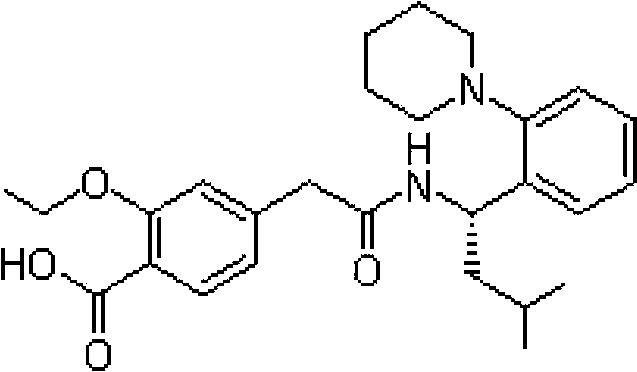
Repaglinide liposome solid preparation
A technology of liposome tablets and liposomes, which is applied in the field of repaglinide liposome solid preparations and new solid preparations of antidiabetic drug repaglinide, which can solve the problem of bioavailability and long-term stability. , the slow onset of the pharmaceutical composition and other problems, to achieve the effects of improving bioavailability, improving drug compliance, and reducing the number of administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
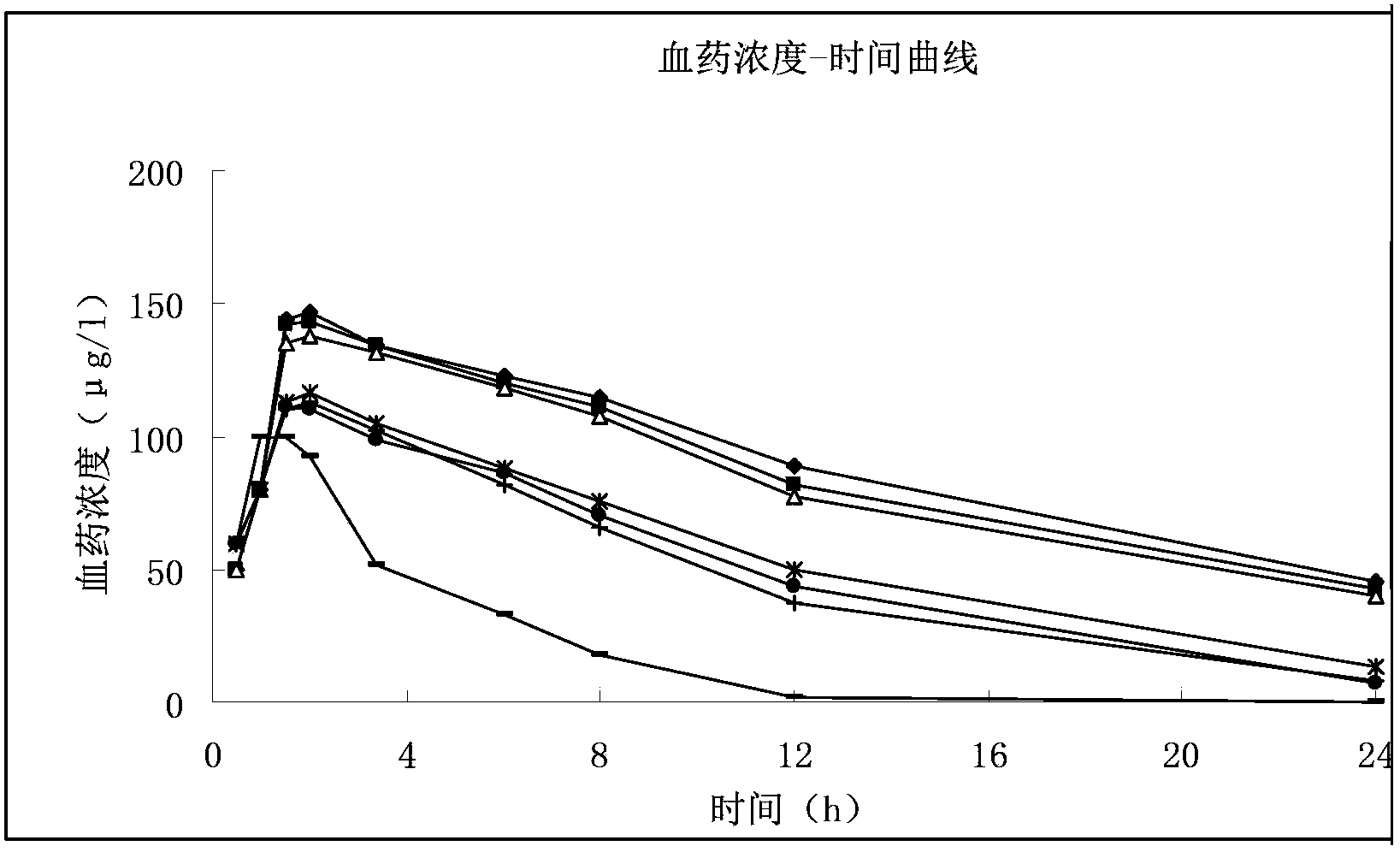
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of Repaglinide Liposome Tablets
[0062] Prescription: (1000 tablets)
[0063]
[0064] Adopt the following production process to prepare repaglinide liposome tablet:
[0065] (1) Dissolve 0.5g repaglinide in a mixed solution of 5ml ethanol and chloroform;
[0066] (2) Dissolve 11g of egg yolk lecithin, 3g of soybean sterol and 3.25g of dilauroylphosphatidylglycerol in a mixed solution of 50ml of ethanol and chloroform, then pour (1) quickly, mix well, and keep warm at 59°C for 30-40 Minutes, then remove the organic solvent under reduced pressure with a rotary vacuum drier to obtain a liposome solid;
[0067] (3) Mix repaglinide liposome solid with 31.95g lactose, 31.95g microcrystalline cellulose and 5.325g low-substituted hydroxypropyl cellulose, pass through an 80 mesh sieve and mix evenly, add 5% povidone K30 50ml of 50% ethanol solution prepares soft materials, passes through 24 mesh sieves, granulates, and dries;
[0068] (4) Mix the...
Embodiment 2
[0069] Example 2 Preparation of Repaglinide Liposome Tablets
[0070] Prescription: (1000 tablets)
[0071]
[0072] Adopt the following production process to prepare repaglinide liposome tablet:
[0073] (1) Dissolve 1g repaglinide in a mixed solution of 5ml ethanol and chloroform;
[0074] (2) Dissolve 20g of egg yolk lecithin, 4g of soybean sterol and 5g of dilauroylphosphatidylglycerol in a mixed solution of 50ml of ethanol and chloroform, then pour (1) quickly, mix well, and keep warm at 55°C for 30-40 minutes , and then remove the organic solvent under reduced pressure with a rotary vacuum dryer to obtain a liposome solid;
[0075] (3) Mix repaglinide liposome solid with 45g lactose, 45g microcrystalline cellulose and 6g low-substituted hydroxypropyl cellulose, pass through an 80 mesh sieve and mix evenly, add 50% of 5% povidone K30 Prepare soft material with 80ml of ethanol solution, pass through a 24-mesh sieve to granulate, and dry;
[0076] (4) Mix the dry g...
Embodiment 3
[0077] Example 3 Preparation of Repaglinide Liposome Tablets
[0078] Prescription: (1000 tablets)
[0079]
[0080] Adopt the following production process to prepare repaglinide liposome tablet:
[0081] (1) Dissolve 2g repaglinide in a mixed solution of 10ml ethanol and chloroform;
[0082] (2) Dissolve 48g of egg yolk lecithin, 16g of soybean sterol and 16g of dilauroylphosphatidylglycerol in a mixed solution of 100ml of ethanol and chloroform, then pour (1) quickly, mix well, and keep warm at 60°C for 30-40 minutes , and then remove the organic solvent under reduced pressure with a rotary vacuum dryer to obtain a liposome solid;
[0083] (3) Mix repaglinide liposome solid with 174g lactose, 174g microcrystalline cellulose and 34g low-substituted hydroxypropyl cellulose, pass through an 80 mesh sieve and mix evenly, add 50% of 5% povidone K30 Prepare soft material with 200ml of ethanol solution, pass through a 24-mesh sieve to granulate, and dry;
[0084] (4) Mix t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com