Liquid crystal gel nano-particle capable of wrapping drugs with different polarities and preparation method of nano-particle
A nanoparticle and gel technology, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, can solve problems such as hemolysis and cytotoxicity, reduce side effects, reduce dosage, The effect of high storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the liquid crystal gel nanometer of the embodiment of the present invention and comparative example, comprises the following steps:
[0034] S1. Weigh the active ingredients of the drug, glyceryl dioleate, phosphatidylcholine, phosphatidylglycerol dilaurate, and nonionic surfactant, and stir to obtain a mixed solution;
[0035] S2, adding ethanol to the mixed solution obtained in S1, and adding it together with ethanol when there are azacarbon nanotubes in the formula, and performing ultrasonic dispersion for 4 minutes to obtain a liquid crystal gel nanoparticle precursor;
[0036] S3. Add water to the liquid crystal gel nanoparticle precursor obtained in S2 to 100wt%, and then disperse it through an ultrasonic probe. The power of the ultrasonic probe is 100W, and the ultrasonic time is 9min. grain.
[0037] The samples of the embodiment of the present invention and comparative example carry out MTT cytotoxicity test in the same way, particle ...
Embodiment 1
[0043] This embodiment provides a liquid crystal gel nanoparticle, which contains the following raw materials in mass percentage: 0.2% by weight of doxorubicin, 1.5% of glyceryl dioleate, 3.0% of soybean phosphatidylcholine, and 0.6% of dilauric acid phosphatidylglycerol , polyoxyethylene 20 sorbitan monooleate 0.2%, ethanol 0.3%, and the balance is water.
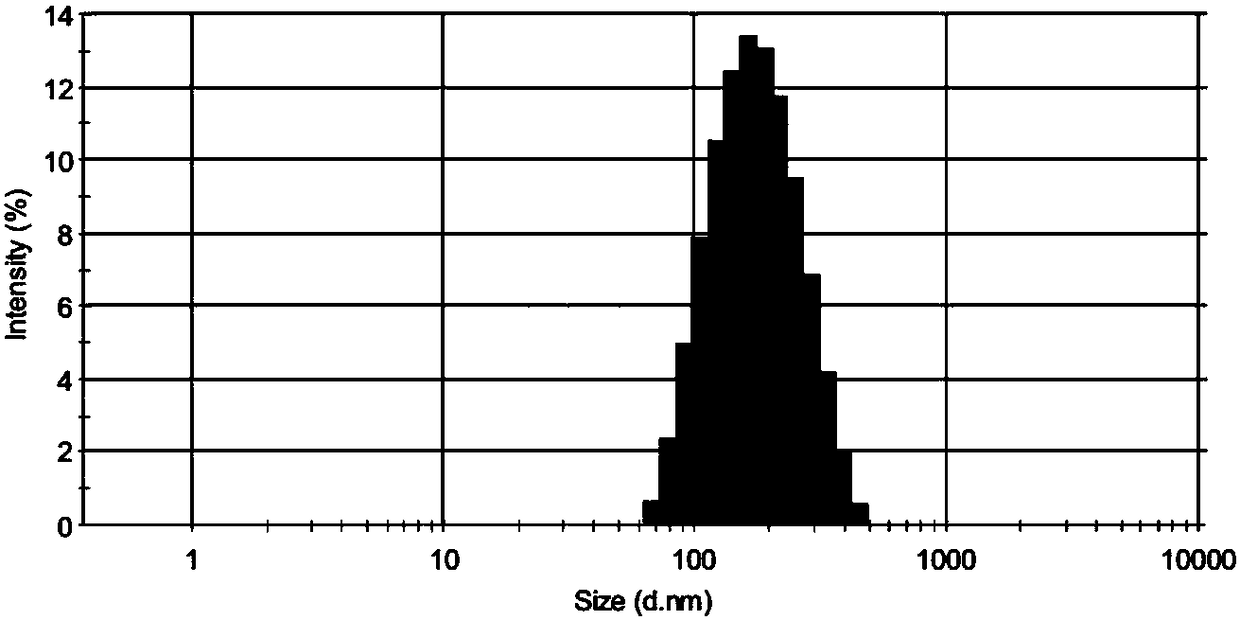
[0044] figure 1 For the particle size distribution figure of the doxorubicin-loaded liquid crystal gel nanoparticles of the present invention, from figure 1 It can be clearly seen from the figure that the particle size distribution of doxorubicin-loaded liquid crystal gel nanoparticles is in the range of 60-500 nm, and the particle size distribution of the doxorubicin-loaded liquid crystal gel nanoparticles is mainly in the range of 100-200 nm. figure 2 It is a polarized light micrograph of the coarse dispersion of doxorubicin-loaded liquid crystal gel nanoparticles of the present invention, image 3 It is a micrograph ...
Embodiment 2
[0046] This embodiment provides a liquid crystal gel nanoparticle, which contains the following raw materials in mass percentage: coumarin 60.2wt%, diolein 1.5%, soybean phosphatidylcholine 3.0%, dilaurate phospholipid Acylglycerol 0.6%, polyoxyethylene 20 sorbitan monooleate 0.2%, ethanol 0.3%, and the balance is water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com