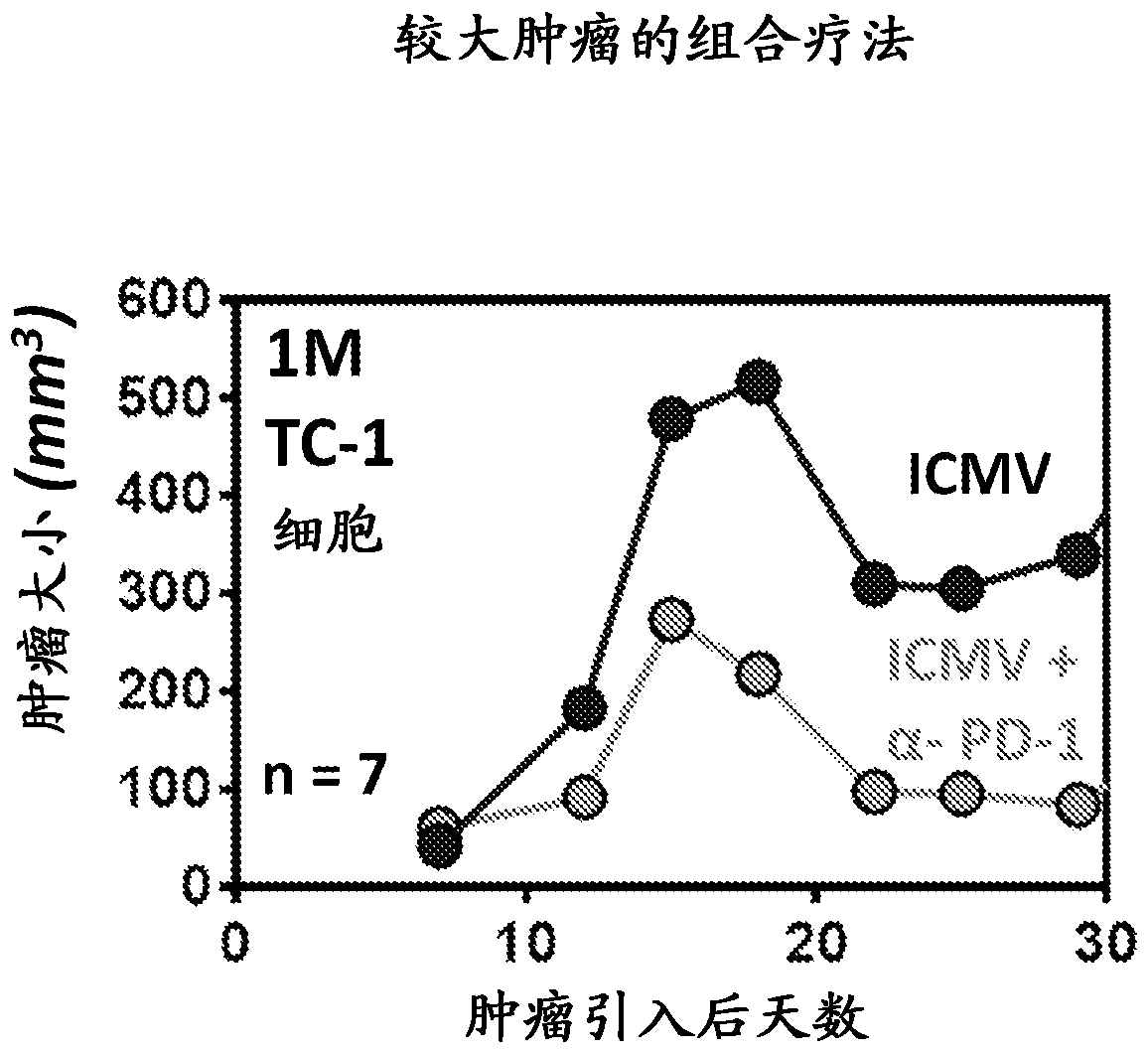
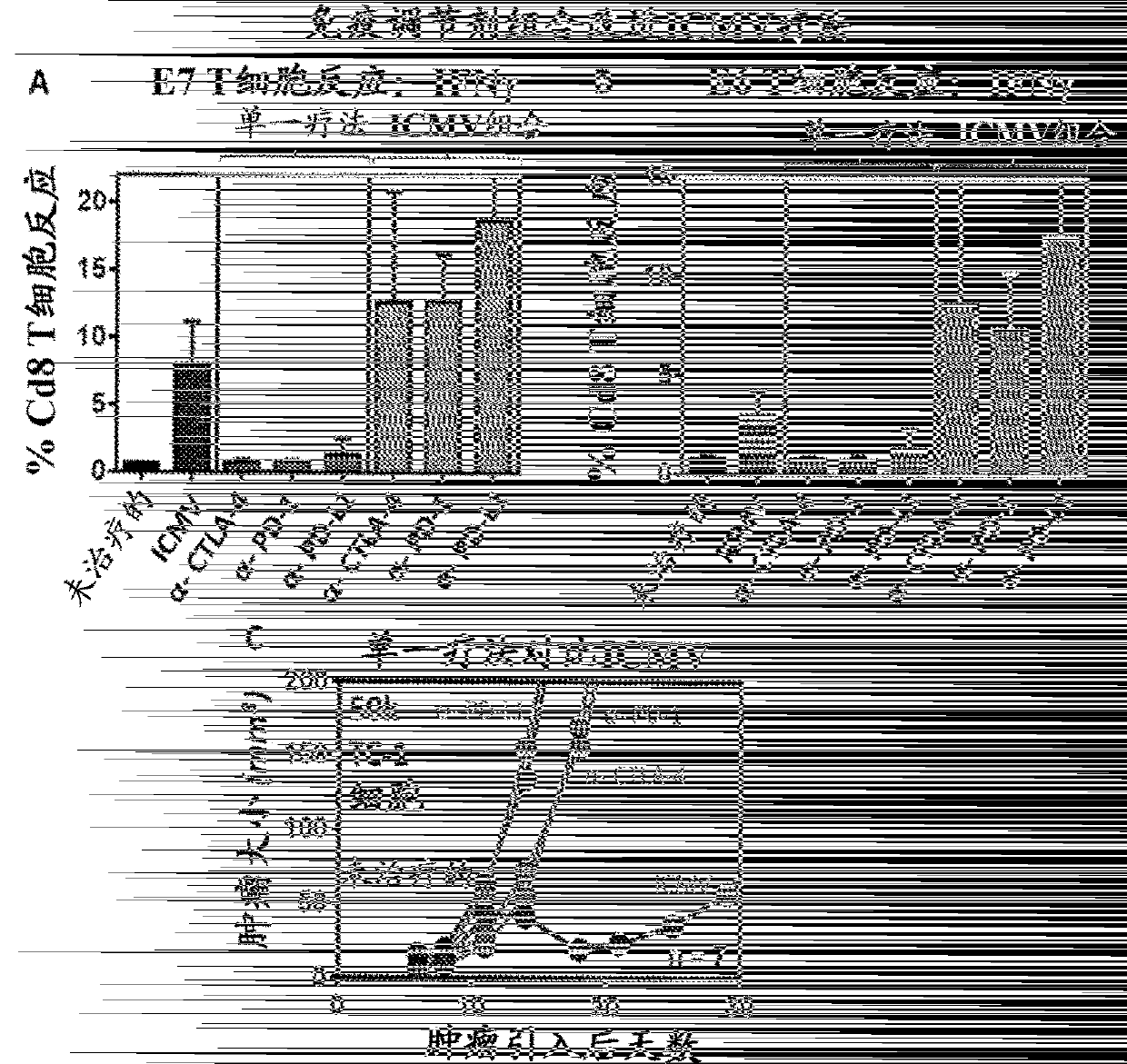
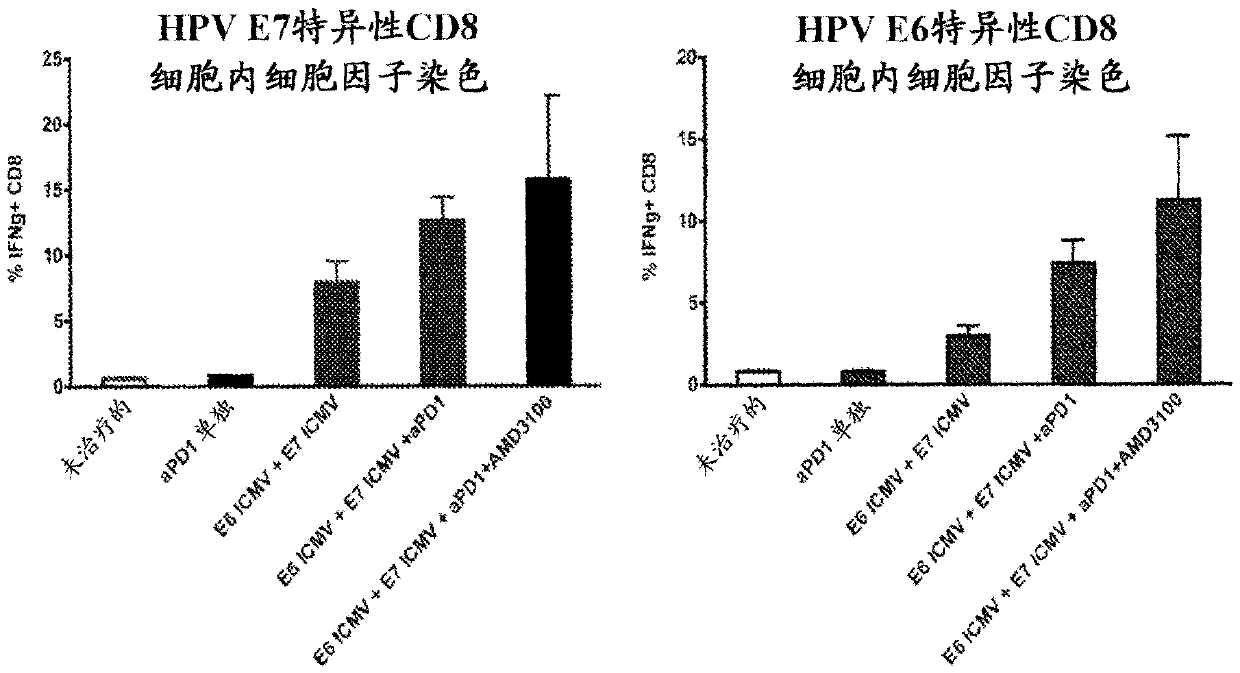
Multilamellar lipid vesicle compositions and methods of use
A technology of lipid vesicles and compositions, which is applied in the field of multilamellar lipid vesicle compositions and uses, can solve problems such as high risks, and achieve the effect of improving encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0346] Example 1. Synthesis of ICMV and Conjugated Reagents
[0347] Material:
[0348] (a) DOPC (5mg / ml in CHCl 3 Medium - Avanti Polar Lipids #850375)
[0349] (b) MPB (10mg / ml in CHCl 3 Medium - Avanti Polar Lipids #870012)
[0350] (c) 3M-052 (0.1mg / ml in CHCl 3 Medium) or MPLA / GLA (0.5mg / ml in MeOH)
[0351] (d) (PEG 2k)-SH (100mg / ml in H 2 Om-Laysan Bio#MPEG-SH-2000)
[0352] (e) dithiothreitol (150mM in H 2 O Medium - Sigma Aldrich #43819)
[0353] (f) CaCl 2 (200mM in H 2 O Medium - Fischer Scientific #BP510)
[0354] (g) Reagents at appropriate concentrations in 20 mM bis-bis(tris-methylol)methylaminopropane (bis-Tris-propane), pH 7.0 (bTp-Sigma Aldrich #B4679)
[0355] (h) Trout's reagent (10 mg / ml in bTp - Thermo Scientific #26101)
[0356] program:
[0357] DOPC (500 μL), MPB (325 μL), and optional adjuvant components were dried under vacuum in scintillation vials for at least 12 hours, followed by vortexing at room temperature at 3000 rpm for 10 seco...
Embodiment 2
[0362] Example 2. Optimization of Reagent Functionalization
[0363] Ovalbumin with different molar equivalents of Trout's reagent resulted in different functionalization ratios. The amount of free thiols was determined using Elman's analysis.
[0364] Material:
[0365] Ellman's reagent (Pierce); Ellman's reaction buffer is 0.1 M sodium phosphate, 1 mM EDTA, pH 8.0; cysteine hydrochloride monohydrate (Pierce)
[0366] Program:
[0367] Ellman's reagent (4 mg) was dissolved in Ellman's reaction buffer (1 mL). Cysteine standards and samples were added to 96-well plates in triplicate at 20 uL / well. Ellman's reagent (20 μL per well) and PBS (60 μL per well) were added and incubated for 15 minutes. Absorbance was read at a fixed wavelength of 412 nm (reference wavelength 540 nm). The results are shown in Table 2.
[0368] Table 2. Functionalization of Ovalbumin
[0369]
[0370] Ovalbumin has an average of 0.35 molecules of added sulfur per molecule when it is ...
Embodiment 3
[0375] Example 3. Optimization of Reagent Conjugation
[0376] Experiments were performed to determine whether increasing the time for reagents to be conjugated to the fluidized lipids would increase encapsulation efficiency. The results are shown in Table 4.
[0377] Table 4. Optimization of Reagent Conjugation Time
[0378] sample
[0379] Due to the high reactivity of the maleimide groups on ICMVs with free thiol groups, the reaction of thiol-containing reagents with ICMVs is usually limited to 1 h, but since functionalization of large protein molecules is slow, it was found to provide More time for this reaction, an increase in protein loading efficiency of up to 47% occurred. Thus, the desired encapsulation efficiency is achieved during the 24 hour incubation period, resulting in increased conjugation of the reagents.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com