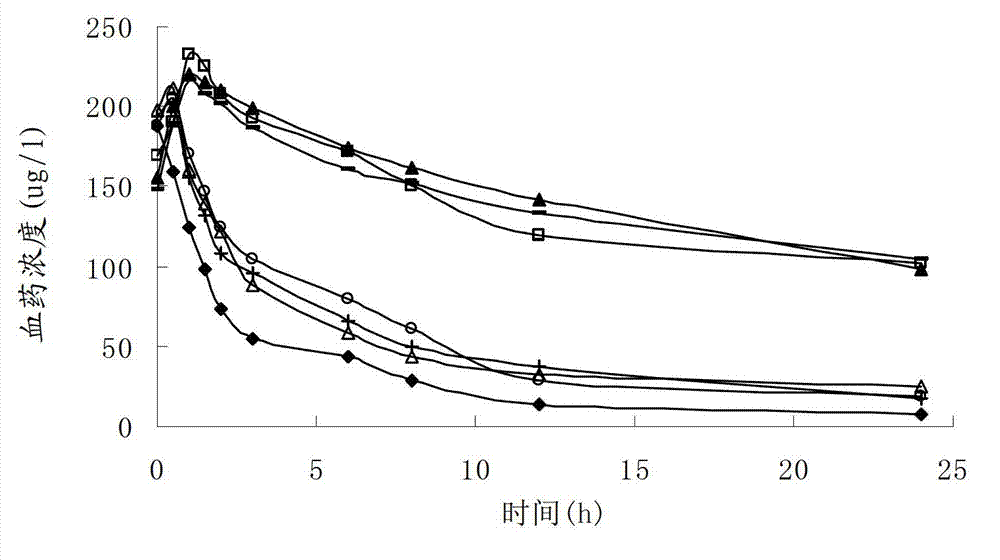
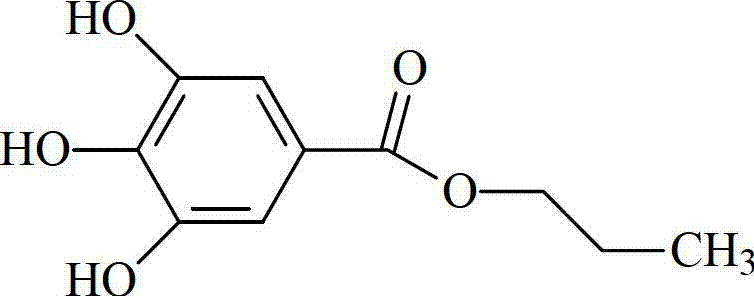
Propyl gallate liposome injection
A technology of propyl gallate and injection, which is applied in the field of medicine, can solve problems such as unqualified clarity, decreased main drug content, instability, etc., and achieve the effects of improving the product quality of preparations, good preparation stability, and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 propyl gallate liposome freeze-dried powder injection
[0053] The ingredients used and their weights are as follows:
[0054]
[0055] The following preparation process is adopted to prepare propyl gallate liposome freeze-dried powder injection:
[0056] (1) Dissolve 60g of dilauroylphosphatidylglycerol, 200g of cholesterol and 40g of Tween 80 in 1500ml of phosphate buffer solution with a pH of 6.2 to make blank liposomes;
[0057] (2) The blank liposomes prepared above were sterilized by circulating steam, and then sonicated twice, each time for 15 minutes;
[0058] (3) Under sterile conditions, add 60 g of propyl gallate to the molten liposome, and add 0.05 g of sodium bisulfite, 0.01 g of EDTA-2Na and 100 g of dextran under constant stirring;
[0059] (4) Filtrate through a 0.45um microporous membrane, subpackage, and freeze-dry to prepare 1,000 bottles of propyl gallate liposome freeze-dried powder for injection.
Embodiment 2
[0060] The preparation of embodiment 2 propyl gallate liposome injection
[0061] The ingredients used and their weights are as follows:
[0062]
[0063] Adopt following preparation process to prepare propyl gallate liposome injection:
[0064] (1) Dissolve 80g of dilauroylphosphatidylglycerol, 50g of cholesterol and 100g of Tween 80 in 1500ml of phosphate buffer solution with a pH of 6.2 to make blank liposomes;
[0065] (2) The blank liposomes prepared above were sterilized by circulating steam, and then sonicated twice, each time for 15 minutes;
[0066] (3) Under sterile conditions, add 60 g of propyl gallate to the molten liposome, and add 0.1 g of sodium bisulfite, 0.05 g of EDTA-2Na and 50 g of dextran under constant stirring;
[0067] (4) Filter through a 0.45um microporous membrane, freeze quickly, then return to room temperature, dilute to 2000ml with water for injection, fill and sterilize to obtain 1000 propyl gallate liposome injections.
Embodiment 3
[0068] The preparation of embodiment 3 propyl gallate liposome injection
[0069] The ingredients used and their weights are as follows:
[0070]
[0071]
[0072] Adopt following preparation process to prepare propyl gallate liposome injection:
[0073] (1) Dissolve 60g of dilauroylphosphatidylglycerol, 150g of cholesterol and 90g of Tween 80 in 3000ml of phosphate buffer solution with a pH of 6.2 to make blank liposomes;
[0074] (2) The blank liposomes prepared above were sterilized by circulating steam, and then sonicated twice, each time for 15 minutes;
[0075] (3) Under sterile conditions, add 120 g of propyl gallate to the molten liposome, and add 0.4 g of sodium bisulfite, 0.2 g of EDTA-2Na and 160 g of dextran under constant stirring;
[0076] (4) Filtrate through a 0.45um microporous membrane, quickly freeze, then return to room temperature, dilute to 5000ml with water for injection, fill, and sterilize to obtain 1000 propyl gallate liposome injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com