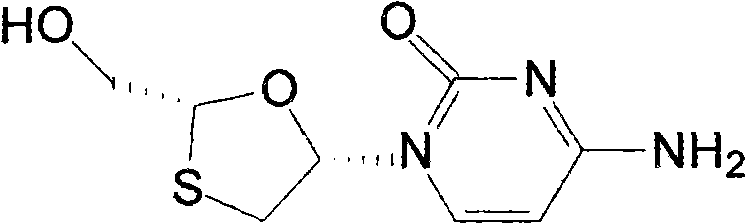
Lamivudine liposome solid preparation
A technology of lamivudine lipid and solid preparations, which is applied in the field of medicine, can solve the problems of liposome stability and poor encapsulation efficiency, and is beneficial to large-scale industrial production, and improves stability, dissolution rate, and utilization high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
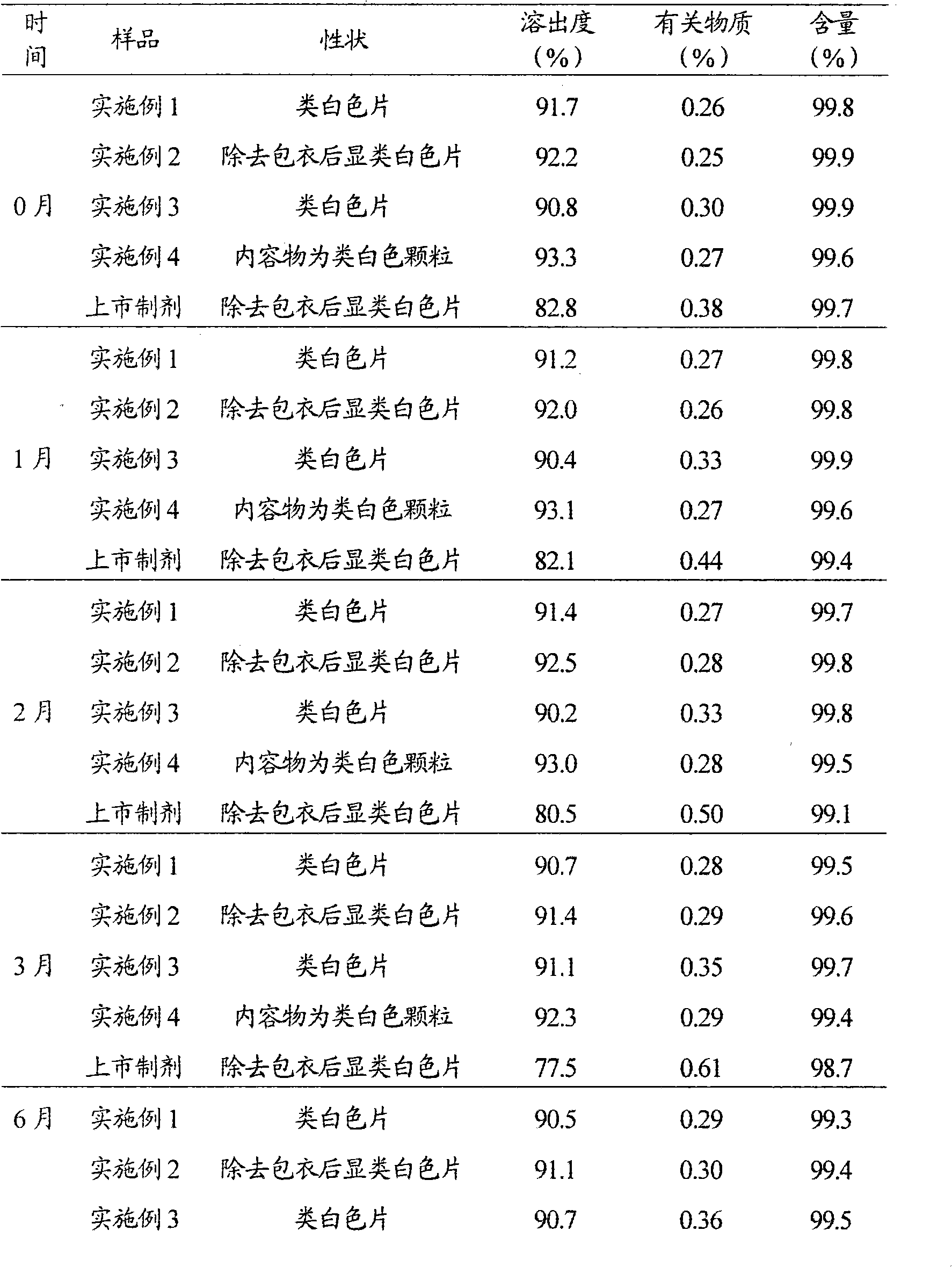
Embodiment 1
[0053] The preparation of embodiment 1 lamivudine tablet
[0054] Prescription: (1000 tablets)
[0055] Component Dosage
[0056] Lamivudine 100g
[0057] Dipalmitoylphosphatidylglycerol 310g
[0058] Sodium deoxycholate 160g
[0059] Cholesterol 100g
[0060] 260g pregelatinized starch
[0061] Mannitol 120g
[0062] Crospovidone 60g
[0063] Povidone K30 25g
[0065] Micronized silica gel 15g
[0066] Preparation Process:
[0067] (a) 310g of dipalmitoylphosphatidylglycerol, 160g of sodium deoxycholate, and 100g of cholesterol were dissolved in 4000ml of methanol and acetonitrile in a mixed solvent with a volume ratio of 1:4, mixed evenly, and removed under reduced pressure on a rotary thin film evaporator. Solvent, obtain phospholipid film;
[0068] (b) Add 2000ml of phosphoric acid-dipotassium hydrogen phosphate buffer solution with a pH value of 5.8, adjust the pH to 5.5 with 10% phosphoric acid solution, shake and stir to completely h...
Embodiment 2
[0073] The preparation of embodiment 2 lamivudine tablets
[0074] Prescription: (1000 tablets)
[0075] Component Dosage
[0076] Lamivudine 100g
[0077] Dipalmitoylphosphatidylglycerol 880g
[0078] Sodium deoxycholate 460g
[0079] Cholesterol 240g
[0080] 320g pregelatinized starch
[0081] Dextrin 150g
[0082] Crospovidone 60g
[0083] Low-substituted hydroxypropyl cellulose 80g
[0084] Sodium Carboxymethyl Cellulose 20g
[0085] Micropowder silica gel v 70g
[0086] Preparation Process:
[0087] (a) 880g of dipalmitoylphosphatidylglycerol, 460g of sodium deoxycholate, and 240g of cholesterol were dissolved in 9000ml of methanol and acetonitrile in a mixed solvent with a volume ratio of 1:4, mixed evenly, and removed under reduced pressure on a rotary thin film evaporator. Solvent, obtain phospholipid film;
[0088] (b) Add 5000ml of phosphoric acid-dipotassium hydrogen phosphate buffer solution with a pH value of 5.8, adjust the pH to 6.6 with 10% potassi...
Embodiment 3
[0094] The preparation of embodiment 3 lamivudine dispersible tablets
[0095] Prescription: (1000 tablets)
[0096] Component Content
[0097] Lamivudine 100g
[0098] Dipalmitoylphosphatidylglycerol 1300g
[0099] Sodium deoxycholate 700g
[0100] Cholesterol 500g
[0101] 340g pregelatinized starch
[0102] Croscarmellose Sodium 150g
[0103] Low-substituted hydroxypropyl cellulose 90g
[0104] Povidone K30 100g
[0105] Micronized silica gel 50g
[0107] Preparation Process:
[0108] (a) Dissolve 1300g of dipalmitoylphosphatidylglycerol, 700g of sodium deoxycholate, and 500g of cholesterol in 15,000ml of isopropanol and acetone in a mixed solvent with a volume ratio of 2:1, mix well, and remove under reduced pressure on a rotary thin film evaporator Mixing solvents to obtain a phospholipid film;
[0109] (b) Add 8000ml of acetic acid-sodium acetate buffer solution with a pH value of 5.6, adjust the pH to 6.0 with 10% sodium citrate solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com