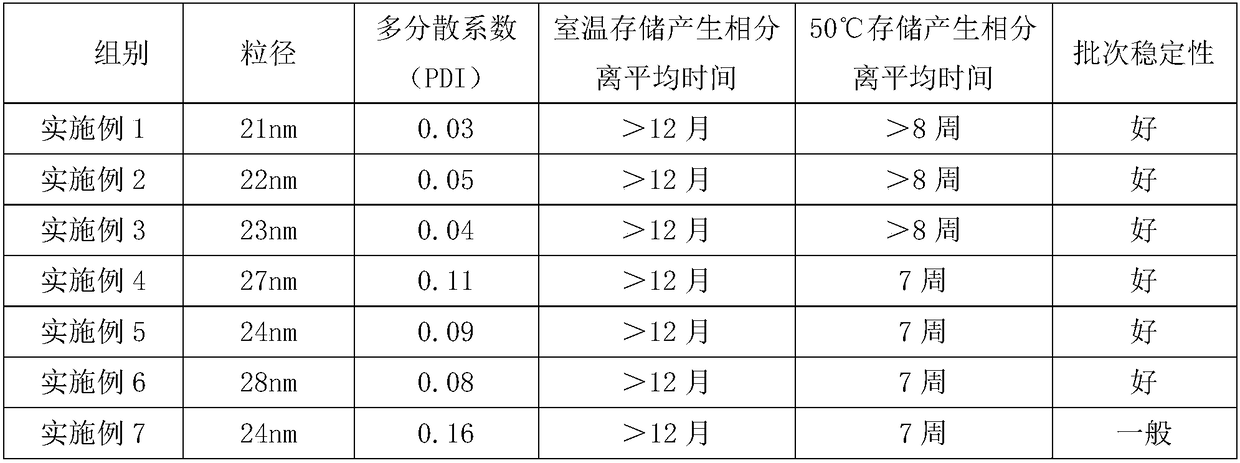
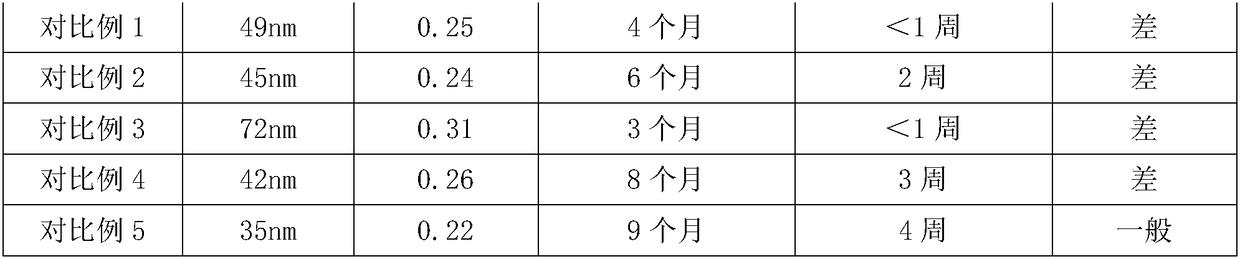
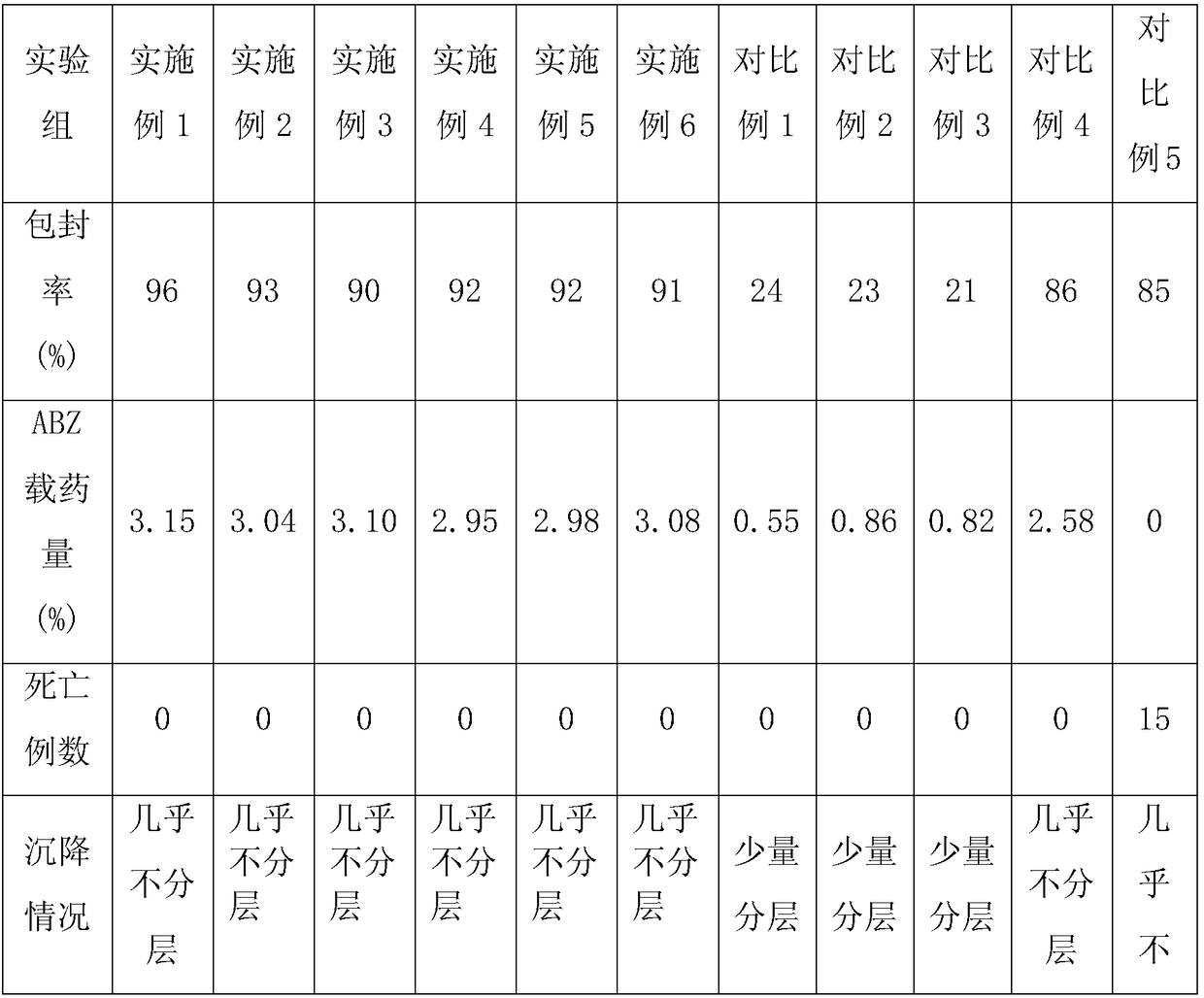
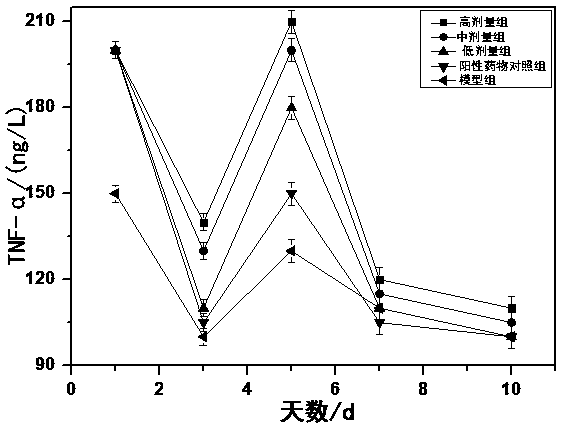
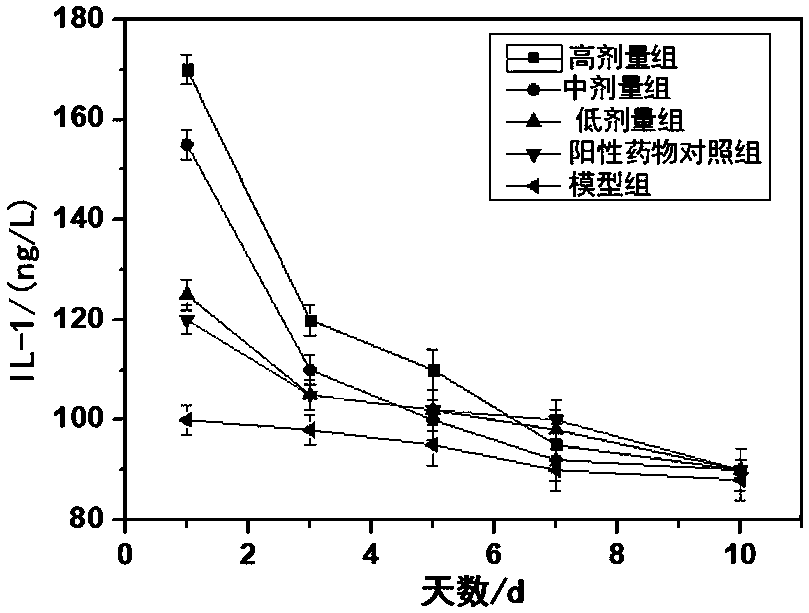
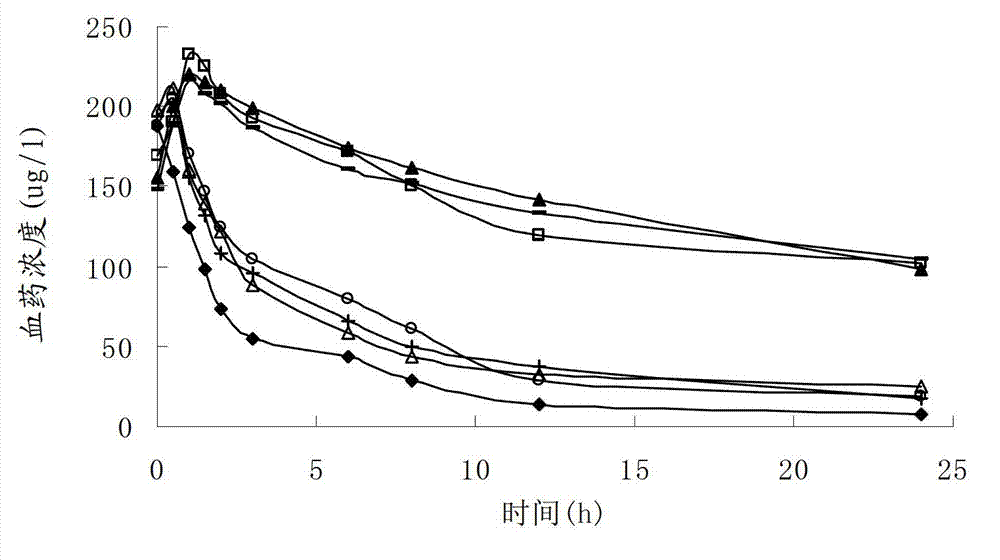
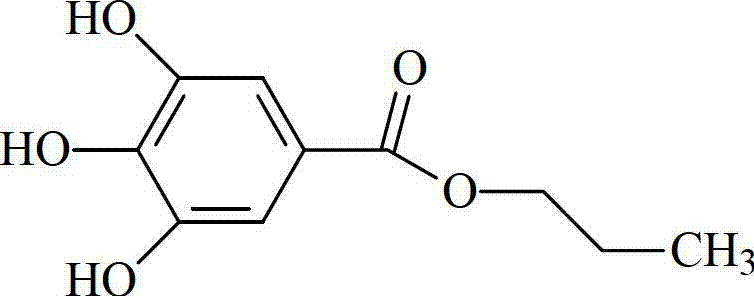
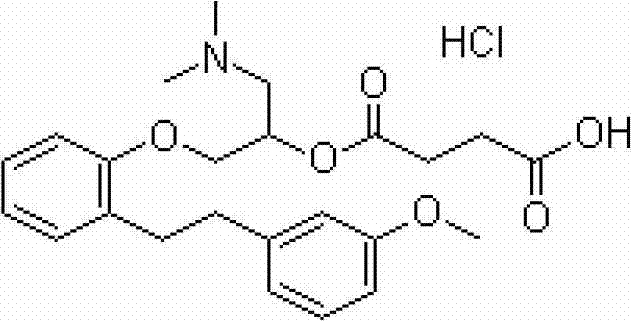
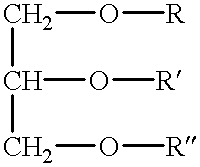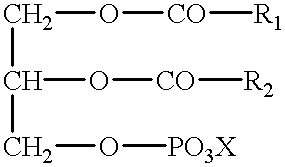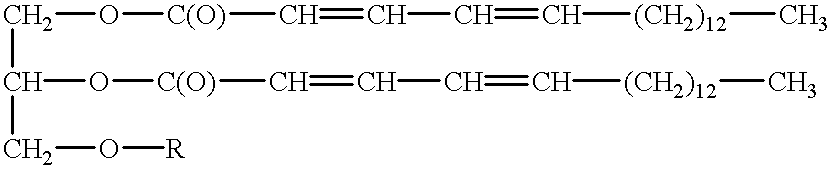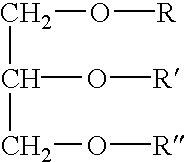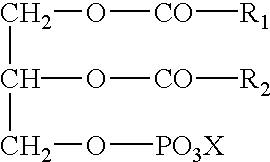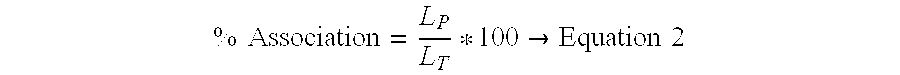Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Phosphatidyl Glycerol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
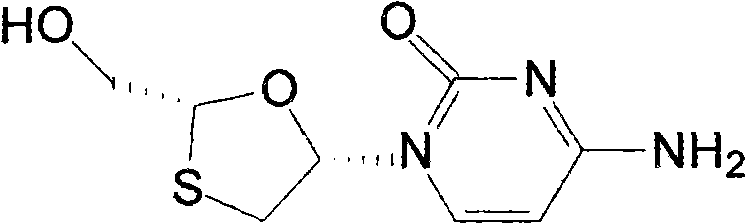
Cardiolipin (IUPAC name "1,3-bis(sn-3’-phosphatidyl)-sn-glycerol") is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition.
Polymerizable fatty acids, phospholipids and polymerized liposomes therefrom
InactiveUS6187335B1Improve stabilityImprove abilitiesFatty acid chemical modificationOrganic chemistryIntestinal structureLipid formation
The invention relates to an oral drug delivery system which delivers biologically active substances to the mucosal tissue of the intestine utilizing novel polymerized liposomes. Novel polymerizable fatty acids having a polymerizable group, a surfactant group, and a functional group, and optionally coupled to ligands which target mucosal tissue in the intestine are disclosed. Novel negatively charged polymerizable lipids which have phosphatidyl inositol (PI), phosphatidyl glycerol (PG) or phosphatidyl serine (PS) groups on a polymerizable backbone are also described.
Owner:DOR BIOPHARMA
Polymerizable fatty acids, phospholipids and polymerized liposomes therefrom
InactiveUS6500453B2Improve stabilityImprove abilitiesBiocideFatty acid chemical modificationIntestinal structureLipid formation
The invention relates to an oral drug delivery system which delivers biologically active substances to the mucosal tissue of the intestine utilizing novel polymerized liposomes. Novel polymerizable fatty acids having a polymerizable group, a surfactant group, and a functional group, and optionally coupled to ligands which target mucosal tissue in the intestine are disclosed. Novel negatively charged polymerizable lipids which have phosphatidyl inositol (PI), phosphatidyl glycerol (PG) or phosphatidyl serine (PS) groups on a polymerizable backbone are also described.
Owner:ORASOMAL TECH
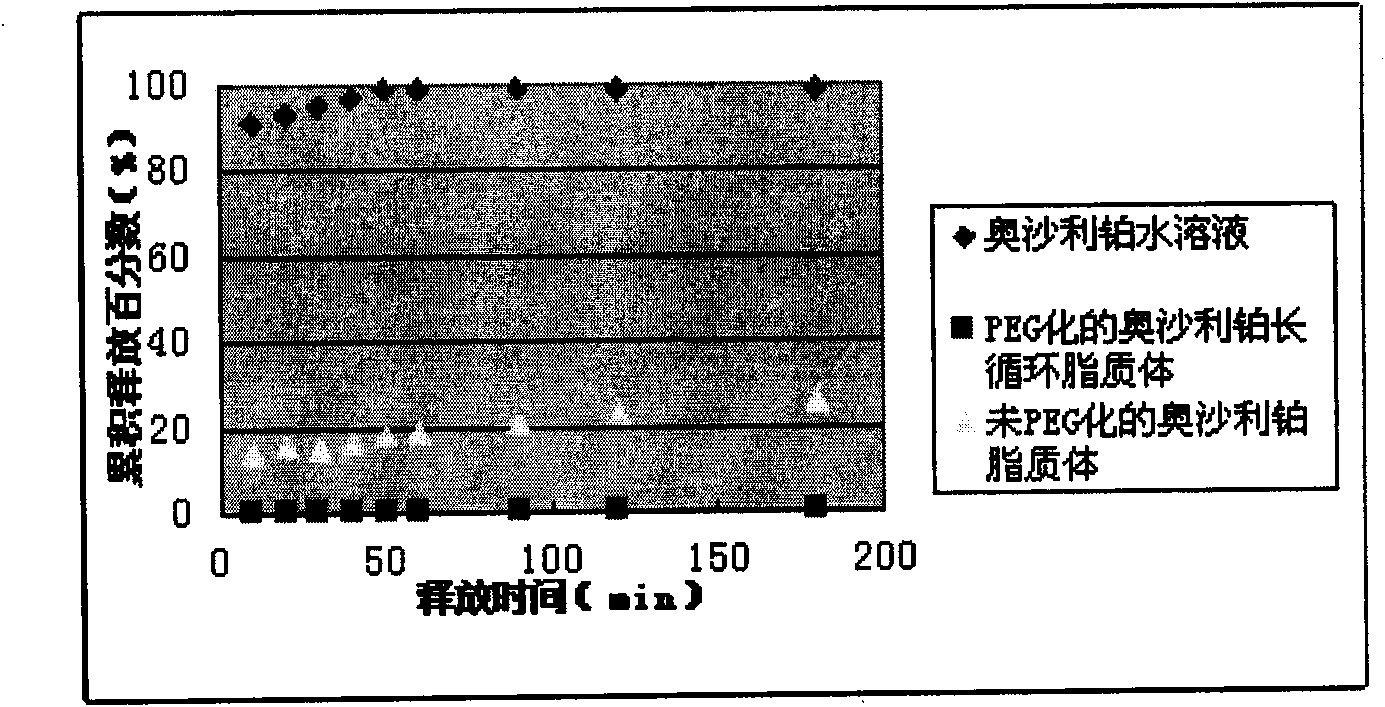
Oxaliplatin liposome, preparation method and application thereof
InactiveCN101897668AHigh encapsulation efficiencyHigh drug loadingAntineoplastic agentsLiposomal deliveryCholesterolLiposomal Oxaliplatin
The invention discloses an oxaliplatin liposome, a preparation method and application thereof. The oxaliplatin liposome contains oxaliplatin, phospholipid and cholesterol, wherein, based on the total weight of the phospholipid, the phospholipid comprises the following components in percentage by weight: 75 to 90 percent of phosphatidylcholine (PC), and 10 to 25 percent of phosphatidyl glycerol (PG) and phosphatidic acid (PA); the weight ratio of the oxaliplatin to the phospholipid is 1:3-20, and the weight ratio of the cholesterol to the phospholipid is 1:2-10.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Pemetrexed disodium liposome injection
InactiveCN103040748AInhibit aggregationLarge particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityMedicine
The invention discloses pemetrexed disodium liposome injection which is mainly made of pemetrexed disodium and a preparation method thereof, distearoyl phosphatidyl glycerol, dipalmitoyl phosphatidyl choline, PEG600, cholesterol and mannitol. The liposome injection has the advantages that the particle size of liposome is small, the liposome is uniformly distributed, the encapsulation efficiency is high, the leakage rate is low, the stability is good, the solubility of the pemetrexed disodium and the quality of injection products are improved, the toxic and side effect is reduced, and the curative effect is improved.
Owner:海南路易丹尼生物科技有限公司
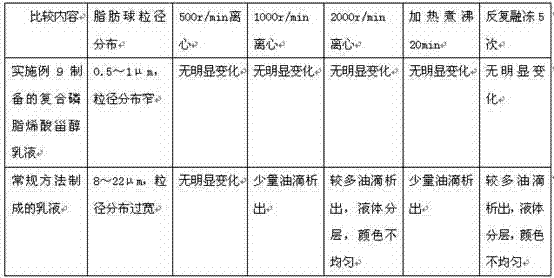
Water-based emulsion containing lecithin and DHA and preparation method and application of water-based emulsion
InactiveCN104490773AImprove bioavailabilityCosmetic preparationsOrganic active ingredientsGlycerolConjugated linoleic acid
The invention provides an emulsion composition containing lecithin, DHA, other fat-soluble substances and purified water. The content of the purified water in the emulsion composition is more than 70% by weight, the weight ratio of the lecithin to the DHA is 1: (0.005-1), the particle size of fat balls in the emulsion is less than 5 mu m, and the other fat-soluble substances comprise one or more of the following components: arachidonic acid, linolenic acid, alpha-linolenic acid, gamma-linolenic acid, linoleic acid, conjugated linoleic acid, conjugated linoleic acid glyceride, phosphatidylserine, polyene phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl inositol, phosphatidyl glycerol, phytosterol, plant sterol ester, plant stanol ester and medium and long-chain fatty acid. The invention further provides a preparation method of the emulsion composition and application of the emulsion composition in the functional aspects of supplementing phospholipids and unsaturated fatty acids by oral administration, protecting heart and brain blood vessels, reducing blood lipids, reducing blood pressure, treating fatty liver and the like.
Owner:FUZHOU QIANZHENG PHARMA
Sorafenib lipidosome freeze-dried injection for injection and preparation method thereof
InactiveCN104523607AImprove bioavailabilitySmall dosePowder deliveryLyophilised deliveryCholesterolFreeze-drying
The invention discloses a sorafenib lipidosome freeze-dried injection for injection and a preparation method thereof. The freeze-dried injection is prepared from the following components in molar ratio: 1 mol of sorafenib, 5-60 mol of phospholipid, 1-30 mol of cholesterol, 1-60 mol of phosphatidyl glycerol, 1-10 mol of a polyethylene glycol surfactant and 50-400 mol of a freeze-drying protecting agent. The preparation method comprises the following steps: sequentially dissolving phospholipid, cholesterol, phosphatidyl glycerol, polyethylene glycol surfactant and sorafenib in an organic solvent in proportion; then, carrying out rotary and vacuum evaporation on the obtained organic solution in a round bottom glass bottle, so that a uniform thin film is formed on the inner wall of the glass bottle; then, adding a buffer liquid with the pH of 7-10 into the glass bottle to be continuously stirred to form a suspension; reducing the grain size of the suspension by an ultrasonic or high-pressure homogenizing method; and adding the freeze-drying protecting agent and freeze-drying. The sorafenib lipidosome disclosed by the invention remarkably improves the bioavailability and is stable in property, and the encapsulation efficiency can reach over 96%.
Owner:TIANJIN ZHONGXI MEIHUA BIOMEDICAL TECH
Liquid crystal gel nano-particle capable of wrapping drugs with different polarities and preparation method of nano-particle
ActiveCN108403664ANo inflammatory responseHigh encapsulation efficiencyPharmaceutical non-active ingredientsMicrocapsulesCrystallographyAlcohol
The invention discloses a liquid crystal gel nano-particle capable of encapsulating drugs with different polarities. The liquid crystal gel nano-particle comprises, in weight percent, 0.1-0.5wt% of drug active components, 1%-3% of dilinolein, 2%-4% of phosphatidylcholine, 0.3%-1% of dilaurate phosphatidyl glycerol, 0.05%-0.5% of non-ionic surface active agents, 0.1%-1% of ethyl alcohol and the rest water, wherein an HLB (hydrophile-lipophile balance) value of the non-ionic surface active agents is 12-18. The liquid crystal gel nano-particle has high encapsulating rate for different drugs and is good in storage stability and low in cytotoxicity.
Owner:武汉百纳礼康生物制药有限公司
Alprostadil freeze-drying cream and preparation method thereof
ActiveCN104173301AEasy to operateImprove stabilityOrganic active ingredientsPowder deliveryFreeze-dryingFreeze dry
The invention discloses alprostadil freeze-drying cream which comprises the following components in parts by weight: 1 part of alprostadil, 2000-20000 parts of injection oil, 400-4000 parts of phosphatidylcholine as emulsifier, 12-120 parts of phosphatidylinositol as phosphatidyl glycerol, and 6000-60000 parts of freeze-drying protecting agent. A prepared product is good in quality stability, the encapsulation efficiency is improved, and a cream grain is not remarkably changed before and after freeze-drying.
Owner:BEIJING LANDAN PHARMA TECH
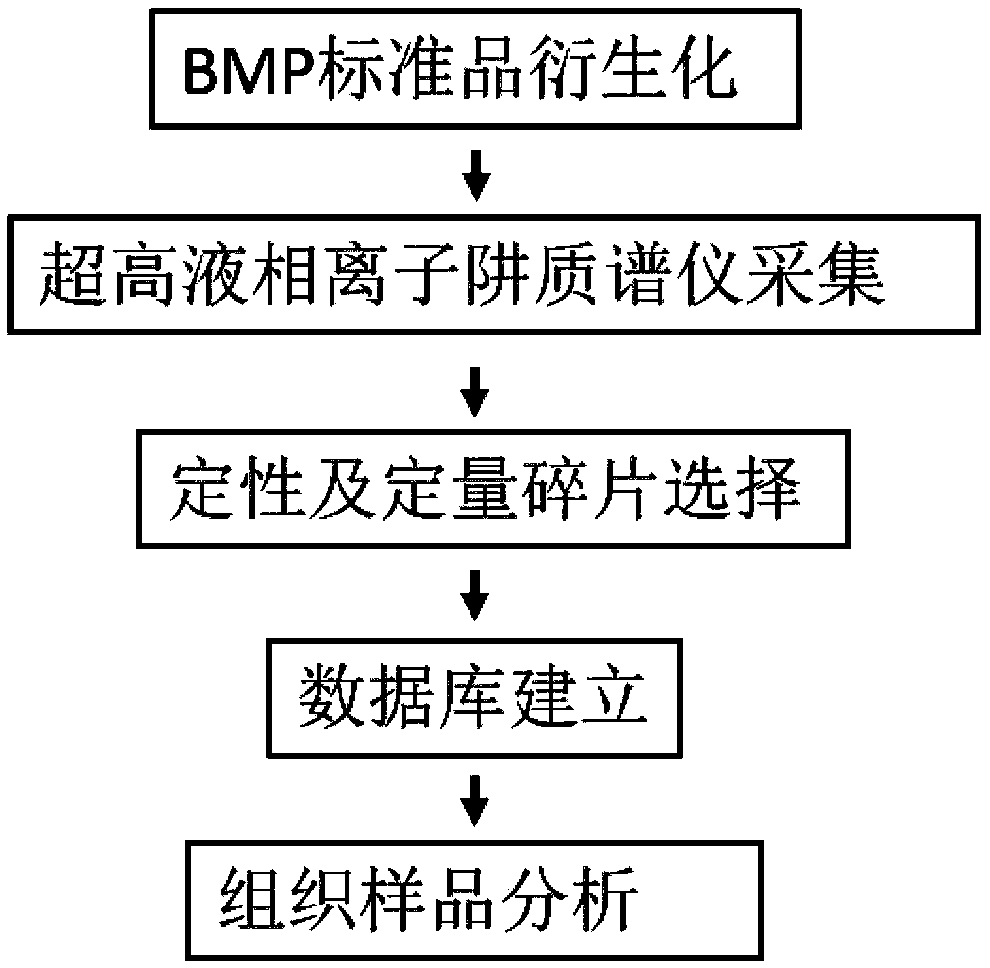
Method for detecting bis-phospholipid in high flux manner
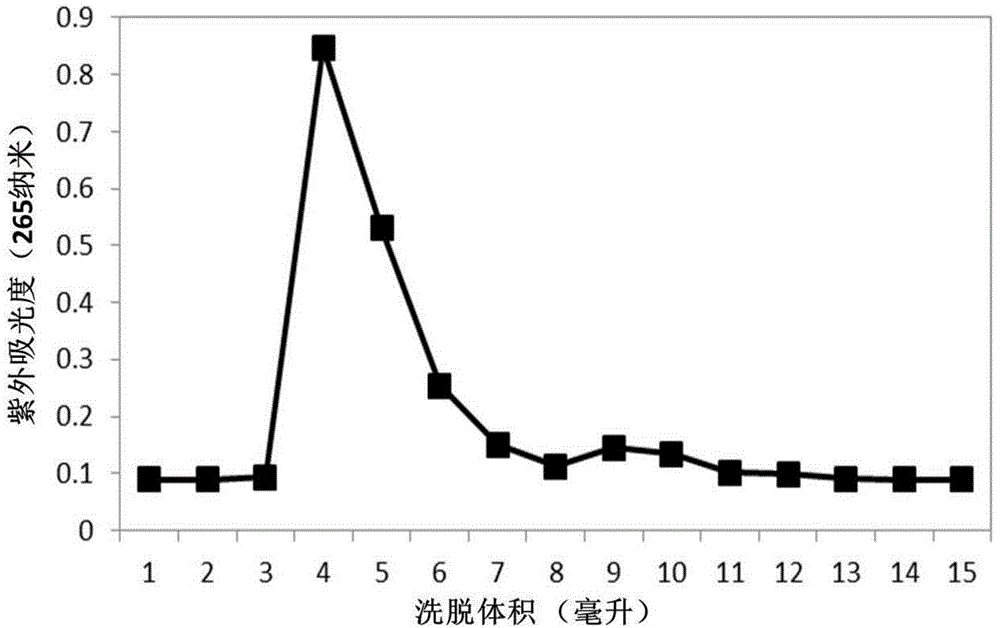
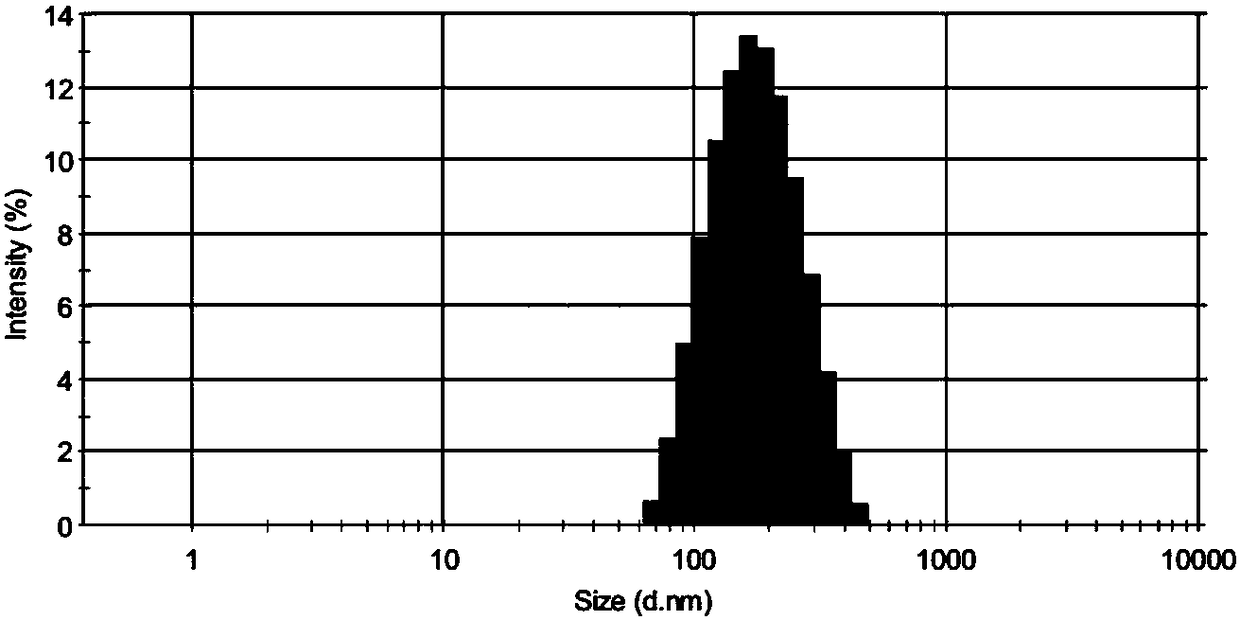
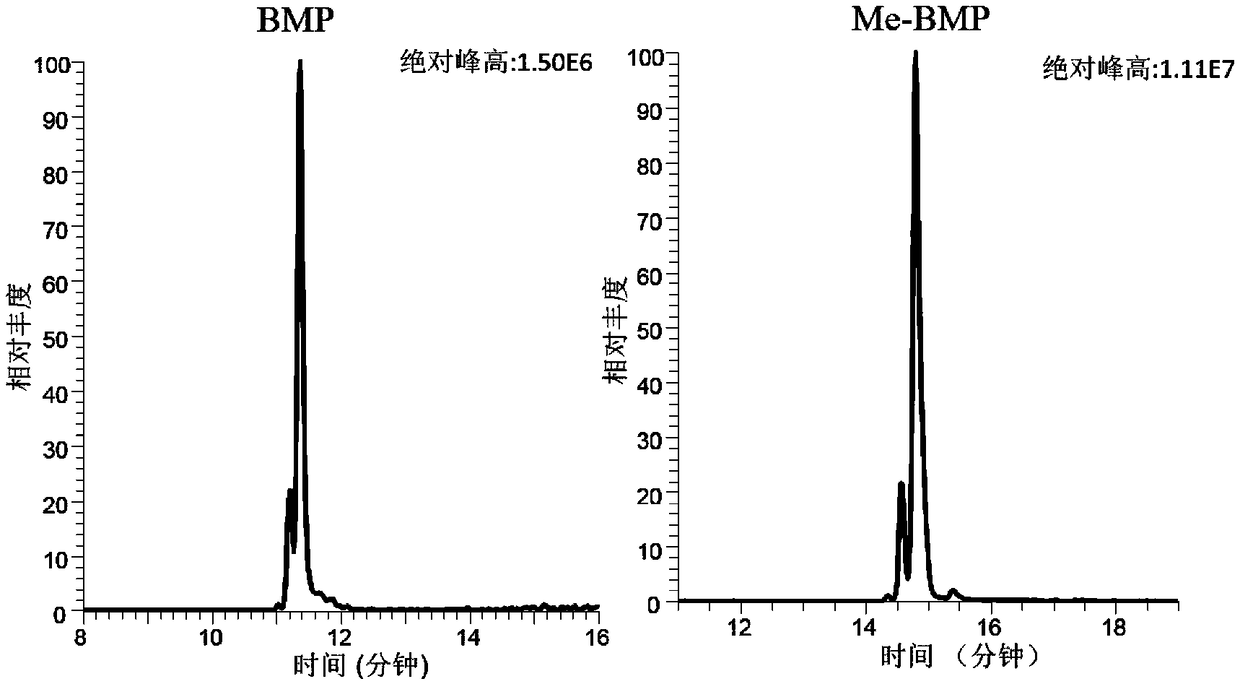
ActiveCN109406687ADerivatization method is stableImprove linearityComponent separationPhospholipidDerivatization
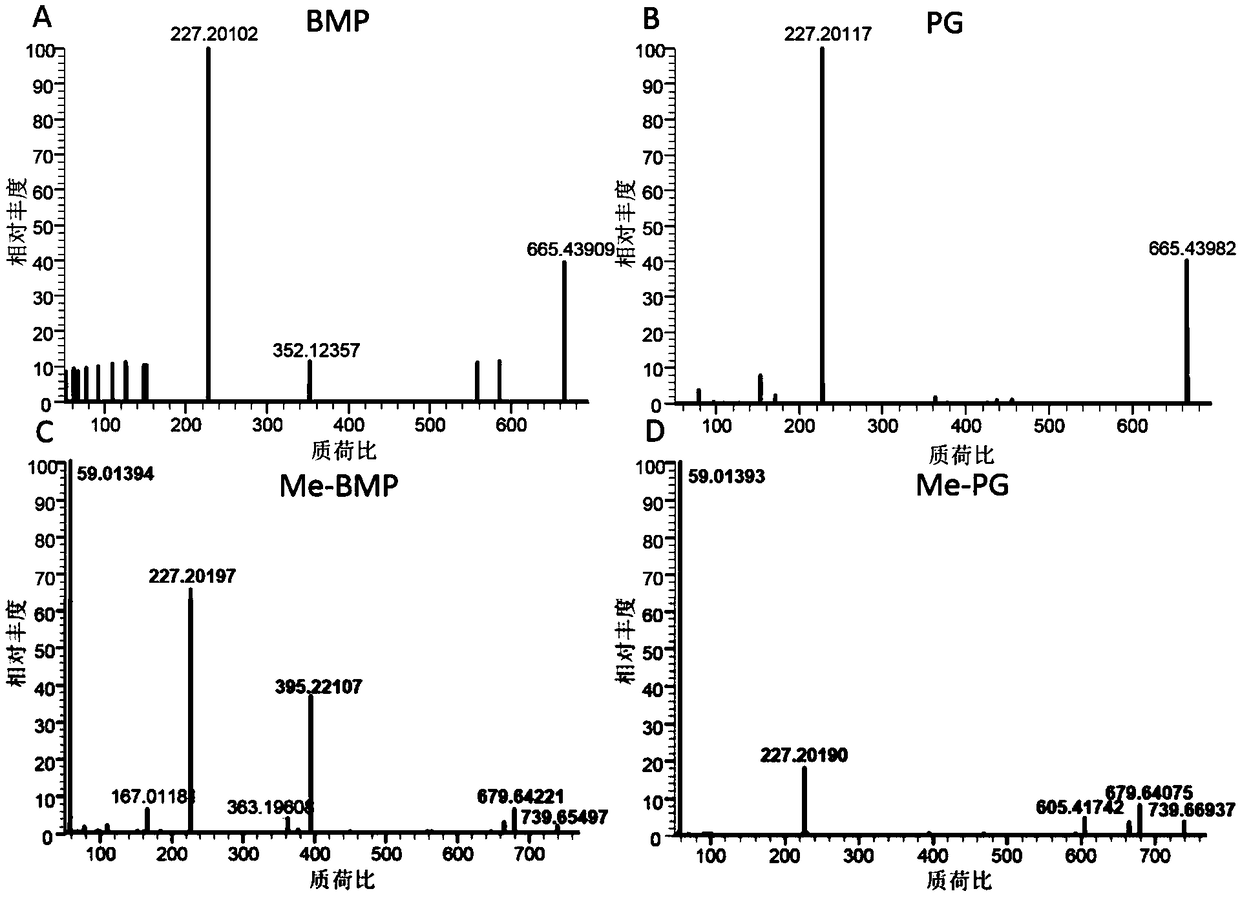
The invention discloses a method for detecting bis-phospholipid in a high flux manner. The method comprises the following steps: separately derivatizating a bis-phospholipid standard product and a phosphatidyl glycerol standard product, separating and detecting the bis-phospholipid and phosphatidyl glycerol standard product by using liquid chromatography tandem mass spectrometry, and establishinga high-flux liquid chromatography tandem mass spectrometry database related to the bis-phospholipid; derivatizating a sample, separating and detecting the sample by using the liquid chromatography tandem mass spectrometry, so as to obtain bis-phospholipid characteristic ions of the sample; and searching the obtained bis-phospholipid characteristic ions in the high-flux liquid chromatography tandemmass spectrometry database, and acquiring the information related to the bis-phospholipid in the sample. According to the method, by establishing the high-resolution database, the high-flux analysisfor BMP can be realized; and by adopting the derivatization method, not only can the signal intensity of the BMP be improved, but also the interference of isomeride can be reduced, and the accuracy ofa data analysis result and the repetition of the data analysis result can be improved.
Owner:TSINGHUA UNIV
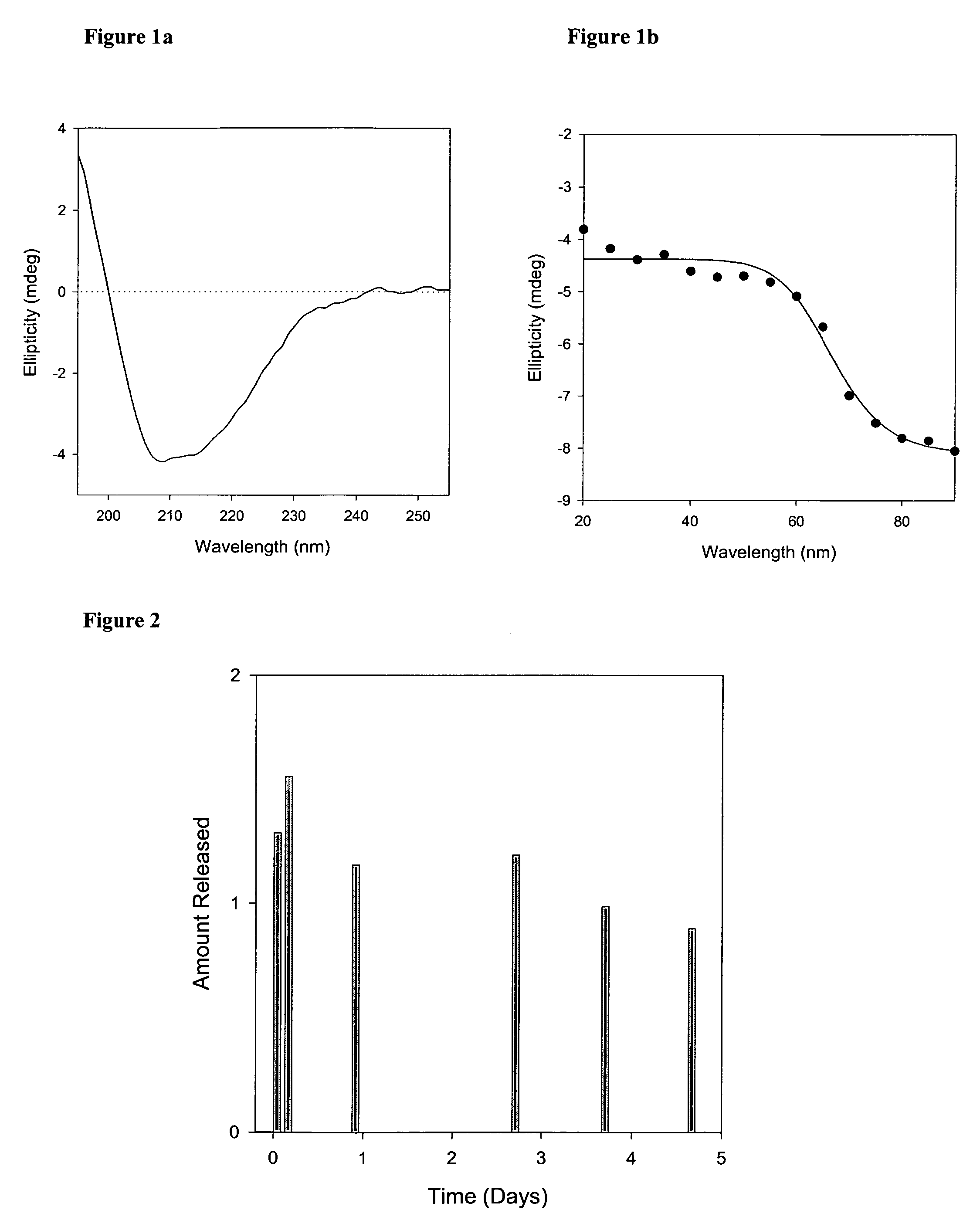
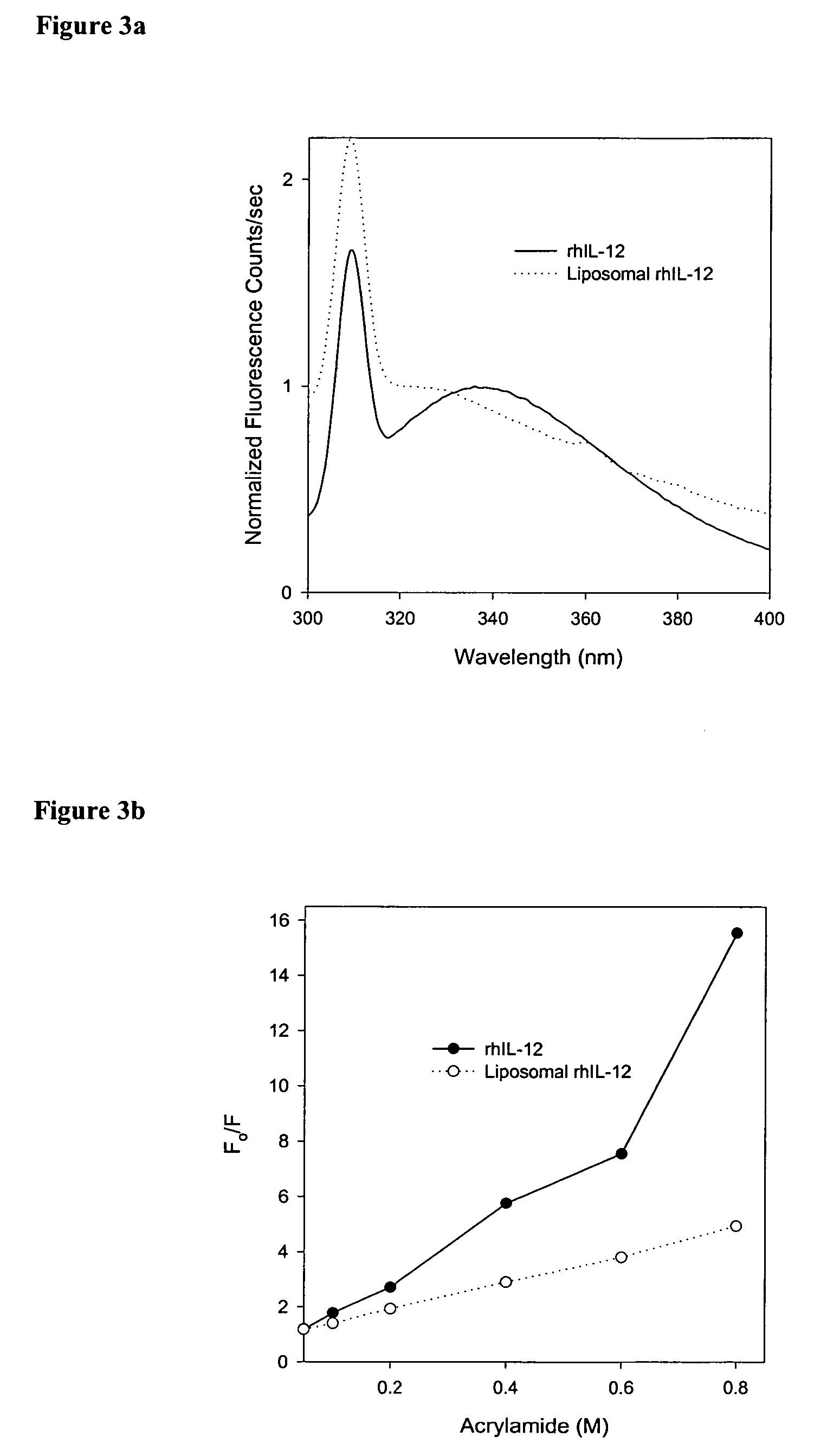
Compositions and methods of preparation of liposomal microparticulate IL-12
ActiveUS7662405B2Enhance electrostatic interactionImprove in vivo stabilityPeptide/protein ingredientsLiposomal deliveryCholesterolLiposome
This invention provides methods and compositions for localized delivery of IL-12 to a desired site. The composition comprises liposomes carrying IL-12. The liposomes comprise phosphatidyl choline, phosphatidyl glycerol and cholesterol. The size and composition of the liposomes is such that there is minimal leakage into the systemic circulation. These compositions can be used for delivery of IL-12 to selected sites such as tumors.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Calcium heparin liposome preparation for injection
InactiveCN102552149ARound shape and not aggregatedImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityCholesterol
The invention discloses a calcium heparin liposome preparation for injection and a preparation method thereof. The liposome injection is made from the following components in a specific weight ratio: calcium heparin, cholesterol, phosphatidyl glycerol, lecithin, polyoxyethylene (40) hydrogenated castor oil, trehalose, sorbitol and polyvinylpyrrolidone. The liposome injection has good stability; the liposomes are not liable to be dehydrated, fused, crystallized or broken during the freezing process; and the liposomes maintain good encapsulation efficiency and stability after long-term storage.The solubility of calcium heparin is increased, the quality of the product is improved, the toxic and adverse effects are reduced, the retention time of drugs in the systemic circulation is increased, the bioavailability of drugs is improved, and the efficacy is greatly improved. Besides, the preparation method is simple and suitable for industrial production.
Owner:灵康药业集团股份有限公司
Lamivudine liposome solid preparation
InactiveCN101953773AImprove stabilityHigh dissolution rateOrganic active ingredientsDigestive systemCholesterolChronic hepatitis
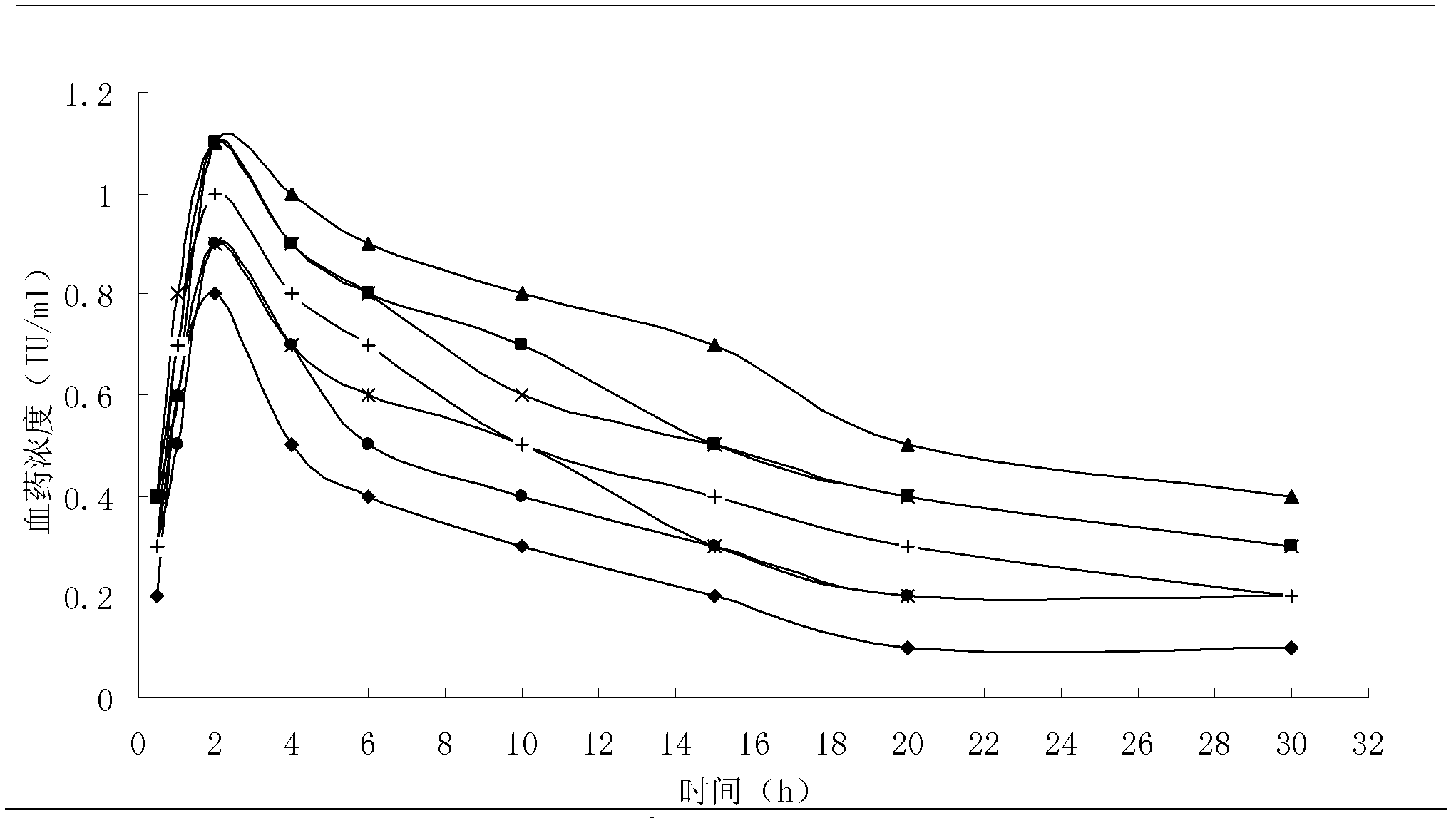
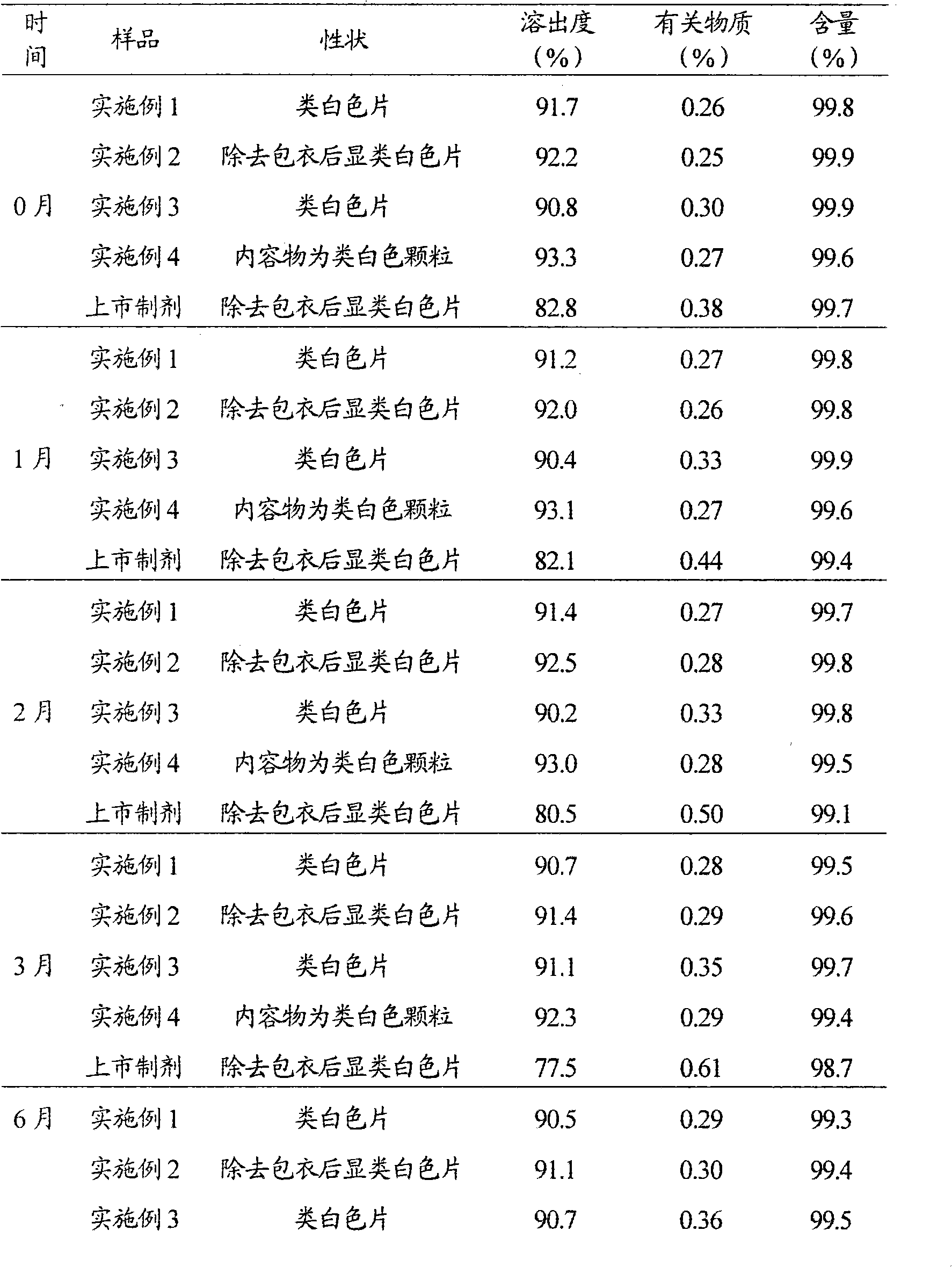
The invention discloses a lamivudine liposome solid preparation and further discloses application thereof in treating chronic hepatitis B. The liposome solid preparation comprises lamivudine, dipalmityl phosphatidyl glycerol, sodium deoxycholate and cholesterol, can be prepared into tablets, dispersible tablets, capsules and the like, has the advantages of high entrapment rate, good stability, improved dissolving rate and the like, and shows favorable safety and drug resistance, better antiviral effects, and lower drug resistant rate and virus bouncing rate on treating the chronic hepatitis B.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Fungal fluorescent staining fluid and its preparation method
ActiveCN107796681AEasy accessHigh fluorescence intensityPreparing sample for investigationBiological material analysisCuticlePotassium hydroxide
The invention discloses a fungal fluorescent staining fluid, which is prepared from, by weight (%), 0.08-0.012% of 5-carboxy flouorescein, 0.045-0.055% of fungal primer ITS4, 8-13% of potassium hydroxide, 28-33% of glycerinum, 0.45-0.55% of diphosphatidyl glycerol, 0.07-0.13% of ascorbic acid, 53.363-63.427% of water. Through adding 5-carboxy flouorescein and fungal primer, the fungal primer ITS4forms a stem ring structure under a free state, thus the fluorescent recession can be effectively avoided; 5-carboxy flouorescein in the of potassium hydroxide solution can penetrate through a fungalcell wall and a cell film to generate specificity combination with alkali group of the ribonucleic acid gene interval zone of fungal ribosome; at the moment, the stem ring structure is opened and shows fluorescence through microscopic examination under a fluorescence microscope; potassium hydroxide can smelt fungal cuticle in the dyeing process, thus the fungal primer ITS 4 can rapidly enter the internal action of the fungal cell; besides, for the fluorescent recession inhibitor is added in the formula, the fluorescence primer can be kept for a long term after the specificity combination withthe alkali group of the ribonucleic acid gene interval zone of fungal ribosome.
Owner:安徽信灵检验医学科技股份有限公司
Formulations for treating bladder cancer
Compositions and methods for making and using proliposomal and liposomal formulations of chemotherapeutic agents are disclosed. The proliposomal and liposomal formulations of chemotherapeutics, as well as medicaments and dosage forms that include such formulations, can be used with treatment regimens for bladder cancer and urothelial cancer. Hence, the formulations, medicaments, and dosage forms of the invention are suitable to treat bladder cancers by intravesical administration and to treat urothelial cancers. The formulations according to the invention include (a) a taxane (e.g., paclitaxel, docetaxel) or cisplatin, (b) a first phospholipid, dipalmitoyl phosphatidylcholine (DMPC), and (c) a second phospholipid, dimyrsityl phosphatidyl glycerol sodium (DMPG). The proliposomal formulations form liposomes upon contact with an aqueous vehicle.
Owner:TESORX PHARMA LLC +1
Preparation method of artificial phospholipid DSPG
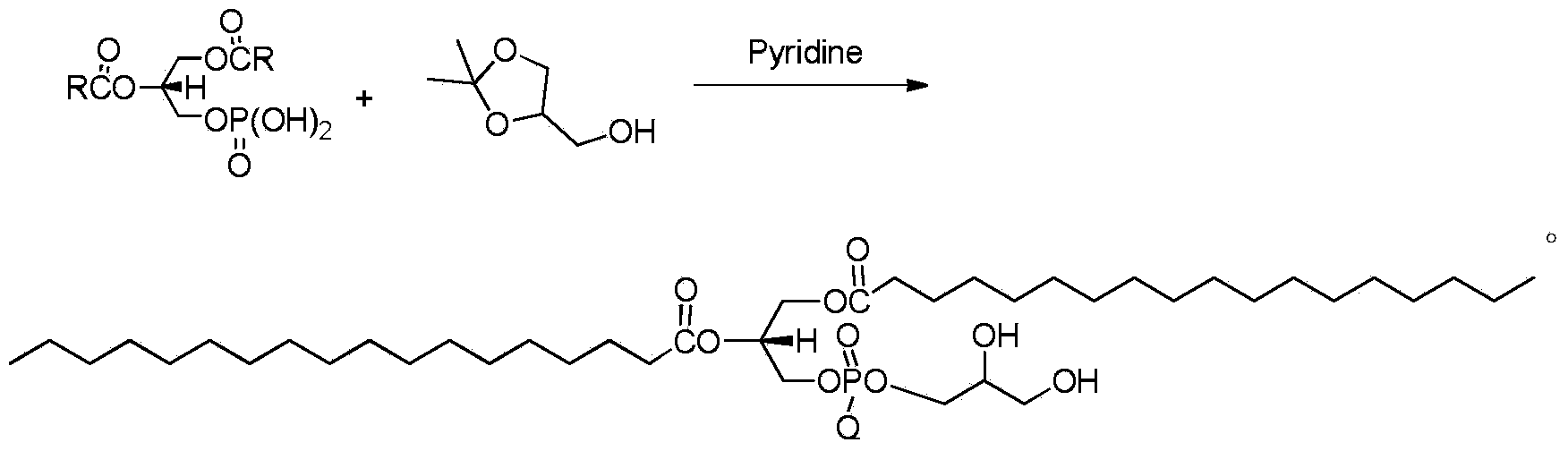
InactiveCN103864840ASimple stepsThe reaction steps are simplePhosphatide foodstuff compositionsBulk chemical productionGlycerolPhosphoric acid
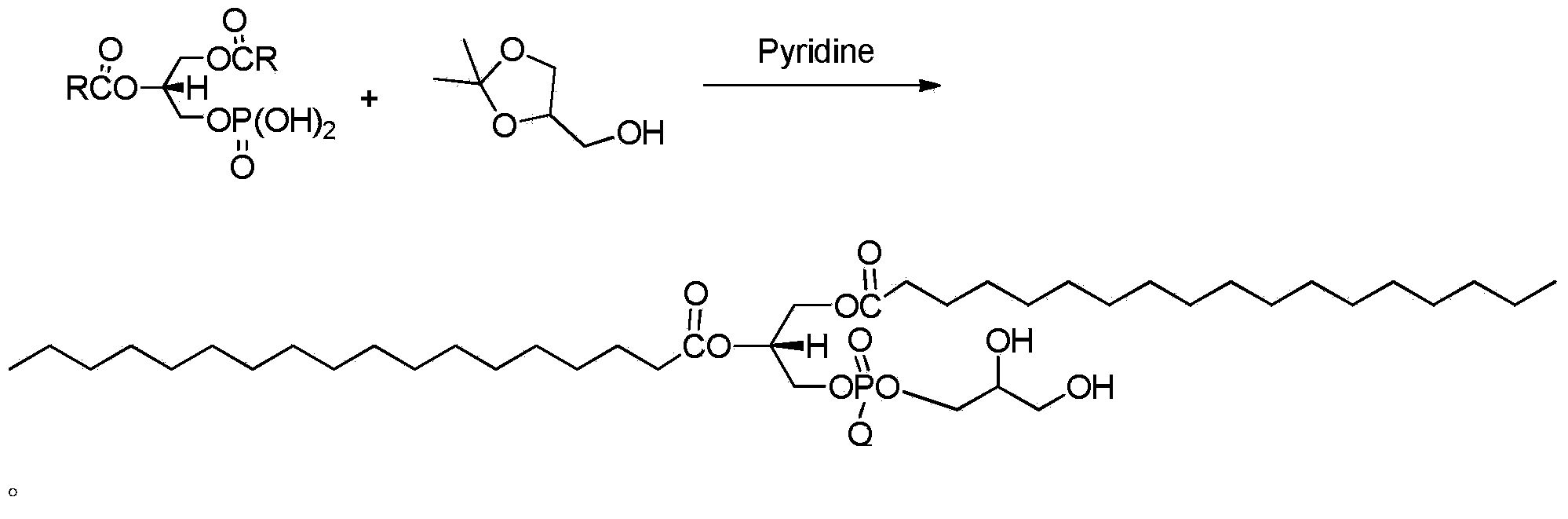
The invention provides a preparation method of artificial phospholipid DSPG. The preparation method comprises the following steps: oxidizing 3-halogenated propylene serving as a raw material so as to obtain (S)-1, 2-diol-3-halogenated propane, then carrying out addition reaction between (S)-1, 2-diol-3-halogenated propane and stearic anhydride or stearoyl halide, introducing phosphate groups with protecting groups, and removing the protecting groups so as to obtain (R)-1, 2-glycerin distearate-glycerin-3-phosphatidic acid; finally carrying out addition reaction between (R)-1, 2-glycerin distearate-glycerin-3-phosphatidic acid and 2, 2-dimentyl-4-methyl alcohol-1, 3-dioxolame in anhydrous pyridine so as to obtain (R)-1, 2-distearoyl phosphatidyl glycerol. The preparation method is simple in steps, mild in reaction and suitable for industrial production.
Owner:苏州东南药业股份有限公司
Cefepime hydrochloride proliposome preparation
InactiveCN101623260AWon't breakEncapsulation efficiency will not decreaseAntibacterial agentsOrganic active ingredientsCefepime hydrochlorideProcess equipment
The invention provides cefepime hydrochloride proliposome which comprises the following components by weight part: 3-20 parts of cefepime hydrochloride, 5-40 parts of dipalmitoyl phosphatidyl glycerol, 1-30 parts of cholesterol and 3-50 parts of proppant which consists of sodium chloride and mannitol with a weight ratio of 1:4. The cefepime hydrochloride proliposome greatly improves the stability, has in-vivo degradation of drug carrier liposome, no toxicity and no immunogenicity and can improve the medical therapeutic index and reduce drug toxicity and side effects. The cefepime hydrochloride proliposome can be prepared by conventional processing equipment and industrially and efficiently produced and has low production cost.
Owner:HAINAN LINGKANG PHARMA CO LTD
Calcium and zinc gluconate liposome oral solution
InactiveCN103070852ASmall particle sizeUniform particle size distributionOrganic active ingredientsMetabolism disorderRetention timeCholesterol
The invention relates to a calcium and zinc gluconate liposome oral solution and its preparation method. The liposome oral solution is prepared from specific weight proportions of calcium gluconate, zinc gluconate, lysine hydrochloride, egg yolk lecithin, soy sterol, phosphatidyl glycerol, cholesterol and mycose. The liposome oral solution has the advantages of good preparation stability, maintenance of the good entrapment rate of liposome after long-term storage, improvement of the preparation product quality, increase of the retention time of medicines in the systematic circulation, improvement of the biological availabilities of the medicines, obvious improvement of the curative effect, simple preparation method and suitableness for the industrialized production.
Owner:海南路易丹尼生物科技有限公司
Olmesartan ester liposome solid preparation
InactiveCN103040777AImprove product qualityUniform particle sizeOrganic active ingredientsPill deliverySide effectCholesterol
The invention discloses an olmesartan ester liposome solid preparation and a preparation method thereof. Active ingredient-olmesartan ester, particularly-combined phosphatidyl inositol, di-stearoyl phosphatidyl glycerol, cholesterol succinate and tween 80 are prepared into liposome, the stability, the dissolution rate and the bioavailability of the preparation is greatly increased, the action is stable and lasting, and the curative effect is significant. The product quality of the preparation is increased, and the toxic and side effects are reduced.
Owner:海南路易丹尼生物科技有限公司
Amino silicone oil emulsion and preparation method thereof
ActiveCN108752935AFast preparationRapid preparation of small particle sizesPolyethylene glycolCocamidopropyl betaine
The invention discloses an amino silicone oil emulsion. The amino silicone oil emulsion comprises, by mass, 42-58% of amino silicone oil, 31-47% of deionized water, 6-10% of a surfactant, 3-1.1% of distearyl phosphatidyl glycerol and 0.5-5% of a pH adjuster. The surfactant comprises, by mass, 10-30% of polyethylene glycol 4000 monooleate, 40-60% of polyoxyethylene sorbitan monolaurate and 20-40% of cocamidopropyl betaine. The viscosity of the amino silicone oil is 2000-15000 mPa.s. The ammonia value is 0.2 to 0.8. The pH of the amino silicone oil emulsion is 6.5 to 7.0. The amino silicone oilemulsion is prepared from glass beads through a double-center mixing disperser, has high content of silicone oil, small particle size distribution and good storage stability and can be used in fabrictreatment.
Owner:江门市润祥纺织科技有限公司
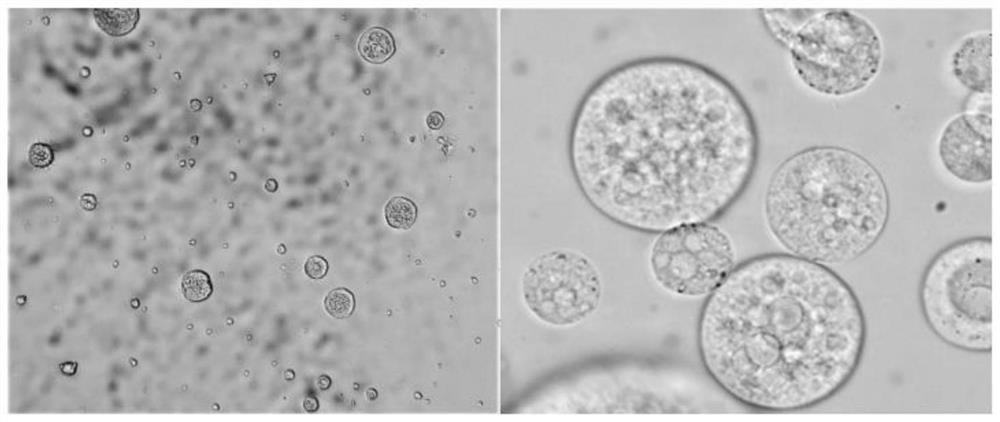
Albendazole liposome and preparation process thereof
InactiveCN109223714APromote absorptionOther effects are smallOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityPhosphatidyl serine
The invention relates to the technical field of veterinary albendazole preparation, in particular to an albendazole liposome and a preparation process thereof. A liposome of albendazole comprise thatfollowing component in parts by weight: 8 to 15 parts by weight of albendazole; 1- Palmitoyl group- 2- 25 to 35 part of oleoyl phosphatidyl glycerol, 6 to 15 part of dipalmitoyl phosphatidyl serine, 10 to 20 part of dierucyl phosphatidylethanolamine, 1 to 5 part of antioxidant, 2 to 6 part of additive. A liposome of albendazole and its preparation method, It can improve the solubility of albendazole, slow down drug release, increase the absorption rate of albendazole, increase the bioavailability of albendazole, increase the targeting and pertinence of the drug, and has high encapsulation efficiency and ABZ drug loading capacity. In addition, the preparation method is simple in production, easy in operation and low in cost.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
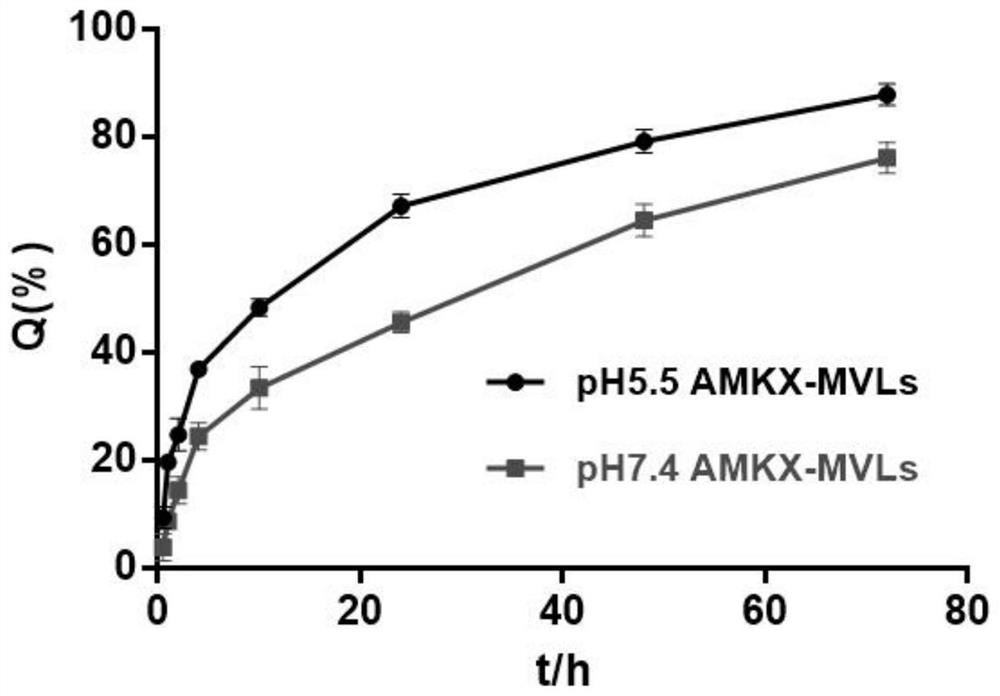
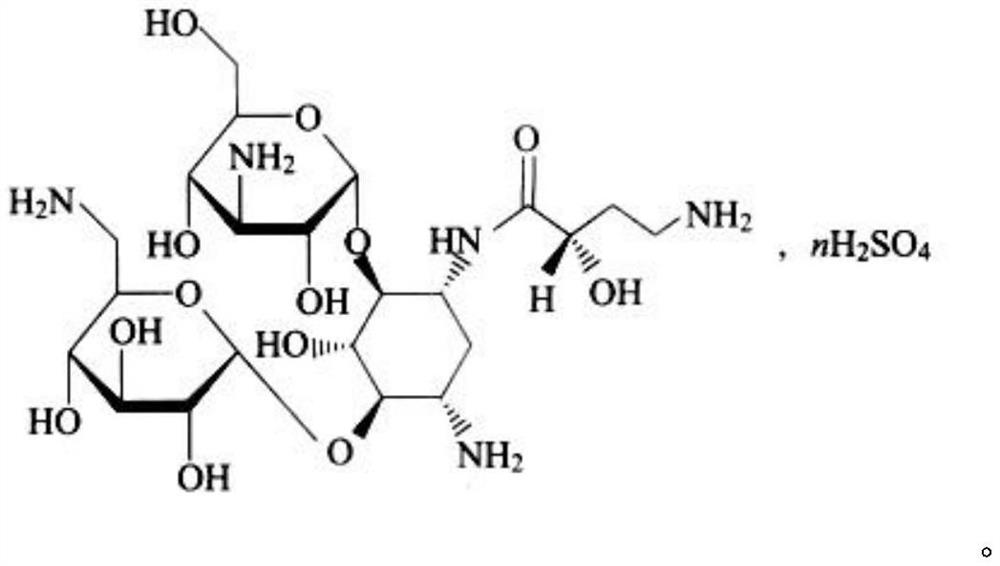
Amikacin sulfate multivesicular liposome for local injection and preparation method of amikacin sulfate multivesicular liposome
InactiveCN113171343AHigh encapsulation efficiencyHigh drug loadingAntibacterial agentsPowder deliveryPhospholipinTG - Triglyceride
The invention provides amikacin sulfate multivesicular liposome for local injection and a preparation method of the amikacin sulfate multivesicular liposome. The amikacin sulfate multivesicular liposome is prepared from the following raw materials in parts by weight: 35 to 180 parts of amikacin sulfate; 35 to 300 parts of a lipid material; 195-410 parts of an osmotic pressure regulator; and 0.50 to 35.50 parts of an auxiliary emulsifier material; and the lipid material is composed of phospholipid, cholesterol, triglyceride and a membrane stabilizer according to the mass ratio of (5-7.5): (3-5.5): (3-6): (1.0-3.0), and the membrane stabilizer comprises at least one of negatively charged phosphatidyl glycerol, fatty acid or stearylamine. The amikacin sulfate multivesicular liposome provided by the invention has the characteristics of high encapsulation efficiency, large drug loading capacity and uniform particle size, can be prepared into a powder injection for injection, can have a better slow release effect, and is beneficial to improving the bioavailability of drugs, so that the curative effect is remarkably improved.
Owner:CHENGDU UNIV
Ginkgolide B lipid microsphere injection
InactiveCN103040742AStable and uniform sustained releaseProtectiveOrganic active ingredientsRespiratory disorderMicrospherePolyethylene glycol
The invention discloses ginkgolide B lipid microsphere injection and a preparation method thereof. The lipid microsphere injection is prepared by ginkgolide B, DOPE (dioleoyl phosphatidyl ethanolamine), polyoxyl (40) hydrogenated castor oil, distearoyl phosphatidyl glycerol and PEG (polyethylene glycol) 400 in a specific weight ratio. The lipid microsphere injection has the advantages of even microsphere particle sizes, good stability and entrapment efficiency, and very low leakage rate; the problems that the ginkgolide B is easy to overoxidize and is prone to hydrolysis failure in vivo are solved; and the preparation method is good in reproducibility and suitable for industrialized production.
Owner:海南路易丹尼生物科技有限公司
Capsaicin liquid crystal nano spray preparation for promoting skin wound healing and preparation method thereof
InactiveCN109820824AModerate viscosityImprove liquidityAntibacterial agentsOrganic active ingredientsCystine knotCapsaicin
The invention discloses a capsaicin liquid crystal nano spray preparation for promoting skin wound healing and a preparation method thereof. The capsaicin liquid crystal nano spray preparation is prepared from, by weight, 0.1-0.5% of capsaicin, 50-70% of a liquid crystal material, 5-10% of chitosan, 5-10% of a surfactant, 10-15% of a cosolvent and the balance water. The liquid crystal material isformed by mixing phosphatidyl glycerol and glyceryl dioleate in the weight ratio of 1:(1-3). The preparation method of the spray preparation comprises the steps that the capsaicin, the liquid crystalmaterial and the chitosan are dissolved and dispersed to form drug-loaded liquid crystal nanoparticles under the action of the cosolvent, the stability of the drug-loaded liquid crystal nanoparticlescan be greatly improved through the above operation, and the slow-release effect is obvious. The chitosan is combined with cysteine in the body to form a film on an affected part, the chitosan also has an antibacterial effect, the effects of isolating and protecting the affected part are achieved, and the action time of the medicine is prolonged, so that the local medicine concentration is obviously increased, the medicine takes effect easily, and wound healing is promoted.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Propyl gallate liposome injection
InactiveCN103040744AHigh encapsulation efficiencyGood formulation stabilityPowder deliveryOrganic active ingredientsEthylene diamineSide effect
The invention discloses propyl gallate liposome injection and a preparation method thereof. The propyl gallate liposome injection with excellent quality is prepared by selecting propyl gallate, dilauroyl phosphatidyl glycerol, cholesterol, tween 80, sodium hydrogen sulfite, ethylene diamine tetraacetic acid (EDTA)-2Na and dextranum. Compared with the conventional preparation, the preparation provided by the invention has the advantages that the stability and the bioavailability of the preparation is greatly improved, the medicine release is stable, the quality of the preparation product is improved, the toxic or side effect is reduced and obvious curative effect is achieved.
Owner:海南路易丹尼生物科技有限公司
Novel target for developing medicaments for treating diabetes, obesity and cardiovascular diseases and biological characteristics thereof
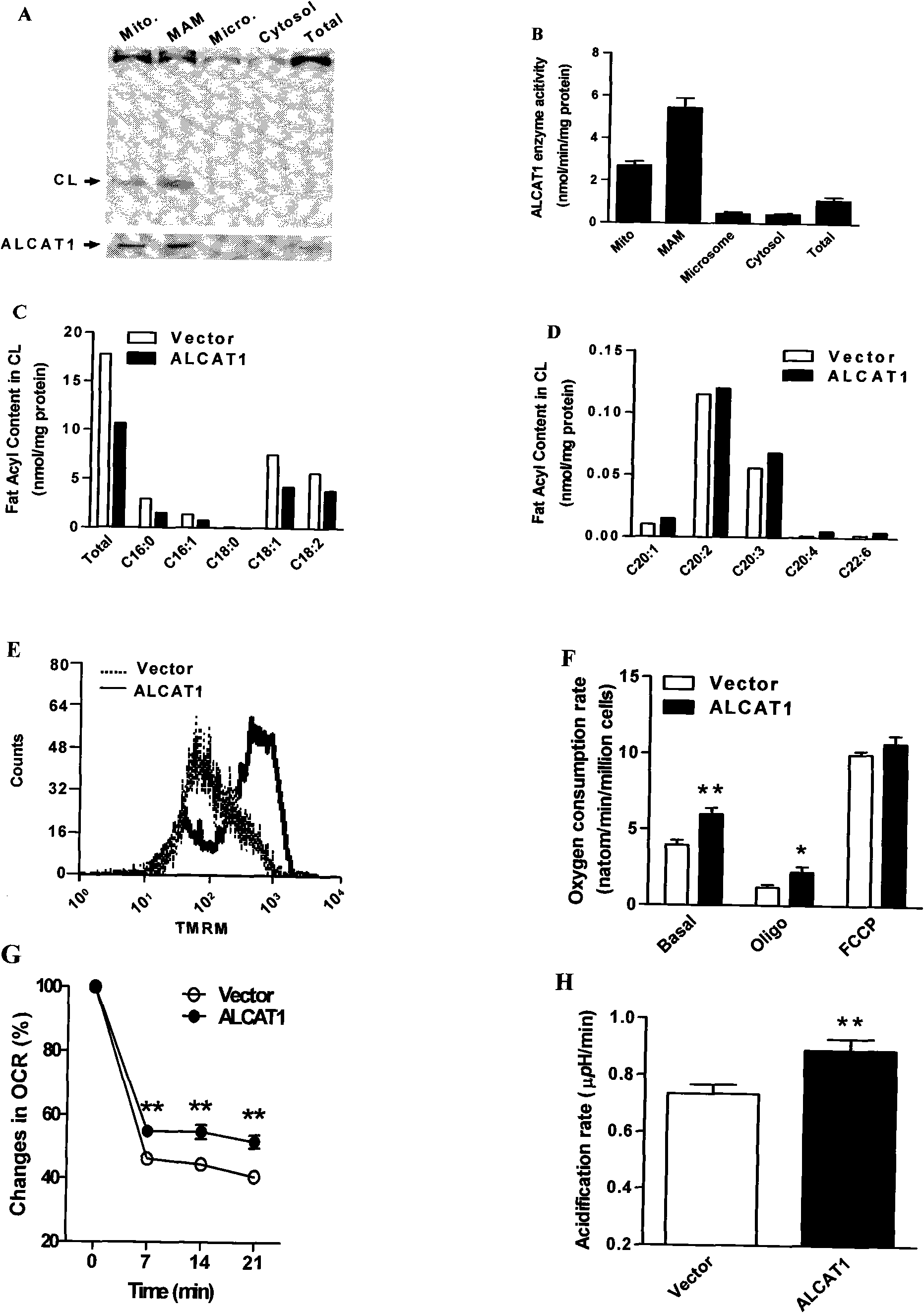
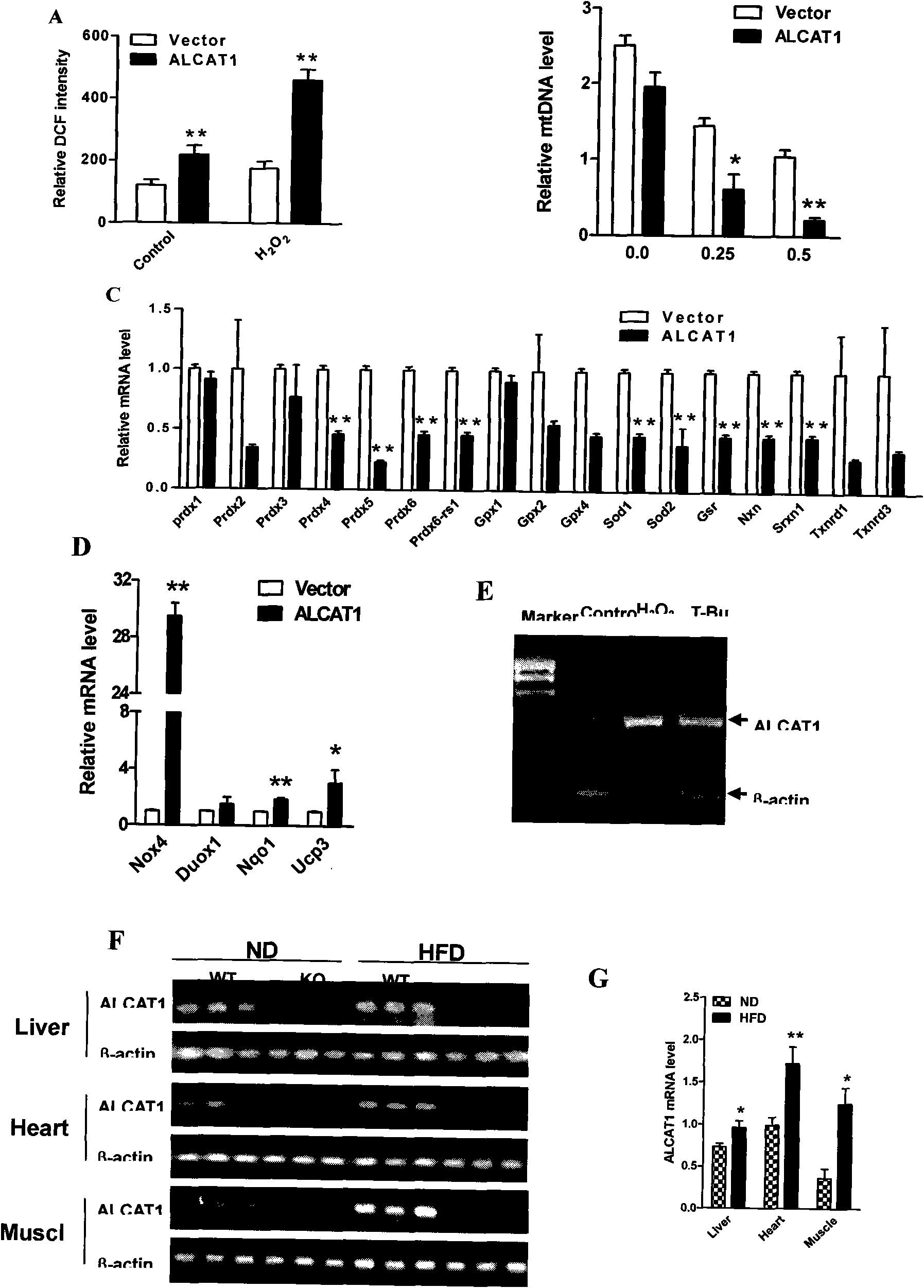
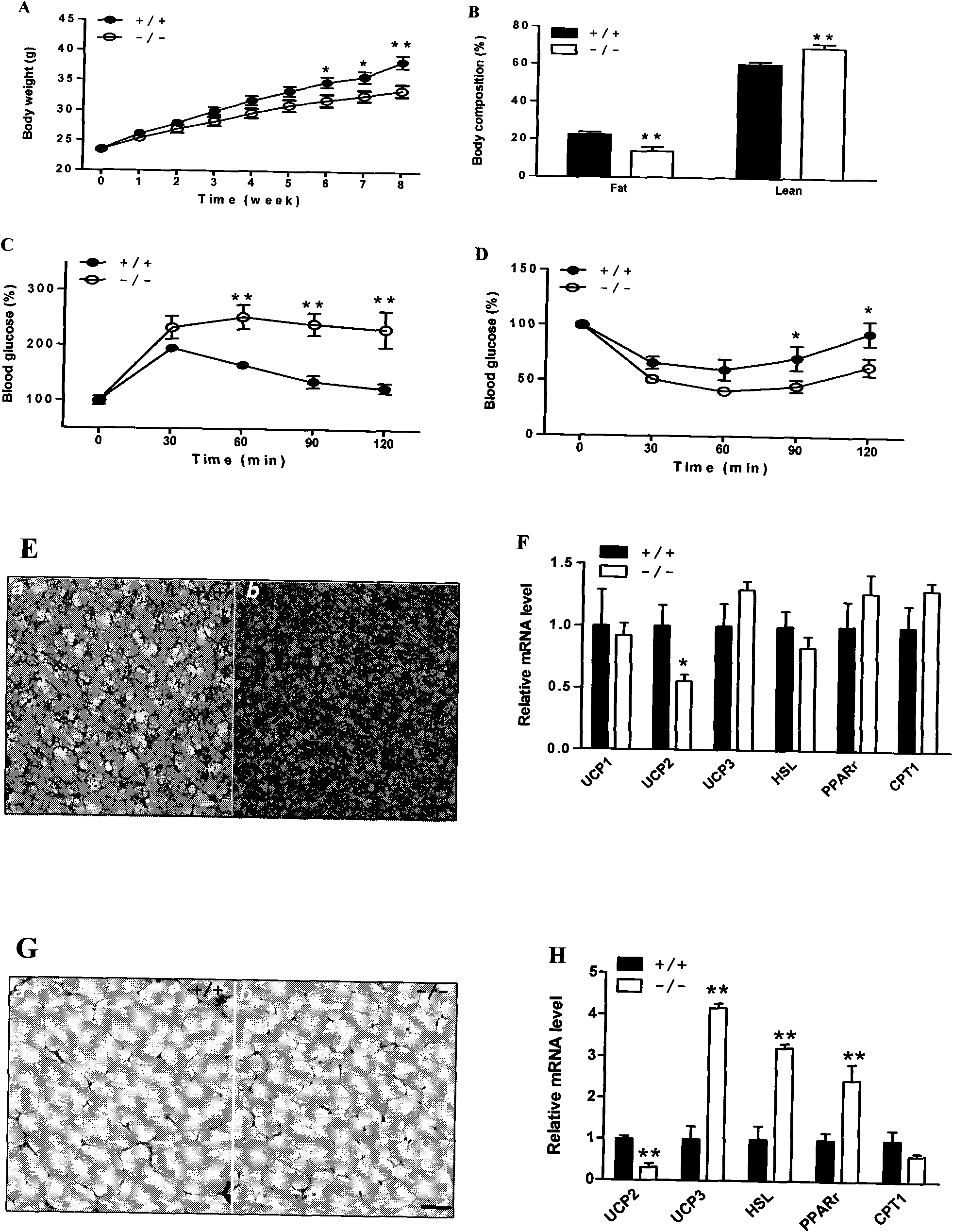
The invention discloses acyltransferase ALCAT1 serving as a target for developing medicaments for treating diabetes, obesity and cardiovascular diseases. The invention discloses that: ALCAT1 causes pathological reconstruction action of diphosphatidyl glycerol under the oxidative stress condition, and thus leads to mitochondria dysfunction, insulin resistance and other symptoms related to diet-induced obesity, wherein the ALCAT1 can be used as a regulatory gene of oxidative stress and mitochondria dysfunction in metabolic diseases and aging; and increased active oxygen causes the change of diphosphatidyl glycerol conformation to increase the ALCAT1, which leads to mitochondrial proton leakage, oxidative stress and mitochondria dysfunction, and further aggravates the insulin resistance and metabolic defects. Therefore, a chemical reagent inhibiting the ALCAT1 can provide a novel method for treating the obesity and related metabolic complications.
Owner:史裕昆
Sarpogrelate hydrochloride lipidosome solid preparation
InactiveCN103040749ASmall particle sizeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolSarpogrelate Hydrochloride
The invention relates to a sarpogrelate hydrochloride lipidosome solid preparation and a preparation method thereof. The preparation method comprises the following steps: selecting sarpogrelate hydrochloride, dilauroyl phosphatidylcholine, phosphatidyl ethanolamine, phosphatidyl glycerol and cholesterol with specific weight ratio to prepare sarpogrelate hydrochloride lipidosome with excellent quality; and preparing the solid preparation by the common preparation method. Compared with the conventional preparation, the preparation provided by the invention improves the stability, the bioavailability and the product quality of the preparation and reduces the toxic or side effect.
Owner:海南路易丹尼生物科技有限公司
Cerebral infarction early diagnosis marker, screening method and application, and construction method and application of cerebral infarction early diagnosis model
ActiveCN114137226AImproved prognosisImprove survival rateComponent separationBiostatisticsCholic acidLipidome
The invention discloses a cerebral infarction early diagnosis marker, a screening method and application, and a construction method and application of a cerebral infarction early diagnosis model. The diagnostic marker is composed of 4-dimethylallyltryptophan, taurochenodeoxycholic acid-3-sulfate, tri-hexose ceramide (d18: 1 / 18: 0), lysophosphatidylcholine (18: 0), arginine-alanine, asparaginic acid-tryptophan, methionine-arginine, sphingomyelin d37: 5, phosphatidyl glycerol (12: 0 / 21: 0) and glucosylceramide (d18: 0 / 18: 0). The diagnostic marker is screened out by simultaneously carrying out metabonomics analysis and lipidomics analysis on serum by utilizing a UPLC-MS (Ultra Performance Liquid Chromatography-Mass Spectrometry) technology; the sensitivity, the specificity, the accuracy and the AUC of the method for constructing the diagnosis model by utilizing the diagnosis marker are all greater than 0.9, and the method can be used for accurately distinguishing cerebral infarction patients from healthy people and can be used for early diagnosis of cerebral infarction.
Owner:CAPITAL NORMAL UNIVERSITY
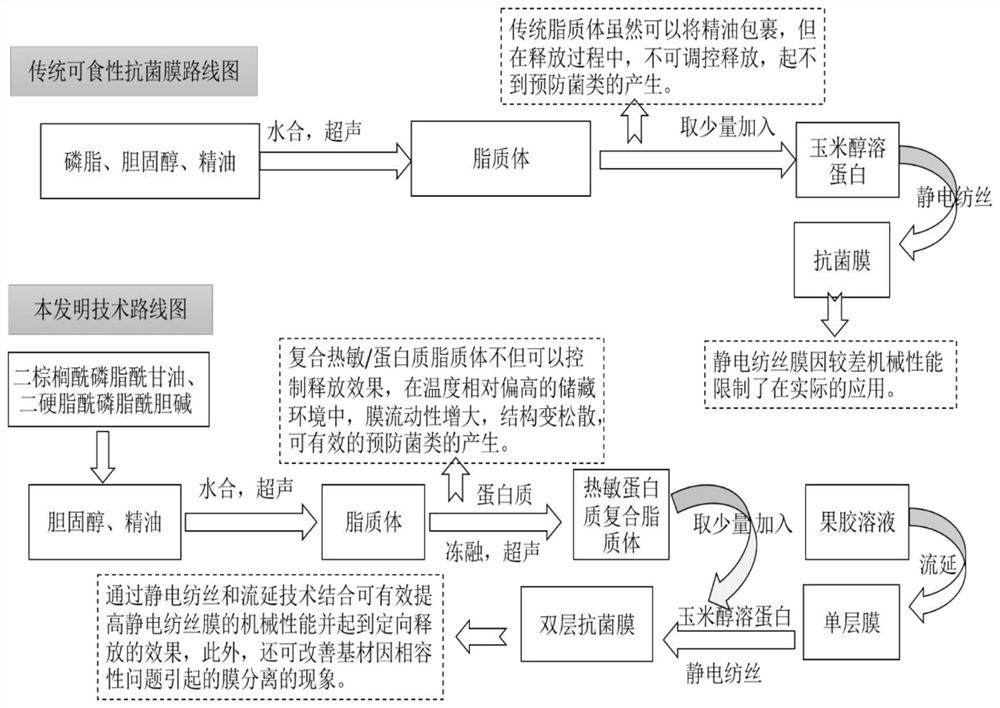
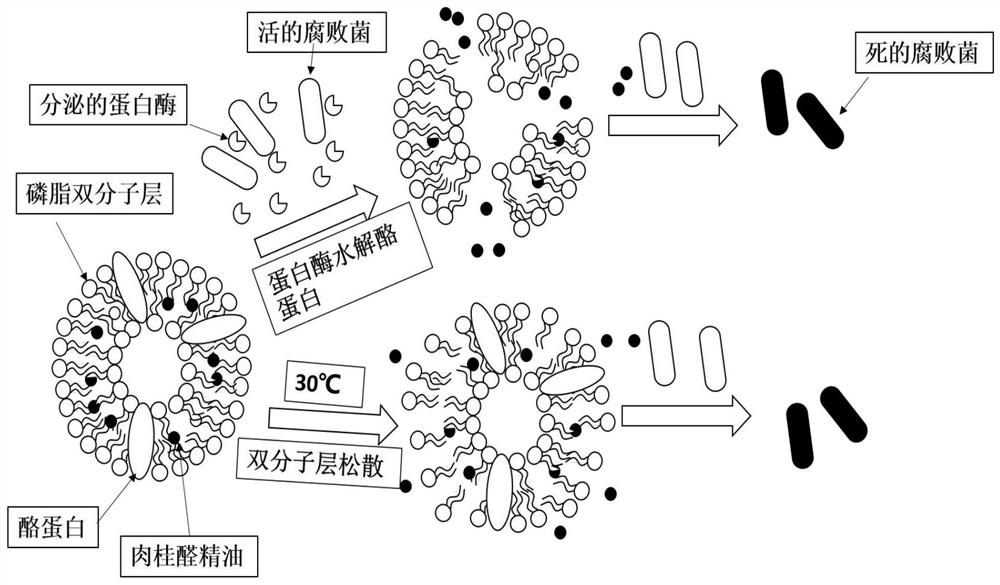
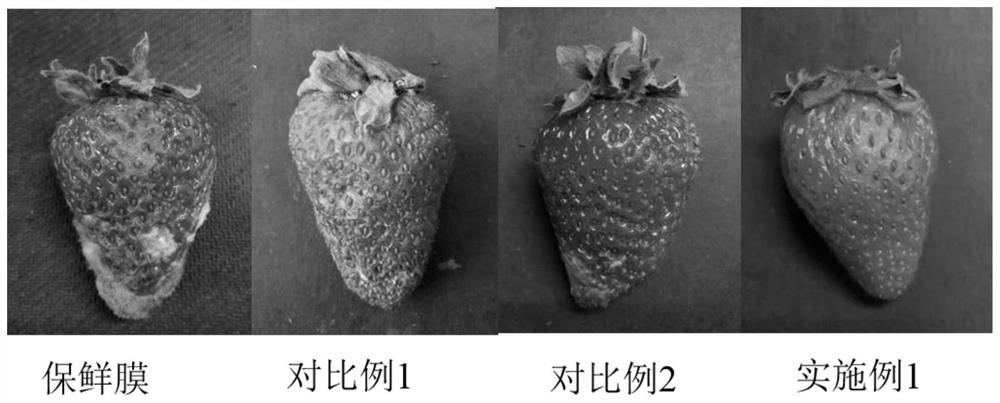
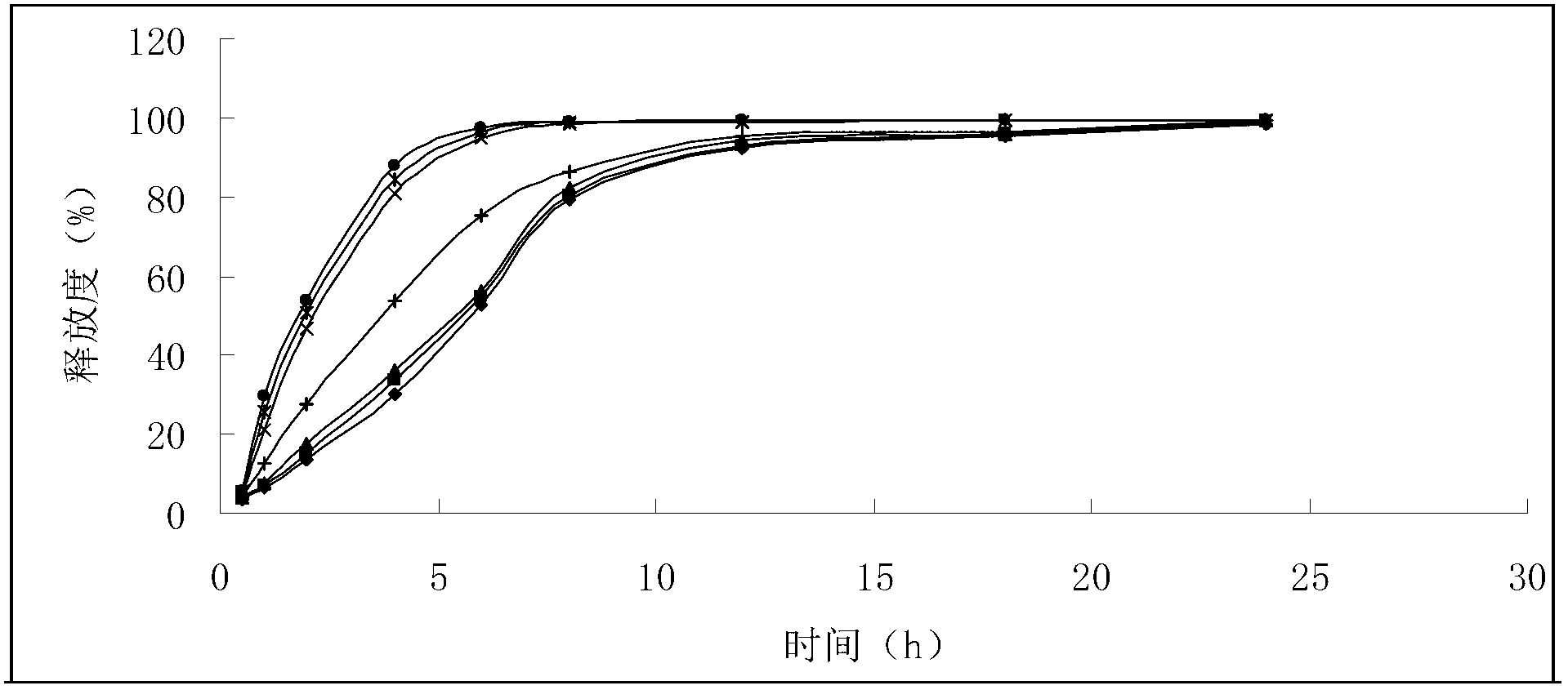
A preparation method of cinnamaldehyde essential oil liposome antibacterial bilayer membrane with adjustable release
ActiveCN109056083BHigh mechanical strengthChange permeabilityFlexible coversWrappersBiotechnologyCholesterol
The invention discloses a preparation method of a cinnamaldehyde essential oil liposome antibacterial double-layer membrane with regulated releasing. The method comprises the steps that (1), cinnamaldehyde heat-sensitive essential oil liposome is prepared taking dipalmitoyl phosphatidyl glycerol, distearoyl phosphatidylcholine, cholesterol and cinnamaldehyde essential oil as raw materials; (2),thecinnamaldehyde heat-sensitive essential oil liposome and casein are taken as raw materials to prepare heat-sensitive protein composite liposome; (3), a pectin cast membrane is prepared; (4), zein powder is taken to be dissolved in an ethanol aqueous solution to obtain a basic spinning solution, the heat-sensitive protein composite liposome is added to the basic spinning solution to obtain a static spinning solution, the pectin cast membrane is taken as a recipient, and static spinning is conducted to obtain the cinnamaldehyde essential oil liposome antibacterial double-layer membrane. By means of the method, the stability of essence oil and the mechanical performance of the membrane can be improved, and the prevention of the generation of putrefying bacteria in the environment with relatively higher temperature and sustained releasing can be achieved.
Owner:ZHEJIANG UNIV OF TECH
Fluoxetine hydrochloride liposome solid preparation
InactiveCN102716084BImprove product qualityUniform particle sizeOrganic active ingredientsNervous disorderSide effectCholesterol
Owner:HAINAN MEIDA PHARMA
Repaglinide liposome solid preparation
InactiveCN102727438BLong retention timeImprove bioavailabilityOrganic active ingredientsMetabolism disorderSide effectBioavailability
The invention provides a repaglinide liposome solid preparation, and a preparation method thereof. According to the invention, repaglinide, ovolecithin, soyasterol, and dilauroyl phosphatidyl glycerol with a certain weight ratio are prepared into repaglinide liposome with excellent quality; and the repaglinide liposome is prepared into a solid preparation with a common preparation method. Compared with existing preparations, the preparation provided by the invention is advantaged in substantially improved preparation stability, bioavailability, product quality, and release-retarding effect, and reduced toxic and side effects.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com