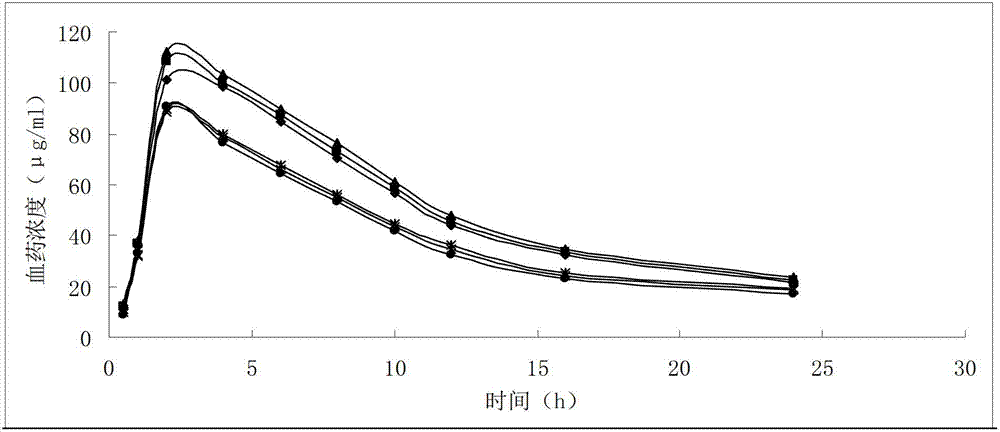
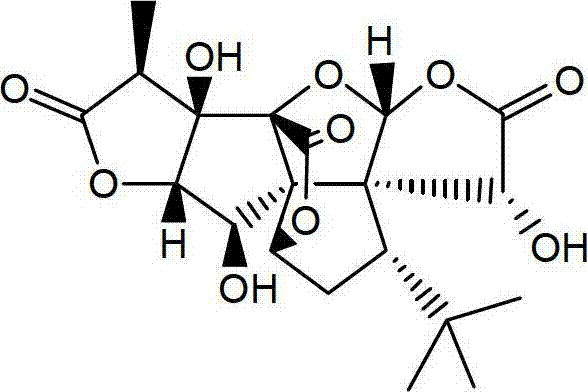
Ginkgolide B lipid microsphere injection
A technology of ginkgolide and lipid microspheres, applied in the field of medicine, can solve the problems of easy aggregation, fusion, poor release controllability of lipid microsphere injection, leakage of encapsulated drugs, etc. The effect of low leak rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] On the other hand, the present invention also provides a preparation method of ginkgolide B lipid microsphere injection, specifically comprising the following preparation steps:
[0064] (1) Dissolve dioleoylphosphatidylethanolamine, distearoylphosphatidylglycerol and ginkgolide B in an appropriate amount of solvent under nitrogen protection, and stir magnetically at about 50°C for 1-2 hours to obtain ginkgo biloba Ester B lipid solution; the above-mentioned phospholipid solution is filtered with a 0.45 μm microporous membrane, and the filtrate is freeze-dried until it is completely dry to obtain a loose ginkgolide B solid;
[0065](2) Dissolve PEG400 and polyoxyethylene 40 hydrogenated castor oil in an appropriate amount of buffered saline solution, stir evenly at 500-800 rpm, and add the above-mentioned ginkgolide B solid under continuous stirring at 60°C to obtain a uniform suspension liquid, and then transferred to a high-speed homogenizer for gradient homogenizatio...
Embodiment 1
[0073] The preparation of embodiment 1 ginkgolide B lipid microsphere injection
[0074] The ingredients used and their weights are as follows (1000 count):
[0075]
[0076] Ginkgolide B lipid microsphere injection was prepared by preparation process:
[0077] (1) Under nitrogen protection, dissolve 100g of dioleoylphosphatidylethanolamine, 20g of distearoylphosphatidylglycerol and 10g of ginkgolide B in 300ml of a mixed solvent of ethanol and tert-butanol with a volume ratio of 2:3 Ginkgolide B lipid solution was obtained under magnetic stirring at 50°C for 1 hour; the above-mentioned phospholipid solution was filtered through a 0.45 μm microporous membrane, and the filtrate was pre-frozen at -60°C for 2 hours, and then - Freezing at 40°C for 6 hours, then sublimating to 20°C for 18 hours, and finally drying at 30°C for 1 hour to obtain loose ginkgolide B solid;
[0078] (2) Dissolve 30g of PEG400 and 25g of polyoxyethylene 40 hydrogenated castor oil in 3000ml of phos...
Embodiment 2
[0080] Preparation of Example 2 Ginkgolide B Lipid Microsphere Injection
[0081] The ingredients used and their weights are as follows (1000 count):
[0082]
[0083] Ginkgolide B lipid microsphere injection was prepared by preparation process:
[0084] (1) Under nitrogen protection, dissolve 250g of dioleoylphosphatidylethanolamine, 50g of distearoylphosphatidylglycerol and 10g of ginkgolide B in 600ml of a mixed solvent of ethanol and tert-butanol with a volume ratio of 2:3 Ginkgolide B lipid solution was obtained by magnetic stirring at 50°C for 1.5 hours; the above-mentioned phospholipid solution was filtered through a 0.45 μm microporous membrane, and the filtrate was pre-frozen at -65°C for 2.5 hours, and then - Freezing at 45°C for 7 hours, then sublimating to 23°C for 20 hours, and finally drying at 32°C for 2 hours to obtain loose ginkgolide B solid;
[0085] (2) Dissolve 45g of PEG400 and 63g of polyoxyethylene 40 hydrogenated castor oil in 5000ml of phosphat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com