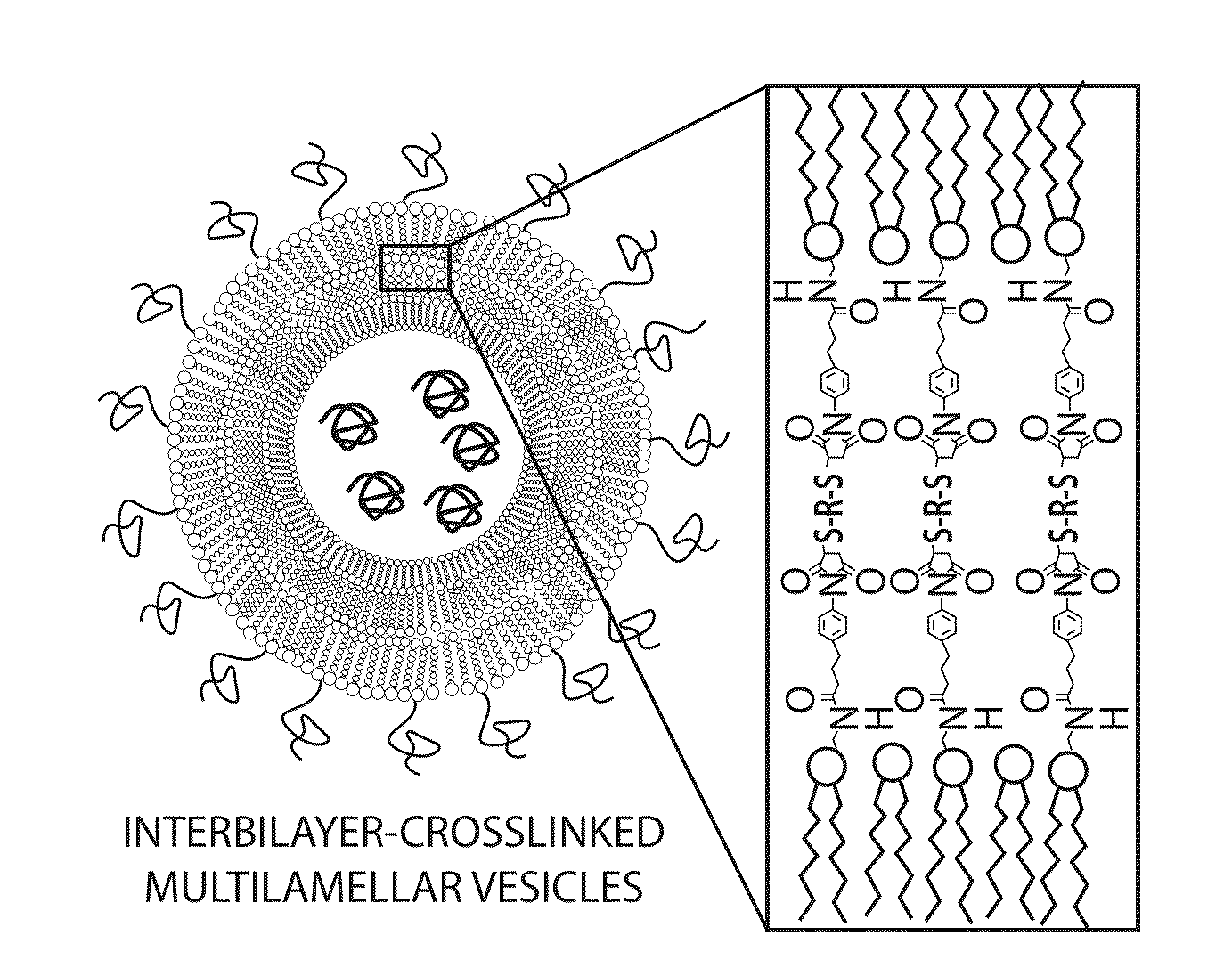
The present invention provides novel and inventive drug delivery systems with higher loading capability, a capacity to sequester high tumors levels of both hydrophobic and hydrophilic agents simultaneously, and longer release profiles. Some aspects of these delivery systems include compositions including stabilized multilamellar lipid vesicles having crosslinked lipid bilayers (referred to herein as inter-bilayer-crosslinked multilamellar vesicles or ICMV) covalently conjugated to an agent (e.g., an antigen).
Owner:VEDANTRA PHARMA
Multilamellar lipid vesicle compositions and methods of use
The present invention provides novel and inventive drug delivery systems with higher loading capability, a capacity to sequester high levels of both hydrophobic and hydrophilic agents simultaneously,and longer release profiles. Some aspects of these delivery systems include compositions including stabilized multilamellar lipid vesicles having crosslinked lipid bilayers (referred to herein as interbilayer-crosslinked multilamellar vesicles or ICMV) covalently conjugated to an agent (e.g., an antigen).
Owner:VEDANTRA PHARMA
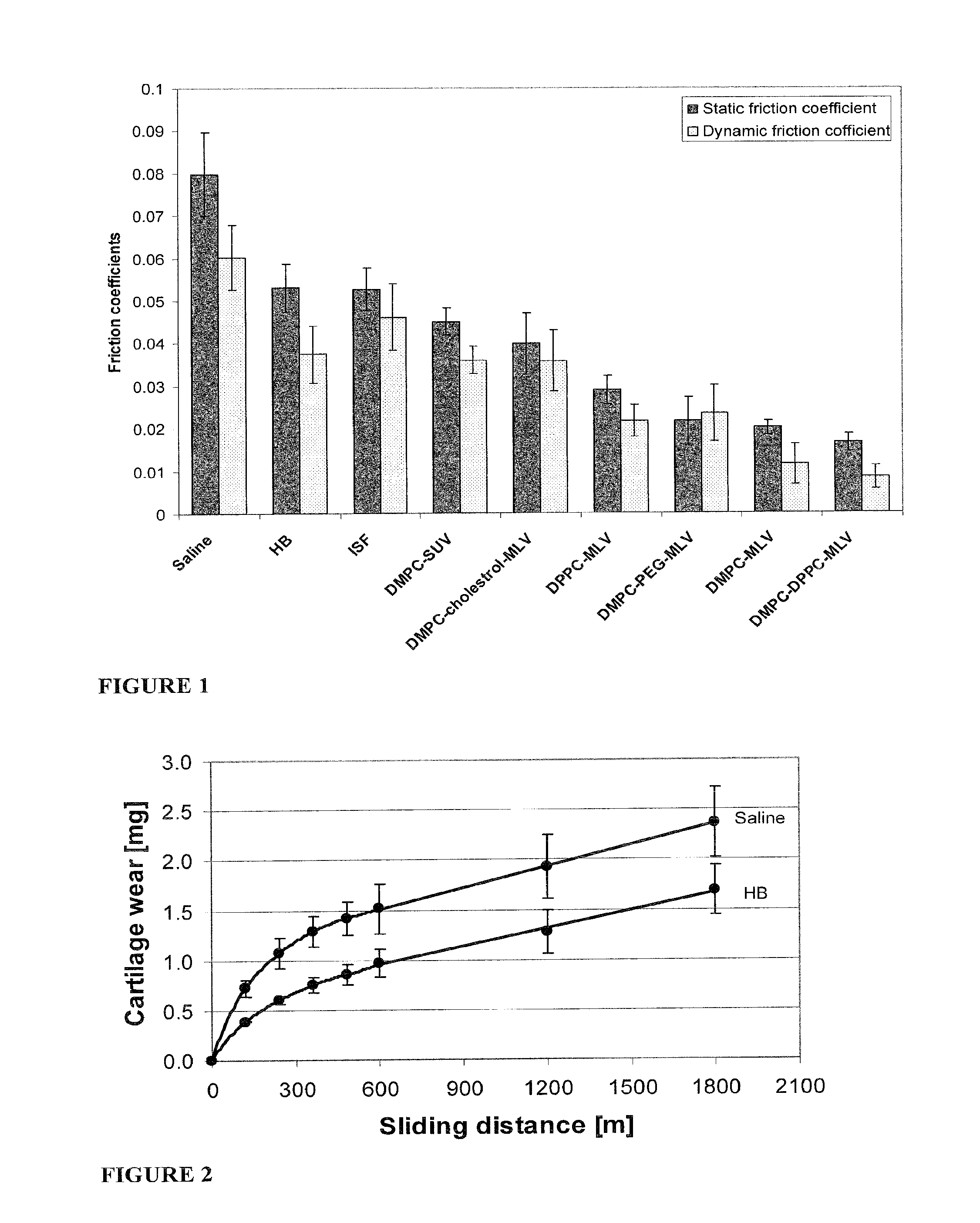
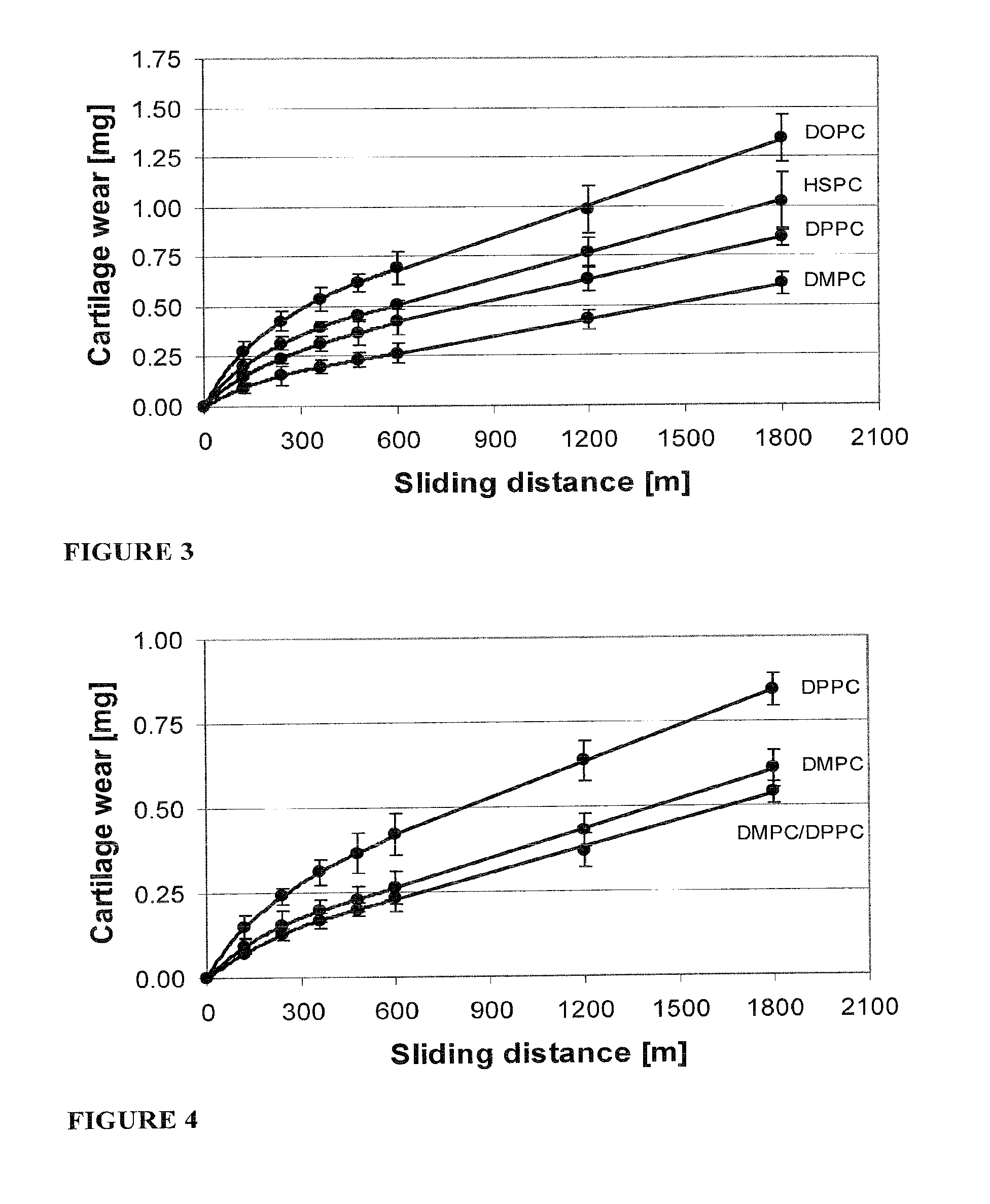
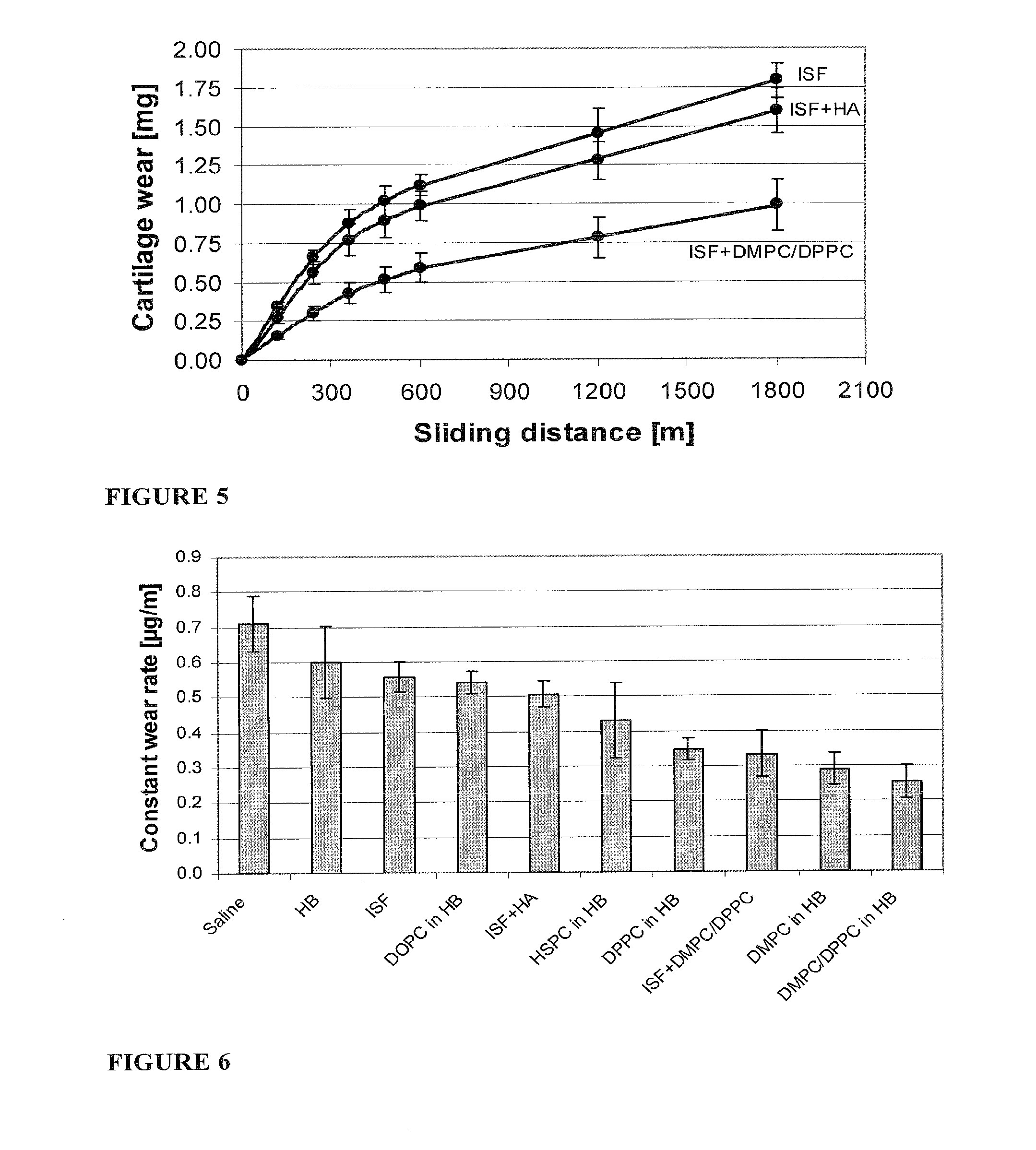
Methods for joint lubrication and cartilage wear prevention making use of glycerophospholipids
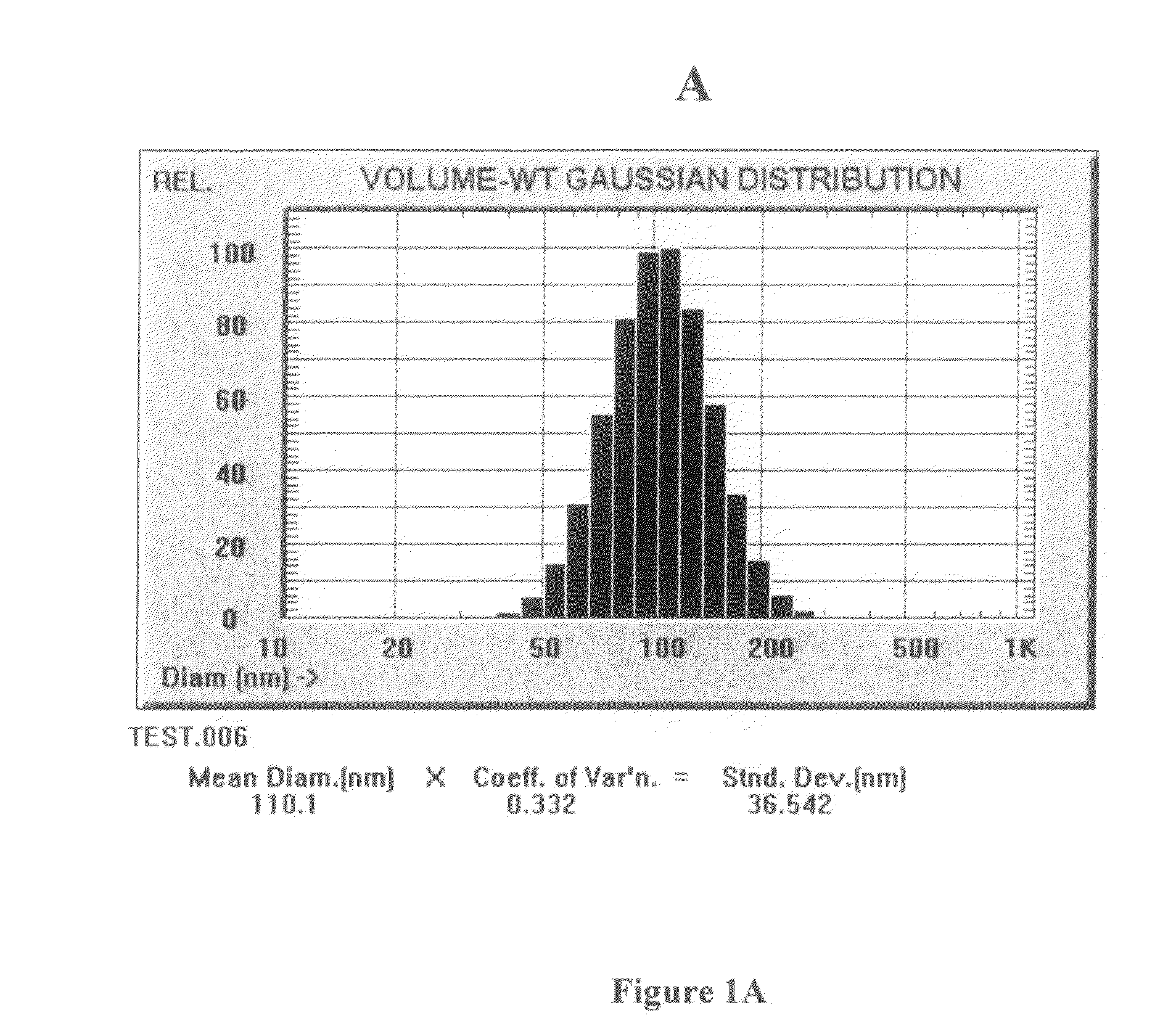
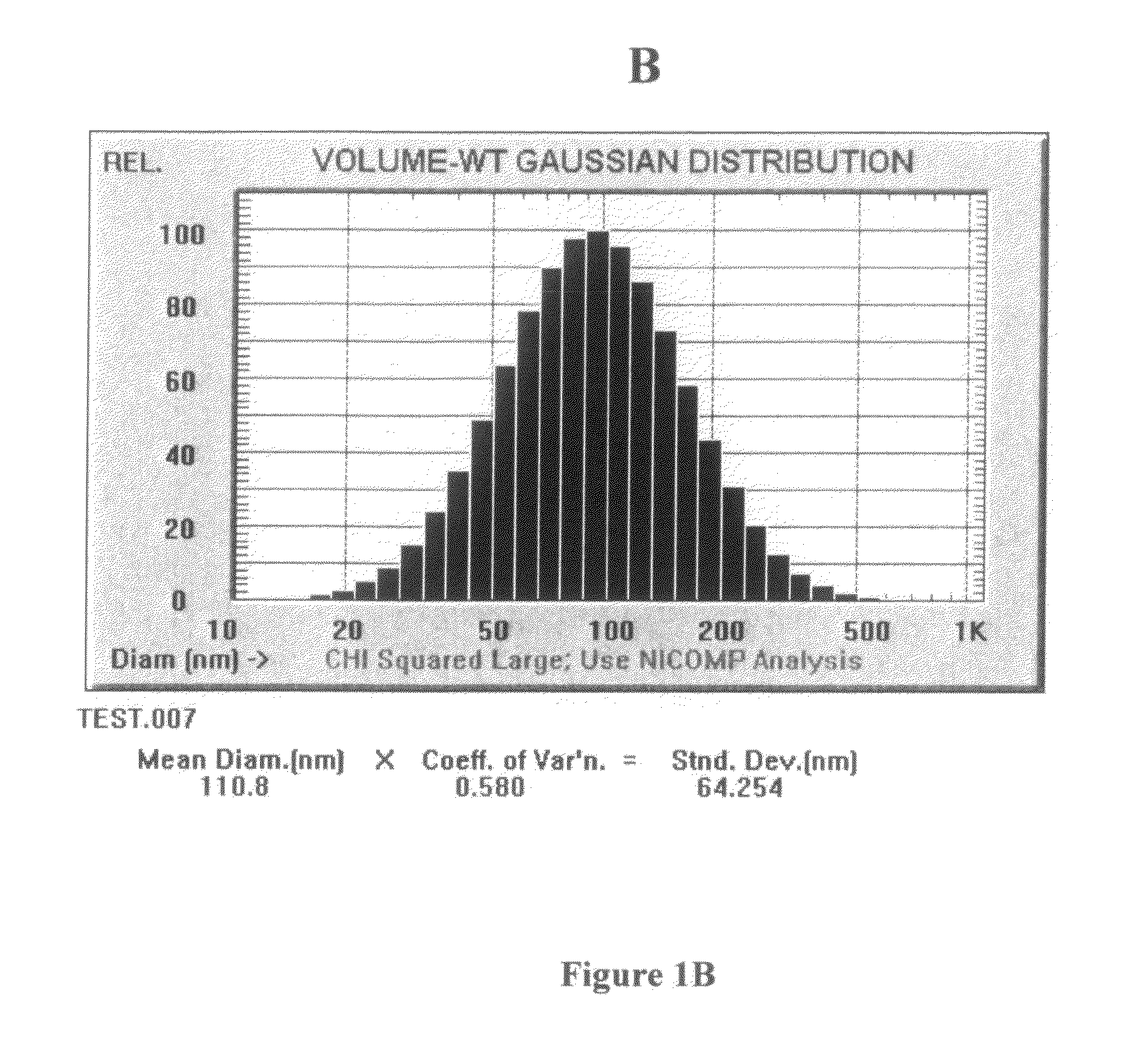
InactiveUS20150037404A1Improve and restore joint mobilityReduce wearBiocideAntipyreticMean diameterMedicine
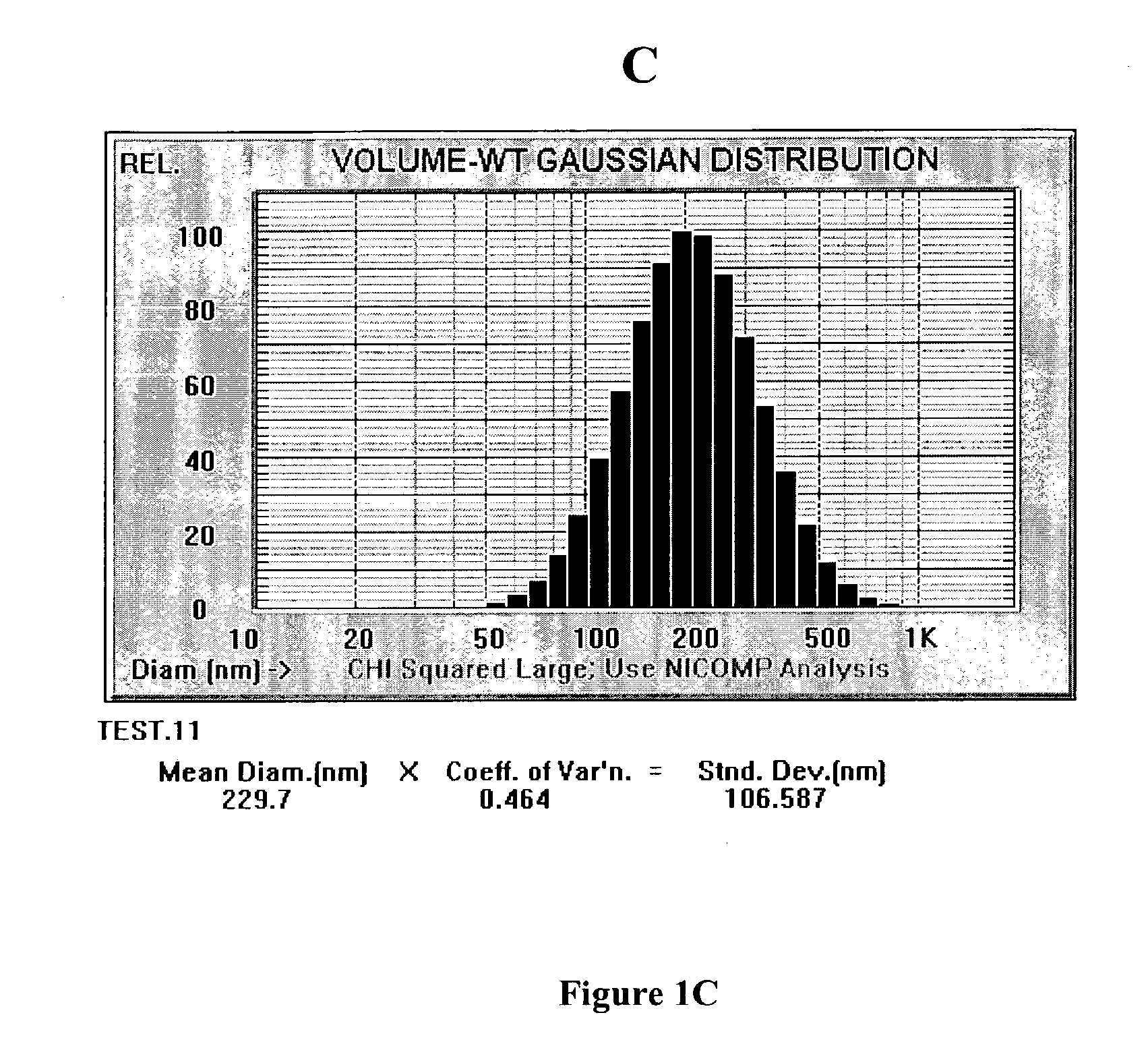
A method for lubricating a joint of a mammal by administering into a cavity of the joint a composition of liposomes that are multilamellar vesicles (MLV) dispersed in a fluid medium, the liposomes having a mean diameter of between about 0.8 μm to about 10 μm and including membranes of at least one glycerophospholipid (GPL) having two C12-C16 hydrocarbon chains which are the same or different. These membranes have a phase transition temperature in which solid ordered (SO) to liquid disordered (LD) phase transition occurs, the phase transition temperature being at a temperature of about 20° C. to about 39° C. and being lower than the temperature of the joint.
Owner:TECHNION RES & DEV FOUND LTD +2
Novel lipophilic compositions and uses thereof
New co-lipids for use in combination with lipophilic compounds as nucleic acid vectors, for preparing a nucleic acid vector composition, and methods for introducing in vitro or in vivo a nucleic acid of interest into host cells, including a step of contacting host cells these nucleic acid vector compositions. The nucleic acid vector compositions have the combination (i) of a nucleic acid, cationic, lipophilic vector with (ii) a co-lipid. These nucleic acid vector compositions, in some embodiments, are present in the form of unilamellar or multilamellar vesicles.
Owner:UNIV DE BRETAGNE OCCIDENTALE +1
Anti-guanosine antibody as a molecular delivery vehicle
PendingUS20190330317A1Improve the effectiveness of treatmentImprove efficiencyPowder deliveryOrganic active ingredientsNanocarriersRegimen
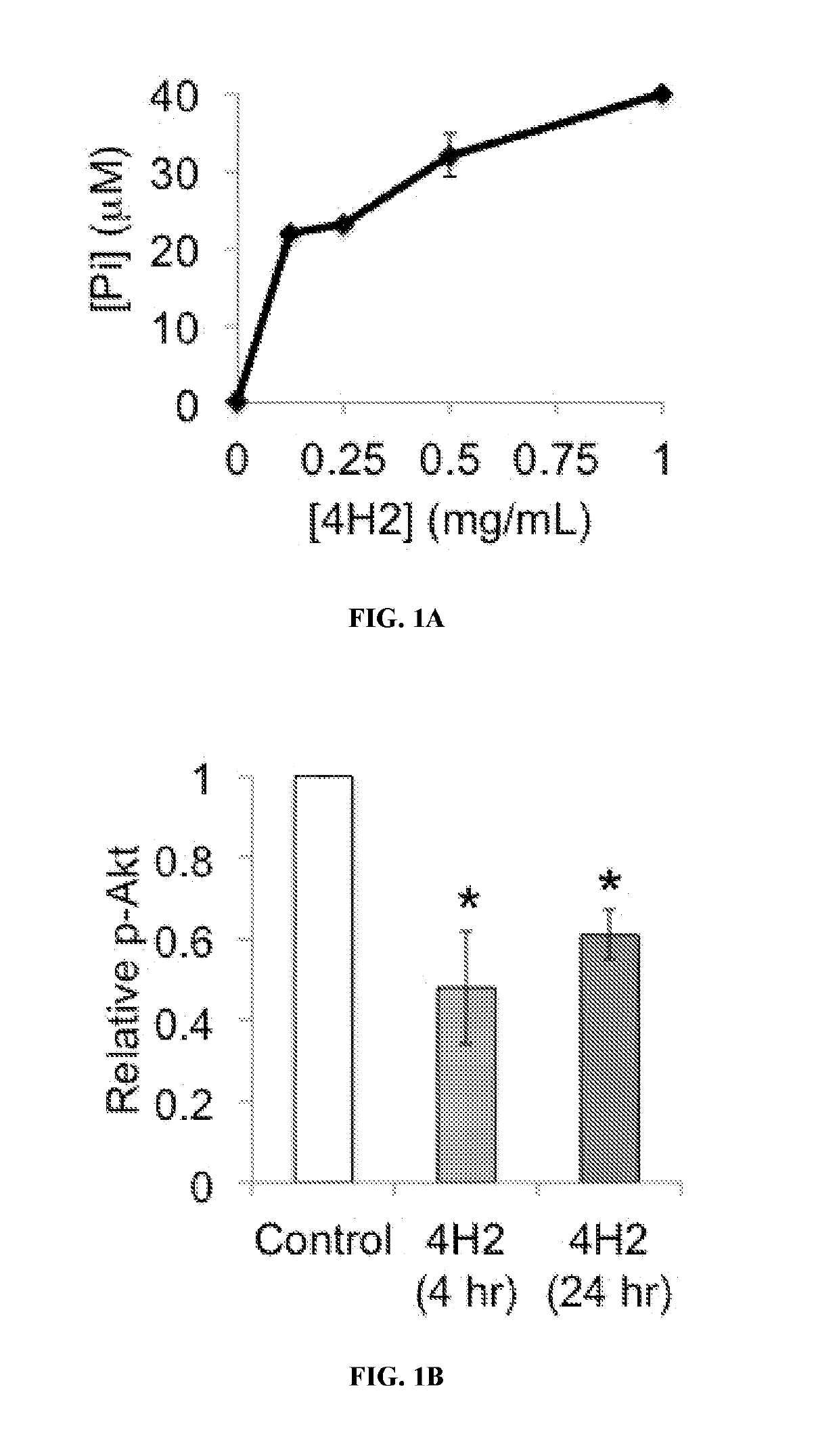
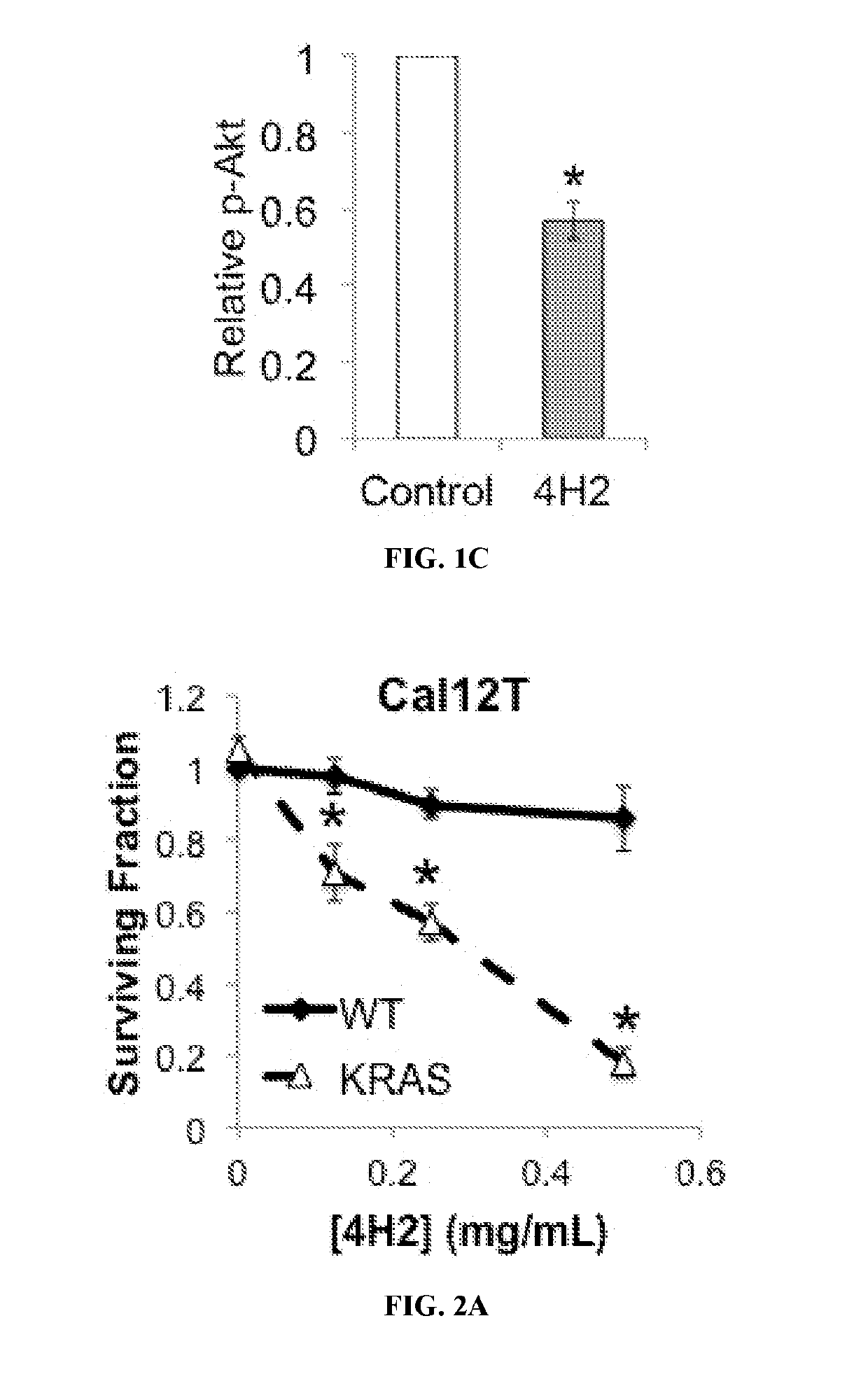
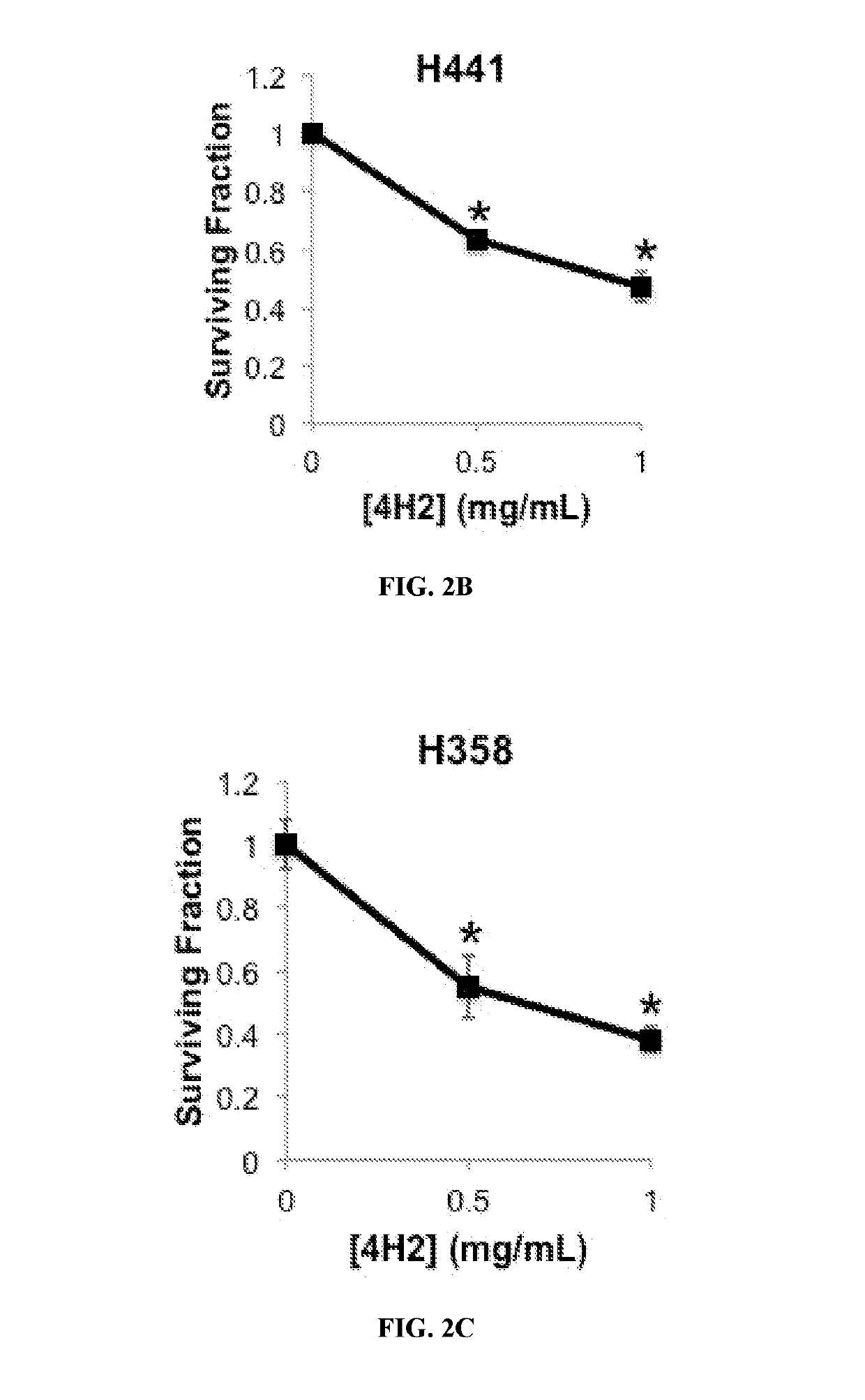
Guanosine-targeted nanocarriers for encapsulating an active agent and delivering it to extracellular guanosine and DNA are provided. The nanocarriers, for example, polymeric particles, liposomes, and multilamellar vesicles have targeting moiety that targets guanosine attached, linked, or conjugated thereto. The targeting moiety that targets guanosine is typically an antibody, or variant, fragment, or fusion protein derived therefrom that binds to guanosine. The targeting moiety can be a circulating autoantibody that binds guanosine such as those commonly found in patients with SLE. Cytoplasmic delivery vehicles that do not localize into endosomes or lysosomes are also provided. The delivery agent is typically an antibody, or variant, fragment, or fusion protein derived therefrom that binds to guanosine. In some embodiments, the targeting moiety or delivery agent is antibody 4H2 or a variant, fragment, or fusion protein derived therefrom. Pharmaceutical compositions, methods of use, and dosage regimens are also provided.
Owner:YALE UNIV
Structured surfactant suspending systems
A structured surfactant system with a very high degree of clarity. The system comprises water and a mixture of at least one surfactant having a HLB (Hydrophilic Lipophilic Balance) value of less than 10, and at least one surfactant having an HLB value of 10 or greater. The structured surfactant system forms multilamellar vesicles and has suspending properties without added electrolytes, carbohydrates, or polymeric thickeners. This makes the structured surfactant system particularly useful in personal care compositions.
Owner:STEPAN COMPANY
Antibody-mediated autocatalytic, targeted delivery of nanocarriers to tumors
ActiveUS20190247515A1Improve the effectiveness of treatmentImprove efficiencyOrganic active ingredientsPowder deliveryRegimenNanocarriers
DNA-targeted nanocarriers for encapsulating an active agent and delivering it to extracellular DNA are provided. The nanocarriers, for example, polymeric particles, liposomes, and multilamellar vesicles have targeting moiety that targets DNA conjugated thereto. The targeting moiety that targets DNA is typically an antibody, or variant, fragment, or fusion protein derived therefrom that binds to DNA or nucleosomes. The targeting moiety can be a circulating autoantibody that binds DNA such as those commonly found in patients with SLE. In some embodiments, the targeting moiety is antibody 3E10 or a variant, fragment, or fusion protein derived therefrom. Pharmaceutical compositions, methods of use, and dosage regimens are also provided.
Owner:YALE UNIV
Multilamellar vesicle preparation containing acyl basic amino acid derivative and physiologically active substance
InactiveUS20170209354A1Easy to makeGood storage stabilityCosmetic preparationsAntipyreticChemical compoundMedicinal chemistry
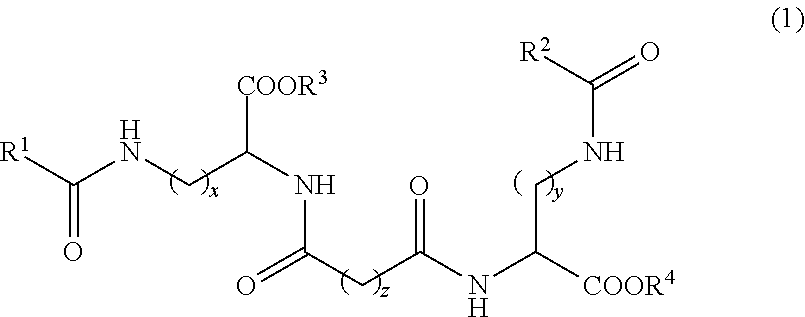
The present invention aims to provide a multilamellar vesicle preparation which can be produced conveniently and is superior in stability. A multilamellar vesicle preparation containing component (A): a compound represented by the formula (1)wherein each symbol is as described in the SPECIFICATION, or a salt thereof, component (B): a physiologically active substance, and component (C): water.
Owner:AJINOMOTO CO INC
Structured surfactant suspending systems
A structured surfactant system with a very high degree of clarity. The system comprises water and a mixture of at least one surfactant having a HLB (Hydrophilic Lipophilic Balance) value of less than 10, and at least one surfactant having an HLB value of 10 or greater. The structured surfactant system forms multilamellar vesicles and has suspending properties without added electrolytes, carbohydrates, or polymeric thickeners. This makes the structured surfactant system particularly useful in personal care compositions.
Owner:STEPAN COMPANY
Compositions and methods for dosing liposomes of certain sizes to treat or prevent disease
InactiveUS20080213351A1Improve the level ofLower levelOrganic active ingredientsSenses disorderDiseaseRegimen
Owner:ESPERION LUV DEV INC
Lipid nanoparticles for transfection and related methods
ActiveUS10342760B2Reduce the amount requiredOrganic active ingredientsMicroencapsulation basedLipid formationNanoparticle
Transfection reagent composition, lipid nanoparticles prepared from the transfection reagent composition, kits that include the transfection reagent composition, and methods for making and using lipid nanoparticles prepared from the transfection reagent composition. Lipids when dispersed in aqueous media readily form liposomes, such as unilamellar vesicles and multilamellar vesicles. Liposomes have been used successfully to encapsulate and deliver a wide range of chemicals including nucleic acids, proteins and small molecule drugs, to cells.
Owner:THE UNIV OF BRITISH COLUMBIA
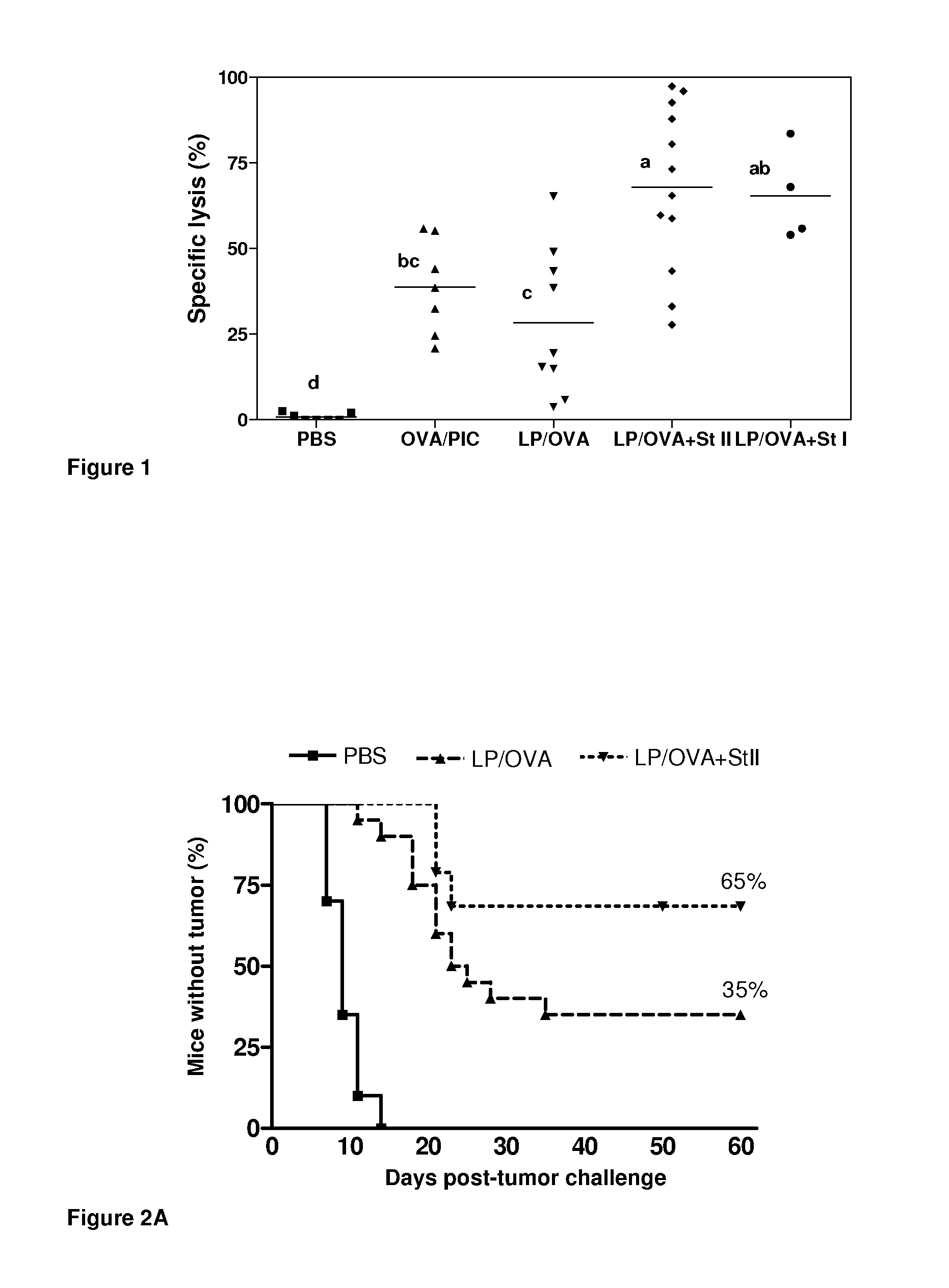
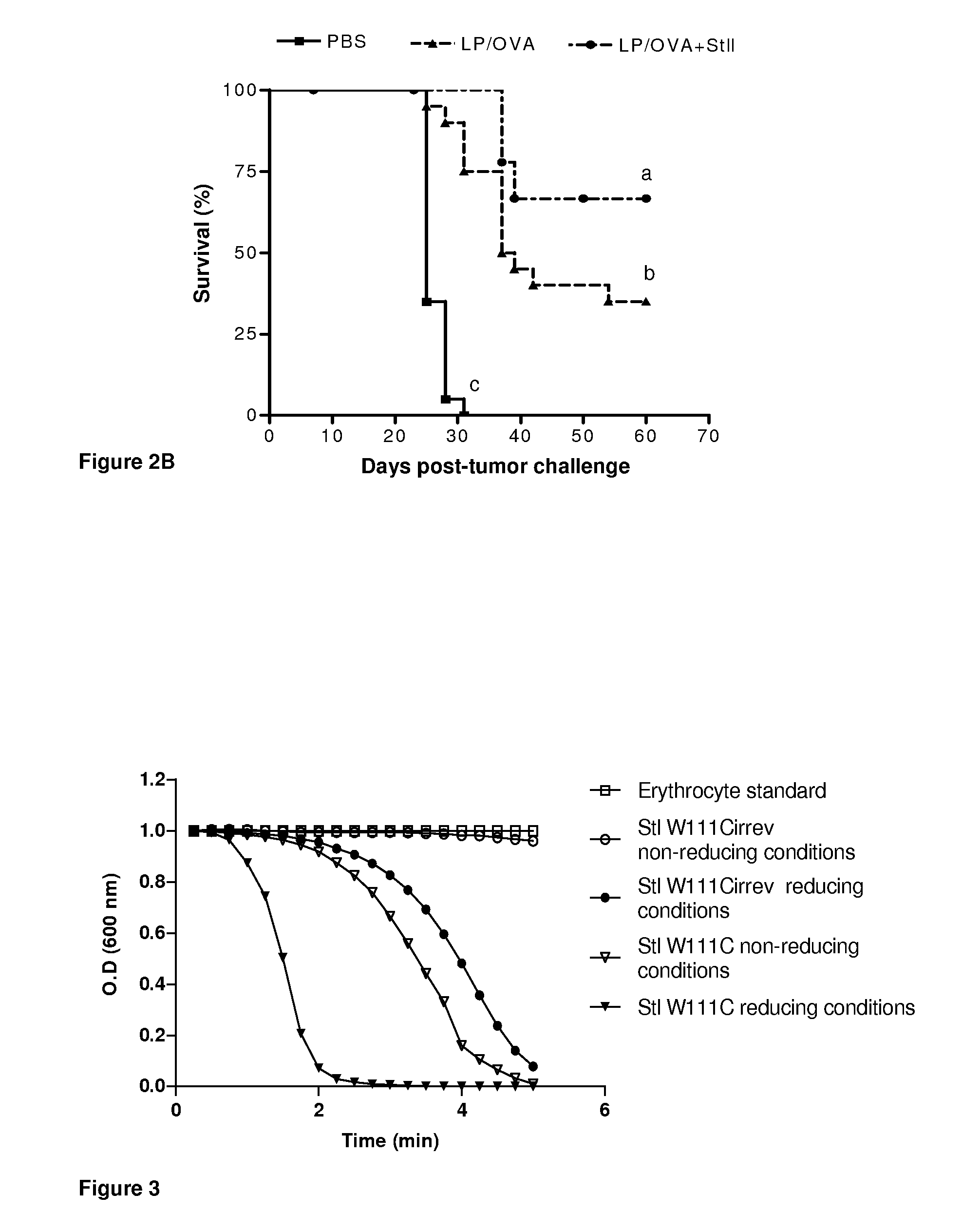
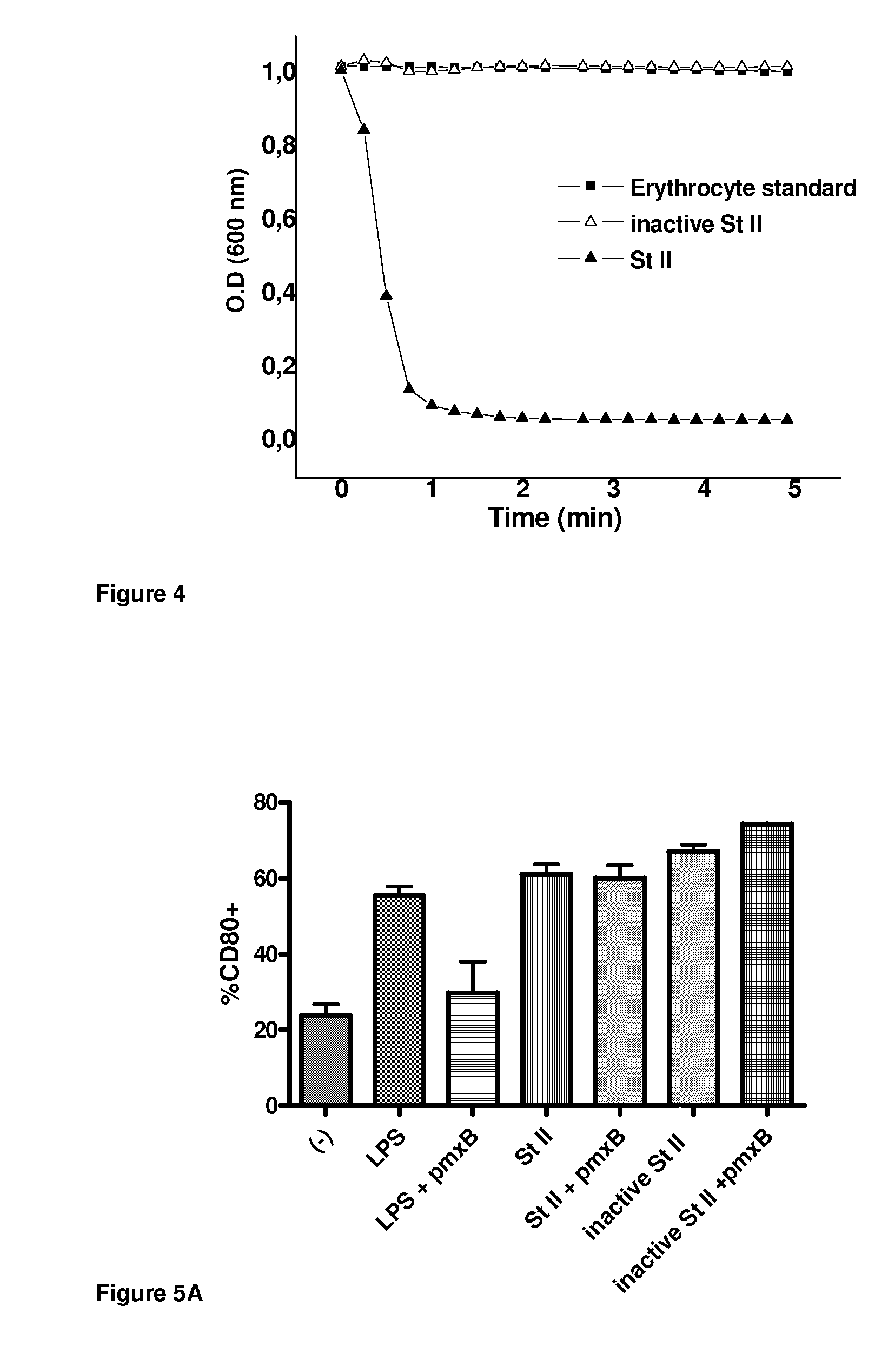
Vaccine composition based on sticholysin encapsulated into liposomes
ActiveUS20130149376A1Increased time-courseImprove survivalAntibacterial agentsBacterial antigen ingredientsAntigenDipalmitoylphosphatidylcholine
The current invention relates to the field of Biotechnology applied to human health. Here it is described a vaccine vehicle wherein toxins from eukaryotic organisms are encapsulated into multilamellar vesicles obtained by the dehydration-rehydration procedure whose lipidic composition is dipalmitoylphosphatidylcholine:cholesterol in a 1:1 molar ratio for subcutaneous or intramuscular administration. These compositions do not require the use of other adjuvants.The disclosed compositions allow modulation of CTL-specific immune response against one or several antigens co-encapsulated into toxin-containing liposomes. The vaccinal vehicle of the present invention shows advantages over others disclosed by the previous art due to the robustness and functionality of the induced immune response as well as its immunomodulating properties.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Vesicles for delayed delivery of fragrance their preparation and use thereof
InactiveCN111655220ACosmetic preparationsNon-ionic surface-active compoundsLipid formationMean diameter
Disclosed is a multilamellar vesicle in the shape of a rotational body comprising two or more concentric lipid double layers and fragrance wherein the vesicle has a mean diameter between 100 and 800 nm, the lipid double layers comprise a) at least one surfactant having a HLB value of greater than 6, and b) an amphiphilic compound having a logP value of 1 or above, and wherein the vesicle comprisesin addition to components a) and b) a fragrance having a log P value of 1 or above. The fragrance is encapsulated in the vesicles. The encapsulated fragrance is stable during storage conditions and the vesicles have long-lasting fragrance release on use thereof. The vesicles may be used in cosmetic formulations or in laundry formulations.
Owner:CLARIANT INT LTD
Micro- or nanoparticular multilamellar vesicles, compositions comprising the same and method for their use in skin care
A micro- or nanoparticular multilamellar vesicle is constituted of concentric membranes comprising at least one non-ionic surfactant of the sucrose ester type comprising at least one chain arising from a linear or branched, saturated or unsaturated, optionally mono- or polyhydroxylated C12 to C22 fatty acid, which vesicle comprises at least a hyaluronic acid (HA), wherein the hyaluronic acid is a crosslinked hyaluronic acid. Also disclosed are a method of manufacture of such vesicle, compositions comprising such vesicle and their uses.
Owner:GALDERMA HLDG SA
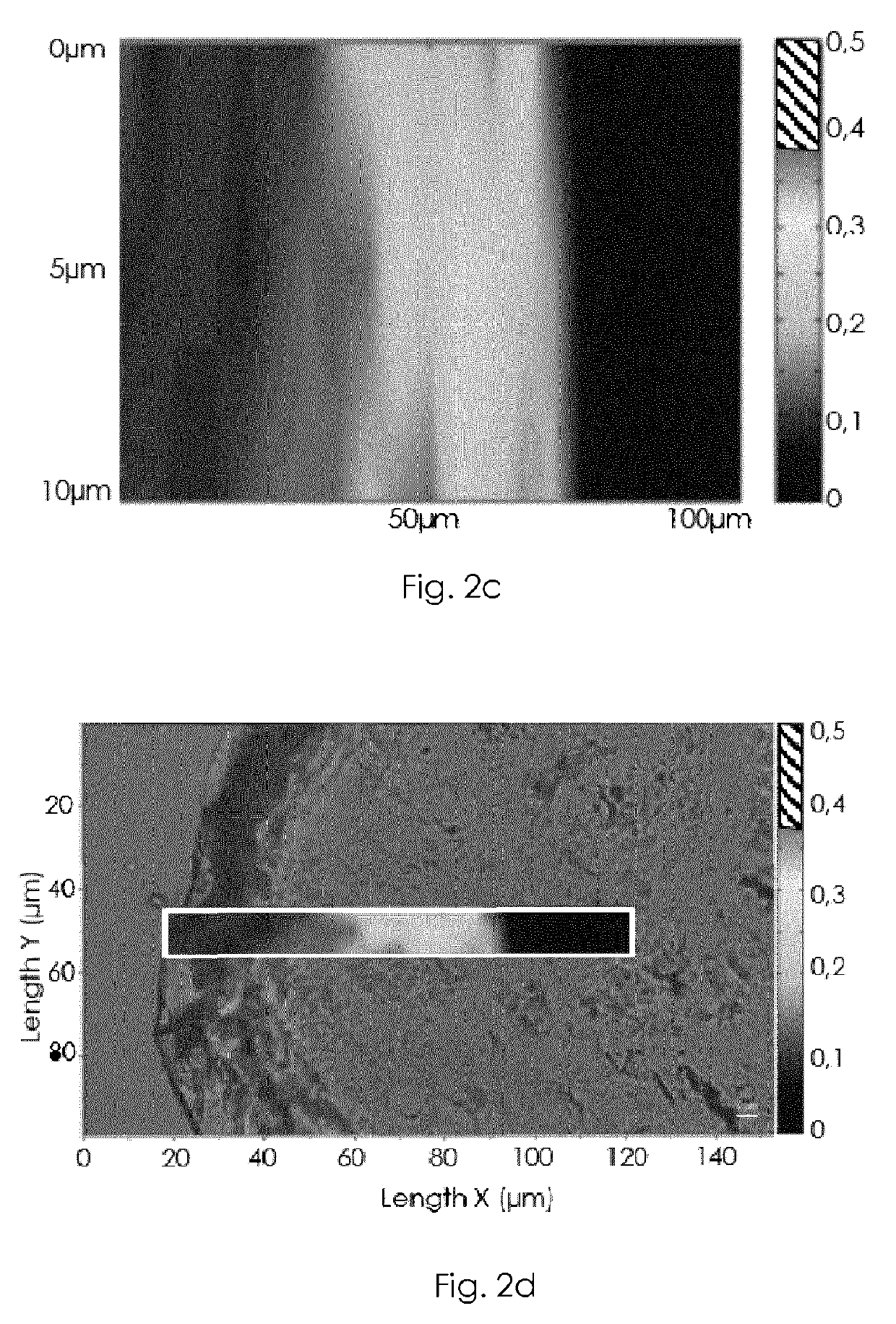
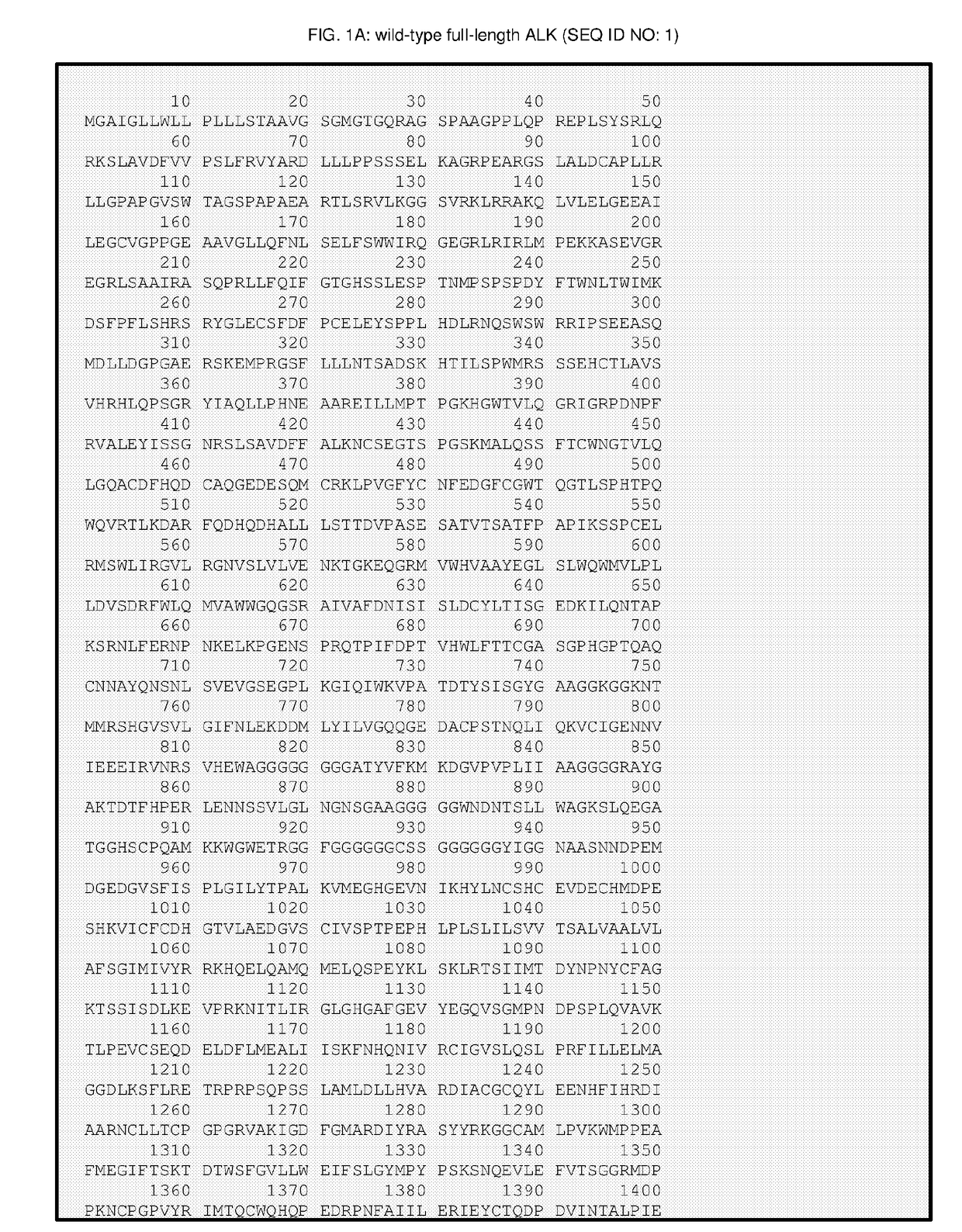
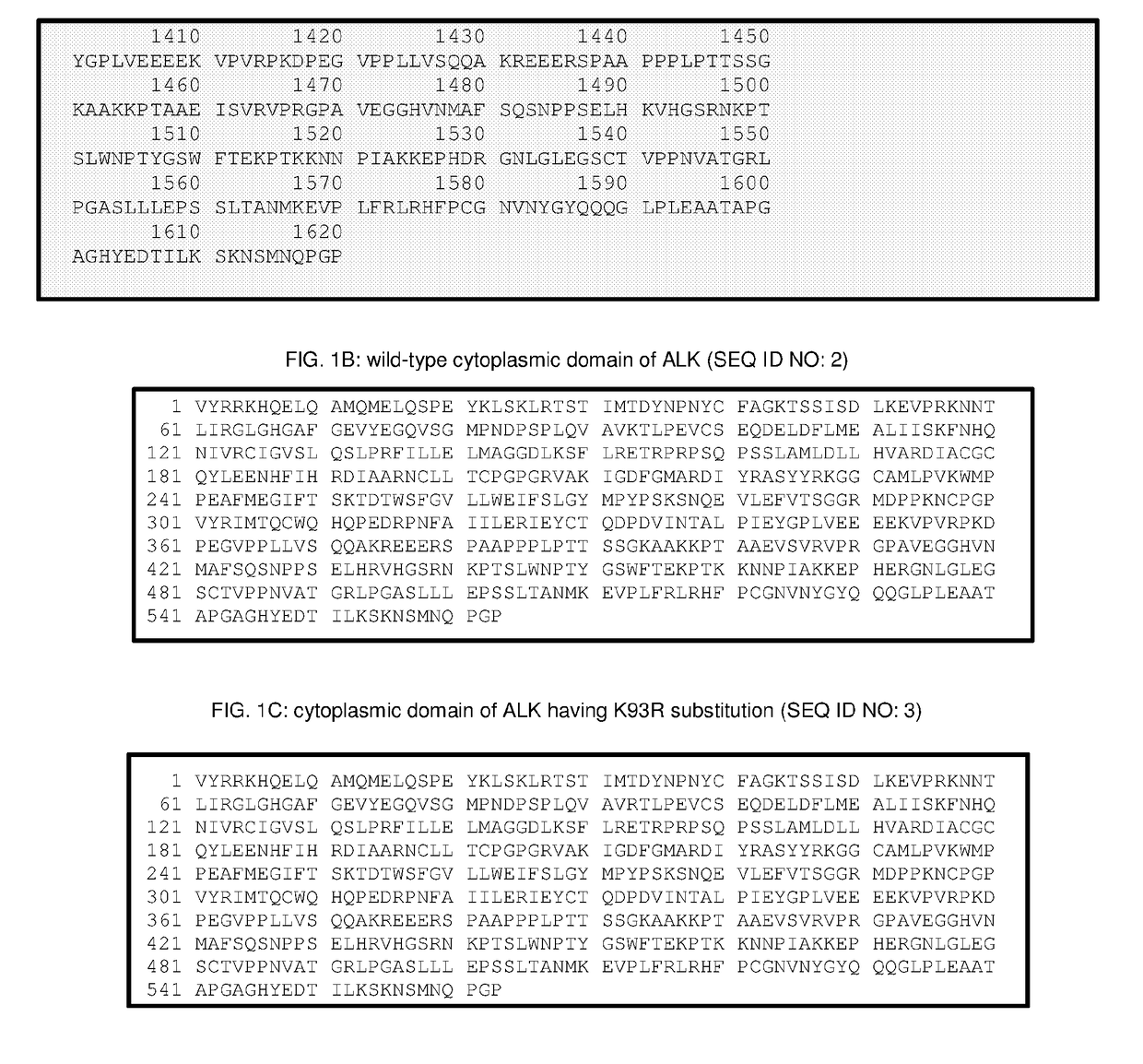
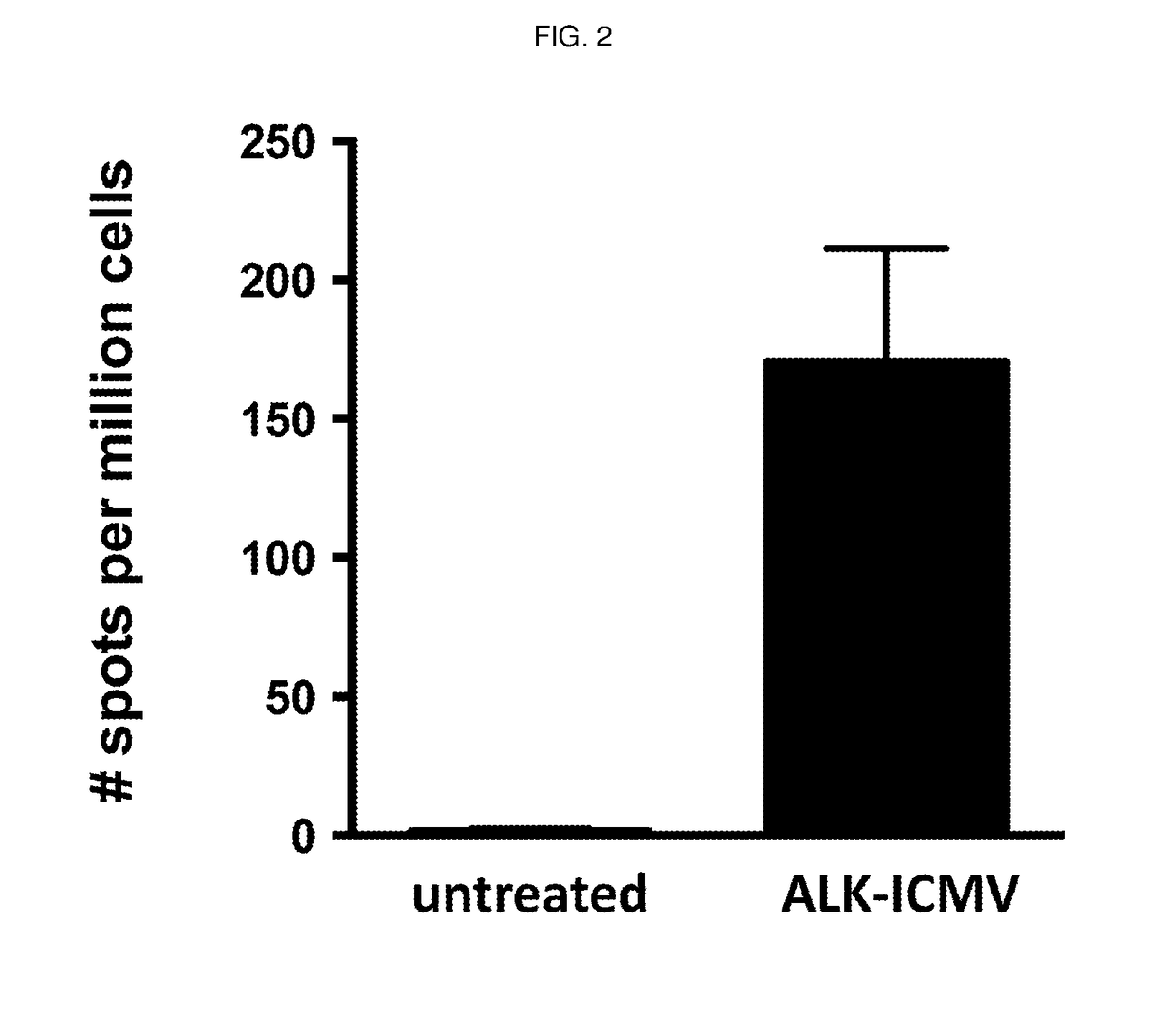
Multilamellar lipid vesicle compositions including a conjugated anaplastic lymphoma kinase (ALK) variant and uses thereof
InactiveUS20180000906A1Polypeptide with localisation/targeting motifOrganic active ingredientsLipid formationAntigen
The invention provides compositions including stabilized multilamellar lipid vesicles having crosslinked lipid bilayers (referred to herein as interbilayer-crosslinked multilamellar vesicles or ICMV) and including an ALK variant, pharmaceutical compositions containing vesicles (e.g., ICMV) including an ALK variant, and methods of treatment using such compositions. The invention provides compositions including stabilized multilamellar lipid vesicles with crosslinked lipid bilayers (e.g., an interbilayer-crosslinked multilamellar vesicle or ICMV) containing an Anaplastic lymphoma kinase (ALK) variant as an antigen that is associated with solid tumor cancers.
Owner:VEDANTRA PHARMA
Retinal-containing multilamellar vesicle and cosmetic composition comprising same
The present invention relates to a retinal-containing multilamellar vesicle and, more specifically, to a retinal-containing multilamellar vesicle comprising 0.01-5% by weight of retinal, 0.1-10% by weight of rapeseed sterol, 0.1-10% by weight of phytosteryl / behenyl / octyldodecyl lauroyl glutamate, 0.1-8% by weight of polyglyceryl-10 oleate, 0.1-10% by weight of cholesterol, 0.01-5% by weight of hydrogenated lecithin, 0.01-5% by weight of ceramide, 1-15% by weight of squalane, 0.1-10% by weight of vegetable butter, 3-30% by weight of oily liquid, 1-20% by weight of glycerin, and 30-70% by weight of water, and to a cosmetic composition comprising same.
Owner:DAREUN COSMETICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com