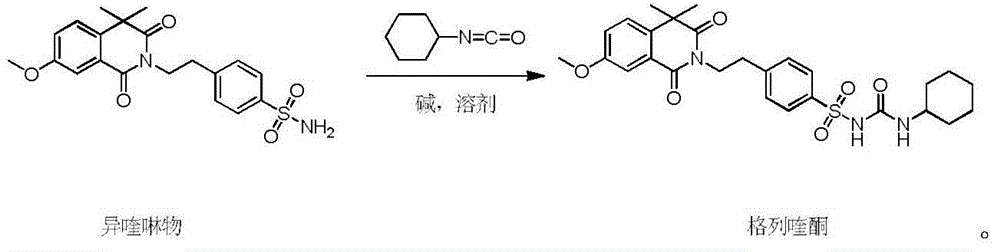
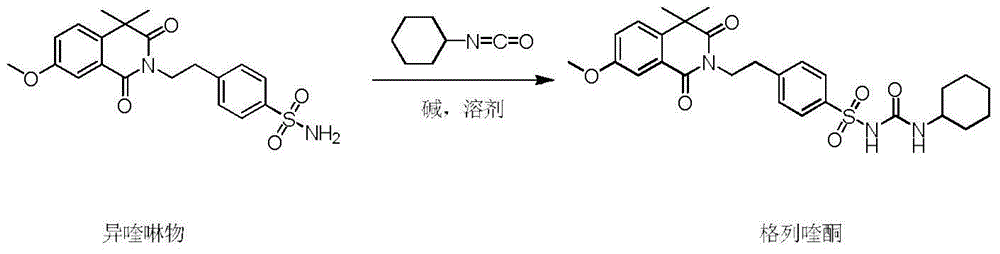
Gliquidone preparation method
A technology for gliquinone and isoquinoline, applied in the field of drug synthesis, can solve the problems of high cost of raw materials, harsh reaction conditions, viscous waste water, etc., and achieve the effects of reducing production cost, easy operation and reaction, and easy recovery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
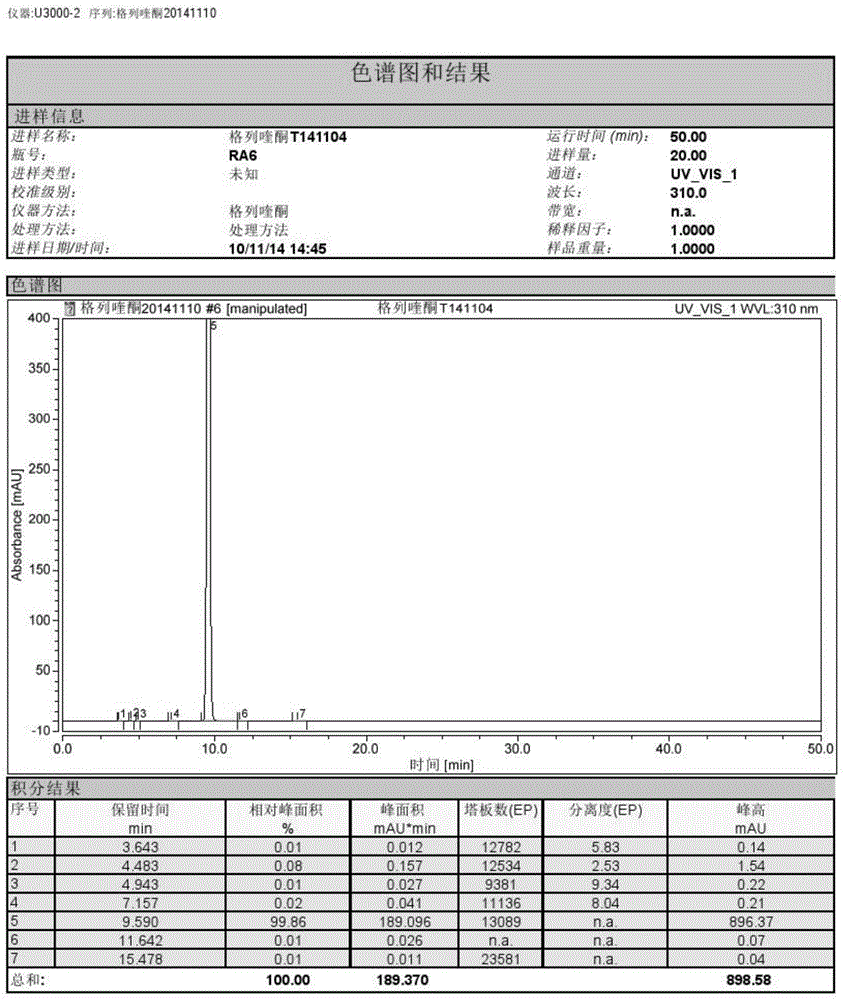
[0032] Embodiment 1: A kind of method for preparing gliquidone
[0033]
[0034] Isoquinoline (30 g, 74.6 mmol), solvent 2,5-dimethyltetrahydrofuran (300 mL) and anhydrous potassium carbonate (21 g, 151.9 mmol) were sequentially added into a 500 mL reaction flask. Turn on stirring and heating, keep warm at 40°C and stir for 30 minutes, then slowly add cyclohexyl isocyanate (11.2g, 89.5mmol), control the dropwise addition in about 30 minutes, raise the temperature after the dropwise addition, and heat to reflux at 90°C for reaction 4 Hour. The solvent 2,5-dimethyltetrahydrofuran was recovered under reduced pressure to obtain a white solid. Add 200 mL of water to the reaction bottle, slowly add hydrochloric acid dropwise to adjust the pH=3, cool down to 0-5°C and stir for 1 hour, and filter to obtain crude gliquidone with a yield of 98%.
[0035] Put the crude gliquidone obtained into a 5L three-necked bottle, add 3L methanol to the bottle, heat to 40°C, add ammonia / methano...
Embodiment 2
[0037] Embodiment 2: A kind of method for preparing gliquidone
[0038]
[0039] Isoquinoline (30 g, 74.6 mmol), solvent dichloromethane (150 mL) and sodium bicarbonate (15 g, 178.6 mmol) were sequentially added into a 500 mL reaction flask. Turn on stirring and heating, keep warm at 40°C and stir for 30 minutes, then slowly add cyclohexyl isocyanate (7.5g, 59.9mmol), control the dropwise addition to be completed in about 30 minutes, raise the temperature after the dropwise addition, and heat to reflux at 90°C for reaction 4 Hour. The solvent dichloromethane was recovered under reduced pressure to obtain a white solid. Add 200 mL of water to the reaction bottle, slowly add hydrochloric acid dropwise to adjust the pH=3, cool down to 0-5°C and stir for 1 hour, and filter to obtain crude gliquidone with a yield of 97.0%.
[0040] Put the crude gliquidone obtained into a 5L three-necked bottle, add 3L methanol to the bottle, heat to 40°C, add ammonia / methanol to the system, a...
Embodiment 3
[0041] Embodiment 3: A kind of method for preparing gliquidone
[0042]
[0043]Isoquinoline (30 g, 74.6 mmol), solvent pyridine (450 mL) and lithium hydroxide (15 g, 626.6 mmol) were sequentially added into a 500 mL reaction flask. Turn on stirring and heating, keep warm at 40°C and stir for 30 minutes, then slowly add cyclohexyl isocyanate (7.5g, 59.9mmol), control the dropwise addition to be completed in about 30 minutes, raise the temperature after the dropwise addition, and heat to reflux at 80°C for reaction 6 Hour. The solvent pyridine was recovered under reduced pressure to obtain a white solid. Add 200mL of water to the reaction flask, slowly add hydrochloric acid dropwise to adjust the pH=3, cool down to 0-5°C and stir for 1 hour, then filter to obtain the crude gliquidone with a yield of 97.1%.
[0044] Put the crude gliquidone obtained into a 5L three-necked bottle, add 3L methanol to the bottle, heat to 40°C, add ammonia / methanol to the system, adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com