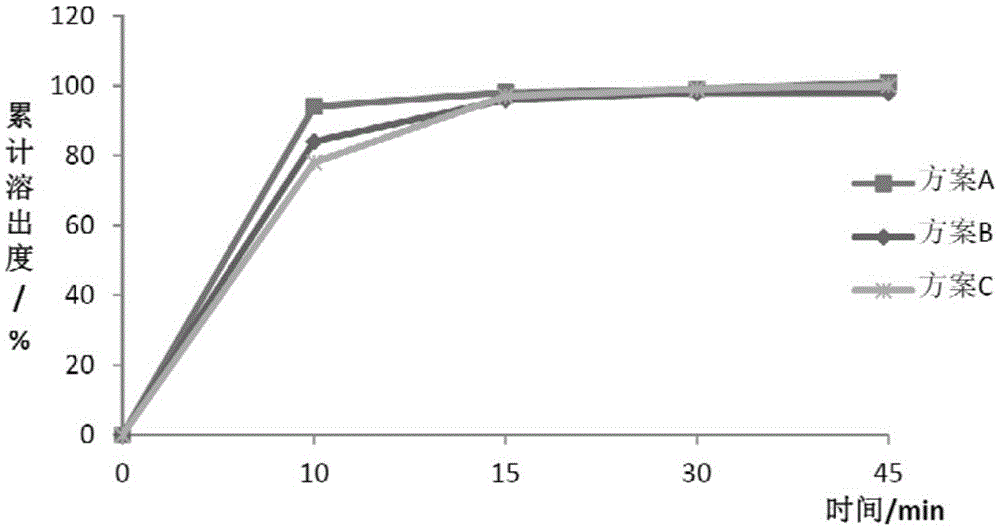
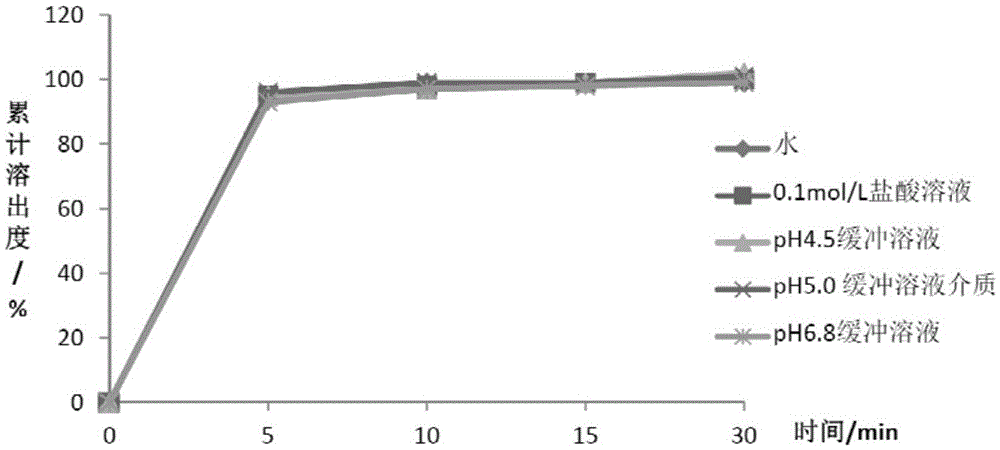
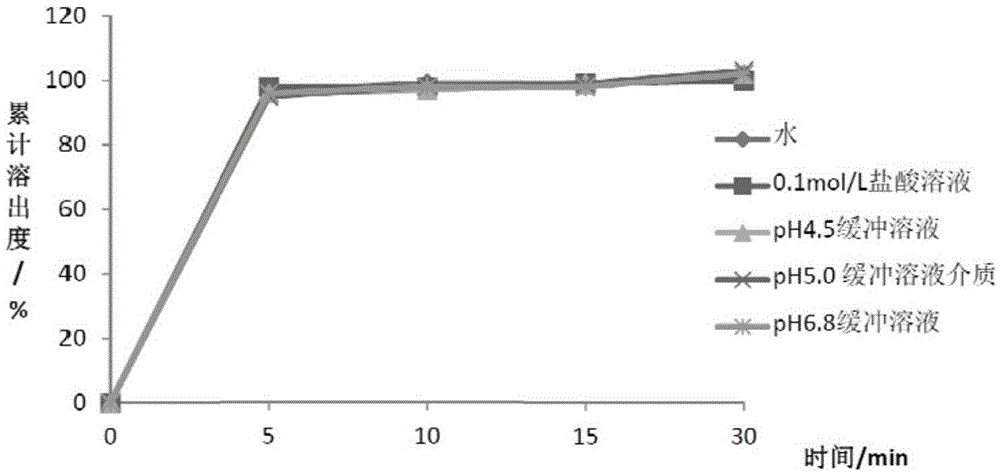
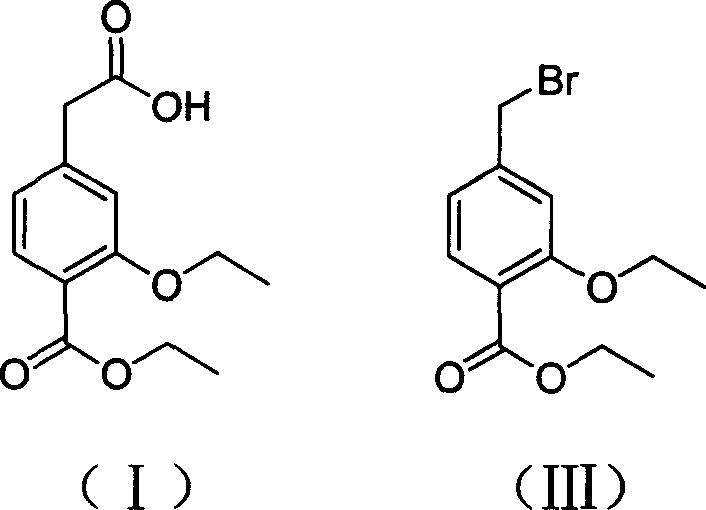
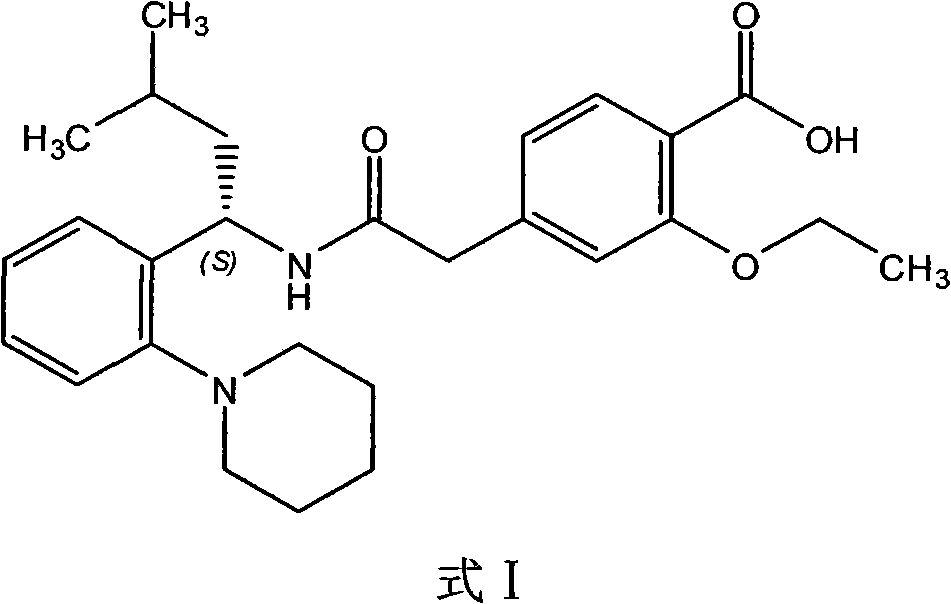
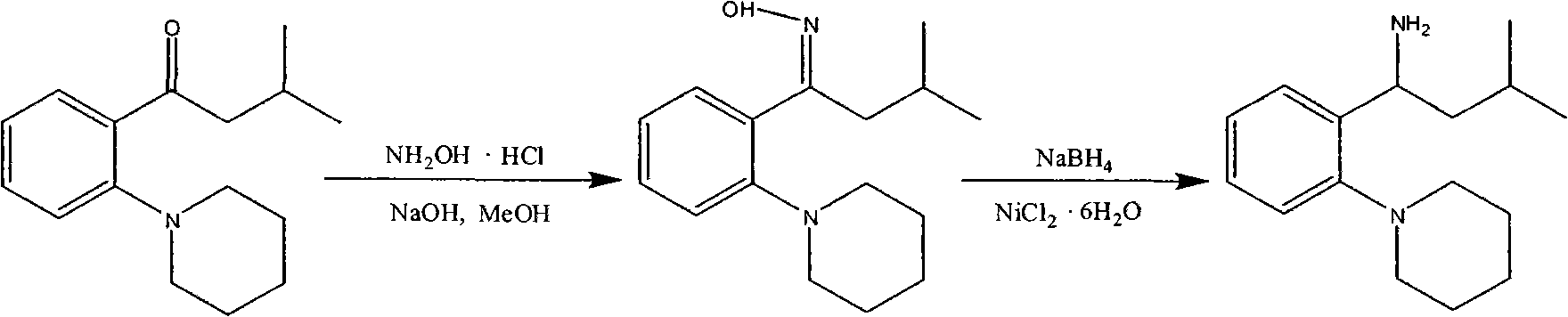
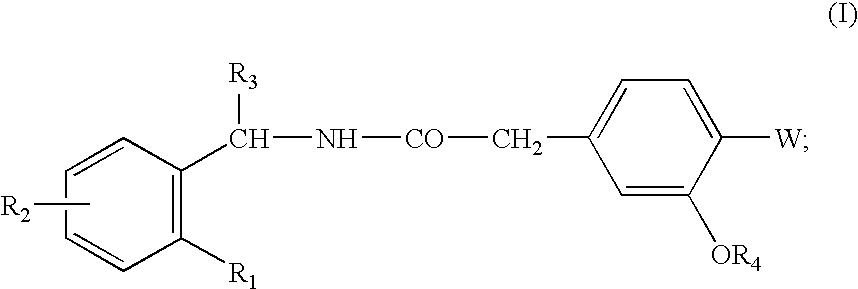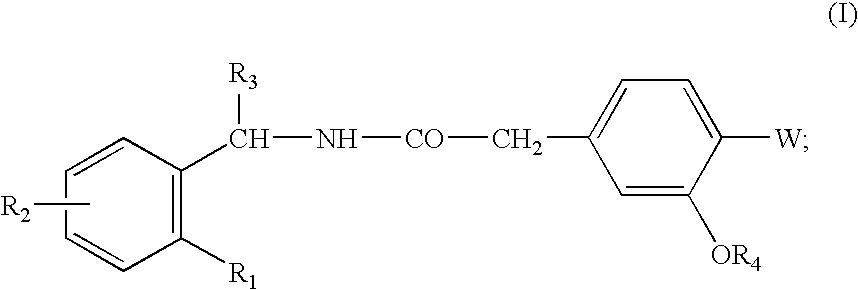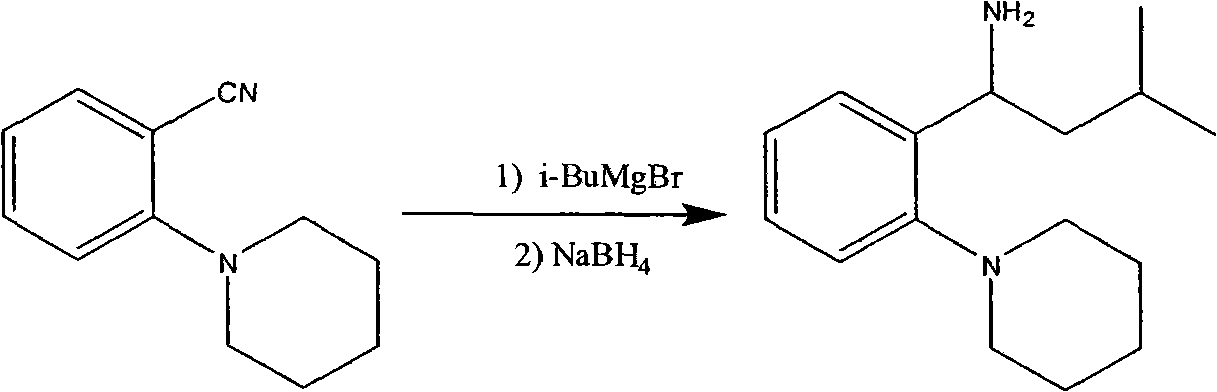Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112 results about "Repaglinide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
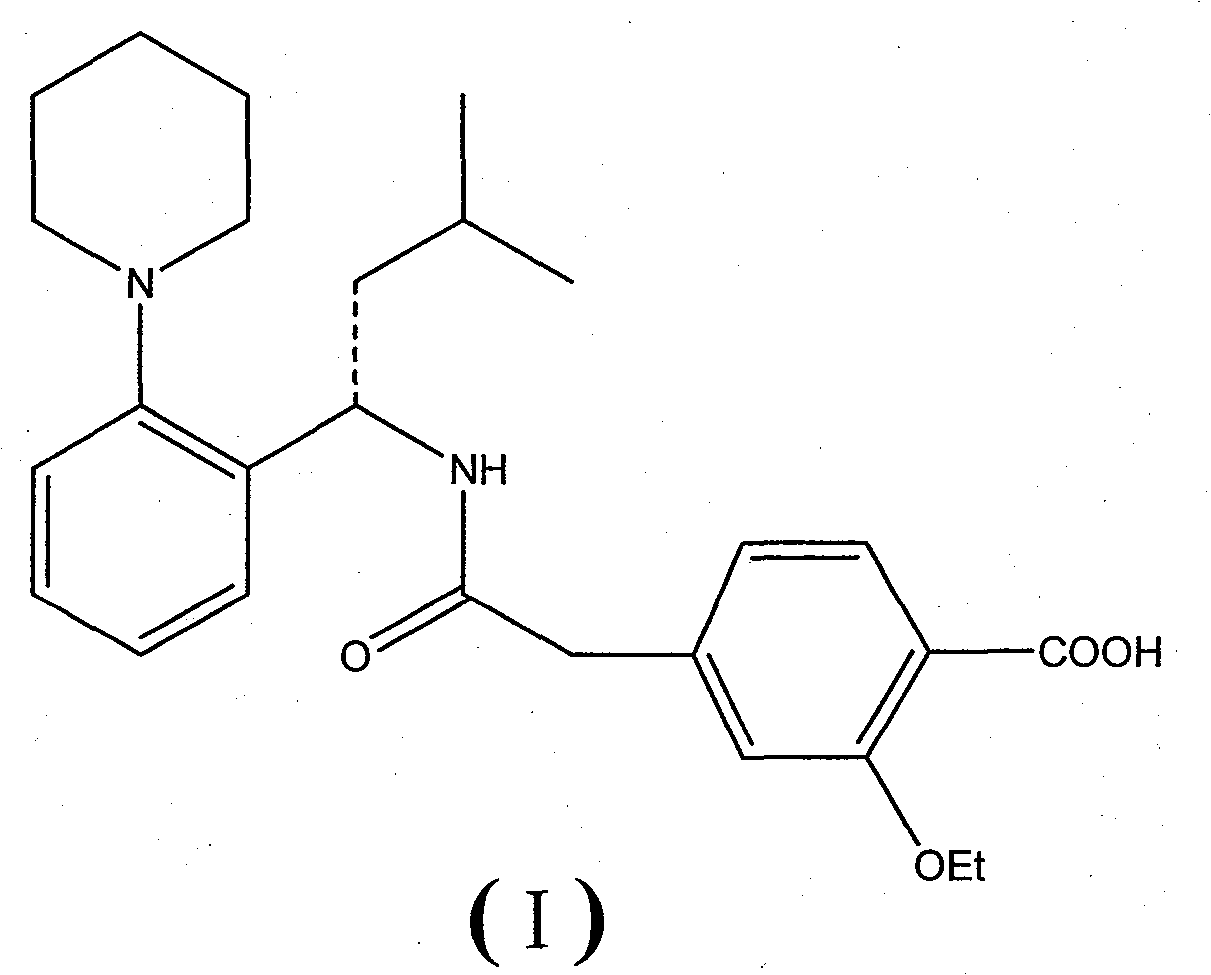
Repaglinide is used alone or with other medications to control high blood sugar along with a proper diet and exercise program. It is used in people with type 2 diabetes.
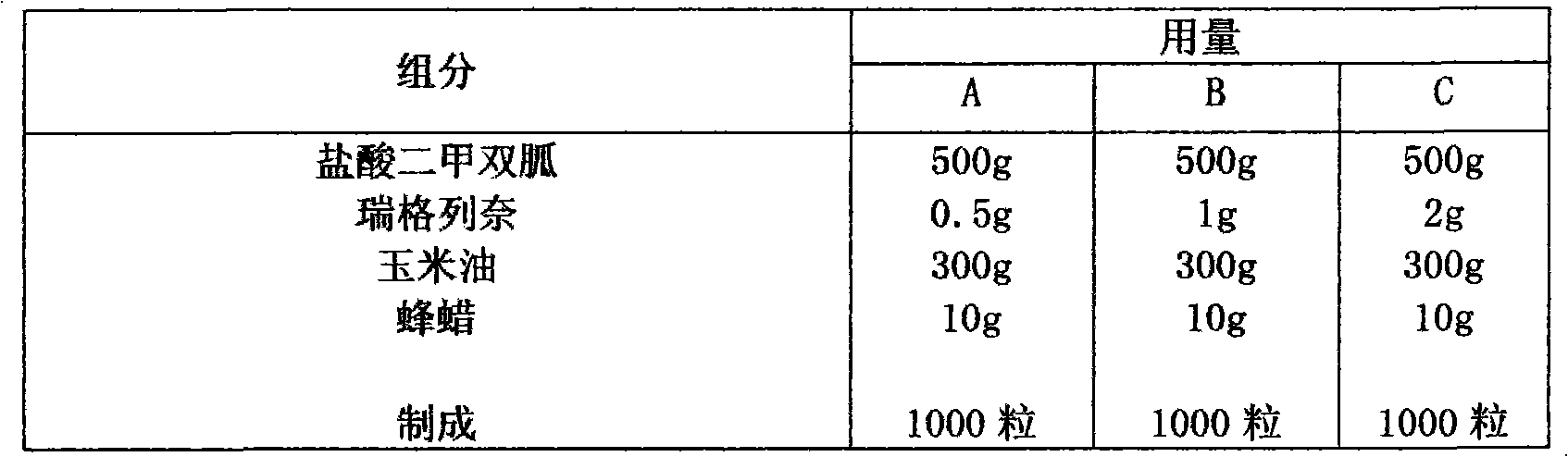
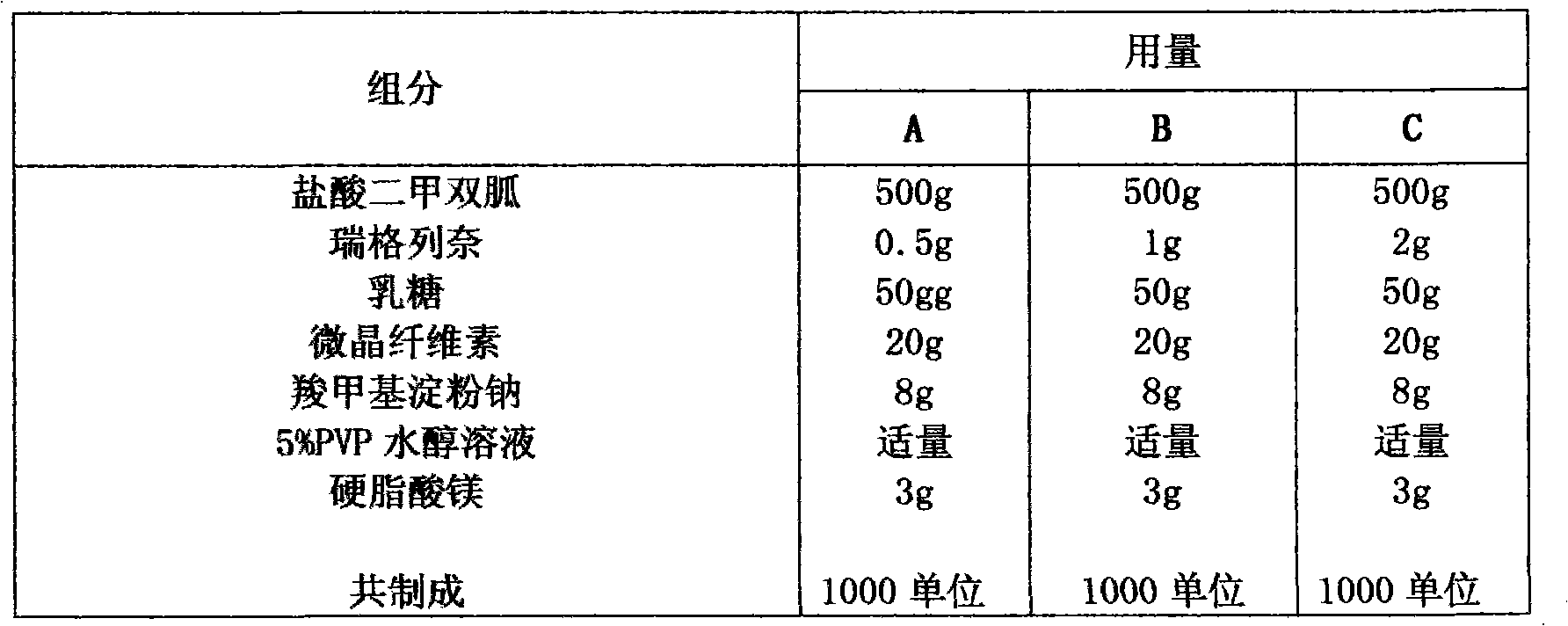
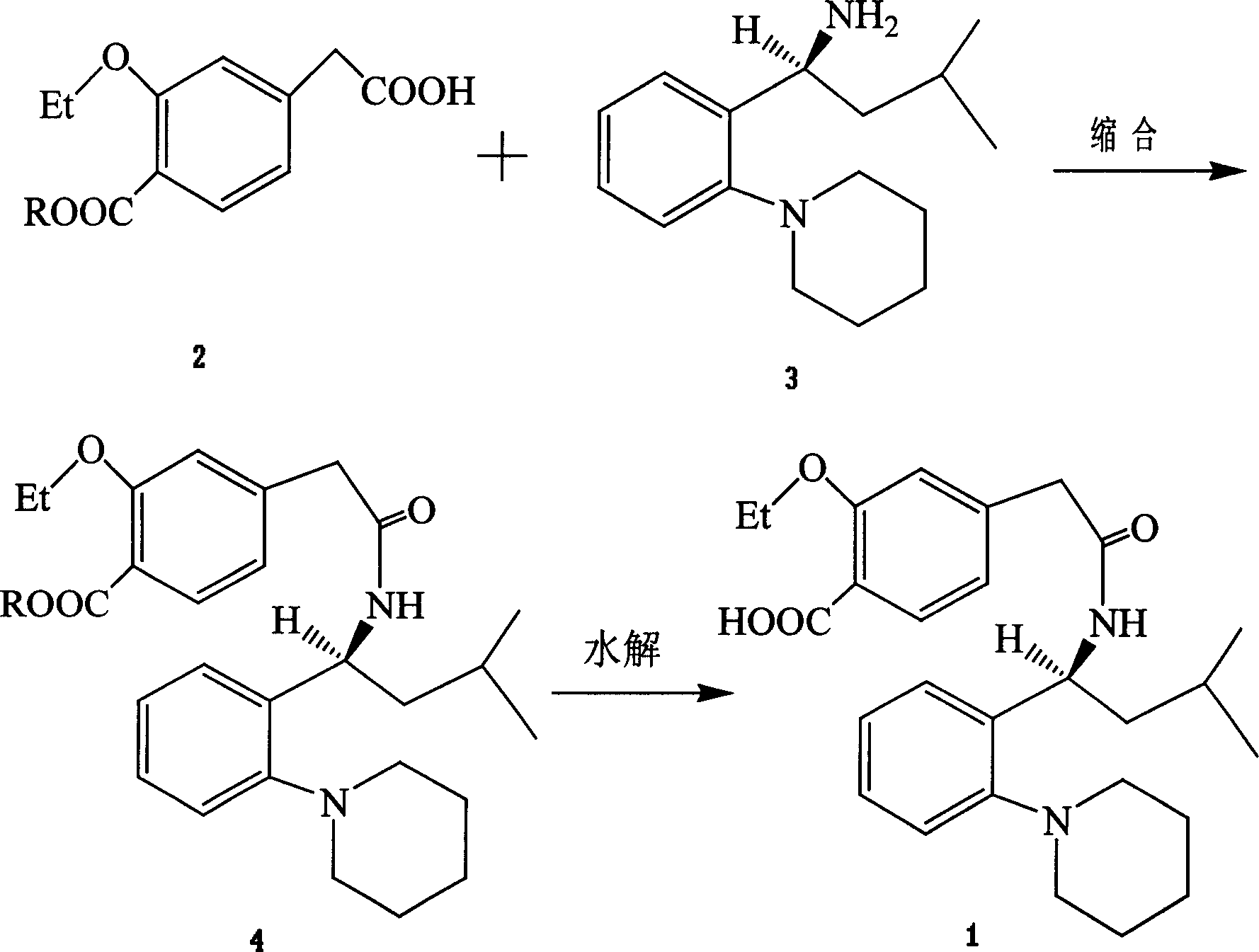
Compound with metformin and repaglinide, preparation method thereof and application thereof
InactiveCN101822672ALower glucose toleranceLower natural responsesOrganic active ingredientsMetabolism disorderInsulin dependent diabetesOrally disintegrating tablet
The invention relates to a composite composition with metformin hydrochloride and repaglinide as active ingredients, a preparation method thereof and application thereof and belongs to the technical field of medicaments. The composite composition is a medicinal composition which is mixed by using the metformin hydrochloride and repaglinide as the active ingredients and by using a carrier and can be prepared into sustained-release tablets, sustained-release granules, sustained-release capsules, common troches and capsules, granules, dispersible tablets, chewable tablets, orally disintegrating tablets, buccal tablets, liquid capsules, soft capsules, drop pills and other oral preparations. The composite composition is used for treating patients with I-type diabetes or II-type diabetes (non-insulin-dependent diabetes) and has synergistic effect on controlling blood sugar.
Owner:深圳南方盈信制药有限公司 +1
Oral solid drug composition of metformin hydrochloride repaglinide
The invention discloses an oral solid drug composition containing metformin hydrochloride repaglinide, which contains pharmaceutically acceptable carriers. The preparation process is simple, the problem of chipping is effectively solved, and simultaneously, the repaglinide has good content uniformity and dissolution; and the oral solid drug composition is used for treating type II diabetes.
Owner:万全万特制药(厦门)有限公司
Repaglinide synthesis process
InactiveCN1865253AHigh yieldRaw materials are easy to getOrganic chemistryEthoxybenzoatesRepaglinide
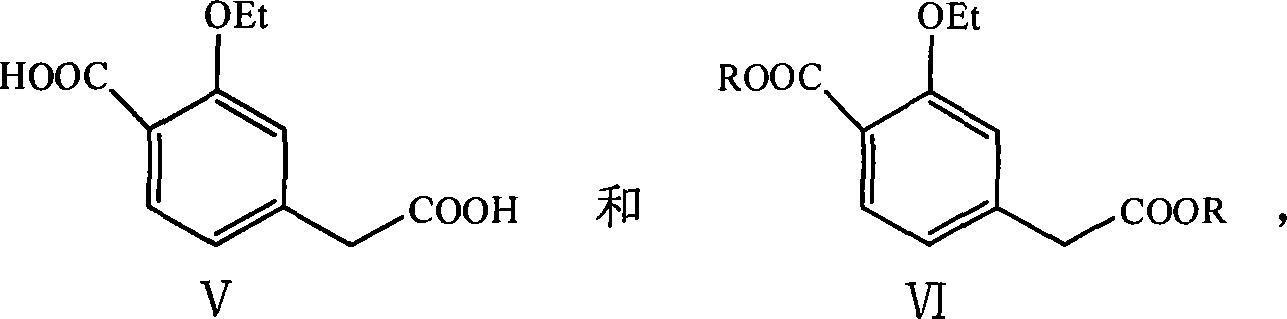
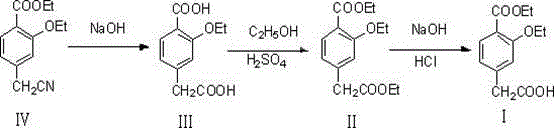
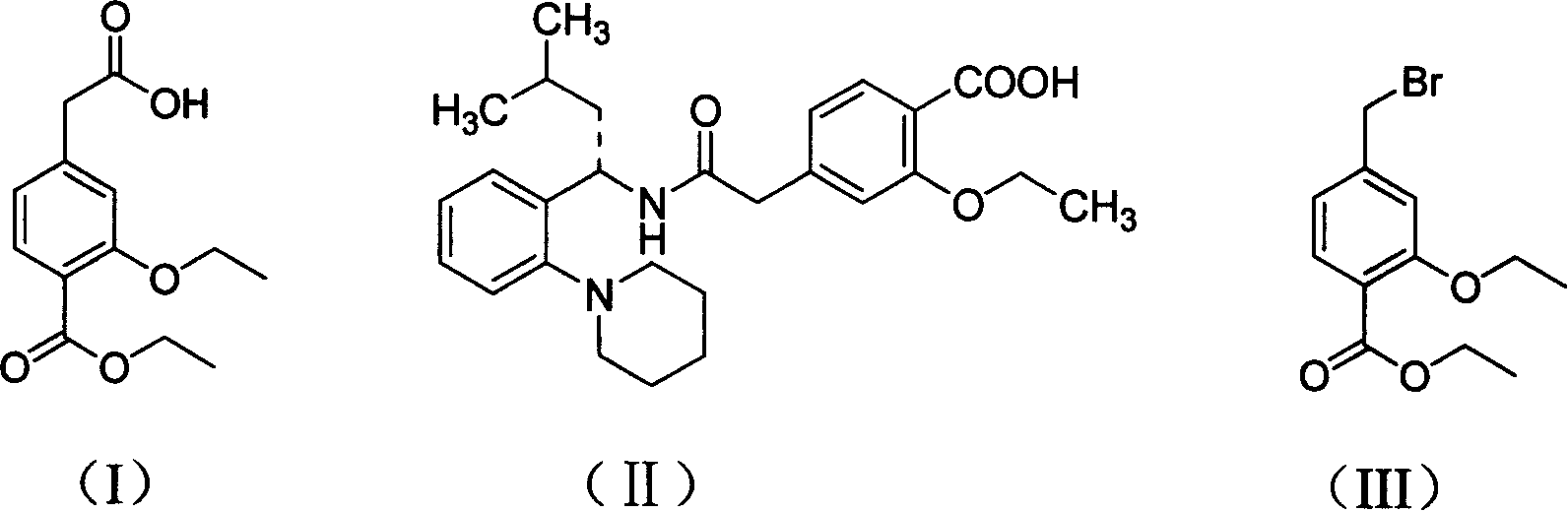
This invention discloses a process for synthesizing repaglinide by using 4-carboxymethyl-3-ethoxybenzoate as raw material to produce acyl chloride. Compared to other process, this invention is characterized of high efficiency, low toxicity, easy obtained and cheap raw material, simple operation, and high yield with 80.9% total yield rate in two-step reaction, which makes it a good repaglinide systhesis process with good industrialization future.
Owner:ZHEJIANG UNIV
Pharmaceutical combination with repaglinide and metformin as active components and preparation method thereof
InactiveCN102218064AEasy to recycleAchieve separationOrganic active ingredientsOrganic chemistryAdhesiveActive component
The invention relates to a pharmaceutical combination with repaglinide and metformin as active components and a preparation method thereof. The pharmaceutical combination comprises 0.1-10 weight portions of repaglinide and 100-1500 weight portions of metformin as active components and pharmaceutic adjuvants, wherein, the pharmaceutic adjuvants comprise filler, disintegrating agent, adhesive, flavoring agent, lubricant and swelling accessory, and the repaglinide is repaglinide crystal. The invention adopts the repaglinide crystal with small particle size, metformin and pharmaceutic adjuvants to prepare the pharmaceutical combination, so as to realize the purpose of simultaneous release. Since the particle size of the repaglinide used in the invention is small, the dissolvability is improved, so that the dissolution is improved and the bioavailability is increased.
Owner:HAINAN JINRUI PHARMA CO LTD
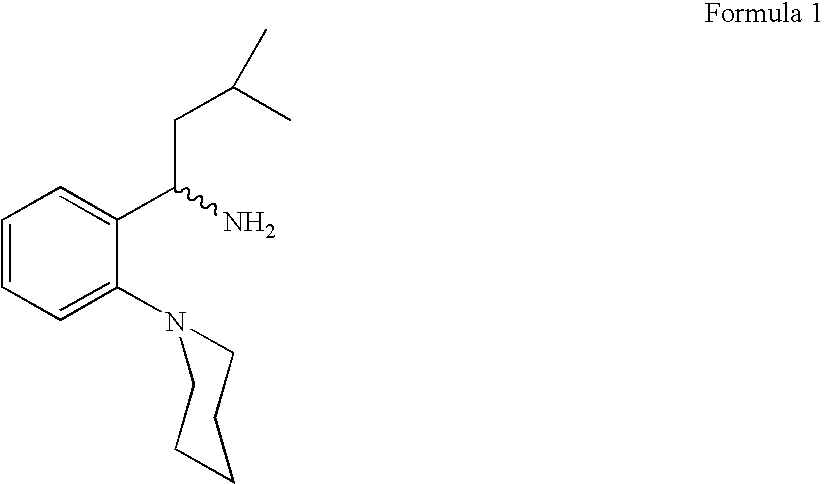
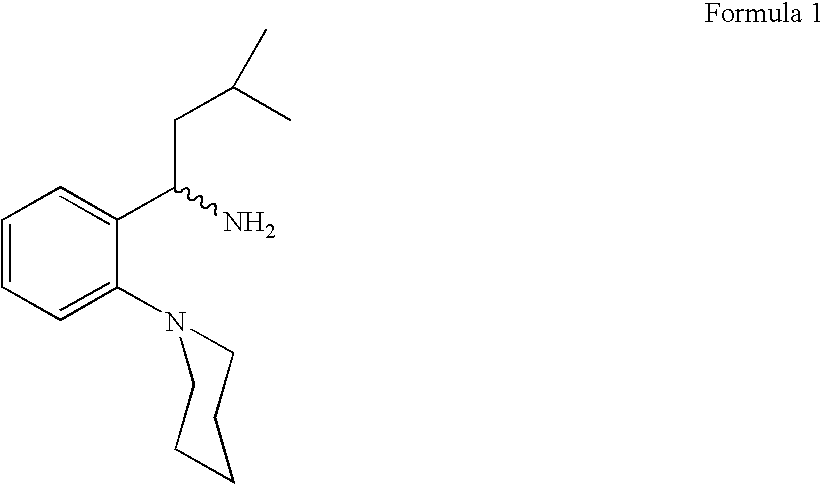
Process for preparing (RS) 3-methyl-1-(2-piperidinyl phenyl) butyl amine
InactiveUS20040192921A1Cheap and easily availableDrawback can be obviatedOrganic chemistryMethyl groupMedicinal chemistry
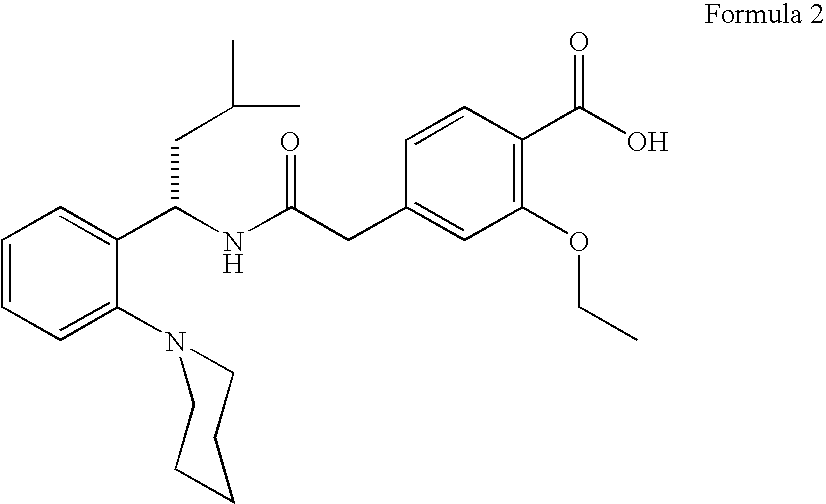
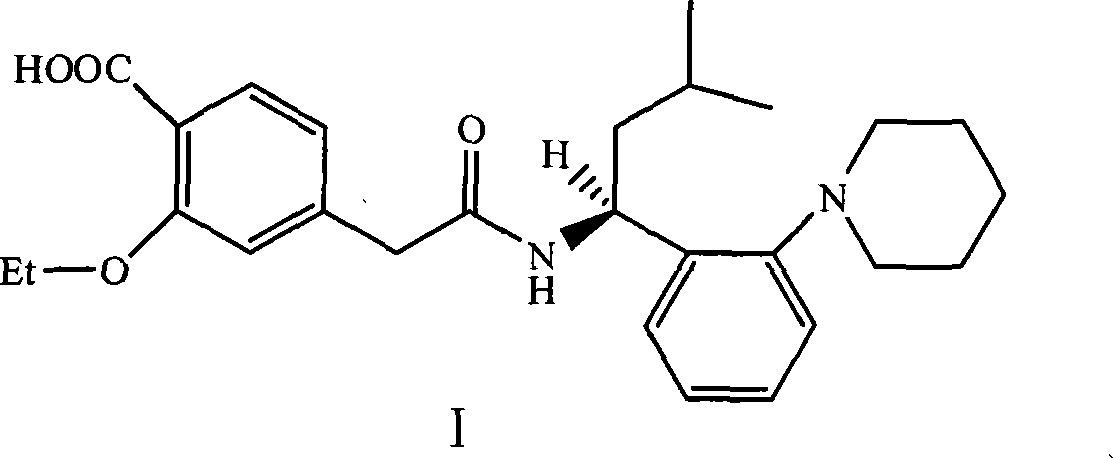
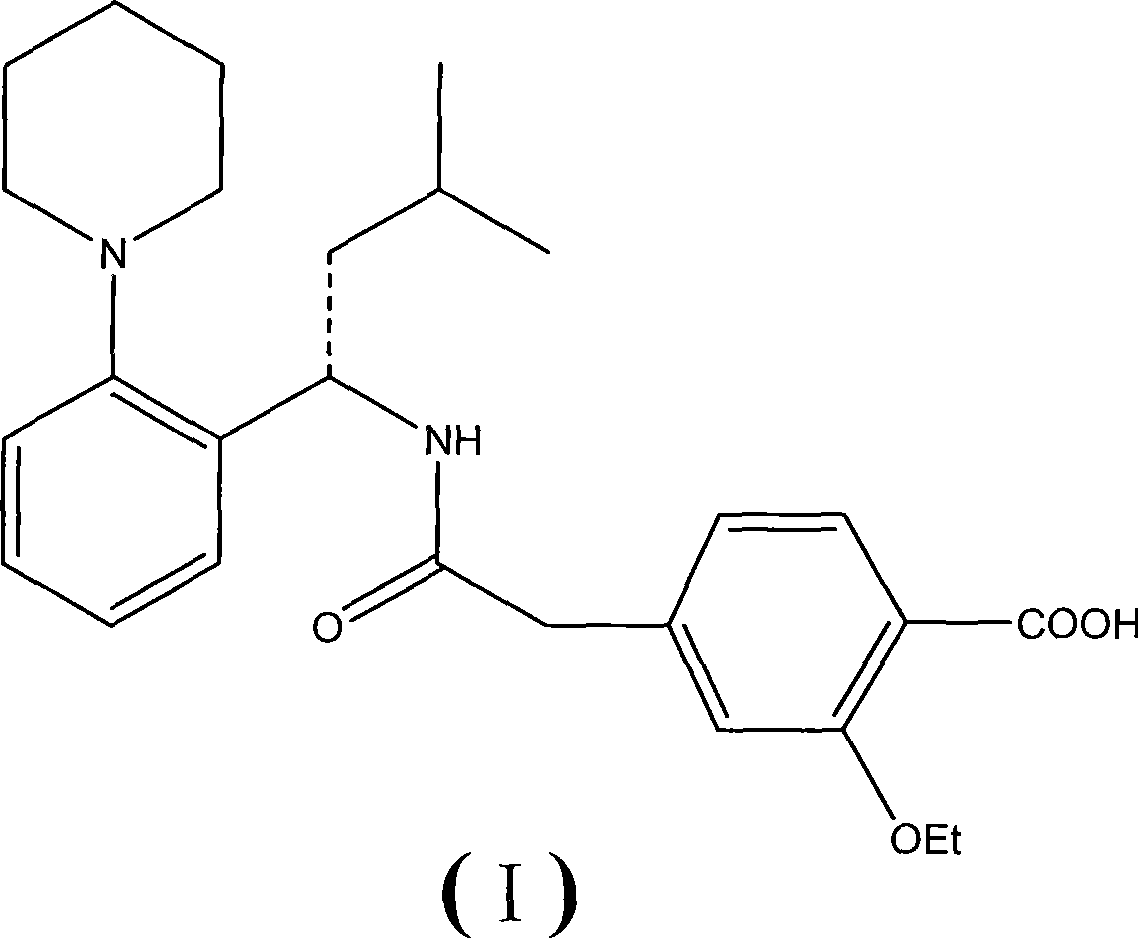
The Present invention relates to a process for the preparation of (RS) 3-methyl-1-(2-piperidinyl phenyl) butyl amine of formula 1. (RS) 3-Methyl-1-(2-piperidinyl phenyl butyl amine having formula 1 is an important key intermediate for the synthesis of repaglinide of formula 2 an oral hypoglycemic agent.
Owner:COUNCIL OF SCI & IND RES
Solid pharmaceutical composition containing repaglinide
The invention discloses a solid pharmaceutical composition containing repaglinide. The solid pharmaceutical composition can exist in various forms of dispersing tablets, oral disintegrating tablets and capsules and is used for curing the type 2 diabetes of which the high blood sugar cannot be effectively controlled by diet control and exercise.
Owner:AVENTIS PHARMA HAINAN
Repaglinide troche and preparation method thereof
ActiveCN103610677AAvoid stickingGood dispersionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention relates to an oral troche which contains repaglinide or pharmaceutically acceptable derivatives of repaglinide as well as a preparation method of the oral troche. According to the preparation method, powder of repaglinide or pharmaceutically acceptable derivatives of repaglinide is directly pressed into troche, so that the production cost is remarkably lowered, and the disintegration and the dissolution rate are greatly improved. The bioavailability and the stability of the medicine can be improved, and the problem of low content uniformity of existing small-dose medicines formed by the direct pressing method is overcome, so that the quality of the troche is better guaranteed.
Owner:华益泰康药业股份有限公司
Method for producing repaglinide
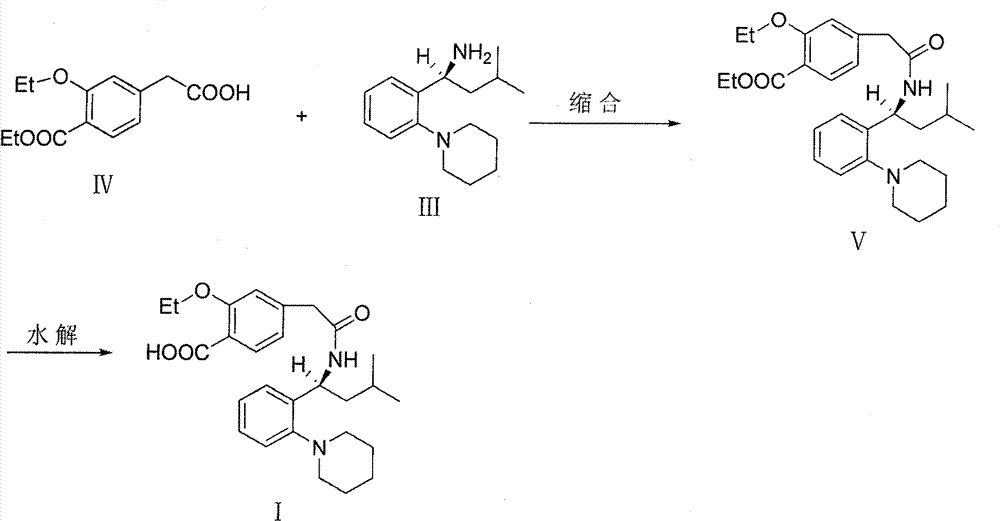
The invention relates to a novel method for preparing repaglinide, which adopts a 4- carboxymethyl-2-ethoxybenzoic acid as raw material and derives a repaglinide intermediate 4-carboxymethyl-2-ethoxy methyl benzoate via esterification and hydrolysis; a product is condensed with an S(+)-1-(2-piperidino-phenyl)-3-methyl n-butylamine to derive an S(+)-2-oxethyl-4-[N-{1-(2-piperidino-phenyl)-3-methyl-1- butyl} amido carbonyl methyl] benzoate; a repaglinide is derived via one more hydrolysis. Compared with the other technologies, the invention has the advantages of simple operation, high efficiency, higher yield, low toxicity, and environmental friendliness. The product repaglinide has quite high optical purity, the ee value is not less than 99.8 percent; the invention is a novel technology for producing repaglinide with industrial prospect.
Owner:ZHEJIANG NEXCHEM PHARMA
Method for preparing repaglinide
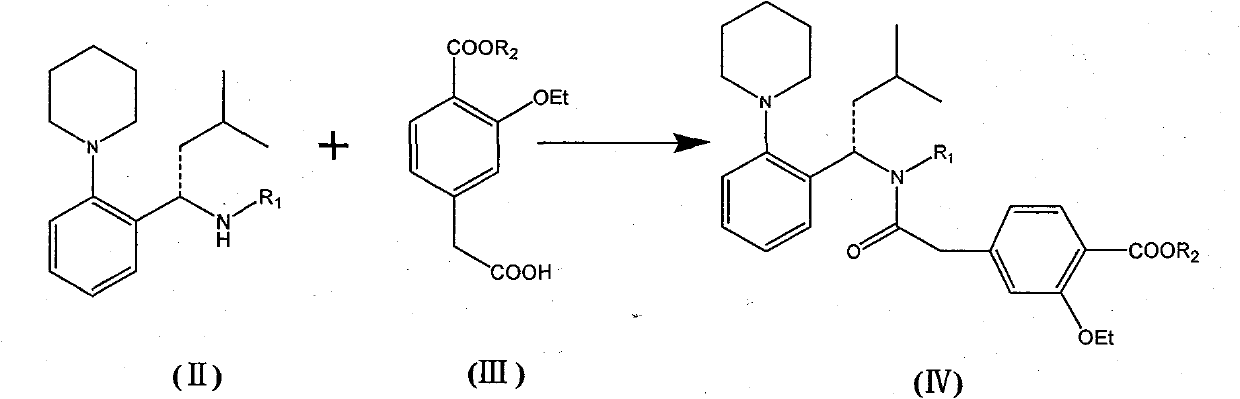
InactiveCN101481363AEnhanced nucleophilicityCondensation reaction is easyOrganic chemistryBulk chemical productionRepaglinidePhotochemistry
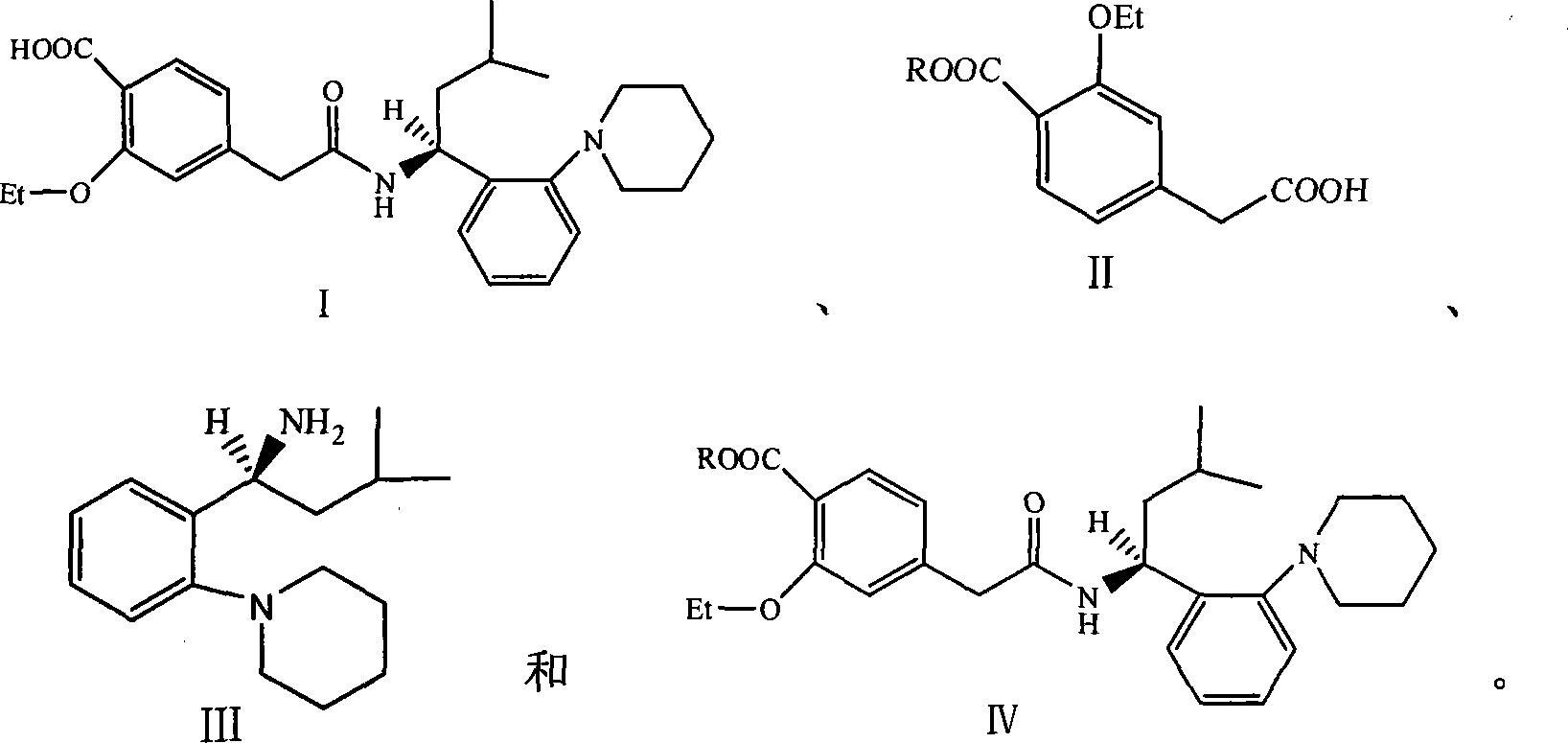
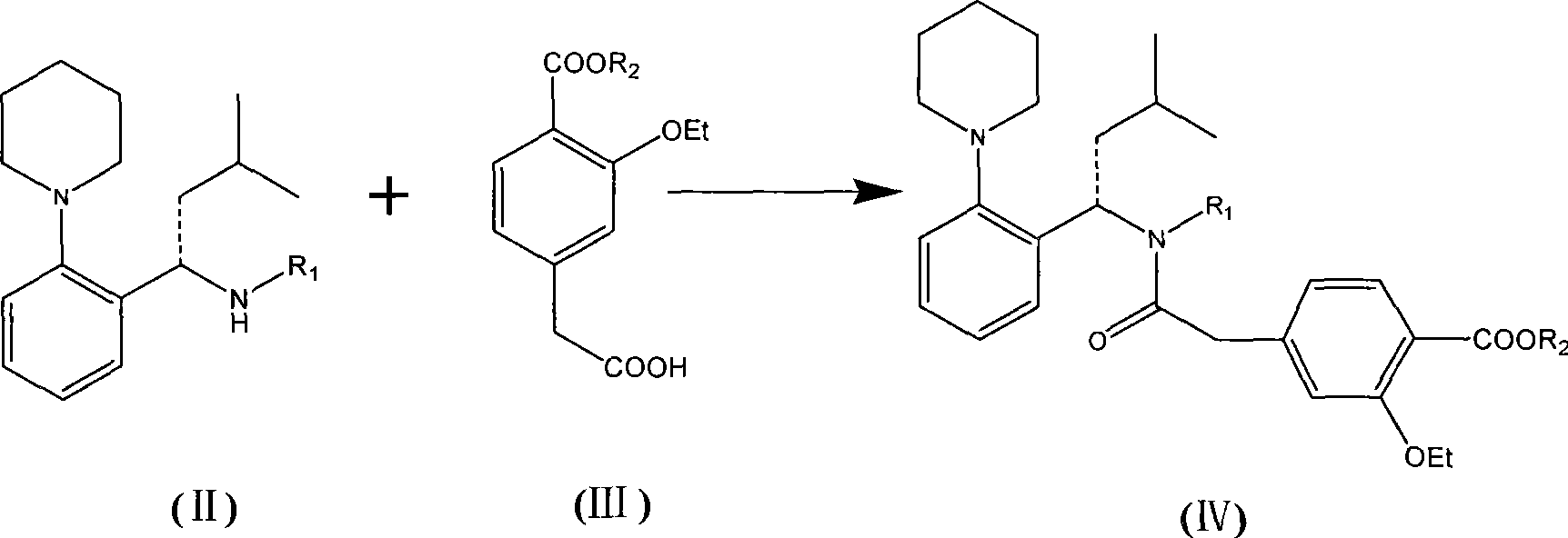
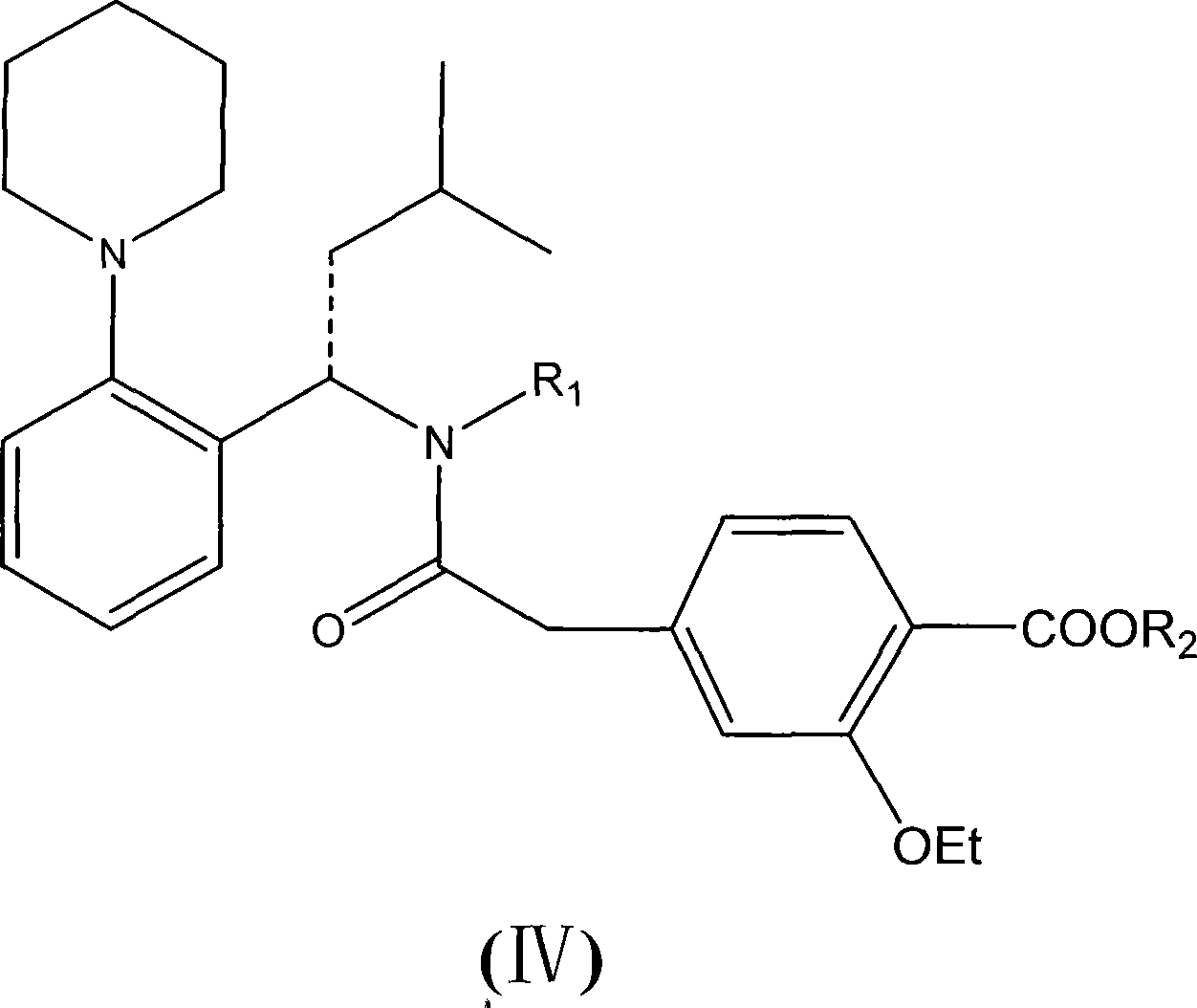
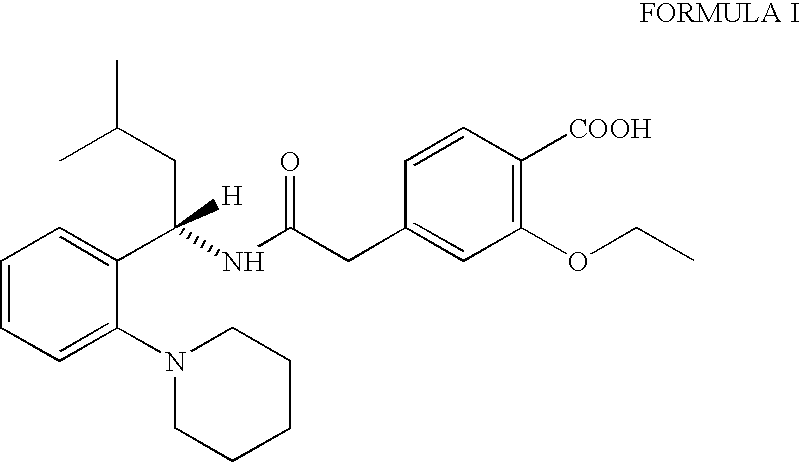
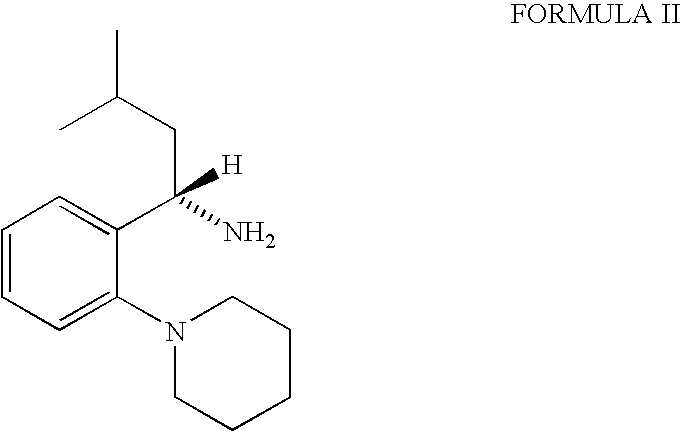
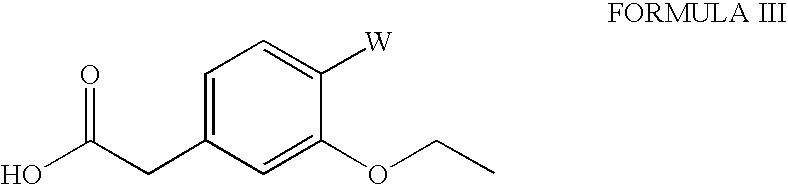
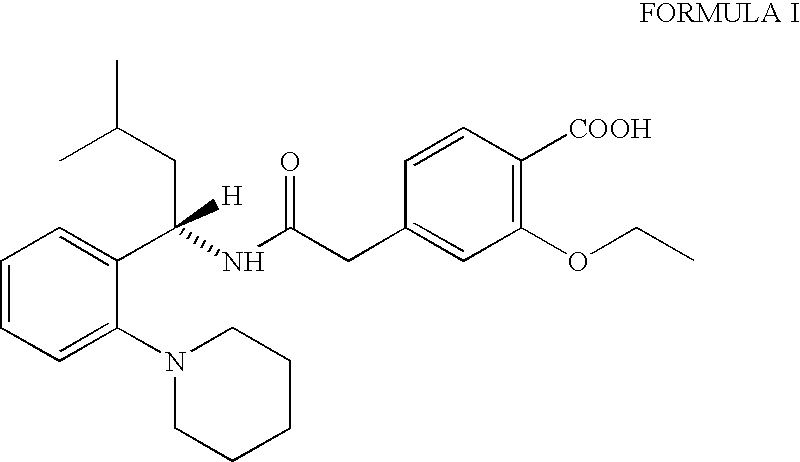
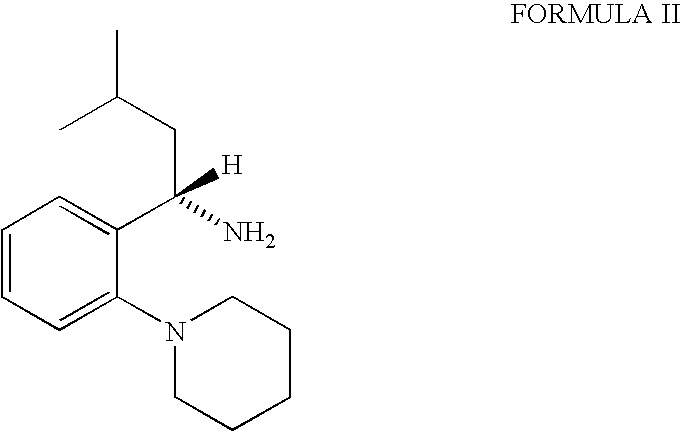
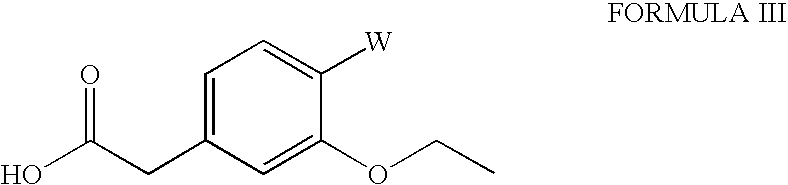
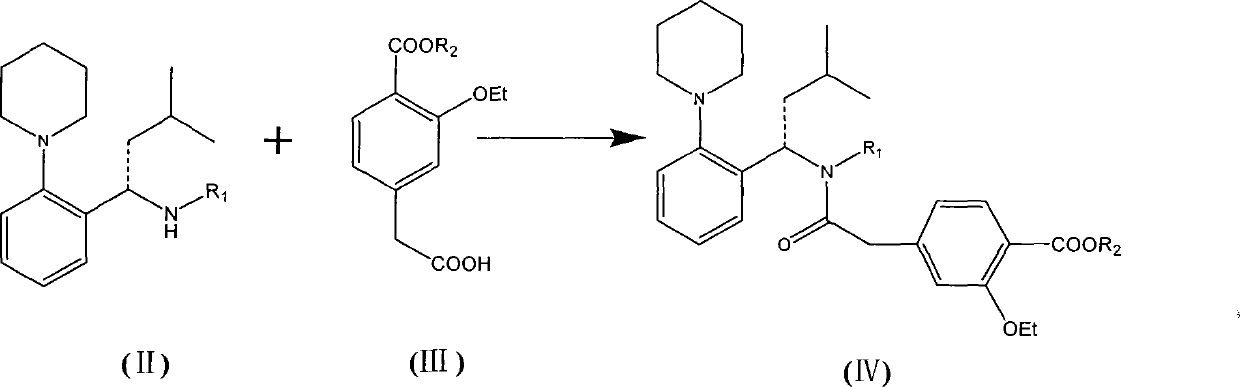
The invention relates to a preparation method of a drug for treating diabetes mellitus, that is, repaglinide. The preparation method comprises the following steps: a (S) type compound as shown in formula (II) and a compound as shown in formula (III) are subject to an amidation reaction in the presence of a condensing agent to generate a (S) type compound as shown in formula (IV); R2 radical is removed from the (S) type compound of the formula (IV) in the presence of alkali, and R1 radical is removed in the presence of acid. The (S) type compounds as shown in the formula (II), the formula (III) and the formula (IV), and the R1 radical and the R2 radical are defined in the specification. In the preparation method, the introduction of the R1 radical and deprotection improvement shorten the reaction time, increase the yield and enhance safety, thus the method is more suitable for industrialized production.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing Repaglinide
This invention relates to a method for preparing repaglinide. Compared with other methods, the method has such advantages as high efficiency, low toxicity, high safety, easy operation, wide raw materials, and low cost, thus is suitable for industrialization.
Owner:JIANGSU HANSOH PHARMA CO LTD
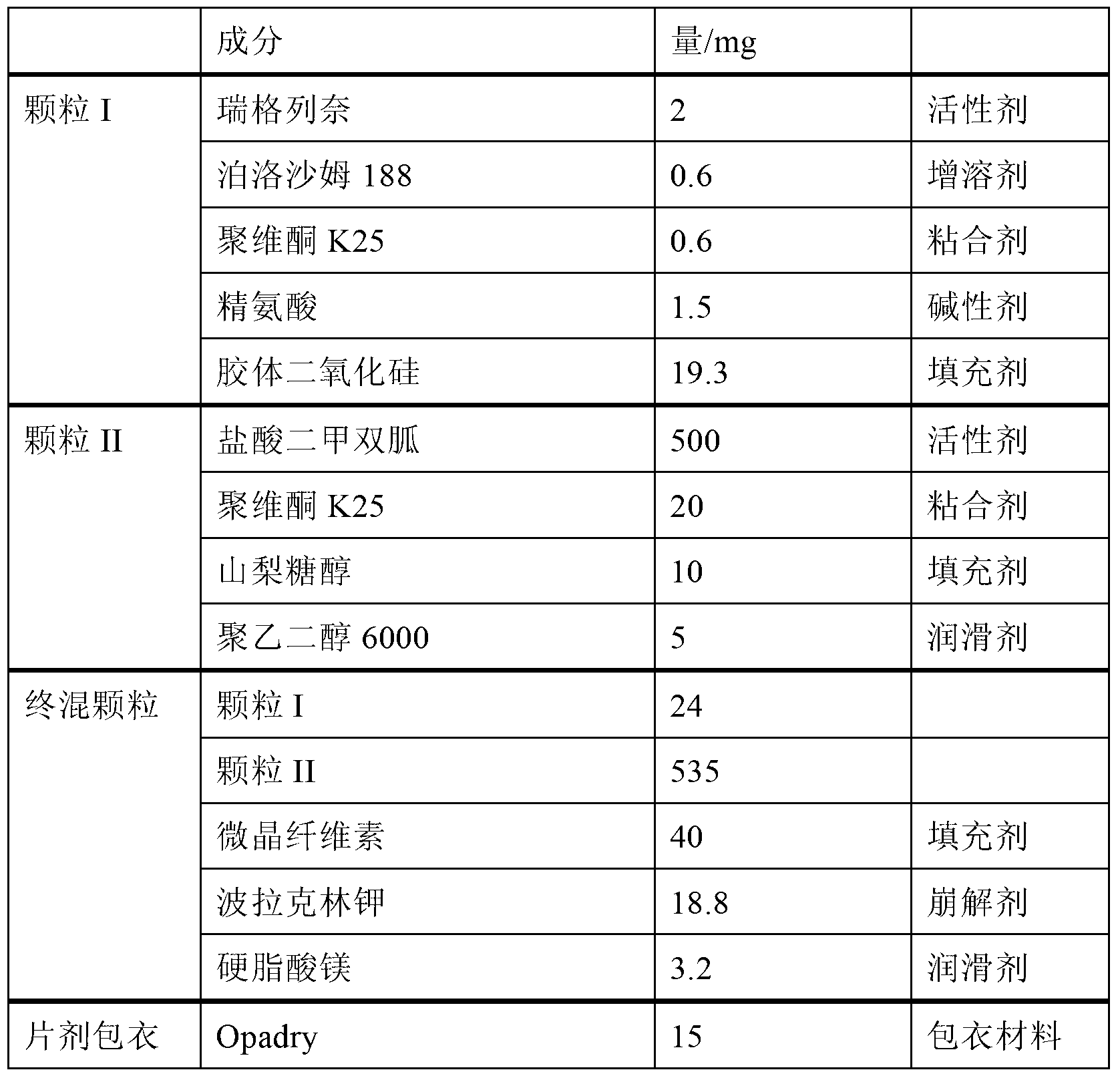
Stable method for preparing repaglinide compressed tablets
InactiveCN102357085AContent Uniformity AdvantageOrganic active ingredientsMetabolism disorderAdditive ingredientFluidized bed
The invention relates to the field of medicine medicinal preparation, in particular to a stable method for preparing repaglinide compressed tablets, which is characterized in that the pelletizing process includes the following steps: adding repaglinide into adhesive solution and making the pellets with fillers through a fluidized bed in ejecting mode. Particles of the repaglinide tablets can rapidly disperse when the epaglinide tablets disintegrate, and active ingredient repaglinide evenly dispersed in the particles can rapidly dissolve out. The prepared repaglinide particles can be evenly mixed with auxiliary materials such as disintegrating agents, lubricating agents and the like and pressed into tablets. Tablet cores can completely disintegrate within two minutes.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
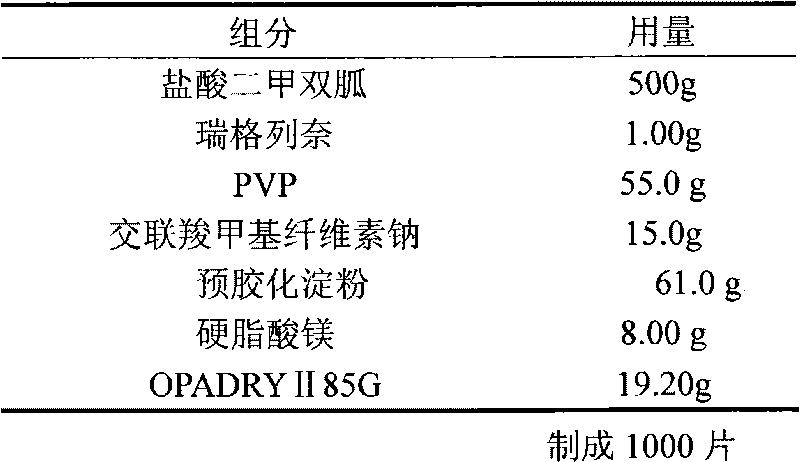
Repaglinide-metformin hydrochloride tablet and preparing method thereof
InactiveCN104337811AUse less excipientsSolve the problem of insoluble in waterOrganic active ingredientsMetabolism disorderMetformin HydrochlorideSolvent
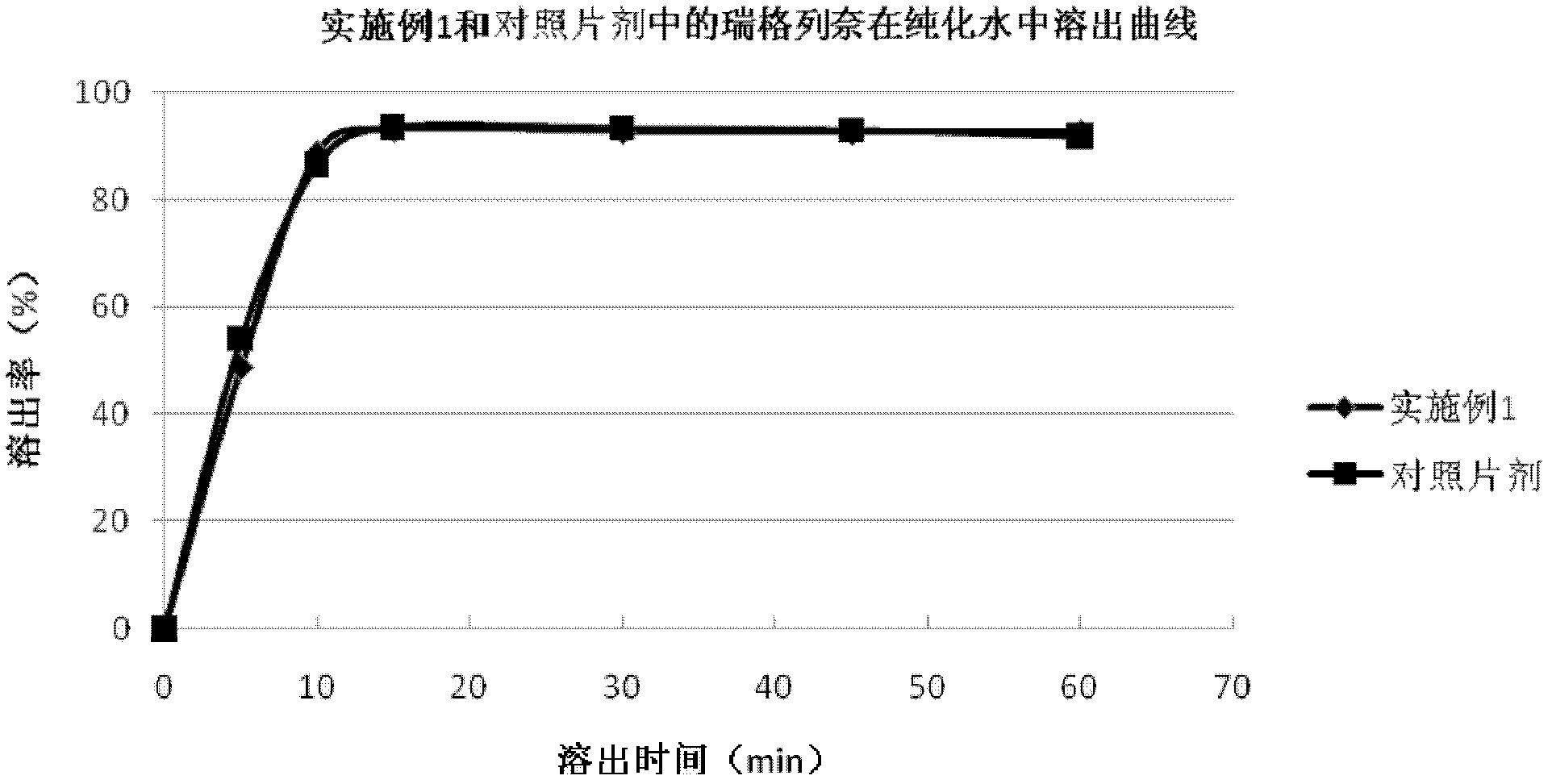
The invention discloses a prescription of a repaglinide-metformin hydrochloride tablet and a preparation technology thereof. In particular, a solid dispersion water solution technology is used; the problem that repaglinide is insoluble in water can be solved only through common wet granulations; dissolved objects and related matter are both superior to those of foreign objects. Microcrystalline celluloses and sorbitol serve as fillers; polyvinylpolypyrrolidone serves as disintegrating agents; PVPk30 serves as bonding agents; silicon dioxide serves as lubricating agents; meglumine serves as saltiness agents; and poloxamer 188 serves as solubilizers. The repaglinide-metformin hydrochloride tablet prepared with the technology is fewer in use auxiliary material; the high dissolving performance and the high stability can be kept; the preparation technology is simple; the technology cost is lower; and the repaglinide-metformin hydrochloride tablet is suitable for industrial production.
Owner:JIANGSU CAREFREE PHARM CO LTD
Process for the preparation of repaglinide
The present invention relates to a cost effective and industrially advantageous process for the preparation of repaglinide.
Owner:RANBAXY LAB LTD
Medicinal preparation containing repaglinide and preparation of medicinal preparation
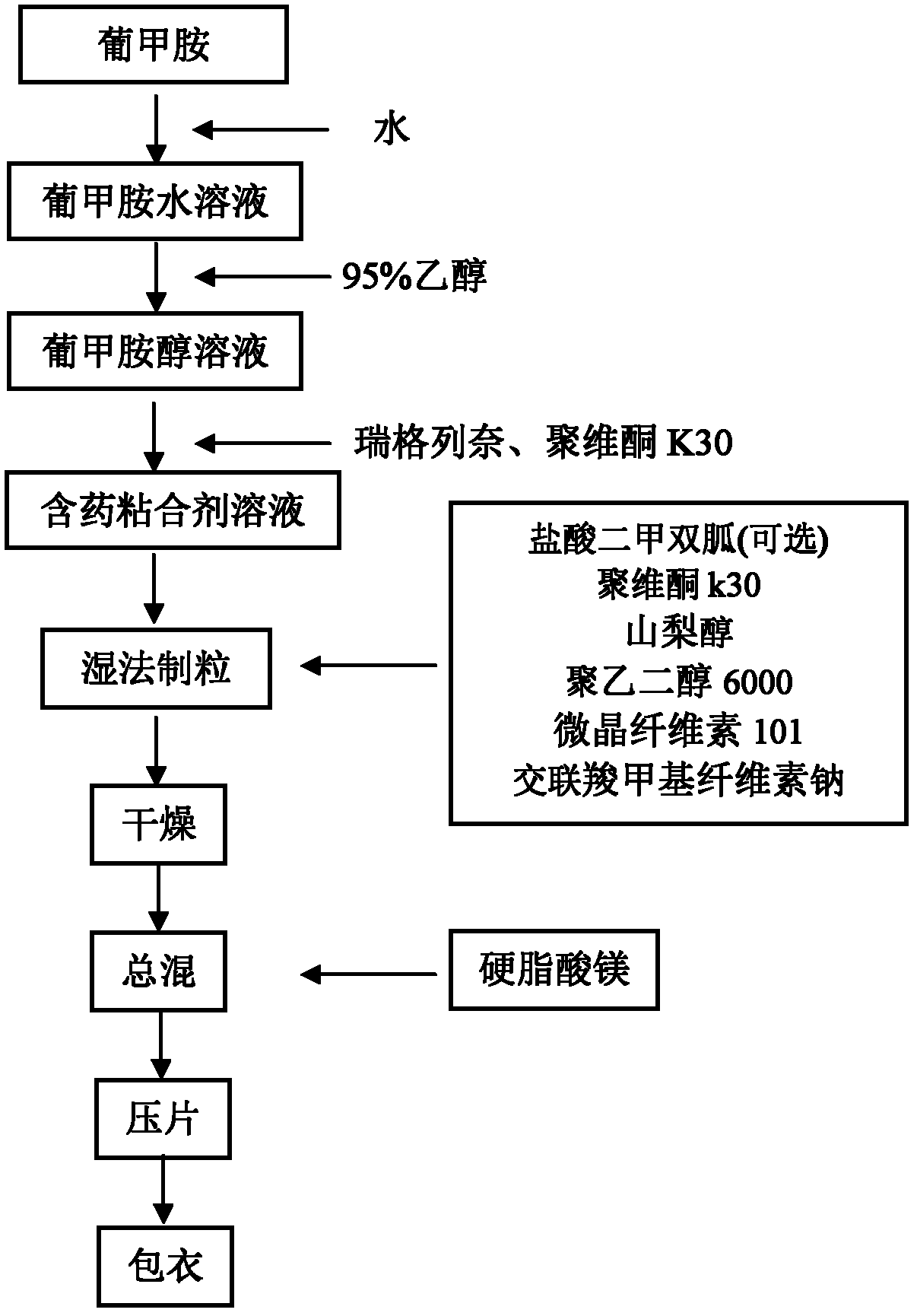
ActiveCN103181923ASimple processSuitable for industrial production needsOrganic active ingredientsMetabolism disorderAdjuvantAlcohol
The invention relates to a preparation method of a medicinal preparation of repaglinide. The preparation method comprises the following steps: (a) dissolving meglumine and repaglinide in alcohol and water to prepare an adhesive solution; (b) mixing pharmaceutically acceptable adjuvant materials to prepare a mixture; (c) adding the adhesive solution to the mixture obtained in the step b, and performing wet granulation; and (d) preparing the medicinal preparation. The invention further relates to application of meglumine as a repaglinide stabilizer in the medicinal preparation containing repaglinide.
Owner:BEIJING HANMI PHARMA CO LTD
Repaglinide and dimethyldiguanide pharmaceutical composition and preparation method thereof
InactiveCN103385878ASolve the splinter problemMaterial excellenceOrganic active ingredientsMetabolism disorderInsulin dependent diabetesMetforminum
The invention belongs to the field of pharmaceutical compositions for treating non-insulin dependent diabetes mellitus (NIDDM), and in particular relates to a novel pharmaceutical composition for repaglinide and dimethyldiguanide tablets and a preparation method thereof. The pharmaceutical composition is equivalent to PrandiMet in dissolving-out characteristic on the basis that good content uniformity of repaglinide is ensured, and related substances are superior to the existing products. The preparation method is suitable for industrialized production.
Owner:SHANDONG INST OF PHARMA IND
Compound repaglinide-metformin hydrochloride solid quick-release preparation and preparation method and application thereof
InactiveCN103371981AImprove securityEasy to solveOrganic active ingredientsMetabolism disorderActive componentCurative effect
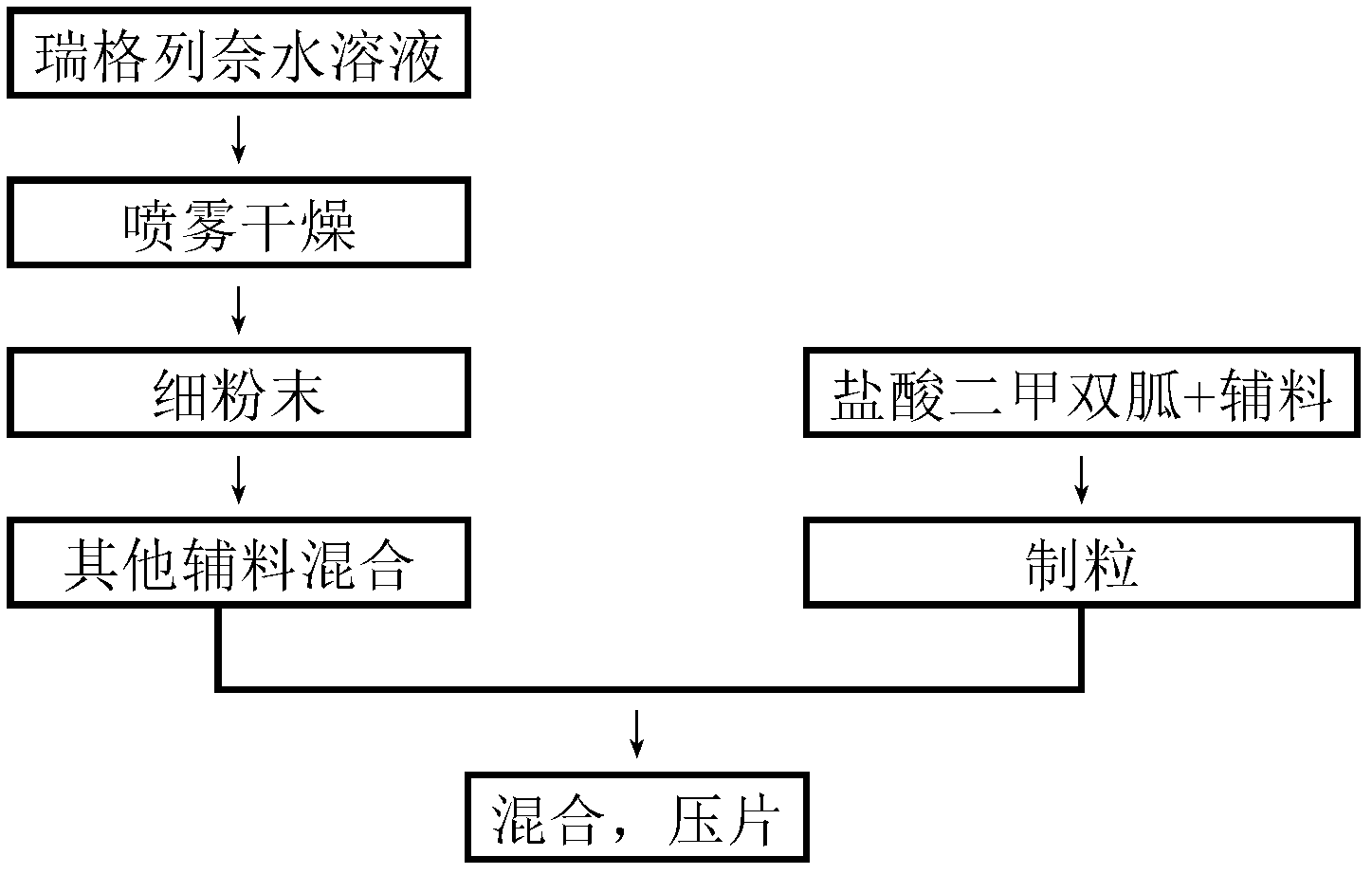
The invention relates to a compound repaglinide-metformin hydrochloride solid quick-release preparation and a preparation method and an application thereof, and belongs to the technical field of medicines. A quick-release tablet is prepared from repaglinide and metformin hydrochloride which are taken as medicinal active components, and suitable medicinal auxiliary materials, and the problems of quick dissolution-out of the indissolvable medicine, namely, repaglinide and the stability of the repaglinide in a tablet placement process are solved. The preparation method comprises the following steps of: respectively granulating the repaglinide and the metformin hydrochloride by selecting the suitable medicinal auxiliary materials; mixing, and then pressing into tablets, or filling into capsules. The prepared compound preparation has a synergetic effect on controlling blood sugar and improving the curative effect and is convenient for patients to take.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
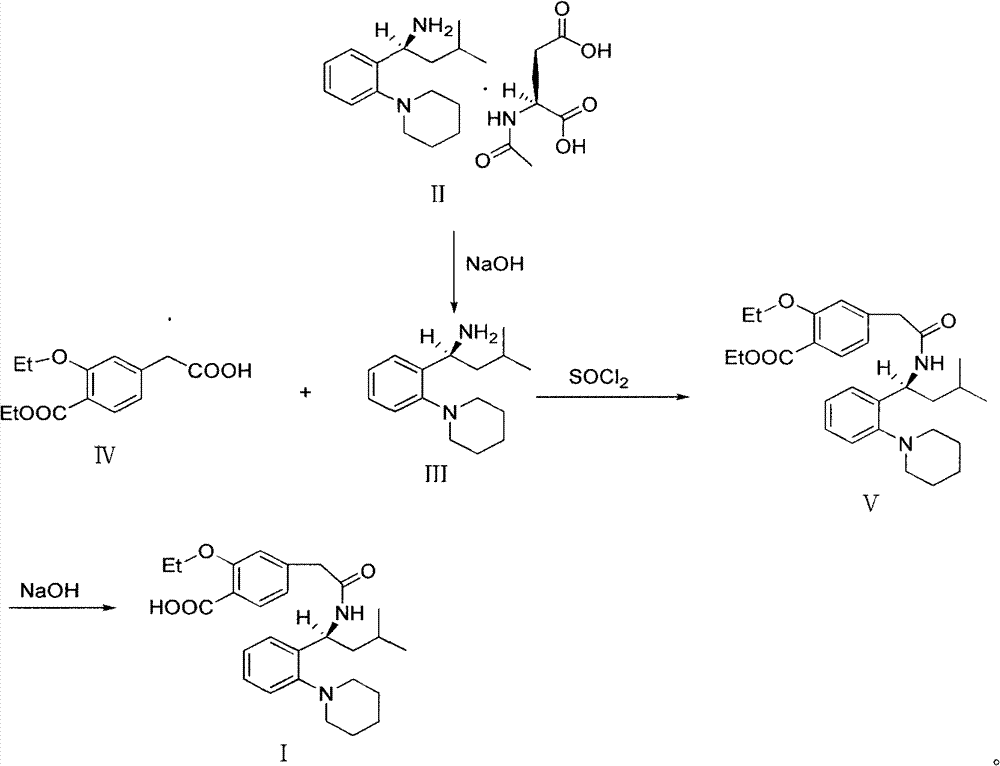
Preparation and refining method of repaglinide
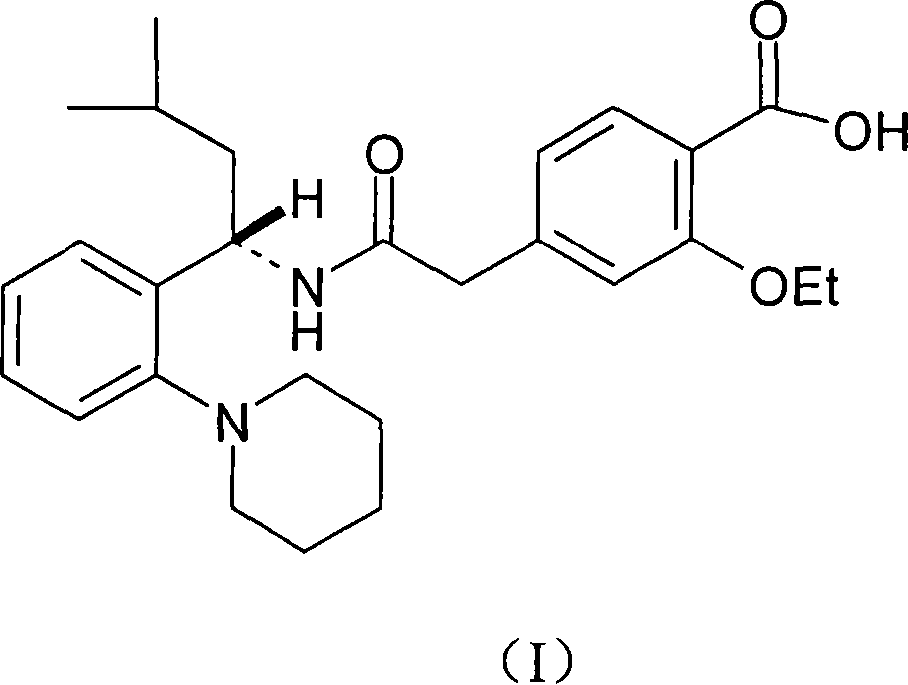
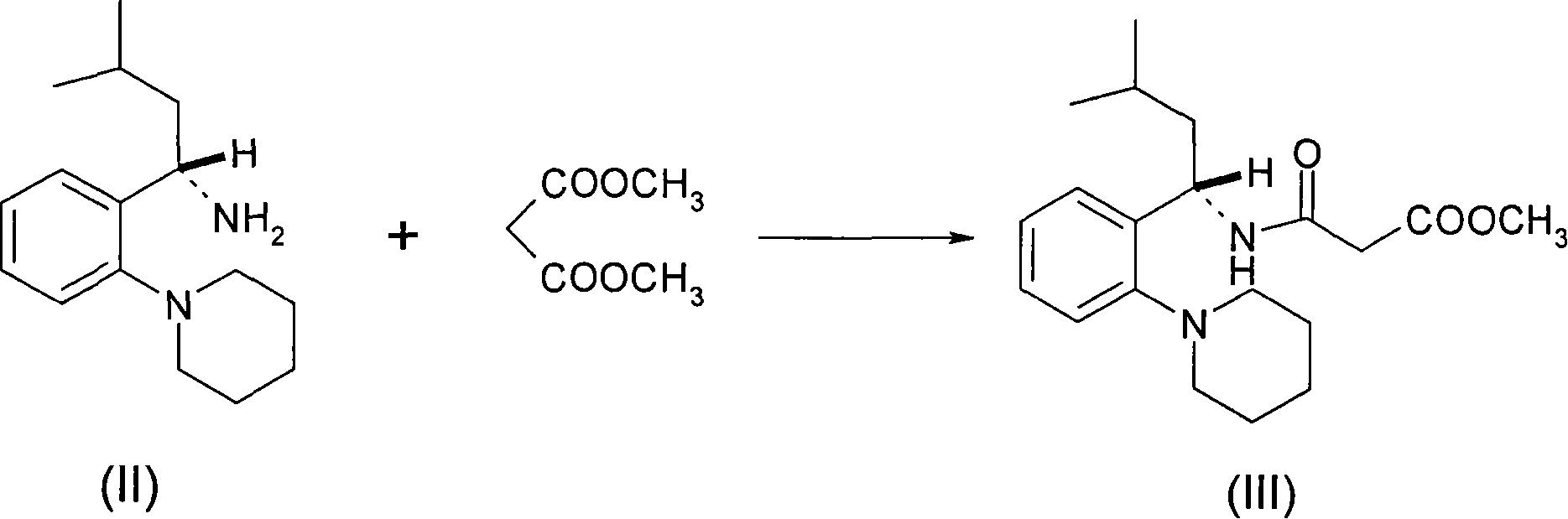
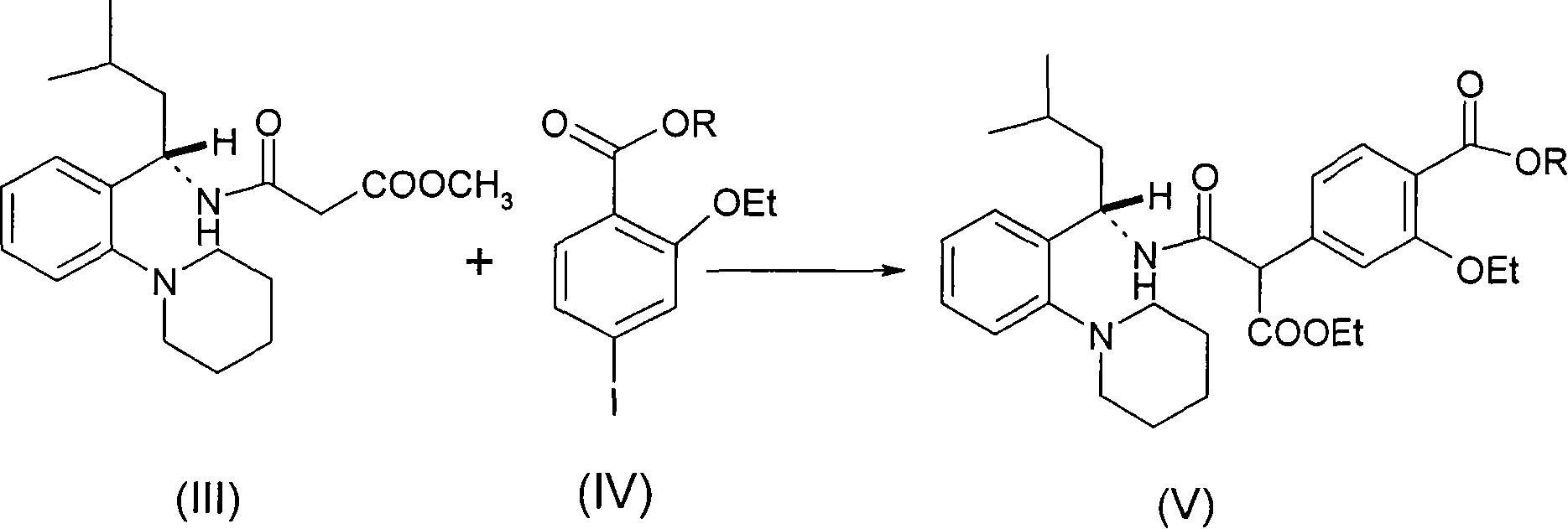
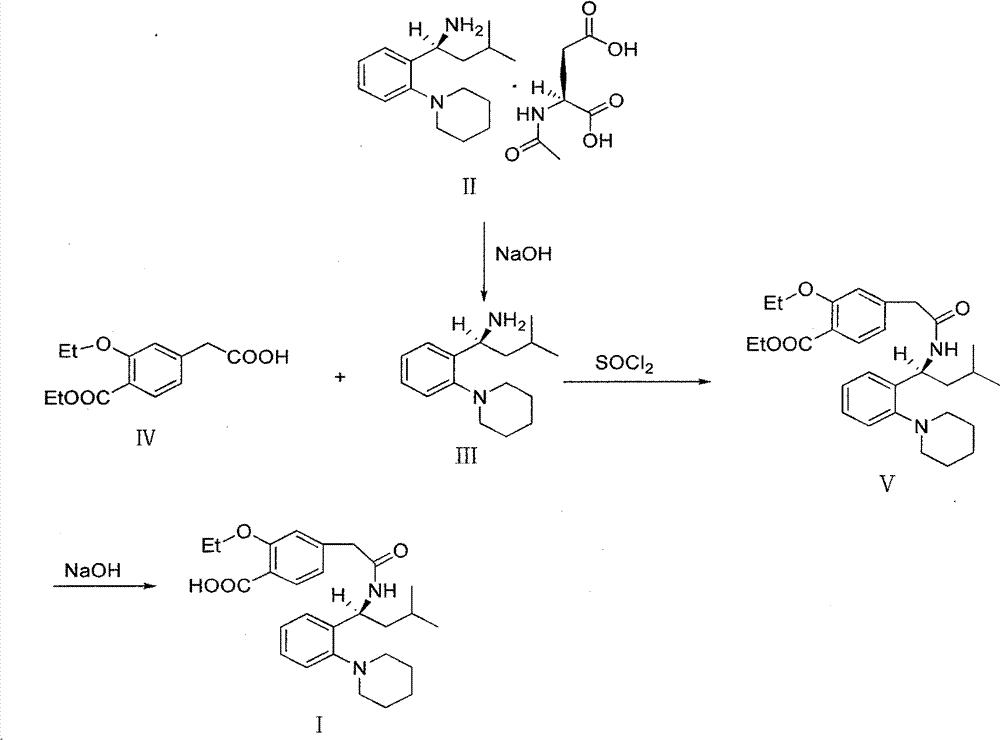
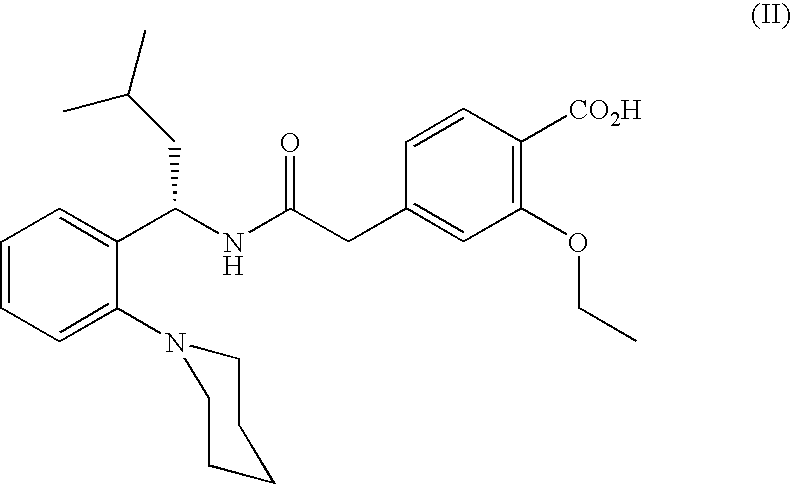
The invention relates to a preparation and refining method of repaglinide. The preparation method comprises the steps that: (S)-3-Methyl-1-(2-piperidinophenyl)butylamine glutamate (II) is adopted as a raw material, and is subjected to hydrolysis and an acylation reaction; an obtained material is subjected to an amidation reaction with 4-ethoxycabonyl-3-ethoxyphenylacetic acid (IV) under the existence of a condensing agent, such that 4-ethoxycabonyl-3-ethoxyphenylacetic ester (V) is obtained; the material V is hydrolyzed under an alkaline condition, such that a repaglinide crude product is obtained; the repaglinide crude product is subjected to ethanol-water solvent crystallization, such that repaglinide (I) is obtained. According to the invention, (S)-3-Methyl-1-(2-piperidinophenyl)butylamine glutamate is adopted as an initial raw material; the reaction conditions are optimized; the yield is improved; and good safety is ensured. An ethanol-water refining method is adopted, such that the product quality is improved, and environment pollution is reduced. Therefore, the method is suitable for industrialized productions.
Owner:双鹤药业(海南)有限责任公司
Repaglinide/metformin composition
ActiveCN103251593AImplement synchronous releaseExcellent in vitro dissolutionOrganic active ingredientsMetabolism disorderMetformin HydrochlorideRepaglinide
The invention relates to a repaglinide / metformin composition, particularly a pharmaceutical composition which comprises the following components in parts by weight: 500 parts of metformin hydrochloride, 0.5-5 parts of repaglinide and 10-200 parts of medicinal auxiliary materials. The medicinal auxiliary materials include, but are not limited to filler, disintegrant, binding agent, alkali and lubricant. The invention also relates to a method for preparing the pharmaceutical composition. The pharmaceutical composition provided by the invention has favorable pharmaceutical properties.
Owner:HANGZHOU ZHUYANGXIN PHARMA
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
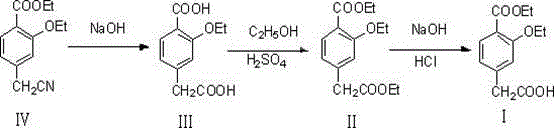
Method for synthesizing repaglinide key intermediate
InactiveCN104628518AHigh yieldAvoid highOxygen-containing compound preparationOrganic compound preparationPhenylacetic acidFirst pass yield
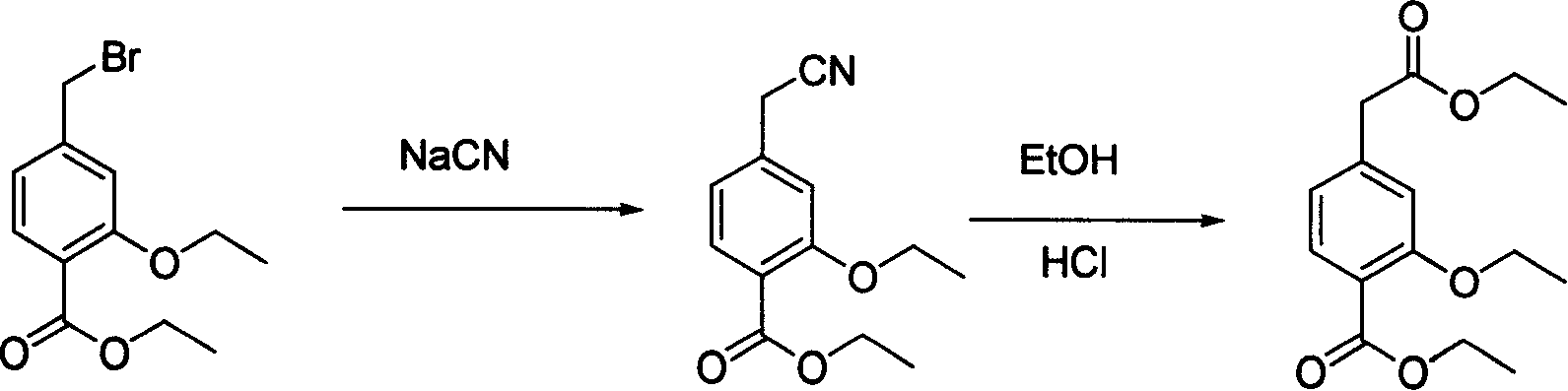
The invention relates to a method for synthesizing a repaglinide key intermediate, namely 4-ethoxycarbonyl-3-ethyoxy phenylacetic acid. The method comprises the following steps: by taking 3-ethyoxy-4-ethoxycarbonyl-benzyl cyanide as an initial raw material, performing hydrolysis, esterification, selective hydrolysis and the like, thereby obtaining the important intermediate 4-ethoxycarbonyl-3-ethyoxy phenylacetic acid of repaglinide. According to the key intermediate, the impurities of the product is less, the purity of the product is high and can reach over 99.7%, so that the quality of the subsequently synthesized repaglinide product is improved, the 100 percent first-pass yield of the subsequently synthesized repaglinide product can be basically reached, a refining step is avoided, and the synthetic yield is effectively improved. The process is easy and convenient to operate, the yield is high, the molar yield is 69.1 percent, the production cost is low, and the method is suitable for industrial production.
Owner:HUBEI YITAI PHARMA
Pharmaceutical Compositions Comprising a Hypoglycemic Agent and Methods of Using Same
The present invention relates to intranasally deliverable compositions comprising a hypoglycemic agent, for example, repaglinide, and to methods of using such compositions in the treatment of various disorders, including, for example, type-2 diabetes.
Owner:UPSHER SMITH LABORATORIES INC
Pharmaceutical composition of repaglinide and metformin hydrochloride and preparation technology of pharmaceutical composition
ActiveCN105534980AImprove stabilityGood dissolution effectOrganic active ingredientsMetabolism disorderParticulatesFiller Excipient
The invention relates to the field of pharmaceutic preparation and in particular relates to a pharmaceutical composition of repaglinide and metformin hydrochloride and a preparation technology of the pharmaceutical composition. The technology comprises the steps of performing dry granulating on repaglinide, performing wet granulation on metformin hydrochloride, then mixing two kinds of particulate matters, and tabletting, so as to solve the problem of compatibility of the repaglinide and the metformin hydrochloride, wherein repaglinide particles take microcrystalline cellulose as a filling agent and a disintegrating agent, meglumine as an alkaline agent, lauryl sodium sulfate as a solubilizing agent, and magnesium stearate as a lubricating agent. The preparation is quick in compound content dissolution, and the dissolution of the repaglinide and the dissolution of the metformin hydrochloride are consistent, and are stable in various dissolution media; related substances of a product are less, the stability and quality are controllable, and the safety of the medicine is guaranteed.
Owner:JIANGSU HANSOH PHARMA CO LTD
Novel method of producing repaglinide key intermediate
InactiveCN101153010AHigh reaction safetyFew reaction stepsOrganic compound preparationCarboxylic acid esters preparationBenzoic acidPhenylacetic acid
The present invention relates to a method of preparing 4-ethoxycarbonyl-3-ethoxy phenylacetic acid. In a ether solvent, the 4-bromomethyl-2-ethoxy-benzoic acid ethyl ester and metal magnesium react at a certain temperature to produce Gers agent preparation of the compound. Without treatment, the reagent is directly dropped into dry ice. At last, a key intermediate 4-ethoxycarbonyl-3-ethoxy phenylacetic acid for preparation of repaglinide can be separated through hydrolysis.
Owner:BEIJING D VENTUREPHARM TECH DEV
Process for the preparation of repaglinide
The present invention relates to a cost effective and industrially advantageous process for the preparation of repaglinide.
Owner:RANBAXY LAB LTD
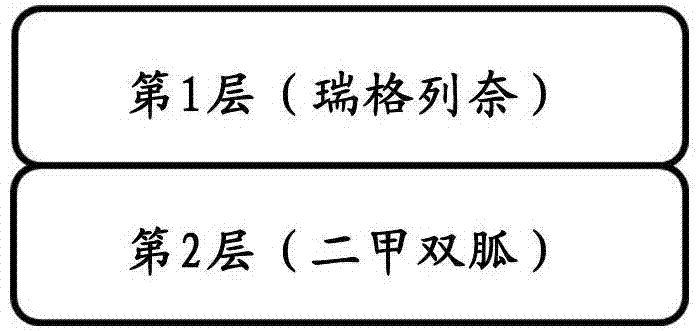
Bilayered tablet comprising repaglinide and meformin and preparation method thereof
InactiveCN104208032AAvoid Layer SeparationOrganic active ingredientsMetabolism disorderMedicineRepaglinide
The invention provides a bilayered tablet comprising repaglinide and meformin and a preparation method thereof. The bilayered tablet is characterized by comprising a first layer containing the repaglinide or other medical salt and a second layer containing the meformin or other medical salt.
Owner:DALIM BIOTECH
One-pot method for synthesizing repaglinide for treating diabetes
InactiveCN102633750AShort reaction pathImprove stabilityOrganic chemistryChromatographic separationPhenylacetic acid
The invention relates to a one-pot method for synthesizing repaglinide. In the method, commercial (S)-3-methyl-1[2-(1-piperidyl) phenyl] butyl amine L-N-acetylglutamate and 4-ethoxycarbonyl-3-ethoxy phenylacetic acid are used as raw materials to prepare repaglinide, thereby greatly shortening the reaction route and facilitating the production stability and continuity; and in esterification reaction, an acylating agent / acid-binding agent replaces triphenyl phosphorus / carbon tetrachloride to perform reaction, the intermediate does not needed to be crystallized, direct hydrolysis is performed according to the one-pot method, the intermediate meeting the requirements is obtained by a crude product recrystallization method, the column chromatographic separation is not needed, waste liquid is easy to treat, the yield is greatly improved, and the industrial production is facilitated.
Owner:ZHEJIANG ANGLIKANG PHARMA
A kind of repaglinide chitosan sustained and controlled release microspheres and preparation method thereof
InactiveCN102266296AUniform qualityWrappedOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaMicrosphere
The invention discloses a preparation of repaglinide chitosan sustained and controlled release microspheres and a preparation method thereof. The core-shell microsphere preparation is formed in the capsule material formed by condensation and cross-linking of polysaccharides. The preparation has slow and controlled release of drugs, and significantly improves the stability of repaglinide. The capsule material prepared by natural cross-linking agent and chitosan has good biocompatibility without any cytotoxicity, and can promote the absorption of drugs , improve the bioavailability of the drug. The invention is used for preparing repaglinide chitosan sustained and controlled release microspheres, and the medicine in dosage form is mainly used for preventing and treating type Ⅱ diabetes mellitus with high blood sugar.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for preparing repaglinide
InactiveCN101481363BEnhanced nucleophilicityCondensation reaction is easyOrganic chemistryBulk chemical productionRepaglinidePhotochemistry
The invention relates to a preparation method of a drug for treating diabetes mellitus, that is, repaglinide. The preparation method comprises the following steps: a (S) type compound as shown in formula (II) and a compound as shown in formula (III) are subject to an amidation reaction in the presence of a condensing agent to generate a (S) type compound as shown in formula (IV); R2 radical is removed from the (S) type compound of the formula (IV) in the presence of alkali, and R1 radical is removed in the presence of acid. The (S) type compounds as shown in the formula (II), the formula (III) and the formula (IV), and the R1 radical and the R2 radical are defined in the specification. In the preparation method, the introduction of the R1 radical and deprotection improvement shorten the reaction time, increase the yield and enhance safety, thus the method is more suitable for industrialized production.
Owner:JIANGSU HANSOH PHARMA CO LTD
Novel NIDDM regimen
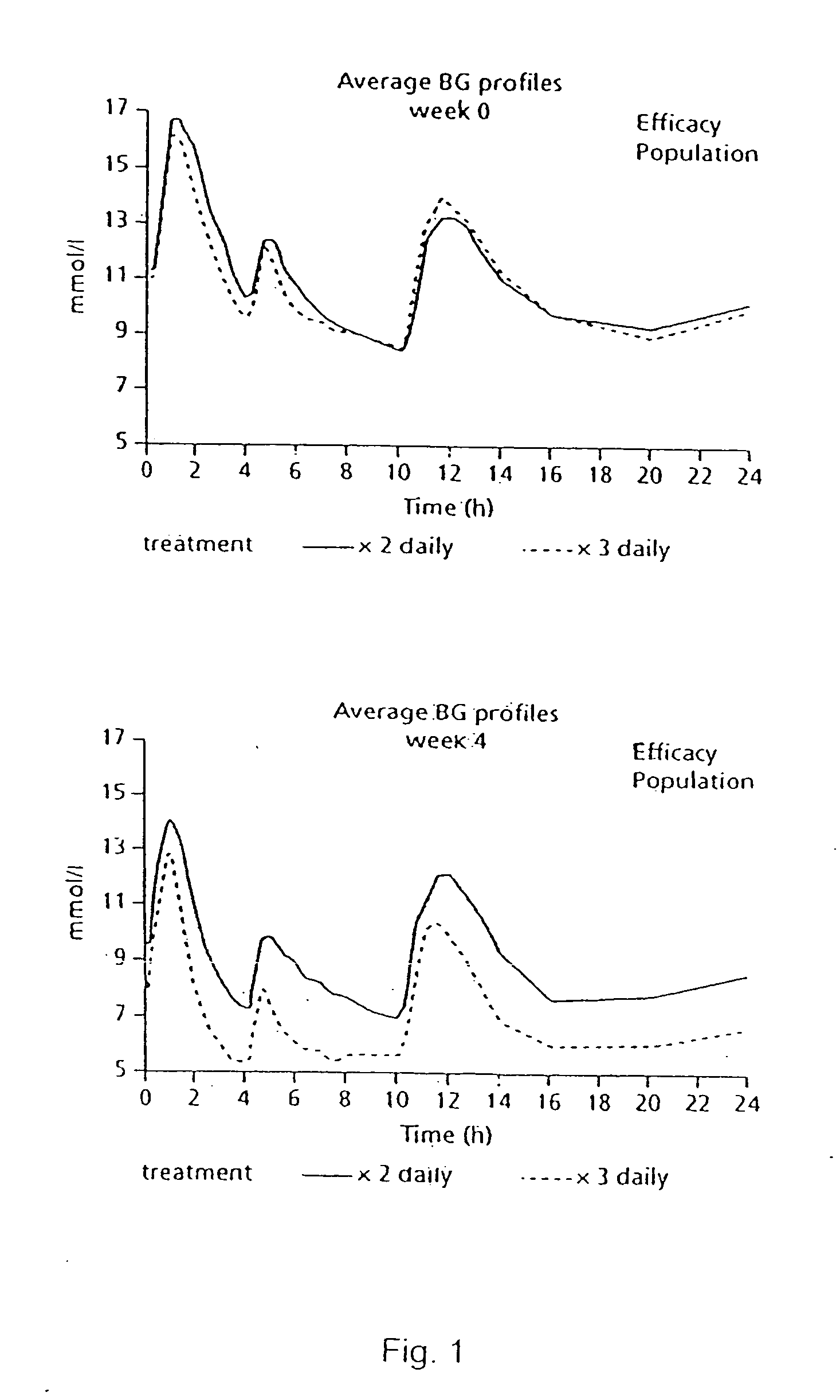
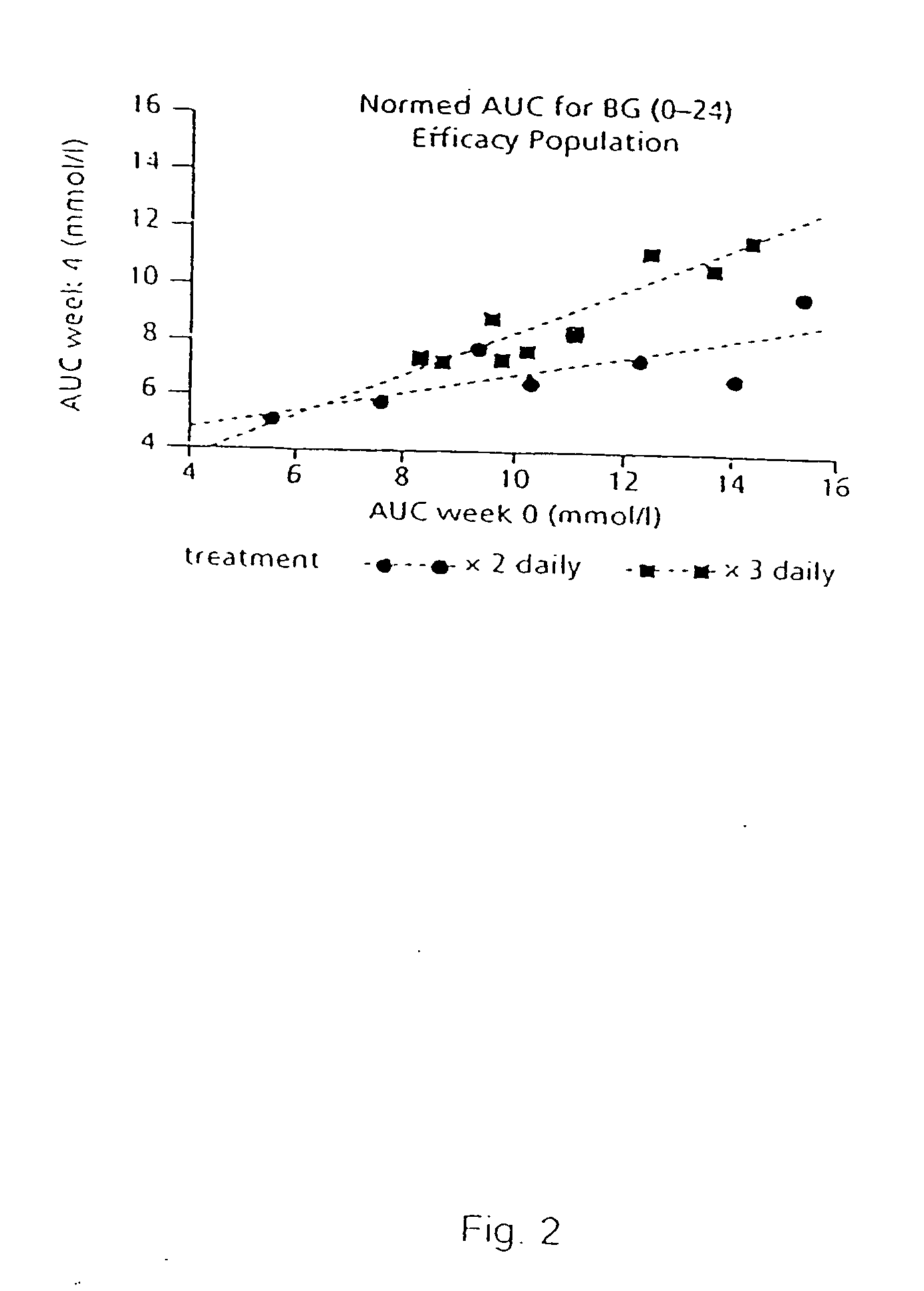
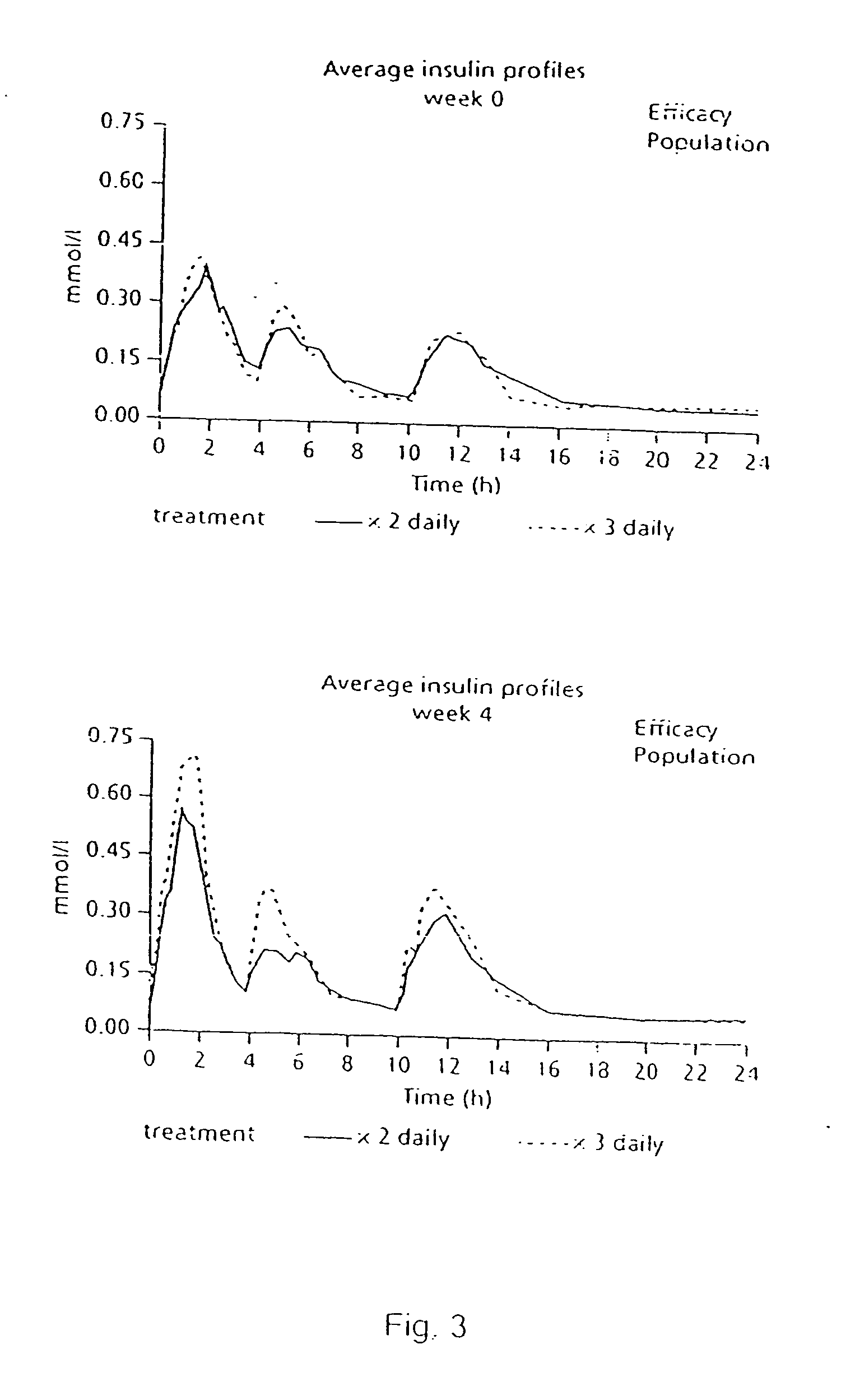
The present invention relates to the use of a short-acting oral hypoglycemic agent and to a novel regimen in the treatment of type 2 diabetes in which the endogenous secretion of insulin is stimulated in connection with meals by administering in connection with the meals a short-acting oral hypoglycaemic agent. Also, the present invention relates to a method of achieving significantly improvement in the glycaemic control by a combined use of repaglinide and metformin in NIDDM patients poorly controlled on metformin alone.
Owner:HEMMINGSEN LISBETH TOFTE +1
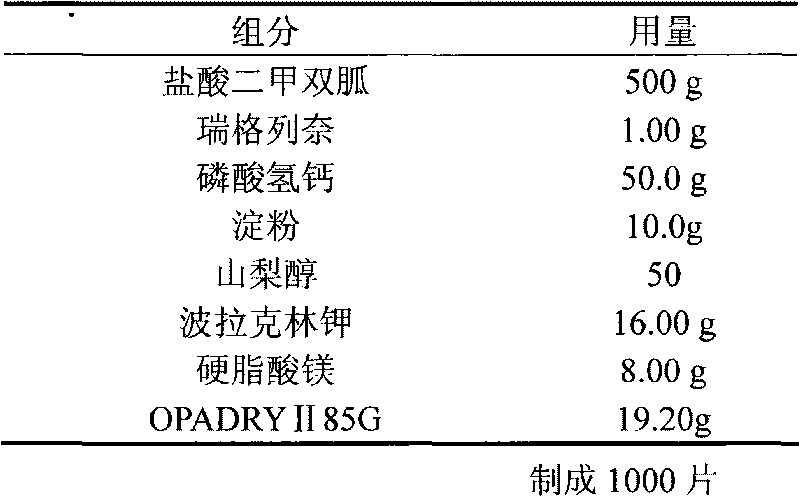
Repaglinide tablet and preparation method thereof
InactiveCN104434840AImprove solubilityUniform contentOrganic active ingredientsMetabolism disorderDissolutionDrug product
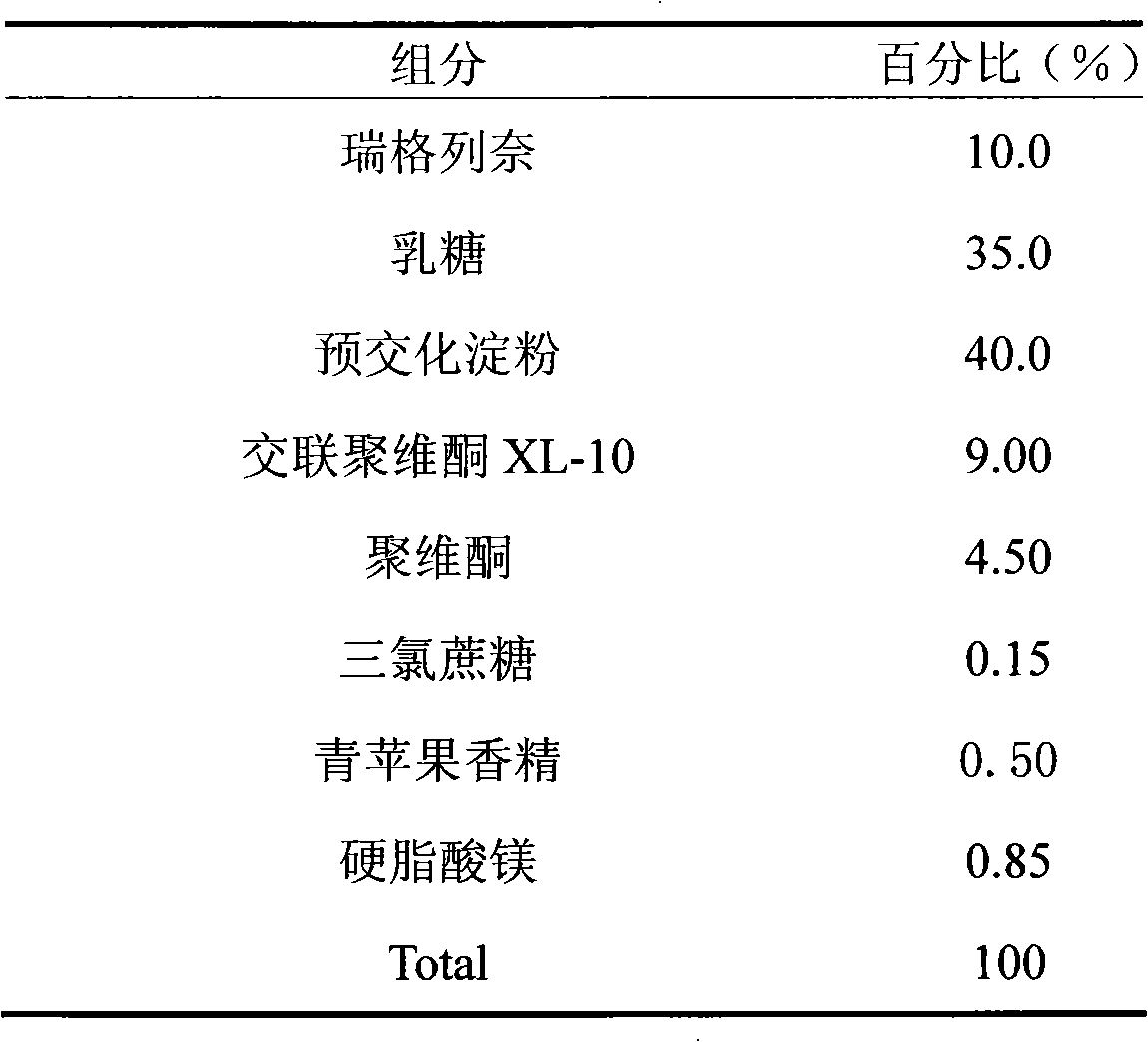
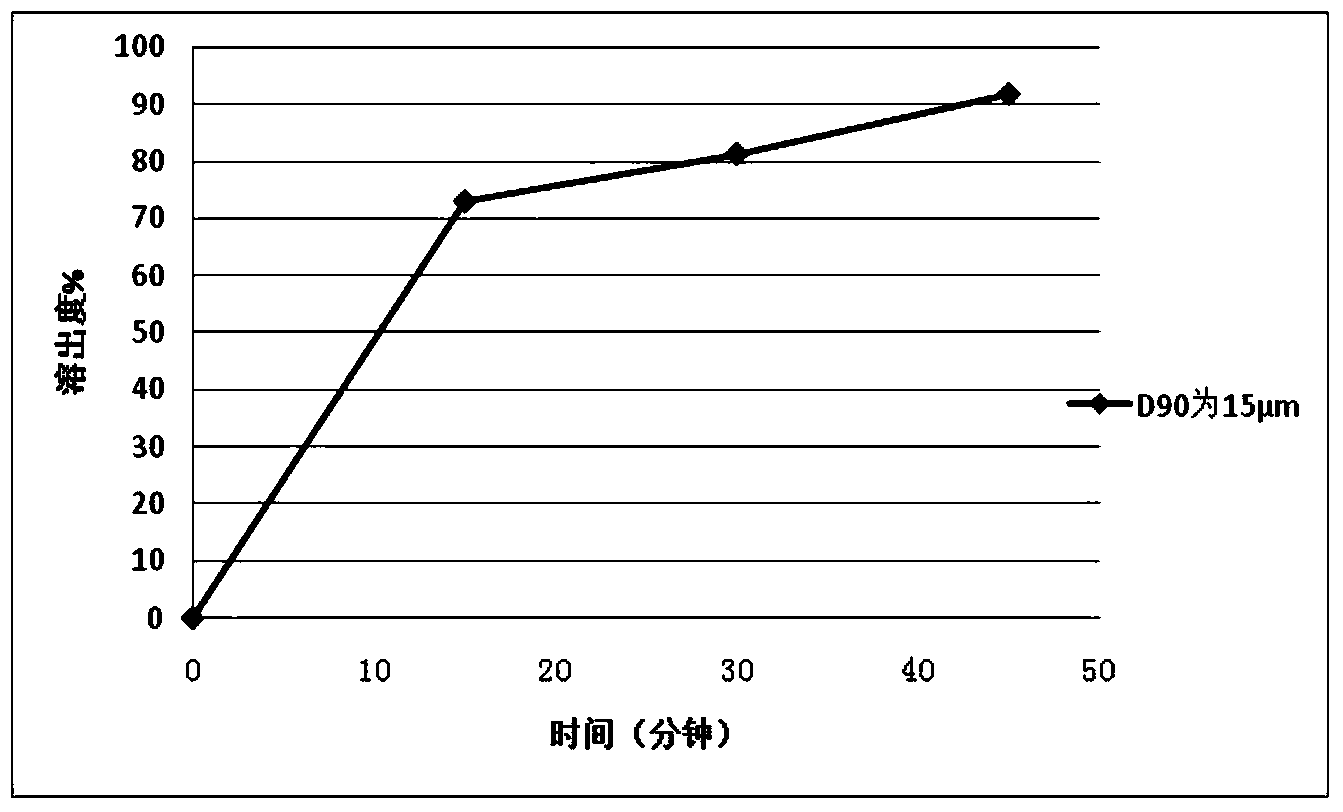
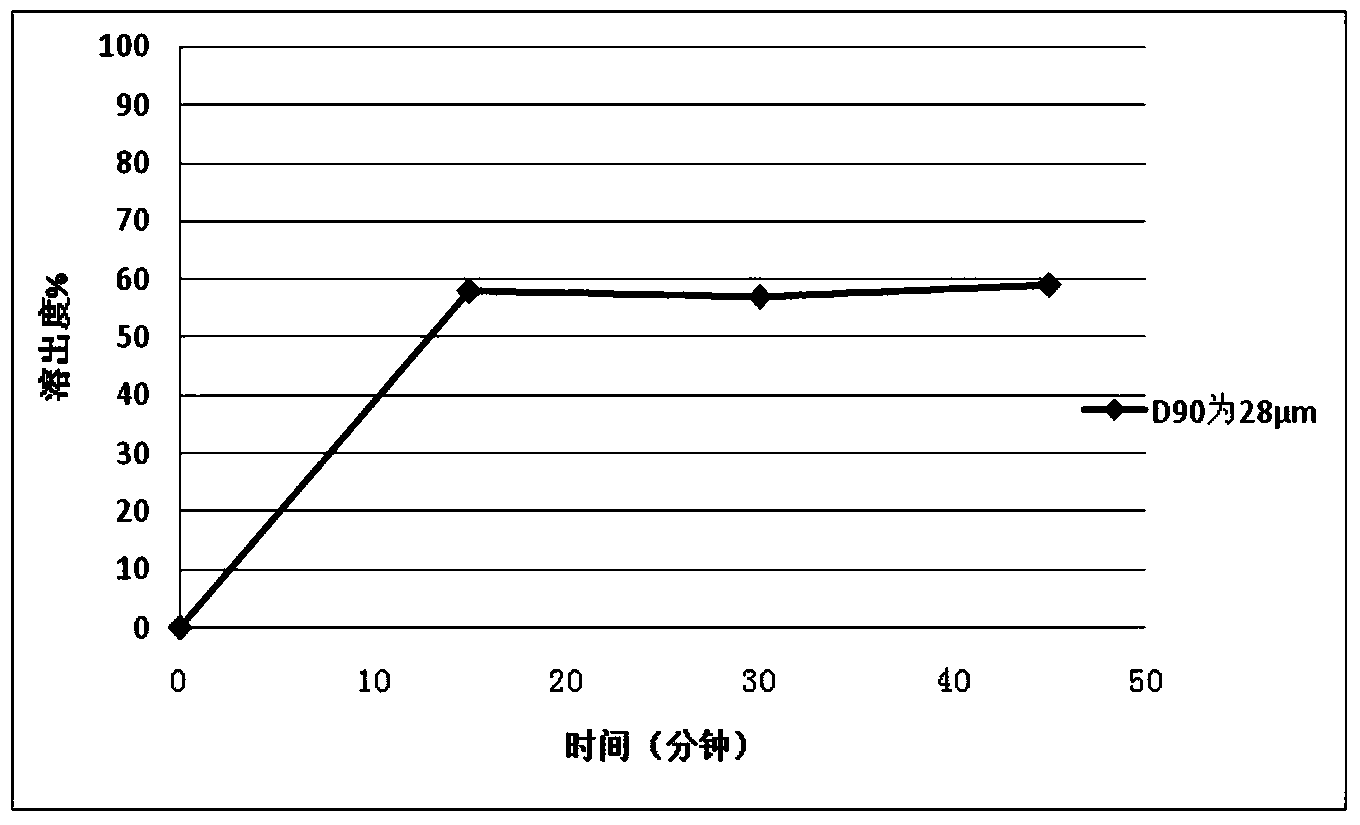
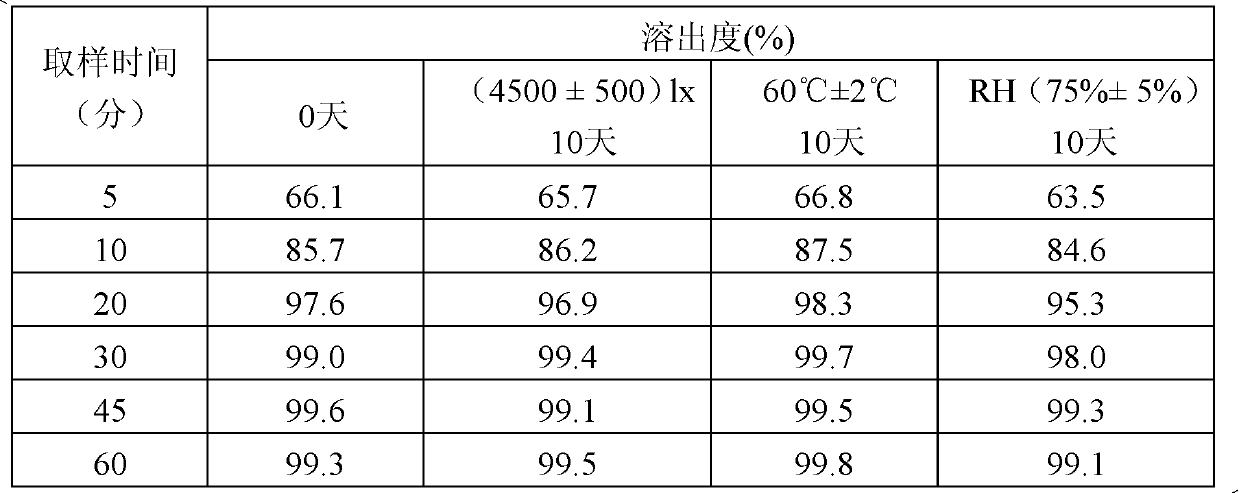
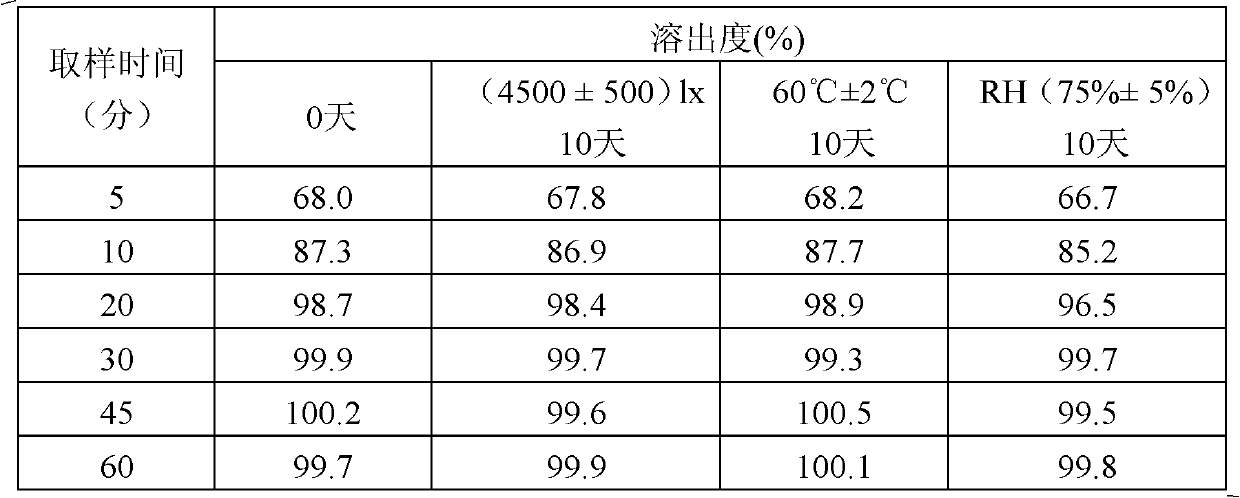
The invention relates to a repaglinide tablet and a preparation method thereof. According to the preparation method, a wet granulation process is used, calcium hydrophosphate, microcrystalline cellulose and pregelatinized starch are used as filling agents, and meglumine, poloxamer and povidone are matched to prepare a binder to carry out wet granulation, so that the prepared repaglinide tablet is uniform in content and favorable to release; compared with the prior art, the wet granulation process is capable of solving the problems of non-uniform content and low dissolution rate in the preparation process; and meanwhile, the dissolution rate and the stability of the prepared preparation are consistent with those of the original contrast drug so that the drug quality is ensured.
Owner:HARBIN PHARMA GROUP TECH CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com