Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Gliquidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gliquidone (INN, sold under the trade name Glurenorm) is an anti-diabetic medication in the sulfonylurea class. It is classified as a second-generation sulfonylurea. It is used in the treatment of diabetes mellitus type 2. It is marketed by the pharmaceutical company Boehringer Ingelheim (Germany).
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Gliquidone compound preparation capable of reducing renal damage
ActiveCN106361791AReduce the burden onExtend quality of lifeMetabolism disorderSulfonylurea active ingredientsFiltrationAmino acid
The invention discloses a gliquidone compound preparation capable of reducing renal damage. The gliquidone compound preparation capable of reducing renal damage is prepared from main drugs and auxiliary materials, and is characterized in that the main drugs are prepared from 20-30% (w / w) of gliquidone, 15-25% (w / w) of ligustrazine and the balance of radix notoginseng extract, wherein the dosage of the radix notoginseng extract is counted based on the mass of dry powder; the radix notoginseng extract is extracted from radix notoginseng, and comprises four types of substances, namely saponin, flavone, amino acid and polyose. The gliquidone compound preparation is supplemented with the radix notoginseng extract and the ligustrazine, so that the gliquidone dosage is reduced, and further the influence of the gliquidone on the renal function of a patient is reduced; the three types of drugs achieve a synergetic effect, so that the blood glucose is reduced, functions of glomeruli are protected, the glomerular filtration capability reduction is prevented, and the blood fat is regulated, so that the gliquidone compound preparation is more applicable to patients suffering from diabetic nephropathy.
Owner:BEIJING WANHUI DOUBLE CRANE PHARMA
Pharmaceutical composition and its application in preparation of medicine for treating type II diabetes
InactiveCN1762356AGood curative effectGood hypoglycemic effectMetabolism disorderAnhydride/acid/halide active ingredientsNephrosisCurative effect
The invention relates a medicinal composition containing Gliquidone, taurine and its medicinal salts, and the use of the composition in preparing medicament for the treatment of diabetes, the treatment and / or prevention of renal disease complication related to type II diabetes. The composition comprises Gliquidone 10-180mg, taurine and its medicinal salts 0.5-10g, and pharmaceutically acceptable carrying agents.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for synthesizing 4-(2-aminoethyl)benzsulfamide
InactiveCN106336366AOvercome the problems of expensive, difficult operation of reaction conditions, etc.Improve responseOrganic compound preparationCarboxylic acid amides preparationGlimepirideHydrolysis
Owner:姜近仁
Gliquidone preparation method
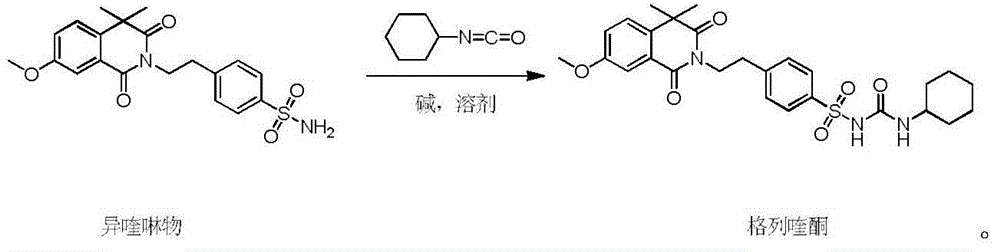
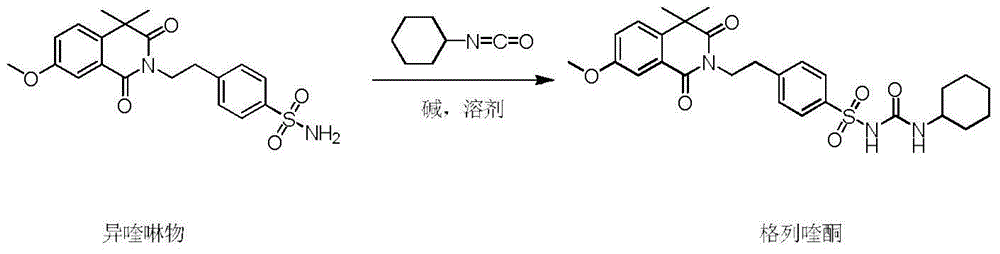
The invention discloses a gliquidone preparation method. According to the preparation method, isoquinoline and cyclohexyl isocyanate carry out condensation reactions in a solvent in the presence of an alkali to generate gliquidone. 2,5-dimethyl tetrahydrofuran is taken as the solvent, isoquinoline can be well dissolved in 2,5-dimethyl tetrahydrofuran, while gliquidone is difficult to dissolve in 2,5-dimethyl tetrahydrofuran; thus, only a little amount of water is needed in the post treatment, the generated wastewater is largely reduced; the used alkali is common inorganic alkalis such as anhydrous potassium carbonate, and the like, is nontoxic, and is easy to process. The boiling point of 2,5-dimethyl tetrahydrofuran is low, 2,5-dimethyl tetrahydrofuran is easy to recover, moreover, the system is not afraid of water, the solvent can be circularly used, the production cost is reduced, the refluxing temperature is adopted, the operation is easy, the reactions last for 6 hours, and the method is rapid and efficient.
Owner:天津市亨必达化学合成物有限公司
Combination containing micronized gliquidone
The invention relates to a pharmaceutical composition taking gliquidone which contains micronization as an active component and a method for preparing the composition. Micronized gliquidone is mixed with a surfactant and a proper excipient, palletized and tabletted to obtain the pharmaceutical composition taking the gliquidone which contains the micronization as the active component. The pharmaceutical composition greatly improves the water solubility of the gliquidone, thereby ensuring that the gliquidone is almost completely dissolved out from the composition.
Owner:北京华禧联合科技发展有限公司
Sulfonylurea compound and metformin salts, preparation method and application
InactiveCN110804017AEasy to operateGood reproducibilityMetabolism disorderOrganic compound preparationDiabetes mellitusFluid phase
The invention relates to sulfonylurea compound and metformin salts, a preparation method and application. The preparation method comprises the following steps: dissolving a sulfonylurea compound and metformin into a solvent according to a mole ratio of (1:0.9):(1:1.1) so as to obtain a mixture; performing reaction crystallization on the mixture under a condition of 25-50 DEG C for 12-48 hours; andperforming solid-liquid phase separation on the obtained product, and performing drying, so as to obtain a salt type solid product. The sulfonylurea compound is gliquidone or glibenclamide. The gliquidone-metformin salt and the glibenclamide-metformin salt provided by the invention are applied to medicines for preventing and treating diabetes. The wet absorption capacities of the salt type products provided by the invention are greatly increased when being compared with that of metformin, that is, the hygroscopy gain of the metformin is 35% when the relative humidity is 75%, and the hygroscopy gains of the salt type products are smaller than 1%; and the dissolubility of the salt type products is greatly improved when being compared with that of the sulfonylurea compound, that is, the dissolubility of the sulfonylurea compound in water is smaller than 0.03mg / ml, and the dissolubility after salt formation is 10.61mg / ml and 16.78mg / ml respectively.
Owner:TIANJIN UNIV
Combination product comprising limonoids and sulfonylurea
The invention relates to a combination product comprising limonoids (pharmacologically acceptable derivatives, esters, steric isomers, salt or prodrugs) and sulfonylurea (such as glibenclamide, gliclazide, glipizide, gliquidone and glimepiride). The invention further relates to an application of the combination product to treatment and / or prevention of diabetes-related diseases and the like.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
Western medicine for treating type II diabetes
PendingCN111346232ARepair functionNo side effectsMetabolism disorderSulfonylurea active ingredientsBULK ACTIVE INGREDIENTGlucose blood
The present invention discloses a western medicine for treating type II diabetes. Active ingredients of the western medicine comprise the following raw materials in mass percentages: 25-35% of a DPP4inhibitor, 30-40% of a SGLT2 inhibitor and 25-45% of gliquidone. The DPP4 inhibitor, SGLT2 inhibitor and gliquidone are compounded, blood sugar level is significantly reduced, and besides, the compounded medicine can effectively relieve symptoms and pathological phenotypes of diabetic nephropathy and reduce production of urine proteins in urine of patients.
Owner:LIAONING INST OF SCI & TECH
Gliquidone-containing medicine combination for treating diabetes and preparation method thereof
InactiveCN105796952AAvoid gastrointestinal adverse reactionsGood treatment effectMetabolism disorderSulfonylurea active ingredientsDiabetes mellitusPharmaceutical drug
The invention belongs to the technical field of medicine and particularly relates to a gliquidone-containing medicine combination for treating diabetes and a preparation method thereof.The gliquidone-containing medicine combination for treating diabetes is prepared from, by weight, 15-45 parts of gliquidone, 4,000-8,000 parts of seedlings of dill, 4,000-8,000 parts of leaf base of chequershaped indocalamus, 4,000-8,000 parts of gum peach and 4,000-8,000 parts of pilea pumila.The traditional Chinese medicine and gliquidone are combined, the common gastrointestinal tract adverse reactions to gliquidone can be avoided, and the treatment effect on diabetes can be improved.
Owner:JINAN BANGWEN MEDICAL TECH
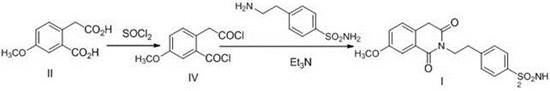
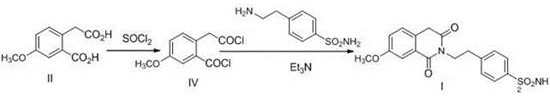
Preparation method of gliquidone intermediate
The invention provides a preparation method of a gliquidone intermediate. A one-step process is employed to replace the traditional two-step process, and the method includes: taking 7-methoxy-2, 4, 4-trimethyl-1, 3(2H, 4H)-isoquinoline dione as the starting raw material for addition with 4-(2-aminoethyl)-benzenesulfonamide in a xylene solution of sodium hydroxide, thus generating 4-(2-(3, 4-dihydro-7-methoxy-4, 4'-dimethyl-1, 3-dioxo-2(1H)-isoquinolyl)ethyl)benzenesulfonamide directly. The method avoids reversible reaction in the two-step process, and significantly improves the product generation rate. In addition, a small dose of sodium hydroxide is added in the reaction process, thereby greatly reducing the output of waste alkali water, and avoiding environmental pollution.
Owner:天津药物研究院药业有限责任公司
Gliquidone crystal, preparation method thereof and drug containing gliquidone crystal
InactiveCN108191759AHigh purityImprove solubilityMetabolism disorderSulfonylurea active ingredientsX-rayCrystal structure
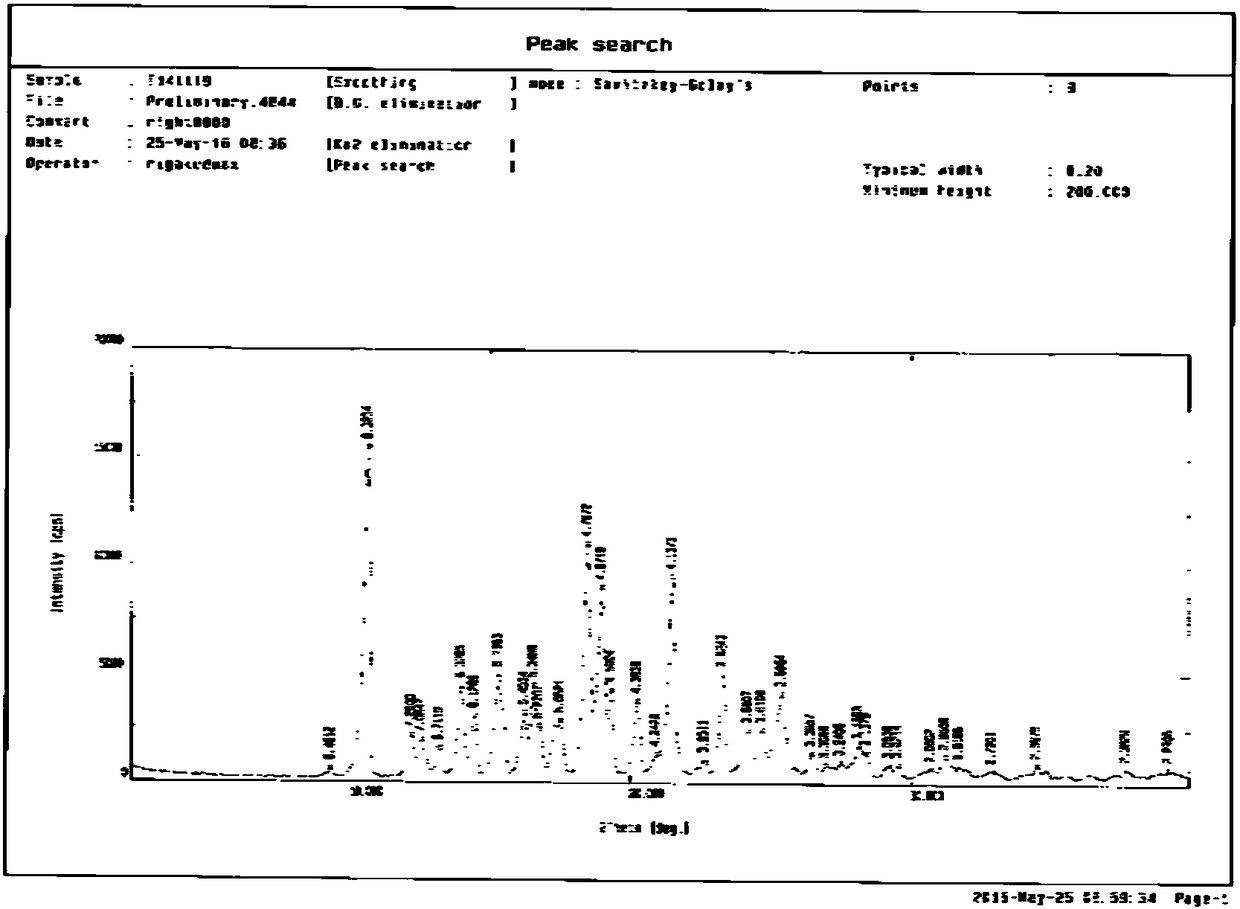
The invention provides a gliquidone crystal, a preparation method thereof and a drug containing the gliquidone crystal. The gliquidone crystal is a crystal form A, a crystal form B or a crystal form Cand is determined by using a powder X-ray diffraction method; according to an X-ray powder diffraction pattern represented by a diffraction angle of 2 theta plus or minus 0.20 degree; a crystal pattern A pattern shows diffraction peaks at 10.60 degrees, 14.00 degrees, 16.10 degrees, 18.98 degrees, 20.24 degrees and 21.46 degrees; a crystal pattern B pattern shows diffraction peaks at 10.60 degrees, 14.00 degrees, 15.30 degrees, 16.10 degrees, 16.54 degrees, 17.54 degrees, 18.53 degrees, 18.98 degrees, 19.33 degrees, 20.24 degrees, 21.46 degrees, 23.24 degrees and 25.35 degrees; and a crystalpattern C pattern shows diffraction peaks at 10.70 degrees, 15.24 degrees, 18.48 degrees, 23.24 degrees and 24.74 degrees. The preparation method comprises the following steps: dissolving a gliquidonecrude product in a solvent and then crystallizing to obtain the gliquidone crystal. The invention discloses a crystal structure of glitaquinone; the glitaquinone in a crystal state has higher solubleness and stronger storage stability than a glitaquinone compound in an amorphous state; and the gliquidone crystal prepared by the preparation method provided by the invention is high in purity, highin yield, simple in preparation process, high in repeatability and suitable for industrial production.
Owner:天津药物研究院药业有限责任公司
Method for detecting content of impurity C in gliquidone
The invention provides a method for detecting the content of impurity C in gliquidone. The detection method adopts high performance liquid chromatography. The detection method provided by the invention has relatively high specificity, sensitivity and accuracy, is simple and convenient to operate, can be used for quickly and accurately detecting the content of the impurity C in the gliquidone, can be used for detecting related substances of the gliquidone, improves the quality standard of the gliquidone by taking the impurity C as a known impurity to be bound into a related substance detection item of the gliquidone, and ensures the medication safety.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Pharmaceutical composition and its application in preparation of medicine for treating type II diabetes
InactiveCN100335056CLight protective effectImprove protectionMetabolism disorderAnhydride/acid/halide active ingredientsNephrosisCurative effect
The invention relates a medicinal composition containing Gliquidone, taurine and its medicinal salts, and the use of the composition in preparing medicament for the treatment of diabetes, the treatment and / or prevention of renal disease complication related to type II diabetes. The composition comprises Gliquidone 10-180mg, taurine and its medicinal salts 0.5-10g, and pharmaceutically acceptable carrying agents.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for treating diabetes by combining traditional Chinese medicine and western medicine
InactiveCN103550227AEnhance physical fitnessImprove microcirculationDipeptide ingredientsMetabolism disorderFormularyHypoproteinemia
The invention relates to a method for treating diabetes by combining traditional Chinese medicine and western medicine. The method specifically comprises the following steps: (1) general treatment; (2) western medicine treatment, namely, controlling blood glucose and blood pressure, and controlling the blood glucose through gliquidone tablets; treating severe patients by cooperatively using insulin; reducing the blood pressure through enalapril maleate tablets; dosing albumin intravenous drip to patients suffering hypoproteinemia, oliguria and severe edema and treating by utilizing equilibrium liquid; and (3) traditional Chinese medicine treatment method, namely mixing 2-5 parts of ginseng, 3-5 parts of lucid ganoderma, 2-5 parts of astragalus root, 4-8 parts of wine-treated rhubarb, 6-9 parts of yam, 3-5 parts of cornus, 4-8 parts of radix rehmanniae, 1-3 parts of leech, 3-5 parts of safflower, 5-8 parts of salviae miltiorrhizae, 10-20 parts of rhizoma smilacis glabrae, 2-6 parts of rhizoma alismatis and 1-4 parts of licorice to a traditional Chinese medicine decoction, and decocting the traditional Chinese medicine in water when in use. The traditional Chinese medicine formulation has the functions of invigorating qi, tonifying kidney, detoxifying, promoting diuresis, promoting blood circulation to remove meridian obstruction, strengthening body resistance and eliminating evil, and the combined treatment manner has a certain curative effect on diabetics.
Owner:李文芝 +1
Primer, application and kit for detecting polymorphic site of sulfonylurea receptor 1 gene
ActiveCN101445824BAvoid blindnessEasy to operateMicrobiological testing/measurementSpecific testSulfonylurea
The invention provides a specific primer for detecting the T1369G polymorphic site (rs757110) genotype of the SUR1 gene by a PCR method, and the specific primer is used in the preparation of reagents for detecting the T1369G polymorphic site of the SUR1 gene by PCR amplification of biological samples The application in the invention, the SUR1 gene T1369G polymorphic site detection kit containing specific primers and the application of the detection kit to predict the effect of sulfonylurea drugs. Sulfonylurea drugs are selected from glibenclamide, glibenclamide, glibenclamide, glibenclamide, glimepiride, glibenclamide, glibenclamide, glibenclamide, glibenclamide , glipizide, gliclazide, glipizide, and gliquidone, preferably gliclazide or glimepiride.
Owner:深圳泰乐德医疗有限公司
Gliquidone dropping pill and preparation method thereof
PendingCN112168799AAvoid Small Pill SituationsImprove pass rateMetabolism disorderSulfonylurea active ingredientsDrip irrigationPharmaceutical drug
The invention relates to the field of medicine processing, and discloses a gliquidone dropping pill and a preparation method thereof. The preparation method of the gliquidone dropping pill comprises the steps that S1, poloxamer and poly-croscarmellose sodium are mixed and slowly heated to be in a molten state; S2, a melt obtained in the step S1 is added into drip irrigation equipment, heating is carried out to liquefy the melt, then gliquidone is added, and full mixing is carried out; S3, dropwise adding is added, specifically, the dropwise adding speed is adjusted by controlling the pressurein the drip irrigation equipment, liquid medicines dropped from the drip irrigation equipment are primarily cooled by cooling gas, then the liquid medicines are dropped into dimethicone cooling liquidfor secondary cooling, dimethicone is drained off, cleaning is carried out by using petroleum ether, and volatilizing is carried out to form a primary dropping pill; and S4, coating is carried out, specifically, the preliminary dropping pill is coated to form a dropping pill finished product. The invention also discloses a gliquidone dropping pill component. The gliquidone dropping pill has the beneficial effects that the gliquidone is prepared into the dropping pill, so that disintegration is easy, and drug absorption is facilitated; and multiple cooling technologies are adopted for the dropped liquid medicines, and the product percent of pass is increased.
Owner:CHENGDU HENGRUI PHARMA
Gliquidone rapid release tablet and preparation method thereof
PendingCN111084761AQuick effectPromote absorptionMetabolism disorderSulfonylurea active ingredientsCarboxymethyl celluloseMagnesium stearate
The invention relates to a gliquidone rapid release tablet and a preparation method thereof. The tablet comprises 15 mg-180 mg of gliquidone, 10 mg-50 mg of cross-linked sodium carboxymethyl cellulose, 20 mg-150 mg of microcrystalline cellulose, and 1 mg-2 mg of magnesium stearate. The gliquidone and the cross-linked sodium carboxymethyl cellulose are passed through a planetary grinder (Model-PM Retsch Haan) at the ratio of 1: 1 by weight to conduct circular grinding at 200 rpm in 4 x 30 minutes. The above mixture is collected into the mixer and is mixed with starch and magnesium stearate evenly. The medicine is pressed directly at 151 mg per tablet, and the hardness is controlled within 60 N-100 N. In the present invention, glitaquinone is made into a rapid release tablet, thereby makingthe glitaquinone more easily to be absorbed by the human body, so that the tablet has a faster effect, and is suitable for quickly alleviating the disease.
Owner:CHENGDU HENGRUI PHARMA
Gliquidone dripping pill and its prepn
InactiveCN1771952ARapid dissolutionQuick effectOrganic active ingredientsMetabolism disorderMedicineBioavailability
The present invention discloses one kind of gliquidone dripping pill and its preparation process. The gliquidone dripping pill is compounded with gliquidone and dripping pill matrix. The present invention has high bioavailability, high stability, convenient taking, fast disintegration and dissolving, high leaching degree, fast medicine releasing, fast acting, simple production process, low cost and other features.
Owner:陈茜
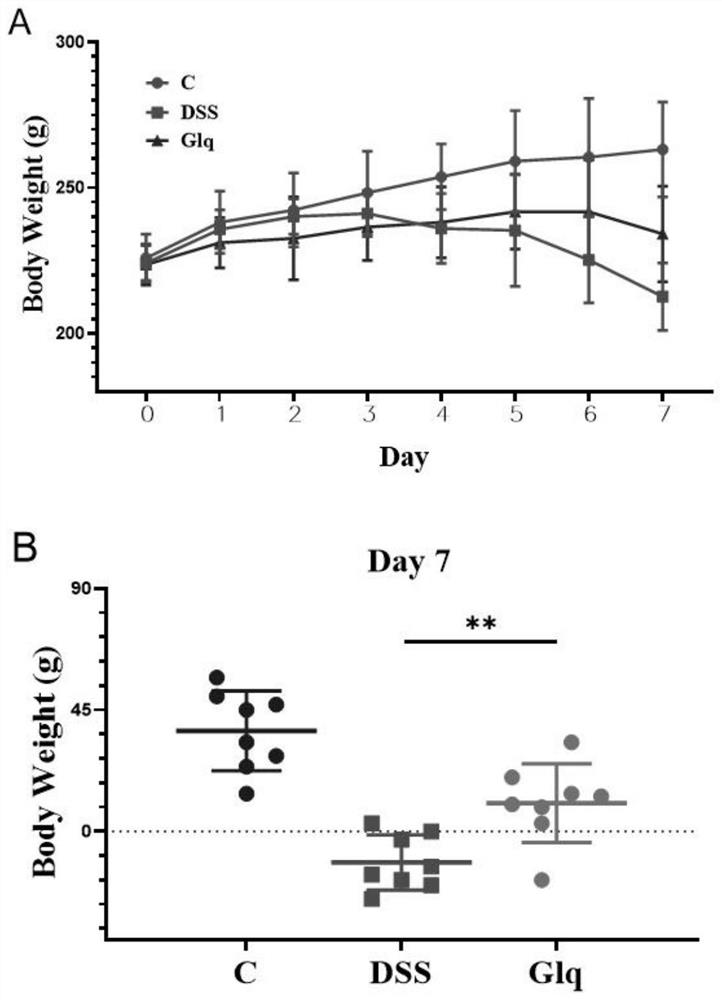
Application of gliquidone in preparation of medicine for treating ulcerative colitis
PendingCN114099520ADisease symptom reliefEasily damagedAntipyreticSulfonylurea active ingredientsUlcerative colitisPharmaceutical drug
The invention discloses an application of gliquidone in preparation of a medicine for treating ulcerative colitis. It is found that gliquidone can effectively relieve disease symptoms of ulcerative colitis model rats and effectively improve intestinal injuries. Therefore, the gliquidone has the prospect of being developed into the medicine for treating ulcerative colitis.
Owner:CHINA PHARM UNIV
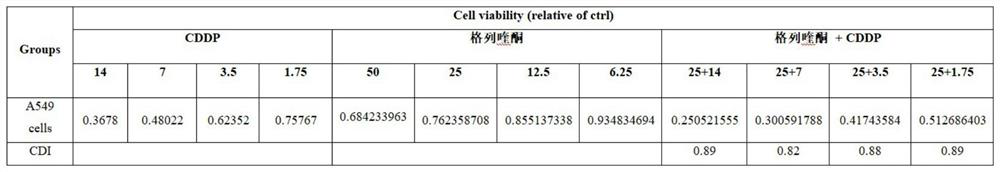
Application of gliquidone and cisplatin combined drug in preparation of antitumor drugs
ActiveCN114469963AReverse drug resistanceInorganic active ingredientsSulfonylurea active ingredientsPharmaceutical SubstancesLung cancer
The invention provides an application of a gliquidone and cisplatin combined drug in preparation of an anti-tumor drug. The invention finds that when the gliquidone and the cisplatin are combined for use, the inhibition effect on lung cancer cells is obviously enhanced, the IC50 of the cisplatin in A549 and A549 / CDDP is 7.29 + / -1.11 mu M and 32.32 + / -0.17 mu M respectively, and the IC50 of the cisplatin and the gliquidone combined for use in A549 and A549 / CDDP is 4.00 + / -0.14 mu M and 18.16 + / -1.03 mu M respectively. The gliquidone can reverse the drug resistance of the drug-resistant cell strain A549 / CDDP to the CDDP to a certain extent.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
Combination product containing limonoid compound and sulfonylurea compound
The present invention relates to a combination product comprising a limonoid compound (or a pharmaceutically acceptable derivative, ester, stereoisomer, salt or prodrug thereof), and a sulfonylurea compound (e.g., glibenclamide, gliclazide, glipizide, gliquidone and glimepiride). The present invention further relates to a use of the combination product for prevention and / or treatment of a disease associated with diabetes and the like.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
A kind of method for preparing gliquidone intermediate
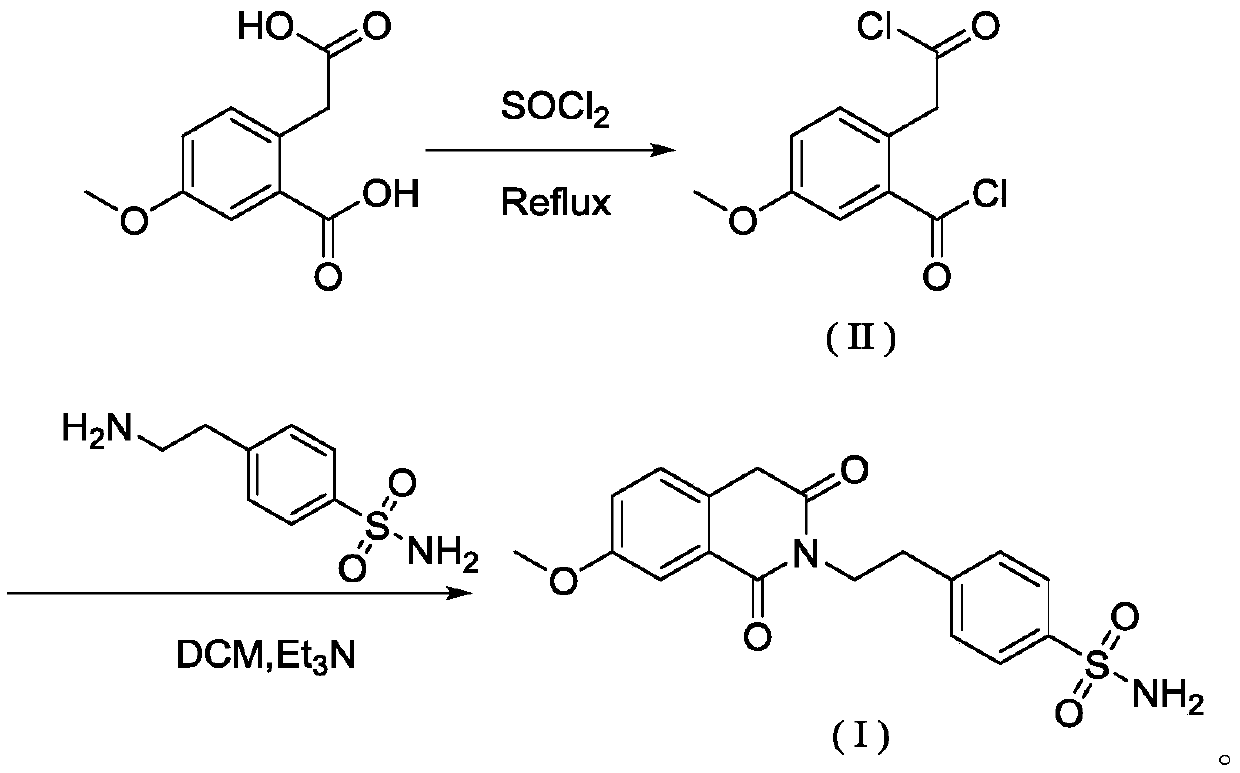
ActiveCN106699658BAvoid severe conditions of high temperature of 200°COrganic chemistryBenzoic acidChemical synthesis
The invention belongs to the technical field of chemical synthesis of medicaments, and particularly relates to a method for preparing a gliquidone intermediate shown as a formula (I). The method comprises the following steps: S1, making 2-carboxymethyl-5-methoxy-benzoic acid react in the presence of thionyl chloride to generate an intermediate shown as a formula (II); S2, making the intermediate shown as the formula (II) react with p-amino ethyl benzene sulfonamide in the presence of triethylamine in a dichloromethane solvent to generate the gliquidone intermediate shown as the formula (I): 4-[2-(3,4-dihydro-7- methoxy-1,3-dioxo-2(1H)-isoquinolyl)ethyl]benzene sulfonamide. All the steps in the method are performed at the temperature of 100 DEG C or lower, so that industrial expanded production is facilitated.
Owner:天津药物研究院药业有限责任公司
Gliquidone composition freeze-dried tablet and preparation method thereof
InactiveCN104523568AReduce typesReduce dosageMetabolism disorderSulfonylurea active ingredientsFreeze-dryingCurative effect
The invention provides a gliquidone composition freeze-dried tablet and a preparation method thereof, and relates to the technical fields of medicines and medicine production. The gliquidone composition freeze-dried tablet contains gliquidone, starch and cane sugar. The starch and the cane sugar are taken as accessories; common corn starch is treated by use of a heating process so that the binding and disintegration effects of the starch in the tablet can be improved and the formability of the tablet can be improved; the gliquidone composition freeze-dried tablet only needs two accessories, namely the starch and the cane sugar. The gliquidone composition freeze-dried tablet is produced by use of a freeze-drying process of twice temperature dropping and twice temperature rising; due to twice temperature dropping and twice temperature rising, the formability of the tablet is better, the dissolubility of the tablet is increased, and therefore, the bioavailability of the tablet is improved; the tablet has the advantages that the defects of the common gliquidone tablet are overcome, the types and the dosage of the accessories in the gliquidone tablet are reduced, the dissolubility and the bioavailability of the tablet are high, and the curative effect and safety of clinical medication are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Low hygroscopicity gliquinone tablet and preparation method thereof
ActiveCN105997913BGood curative effectReduce adverse reactionsMetabolism disorderSulfonylurea active ingredientsMoisture absorptionDissolution
The invention discloses a low-moisture-absorption gliquidone tablet and a preparation method thereof. The low-moisture-absorption gliquidone tablet comprises the main material (gliquidone) and auxiliary materials, wherein the auxiliary materials comprise lactose, microcrystalline cellulose, ethylene cellulose, pluronic, magnisium aluminometasilicate and talcum powder. The preparation method comprises the following steps: weighing the raw drug of gliquidone according to the prescription; uniformly mixing gliquidone, lactose and microcrystalline cellulose by adopting the equivalent progressive increase method; then adding ethylene cellulose and magnisium aluminometasilicate, and fully mixing; atomizing to add a pluronic ethanol solution under stirring to prepare a soft material; sieving and pelletizing; drying; granulating; adding talcum powder, uniformly mixing, and tabletting. Through improvement on the auxiliary material formula, the consumption of microcrystalline cellulose is reduced while ethylene cellulose and magnisium aluminometasilicate are added, pluronic is adopted as a wetting raw material of a wetting agent to prepare the soft material, and talcum powder is added to prevent excessive adhesion and realize easy tabletting. The obtained low-moisture-absorption gliquidone tablet is effectively reduced in moisture absorption, good in stability and easy to disintegrate, and dissolution release of gliquidone is not influenced.
Owner:BEIJING WANHUI DOUBLE CRANE PHARMA
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample tobe tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Gliquidone and rosiglitazone composition and preparation method thereof
PendingCN112245439AHigh single drug doseRich choiceMetabolism disorderSulfonylurea active ingredientsCelluloseIndividualized treatment
The present invention belongs to the field of medicine processing and discloses a gliquidone and rosiglitazone composition and a preparation method thereof. The gliquidone and rosiglitazone composition is composed of the following raw medicines in parts by weight: 30 mg-180 mg of gliquidone, 1 mg-8 mg of rosiglitazone, 10 mg-30 mg of carboxymethyl starch, 20 mg-50 mg of hydroxypropyl cellulose, 40mg-240 mg of lactose, and 1 mg-10 mg of magnesium stearate. The gliquidone and rosiglitazone are combined together, an adhesive, a diluent, a disintegrating agent and a lubricant are added as auxiliary materials, a part of the auxiliary materials are premixed, and finally dry tabletting or direct filling of capsules is carried out. Aiming at facts that diabetes is mainly treated by medicines currently domestically and individualized treatment is required, in order to avoid over-high dosage of a single medicine when a medicine effect of the single medicine is poor, the novel diabetes treatmentmedicine composition is provided to provide more choices for patients.
Owner:CHENGDU HENGRUI PHARMA
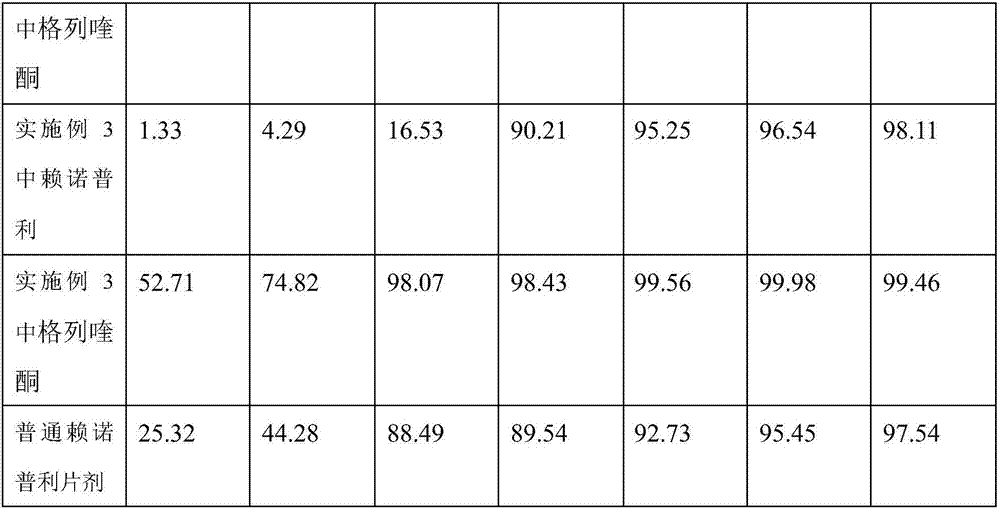
Lisinopril compound preparation and preparation method thereof
InactiveCN107184952ASolve the problem of high blood pressureBlood pressure controlDipeptide ingredientsMetabolism disorderNormal blood glucoseMedicine
The present invention belongs to the technical field of biomedicine, and particularly relates to a lisinopril compound preparation and a preparation method thereof. In the prior art, the blood pressure lowering effect is not good in the application of the existing lisinopril tablet in the diabetes patient with hypertension. Based on the problem in the prior art, the invention provides a lisinopril compound preparation, which comprises, 10-20 parts of lisinopril, 10-20 parts of gliquidone, and 100-500 parts of an auxiliary material. The preparation method comprises: preparing a soft material A from lisinopril and materials, preparing a soft material B from gliquidone and an auxiliary material, taking the same doses of the soft material A and the soft material B, uniformly tableting to form a tablet, placing into a lactose solution with a concentration of 80-90%, soaking for 3-5 h, and drying to obtain the lisinopril compound preparation. According to the present invention, the prepared lisinopril compound preparation can lower the blood glucose concentration, and can provide the good blood pressure lowering effect in the case of the normal blood glucose, and the preparation method is simple, and is suitable for promotion use.
Owner:江苏黄河药业股份有限公司
A kind of preparation method of gliquidone intermediate
ActiveCN111825614BReduce consumption costEvenly dispersedOrganic chemistryDrugs synthesisReaction temperature
The invention belongs to the technical field of pharmaceutical synthesis chemistry, and in particular relates to a method for preparing a gliquidone intermediate. The technical scheme of the present invention is: step 1. join compound II and compound III in the reactor by certain molar ratio, add the hydrophilic solvent of 5-10 times of compound II quality, airtight, reaction temperature is 150~170 °C; step 2. After the reaction material in step 1 was cooled to room temperature, the air valve was gradually opened, the reaction material liquid was centrifuged, washed with a reaction solvent, and dried at 60 °C to obtain gliquidone intermediate I. The invention provides an energy-saving and safe preparation method of gliquidone intermediate I.
Owner:迪嘉药业集团股份有限公司
Preparation method of gliquidone intermediate
ActiveCN111825614AReact energy saving and safetyReduce consumption costOrganic chemistryReaction temperatureCombinatorial chemistry
The invention belongs to the technical field of pharmaceutical synthetic chemistry, and particularly relates to a method for preparing a gliquidone intermediate. According to the technical scheme, themethod comprises the steps that 1, a compound II and a compound III are added into a reaction kettle according to a certain molar ratio, a hydrophilic solvent with the mass 5-10 times that of the compound II is added, the reaction kettle is sealed, and the reaction temperature is 150-170 DEG C; and 2, the reaction material in the step 1 is cooled to room temperature, an air valve is gradually opened, the reaction material liquid is centrifuged, followed by washing with a reaction solvent and drying at 60 DEG C to obtain a gliquidone intermediate I. The invention provides an energy-saving andsafe preparation method of a gliquidone intermediate I.
Owner:迪嘉药业集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com