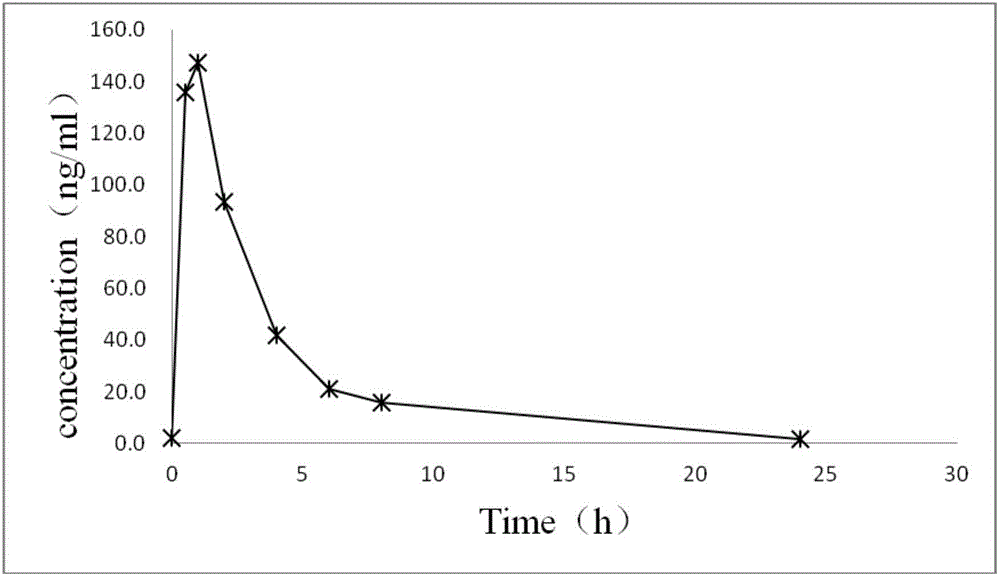
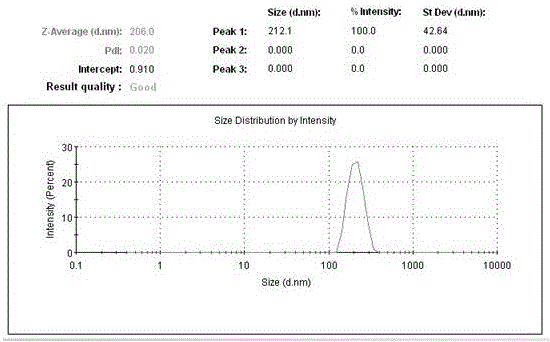
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
110 results about "Glibenclamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glyburide is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes. It may also be used with other diabetes medications.
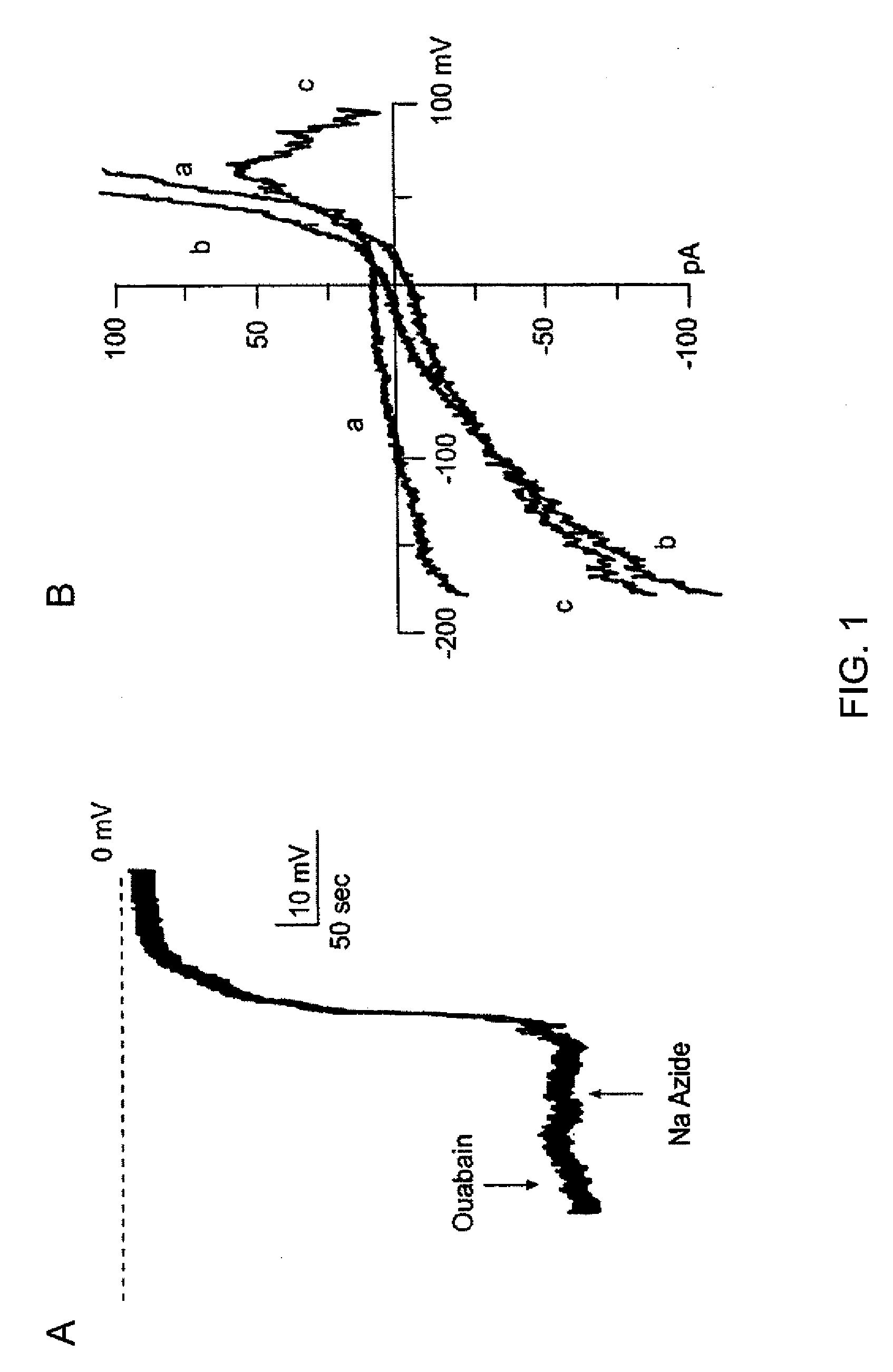
Novel non-selective cation channel in neuronal cells and methods for treating brain swelling
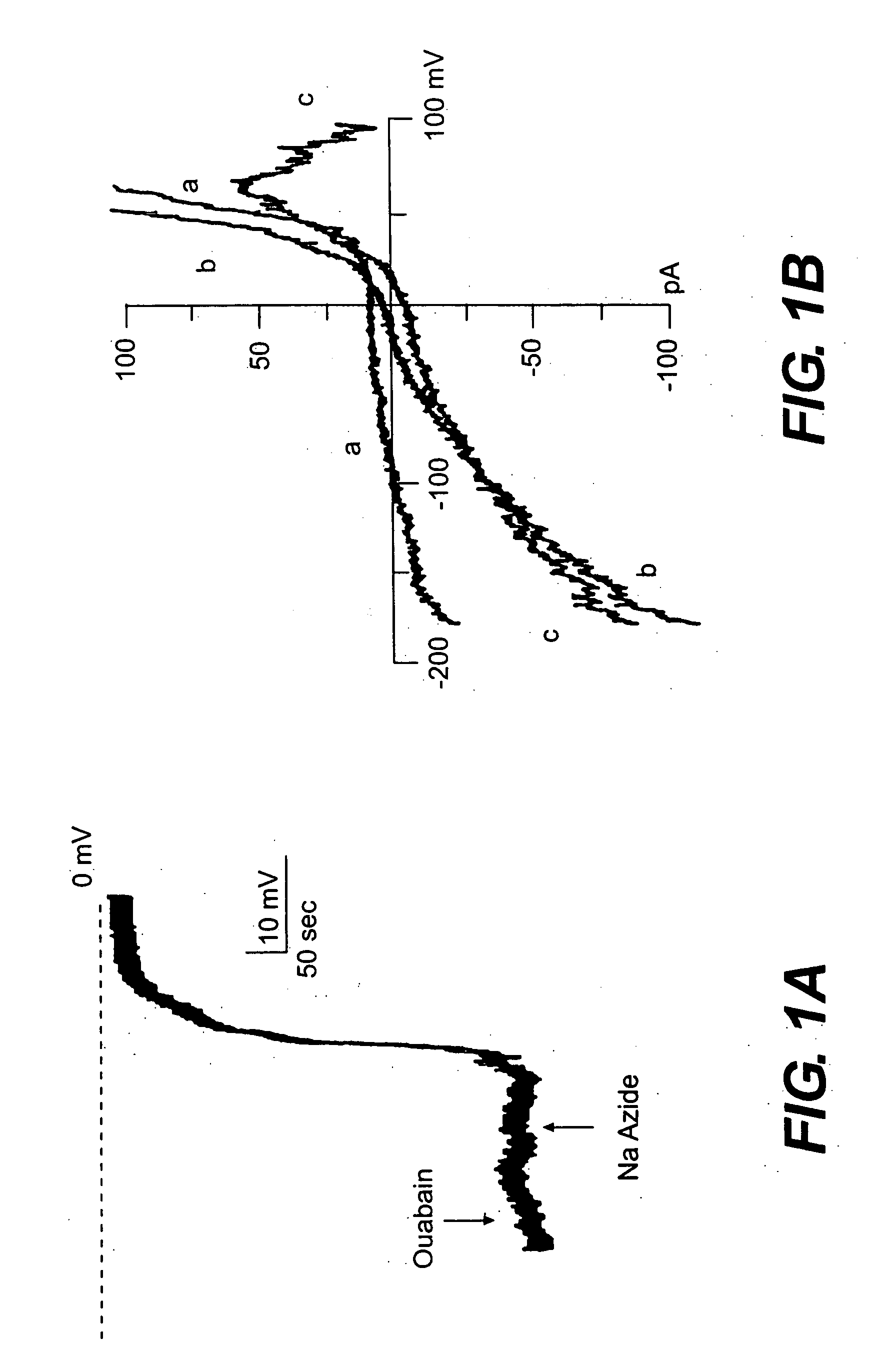
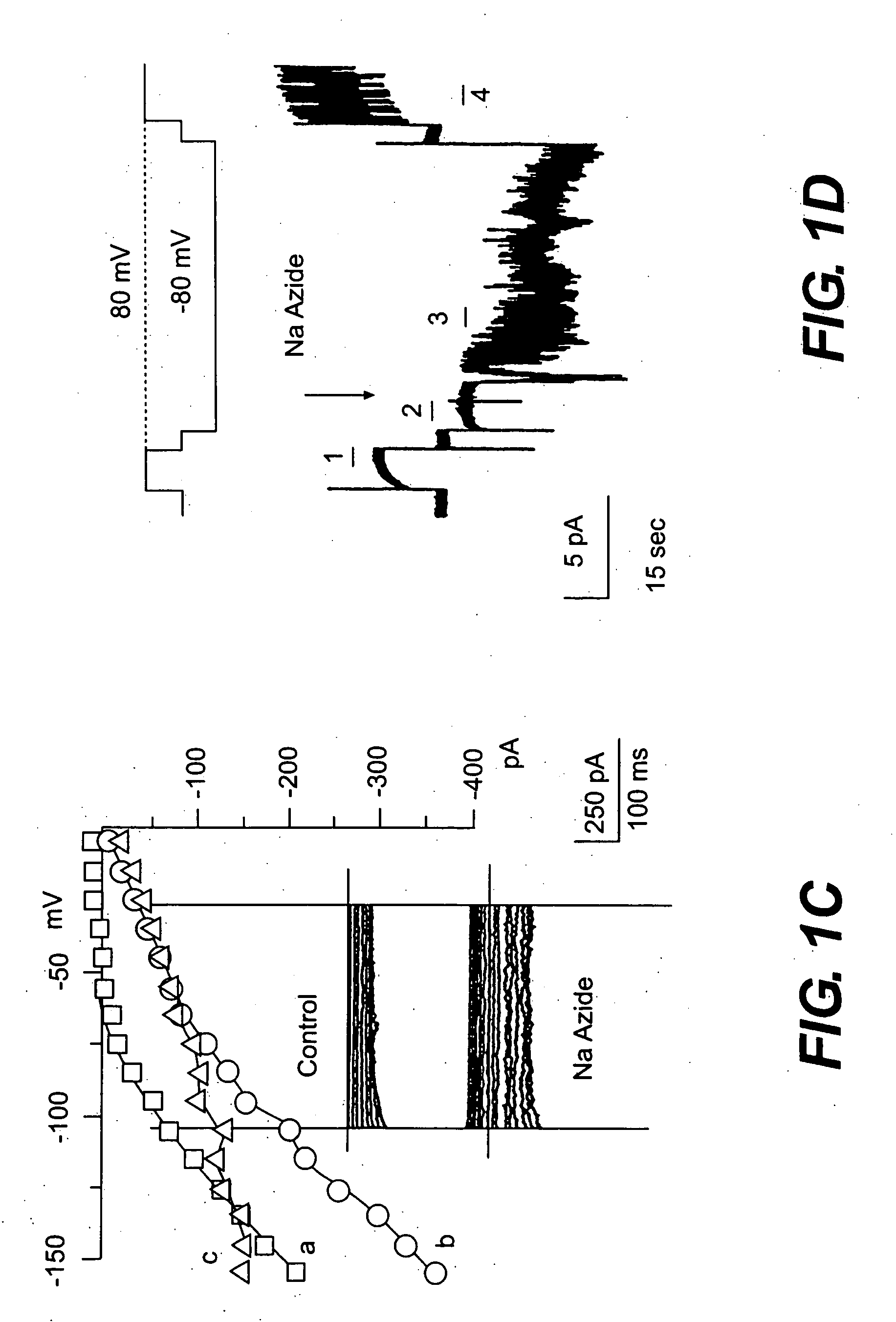
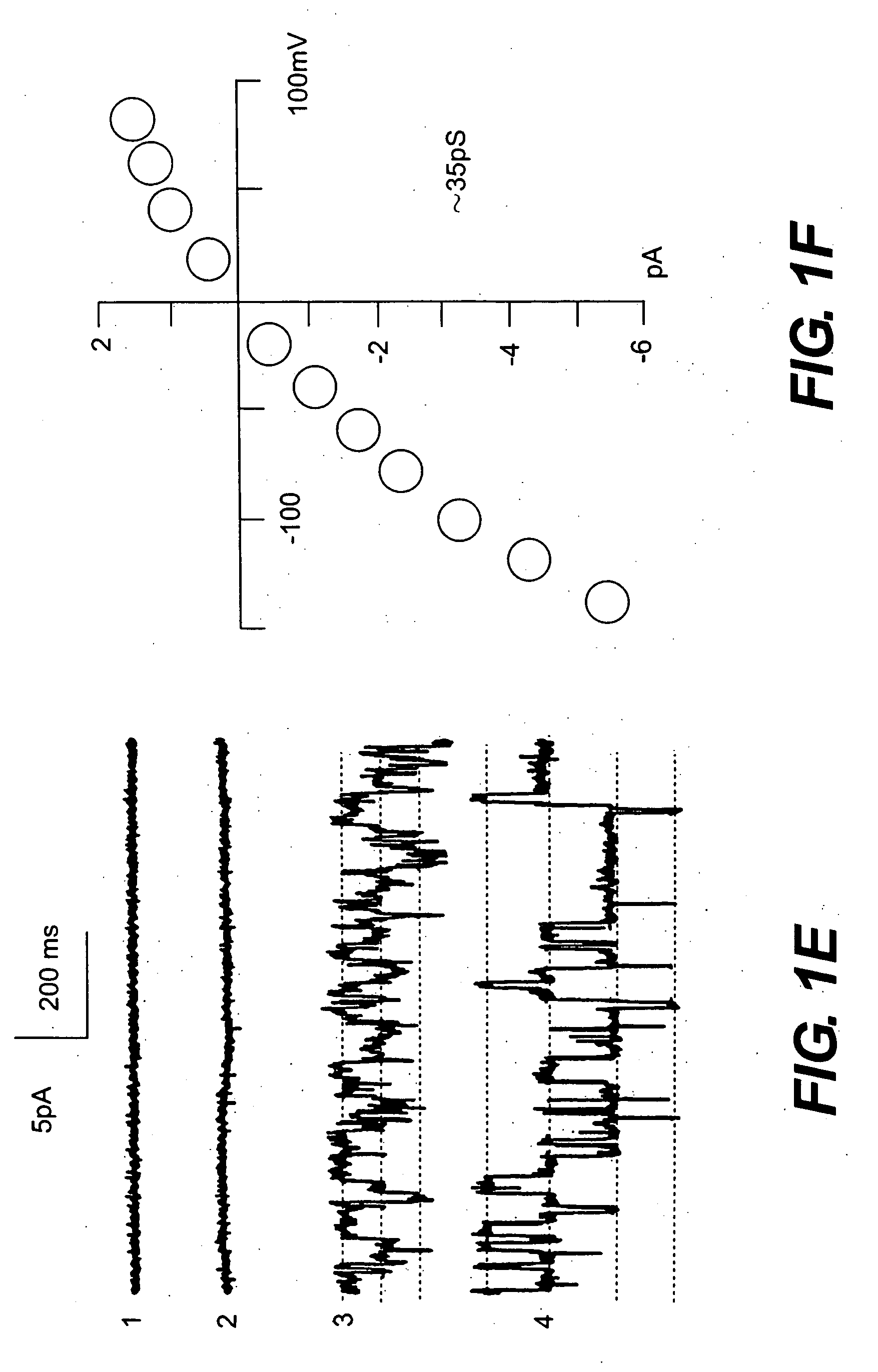
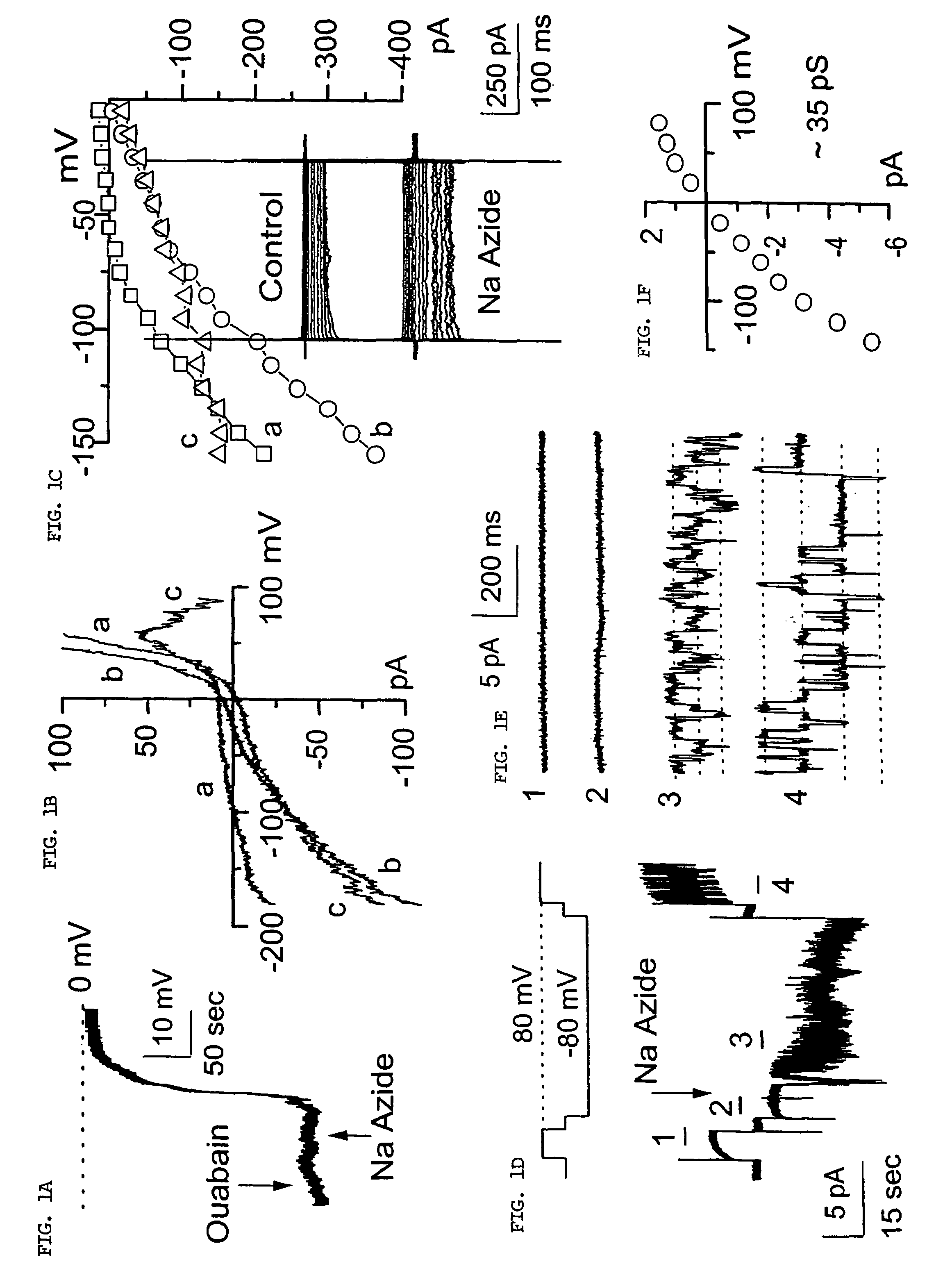
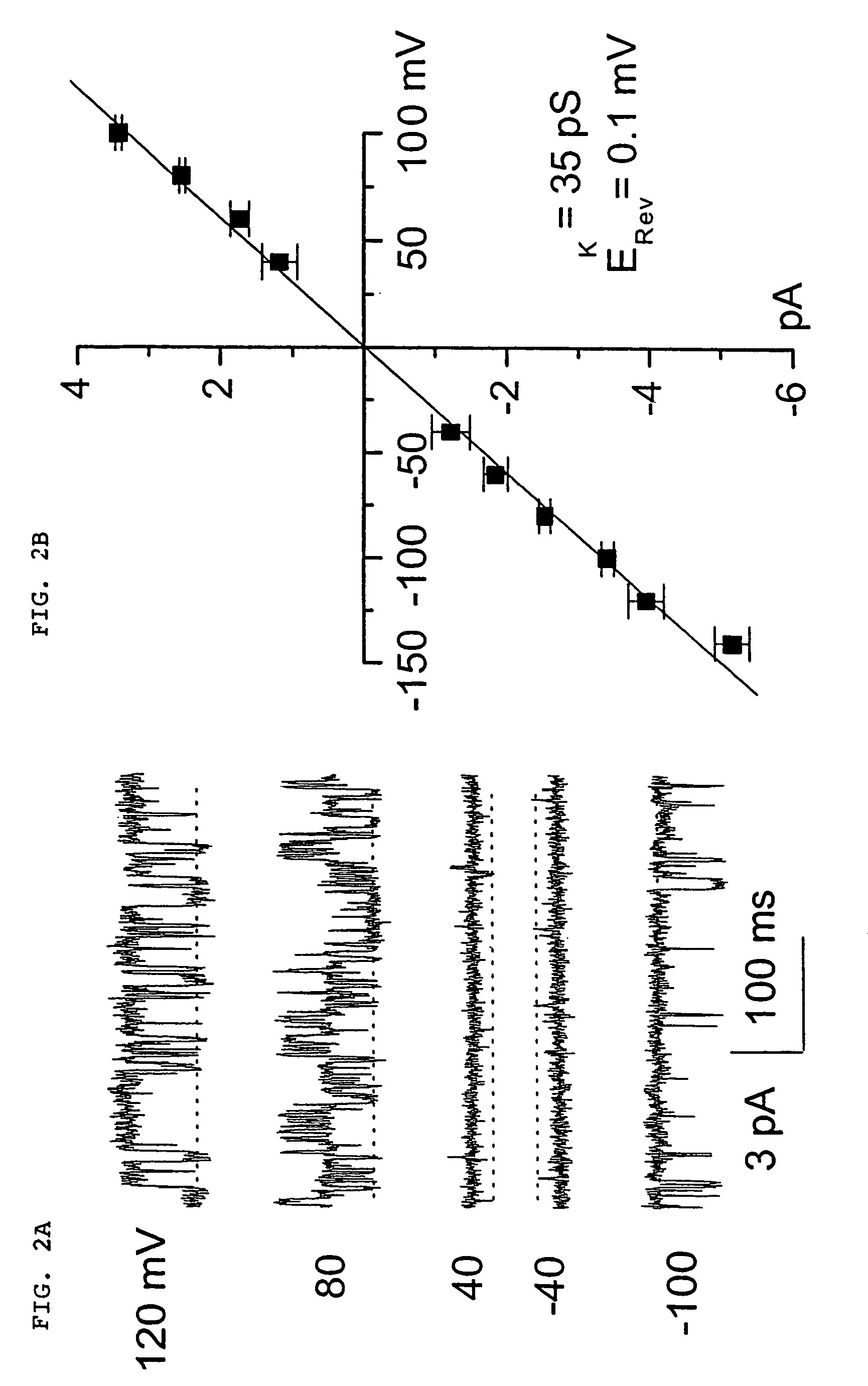
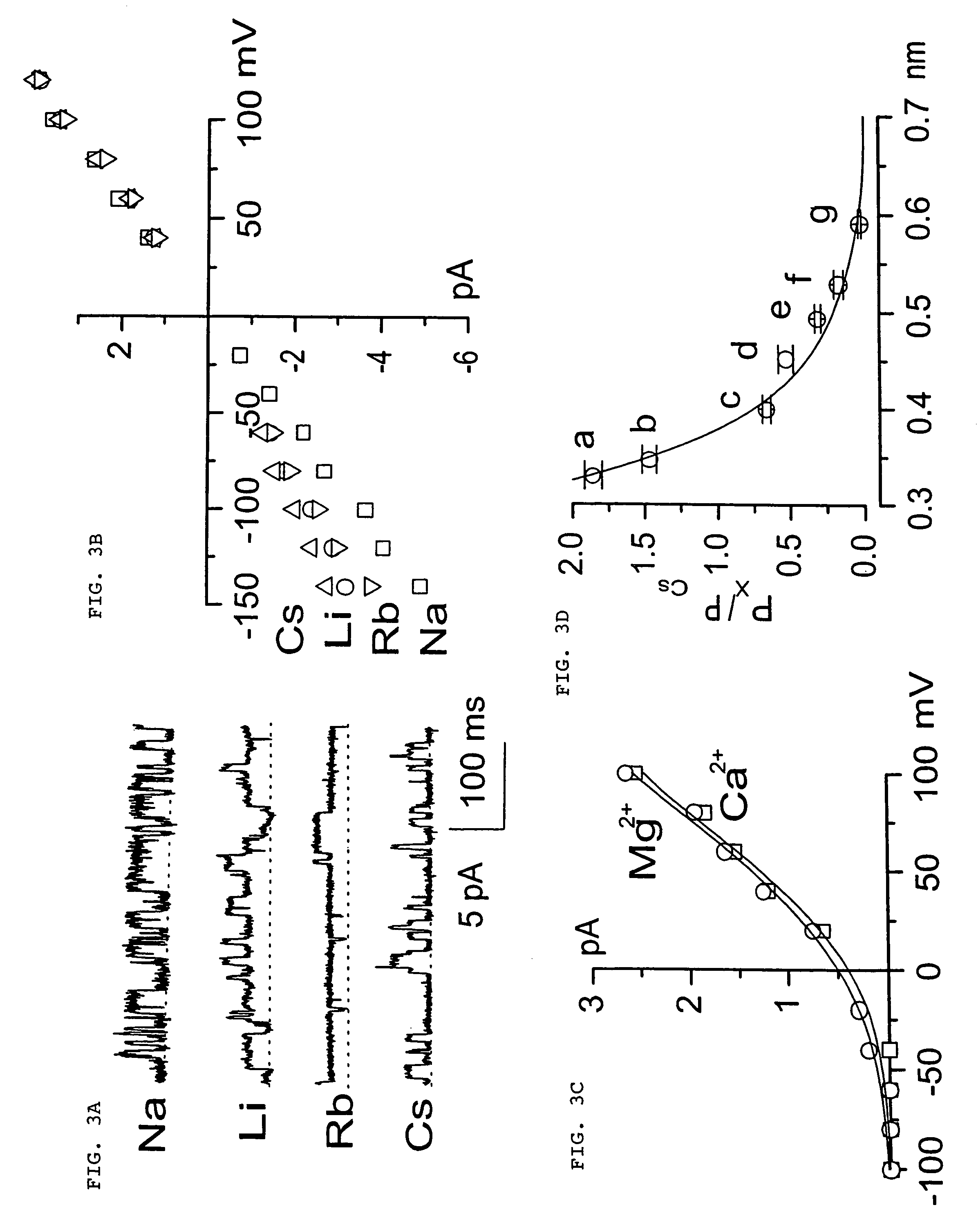
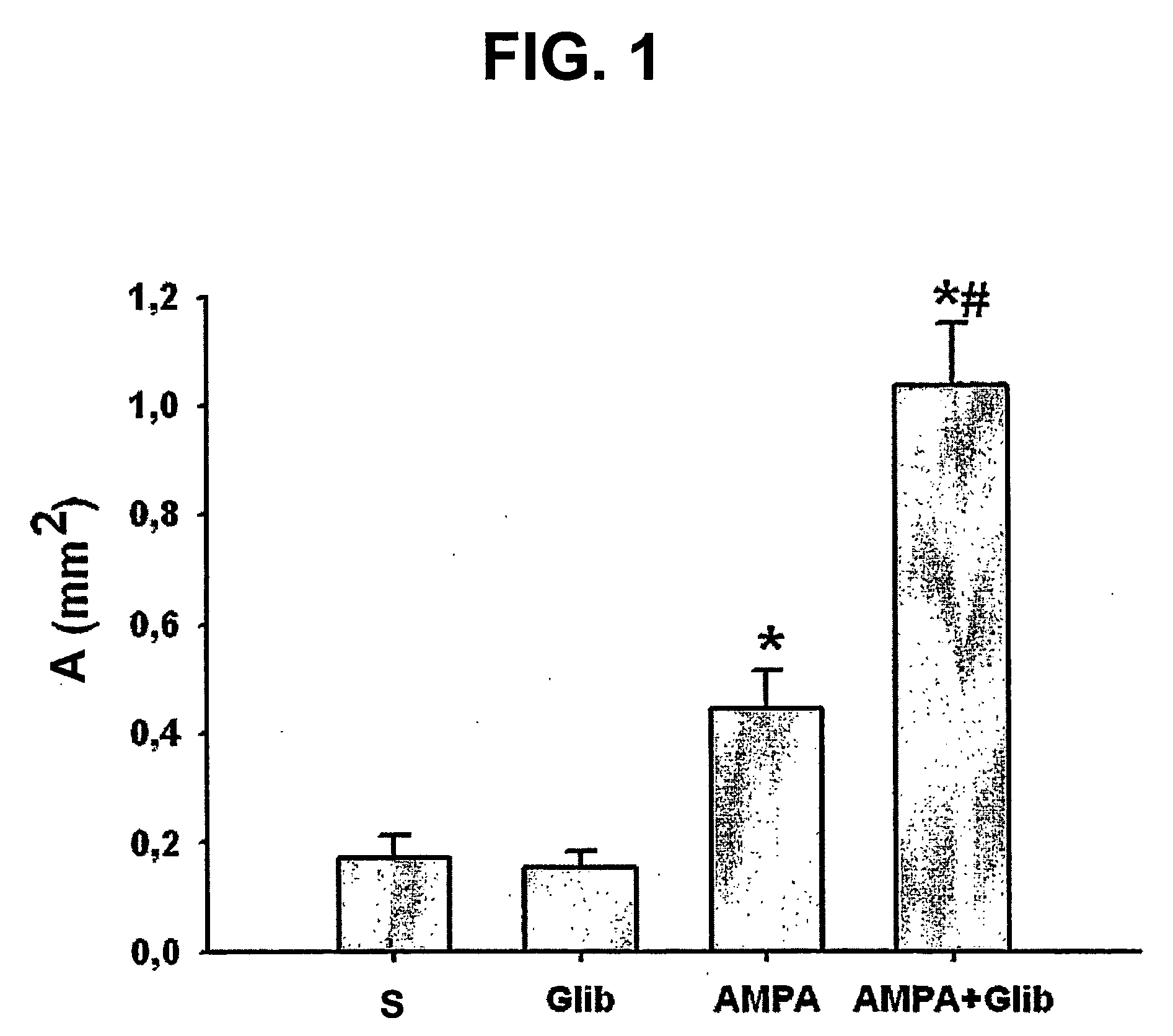
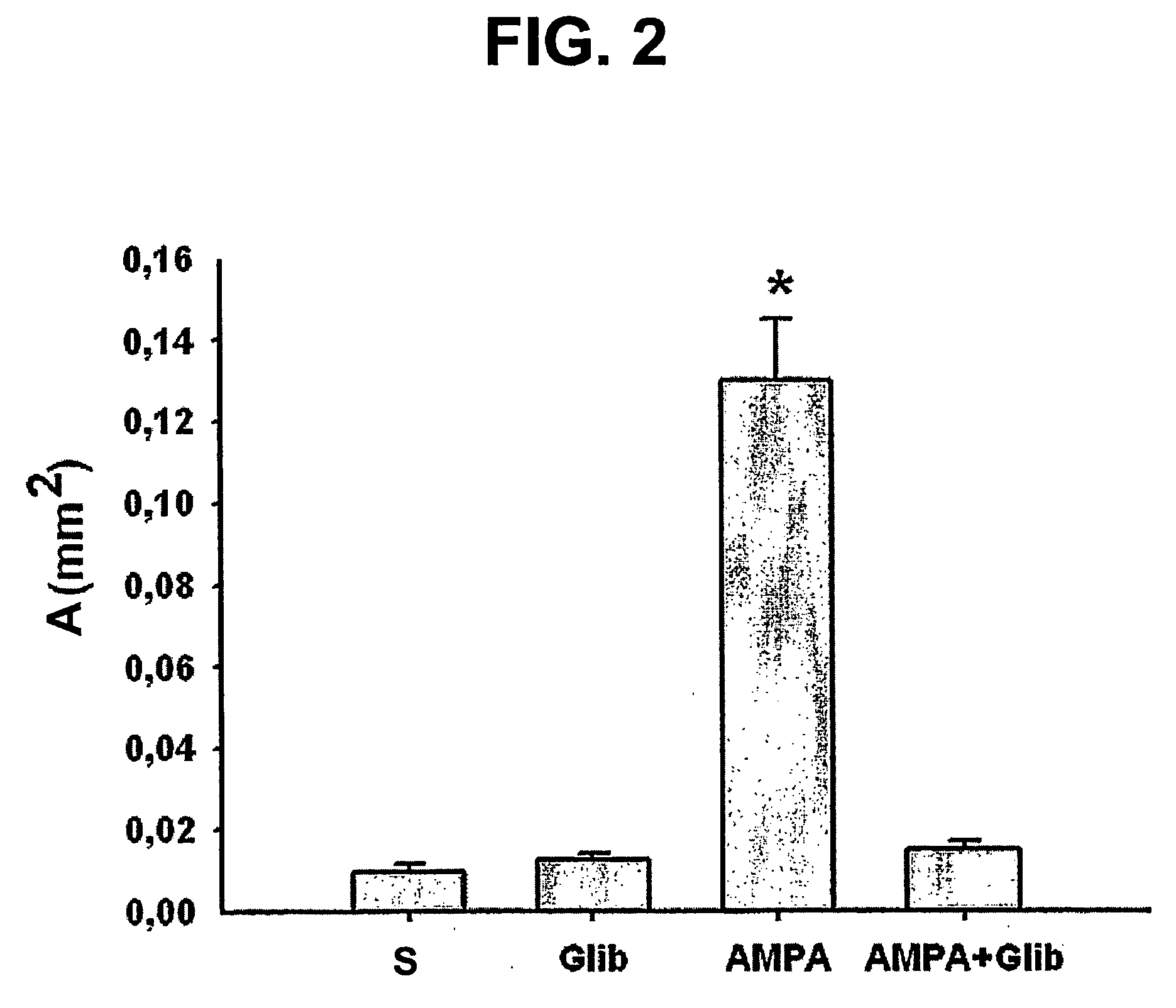
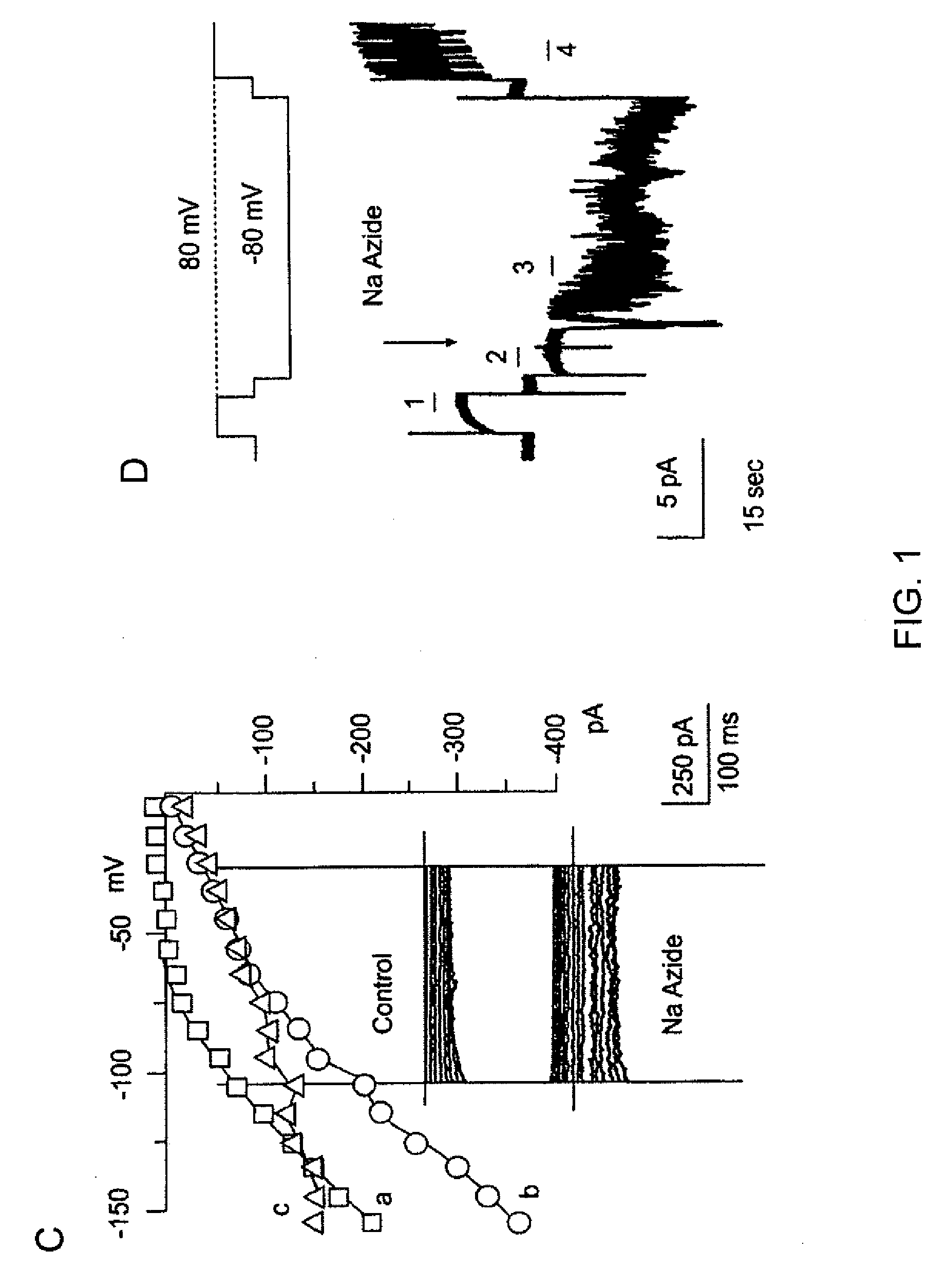
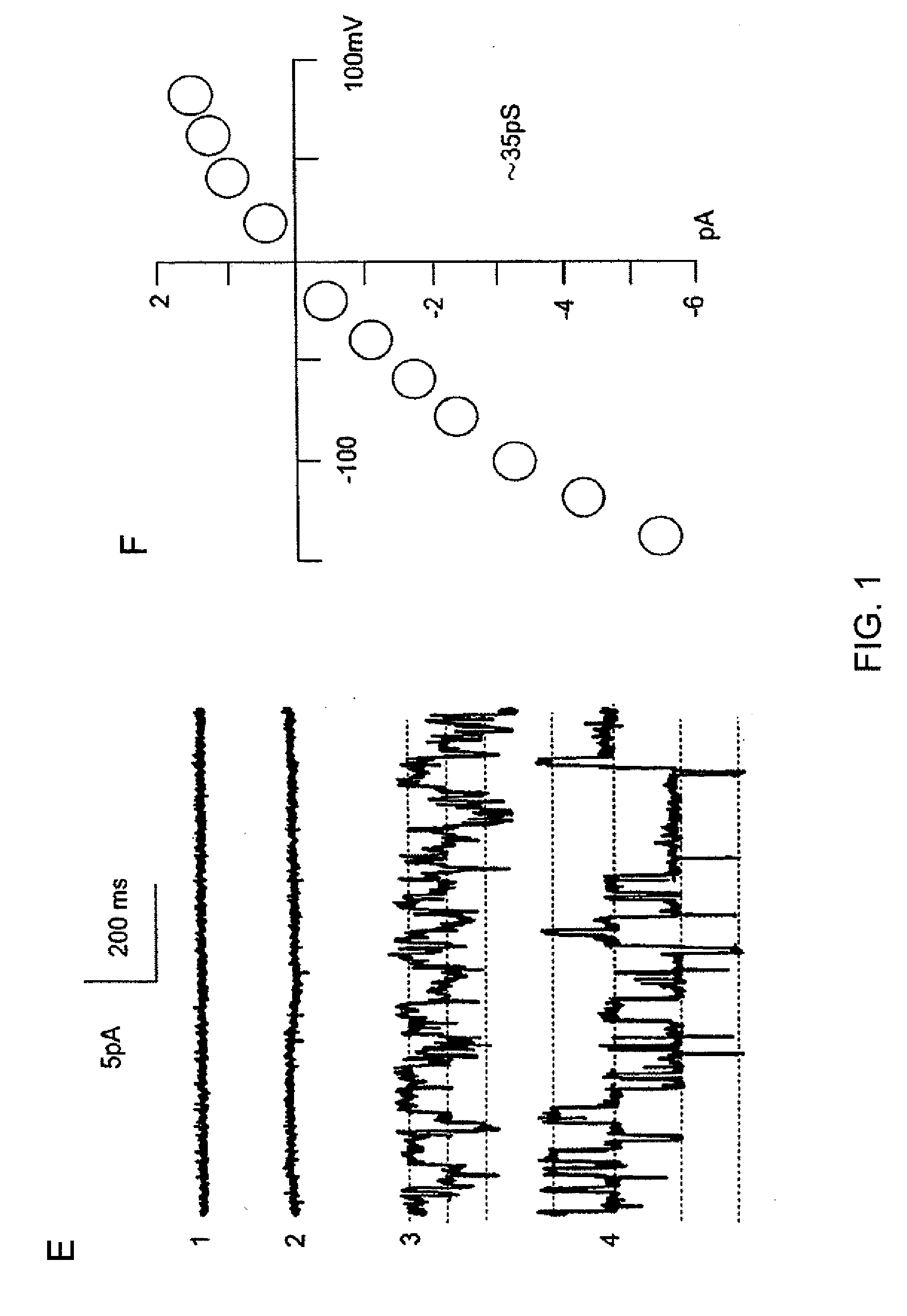
The present invention is directed to therapeutic compounds, treatment methods, and kits affecting the NCCa-ATP channel of neural tissue, including neurons, glia and blood vessels within the nervous system, and methods of using same. The NCCa-ATP channel is newly expressed in neural tissue following injury such as ischemia, and is regulated by the sulfonylurea receptor SUR1, being inhibited by sulfonylurea compounds, e.g., glibenclamide and tolbutamide, and opened by diazoxide. Antagonists of the NCCa-ATP channel, including SUR1 antagonists, are useful in the prevention, diminution, and treatment of injured or diseased neural tissue, including astrocytes, neurons and capillary endothelial cells, that is due to ischemia, tissue trauma, brain swelling and increased tissue pressure, or other forms of brain or spinal cord disease or injury. Agonists of the NCCa-ATP channel may be are useful in the treatment neural tissue where damage or destruction of the tissue, such as a gliotic capsule, is desired.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Methods for treating neural cell swelling
A composition comprising a novel Ca2+-activated, [ATP]i-sensitive nonspecific cation (NCCa-ATP) channel is described. The channel is found in mammalian neural cells and exhibits a different sensitivity to block by various adenine nucleotides, and is activated by submicromolar [Ca]i. The NCCa-ATP channel is activated under conditions of ATP depletion, which causes severe cell depolarization, followed by cell swelling. The NCCa-ATP channel is regulated by a sulfonylurea receptor and is inhibited by sulfonylurea compounds glibenclamide and tolbutamide. Methods employing compositions comprising the NCCa-ATP channel to screen for compounds that block the channel and the use of such antagonists as therapeutics in preventing brain swelling and damage are described. In addition, methods employing compositions comprising the Kir2.3 channel to screen for compounds that open the channel and the use of such antagonists as therapeutics in preventing brain swelling and damage are described.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Compounds For The Treatment Of An Acute Injury To The Central Nervous System
InactiveUS20070203239A1Easy to disassembleBiocideNervous disorderNervous systemTherapeutic treatment
KATP channel closers (KCCs) are useful for the prophylactic and / or therapeutic treatment of a CNS acute damage in a mammal, including a human, because their administration, particularly in the case of glibenclamide, potientates the neuroprotector microglial effect. Therefore, they may be useful in treating the acute phase of CNS diseases such as stroke, seizure, axonal injury, traumatic damage, neurodegeneration, spinal cord injury, infectious and autoimmune CNS diseases. KCCs, isotopically modified, are also useful for the preparation of diagnostic agents for detection and follow-up of CNS acute damage.
Owner:NEUROTEC PHARMA
Preparation process for ultrafine glibenclamide particles
InactiveCN102397257ASimple processEasy to operateMetabolism disorderSulfonylurea active ingredientsSlurryGlibenclamide
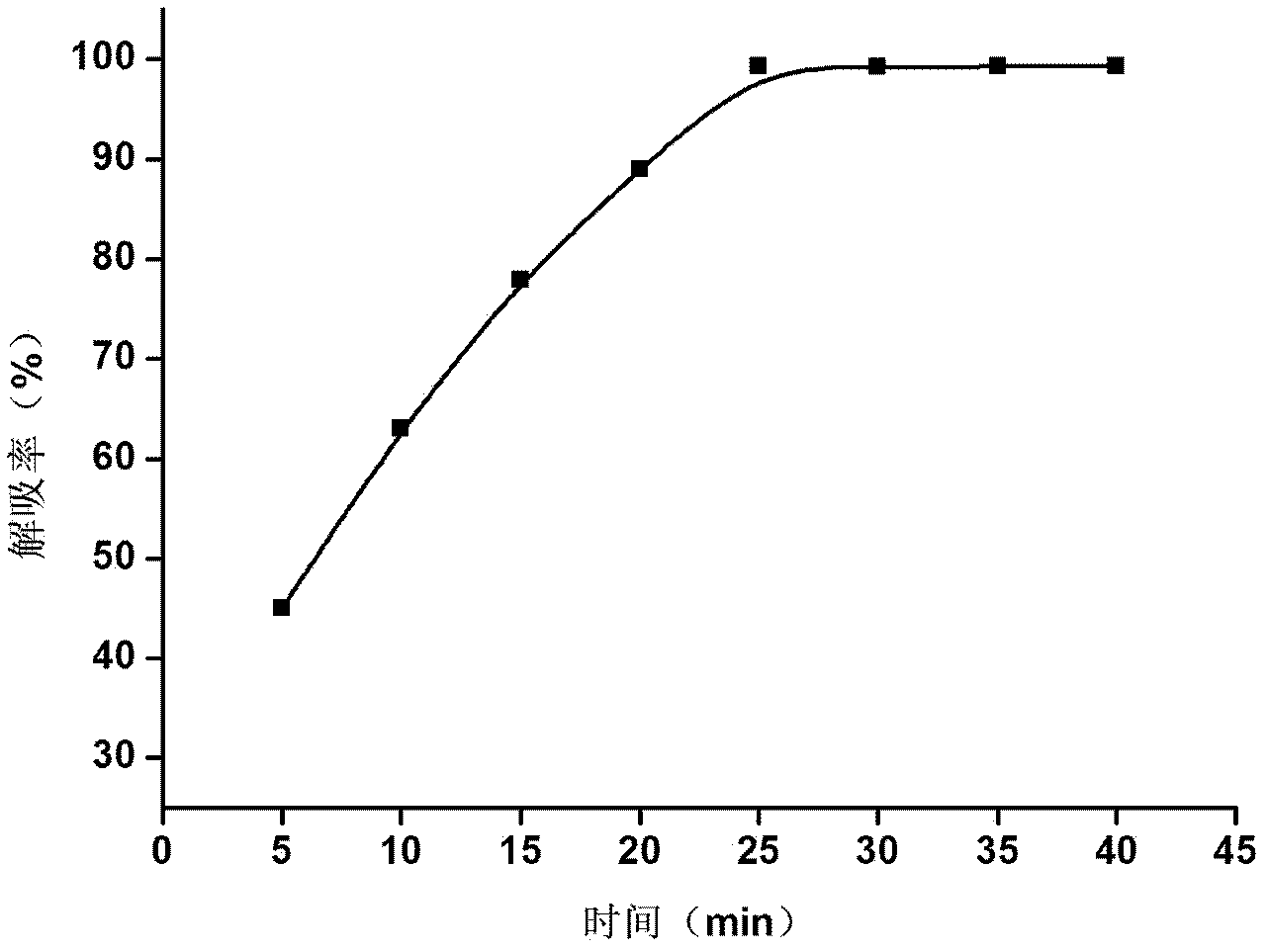
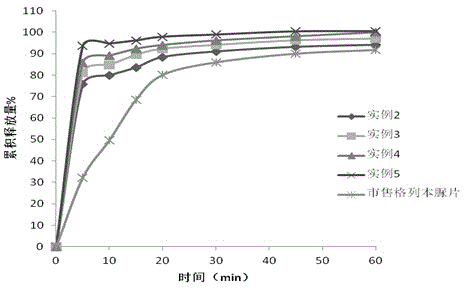
The invention discloses a preparation process for ultrafine glibenclamide particles, which belongs to the field of micronization of drugs. The process comprises the following steps: preparing an organic solution of glibenclamide; dissolving pharmaceutic adjuvants in water to form an aqueous solution and controlling the temperature of the aqueous solution to be 2 to 50 DEG C; mixing the above mentioned two solutions to prepare medicinal slurry, carrying out spray drying on the medicinal slurry, and controlling inlet temperature to be 100 to 170 DEG C, outlet temperature to be 60 to 95 DEG C, a feeding speed to be 5 to 40 ml / min and compressed air pressure to be 0.4 to 0.8 MPa so as to obtain ultrafine glibenclamide powder. The ultrafine powder provided in the invention has good stability, and more than 85% of the powder can be dissolved within 2.5 minutes; the process is simple and is easy to operate.
Owner:BEIJING UNIV OF CHEM TECH
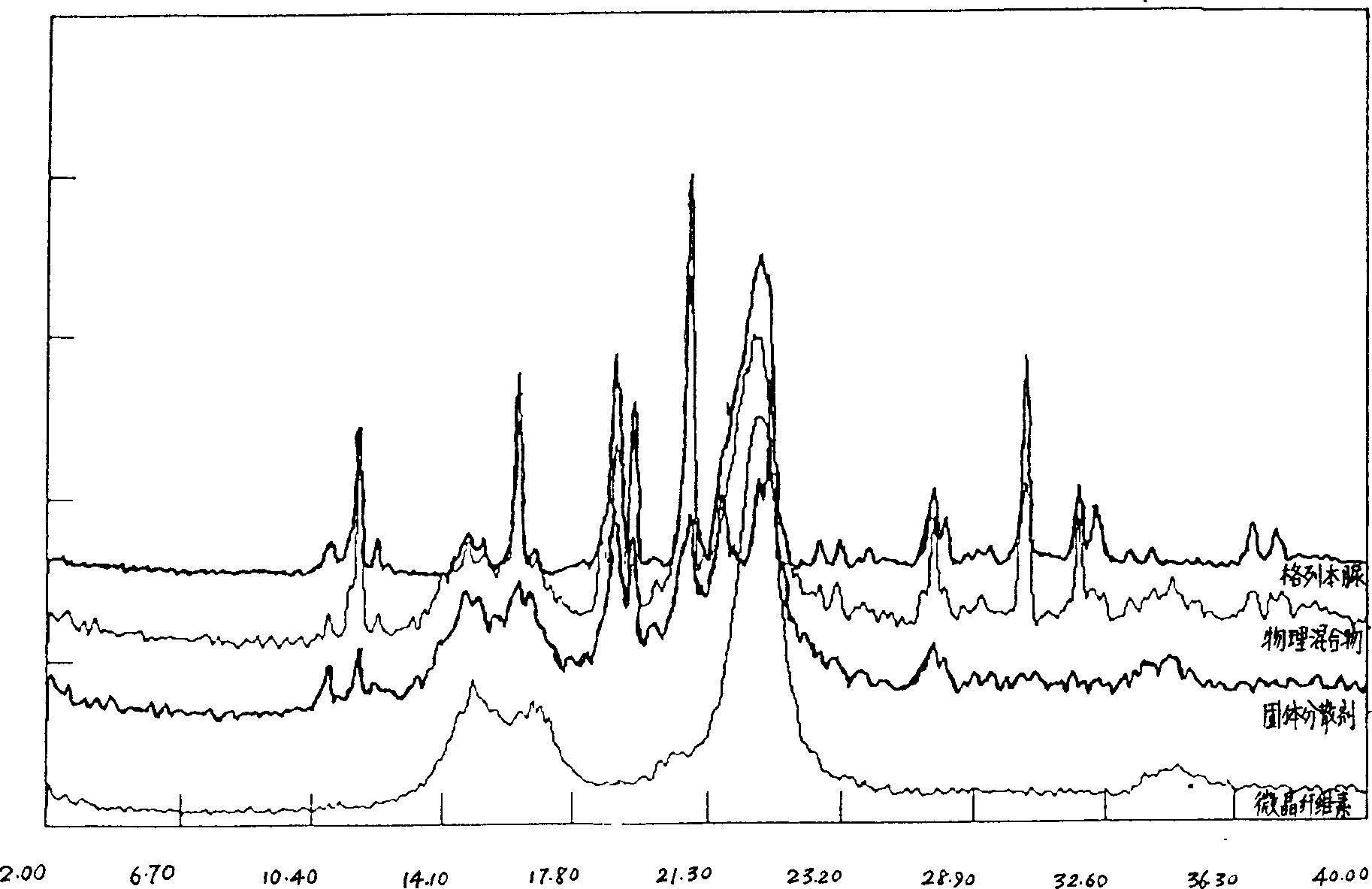
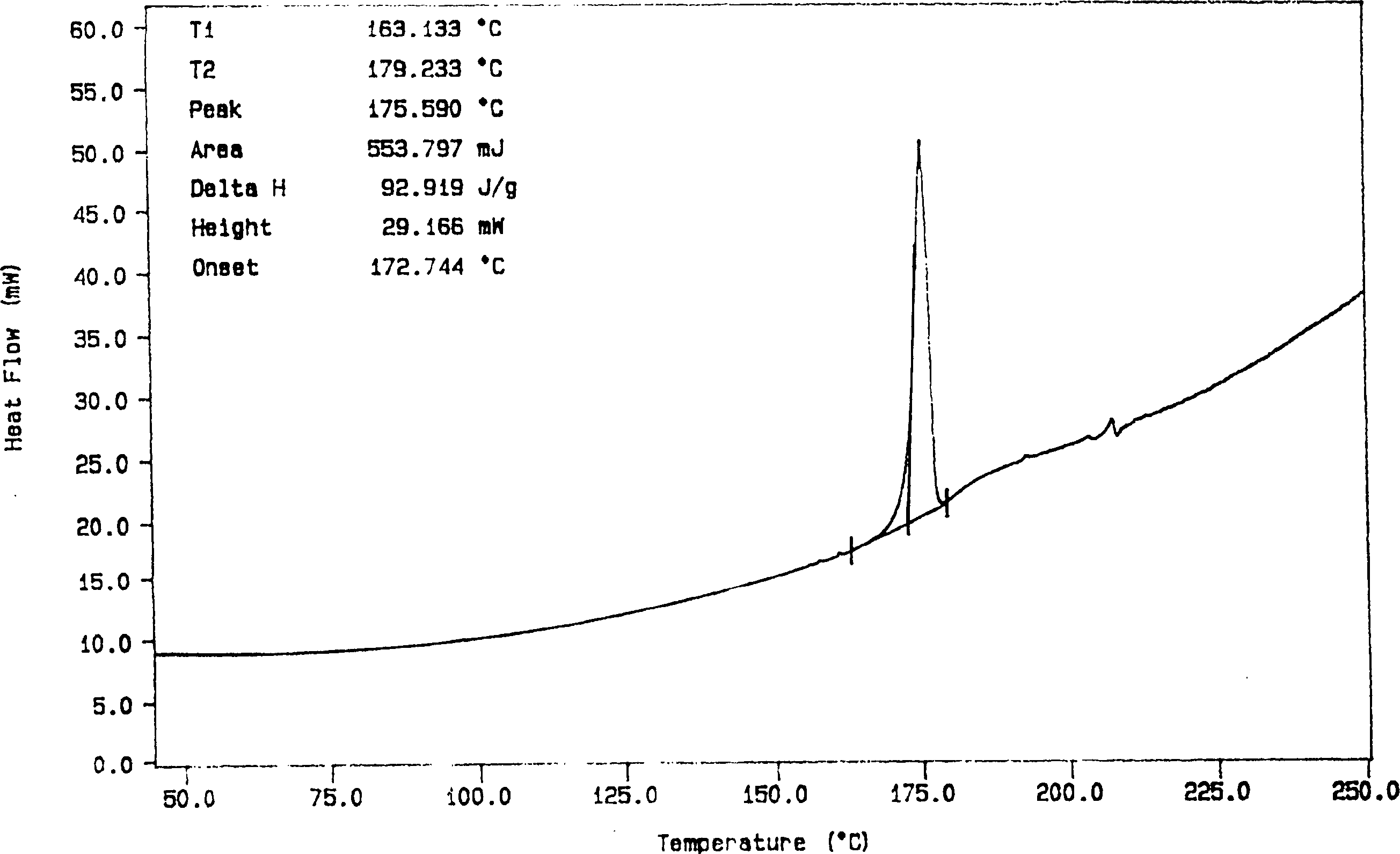
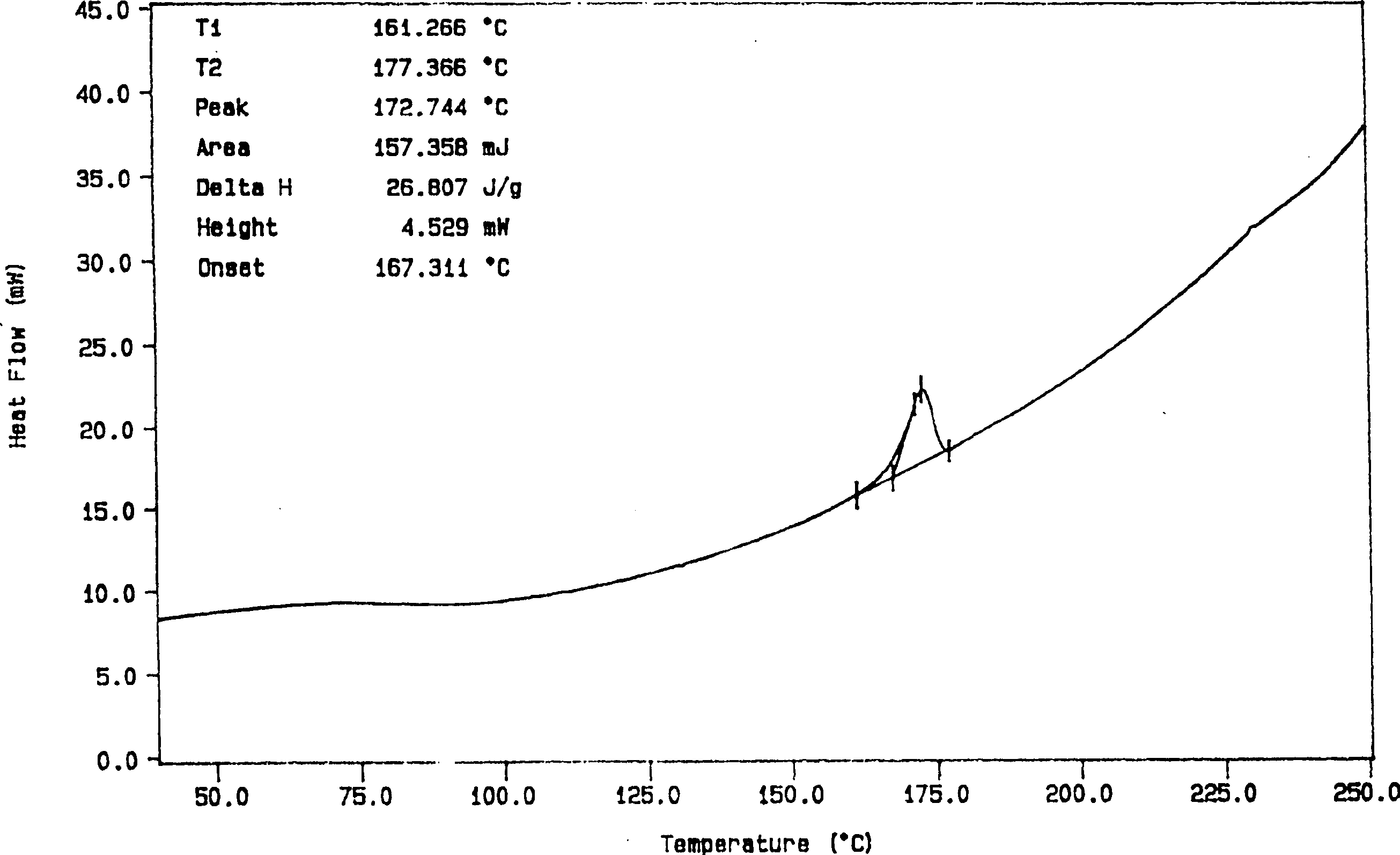
Solid dispersion and preoral combination of glibenclamide and preparation method
ActiveCN1660057AInhibition of dissolution rateDoes not change the chemical structureMetabolism disorderSulfonylurea active ingredientsSolubilityDispersed media
A dispersed solid or orally taken composition of gliben clamide clamide is prepared from glibenclamide and dispersing medium proportionally through mixing, grinding and fusing or spray drying.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
Novel non-selective cation channel in neuronal cells and method for treating brain swelling
The present invention is directed to therapeutic compounds, treatment methods, and kits affecting the NCCa-ATP channel of neural tissue, including neurons, glia and blood vessels within the nervous system, and methods of using same. The NCCa-ATP channel is newly expressed in neural tissue following injury such as ischemia, and is regulated by the sulfonylurea receptor SUR1, being inhibited by sulfonylurea compounds, e.g., glibenclamide and tolbutamide, and opened by diazoxide. Antagonists of the NCCa-ATP channel, including SUR1 antagonists, are useful in the prevention, diminution, and treatment of injured or diseased neural tissue, including astrocytes, neurons and capillary endothelial cells, that is due to ischemia, tissue trauma, brain swelling and increased tissue pressure, or other forms of brain or spinal cord disease or injury. Agonists of the NCCa-ATP channel may be are useful in the treatment neural tissue where damage or destruction of the tissue, such as a gliotic capsule, is desired.
Owner:UNIV OF MARYLAND
Preparation method of glibenclamide magnetic nano silica gel surface polyamide-amide dendritic molecular imprinting polymer
InactiveCN102489269ACheap manufacturingApplication economyOther chemical processesCross-linkFunctional monomer
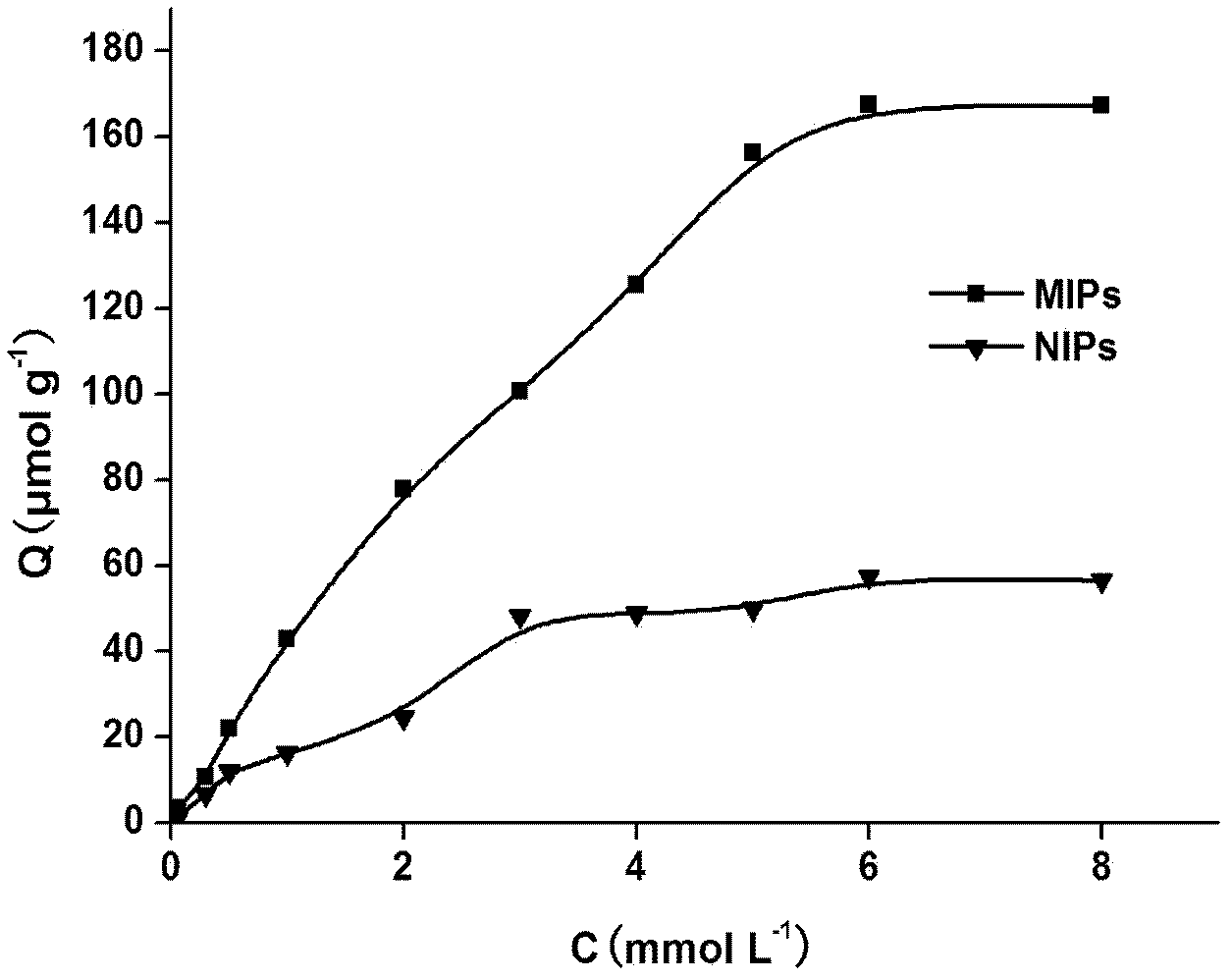
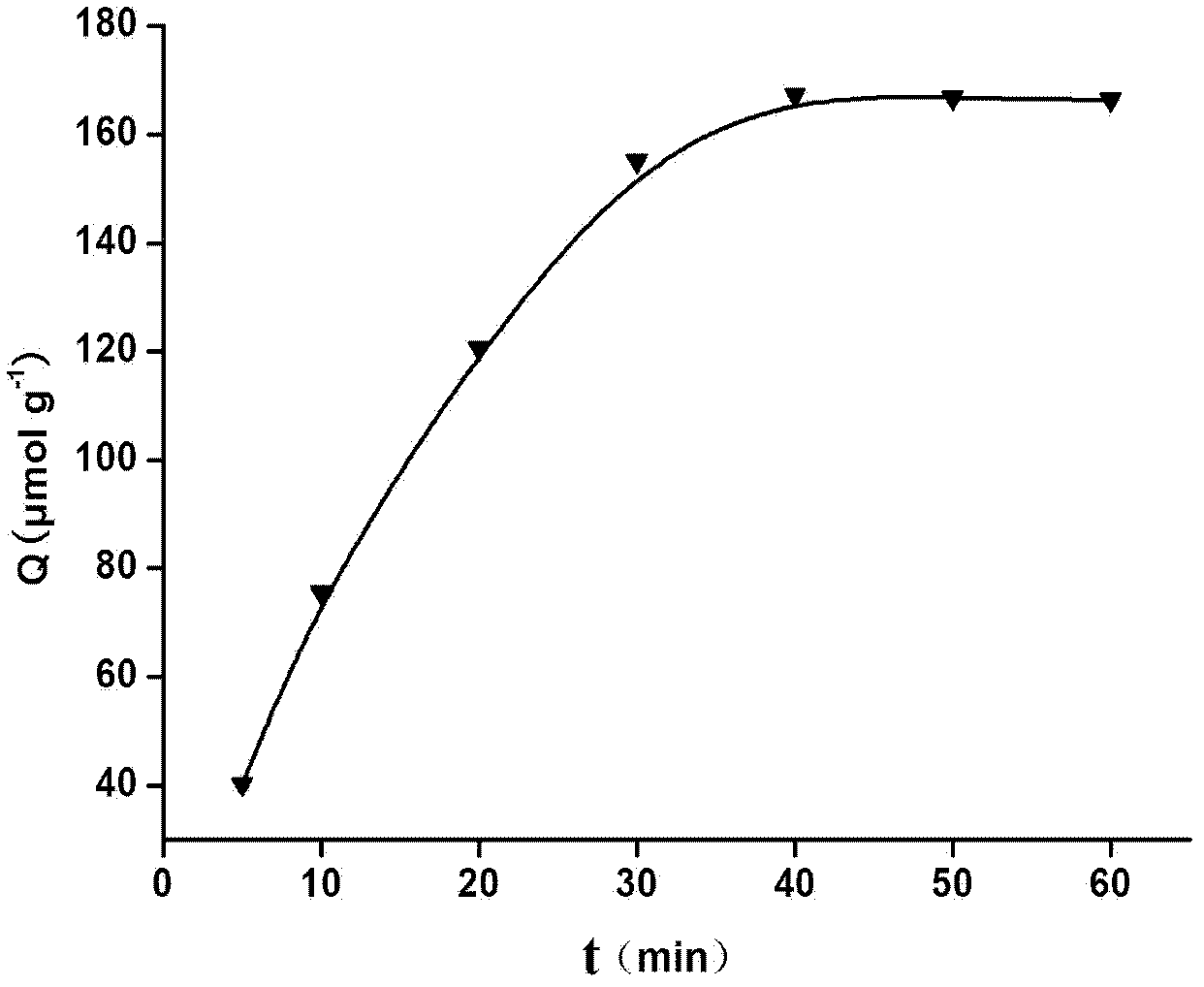
The invention discloses a preparation method of a glibenclamide magnetic nano silica gel surface polyamide-amide (PAMAM) dendritical molecular imprinting polymer, which comprises the following steps of: (1) dendritically and functionally grafting Fe3O4@SiO2; (2) placing a template molecule, a functional monomer and the functionally grafted Fe3O4@SiO2 in a porogenic agent for prepolymerization, adding a cross-linking agent and an initiating agent, and polymerizing the materials under the reflux of nitrogen; and (3) eluting the polymer to remove a template molecule using a methanol hydrochloricacid solution. The glibenclamide magnetic nano silica gel surface polyamide-amide dendritical molecular imprinting polymer prepared in the invention has specific recognition capability on glibenclamide and drugs with similar structure thereof. The glibenclamide magnetic nano silica gel surface polyamide-amide dendritical molecular imprinting polymer is used together with chromatography and can beused for the fast separation, enrichment and measurement of glibenclamide illegally added into health foods and drugs.
Owner:NANJING MEDICAL UNIV
Metformin hydrochloride and glibenclamide capsule and preparation method thereof
InactiveCN105030793AHigh dissolution rateLittle difference in loadingMetabolism disorderSulfonylurea active ingredientsMedicineAdhesive
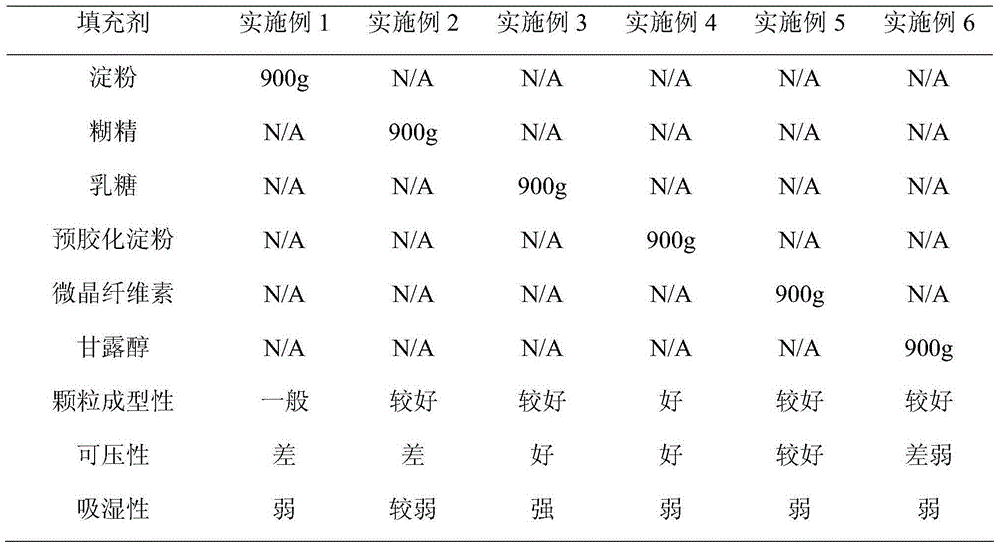
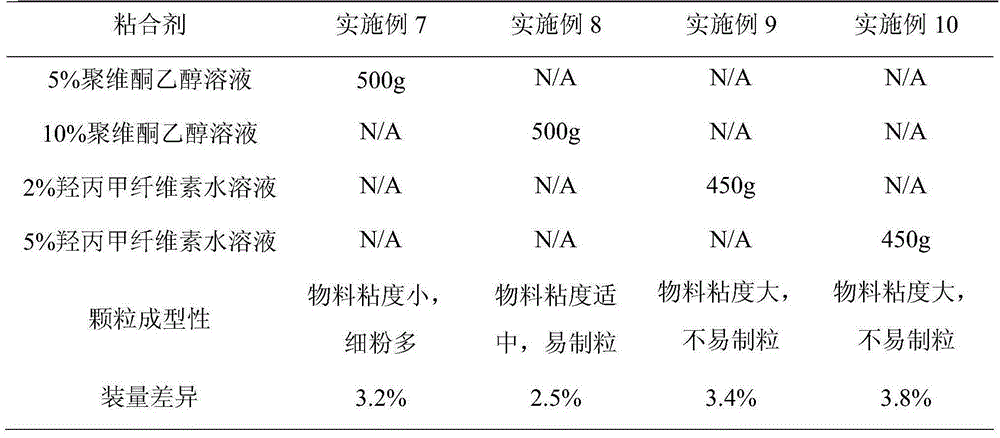
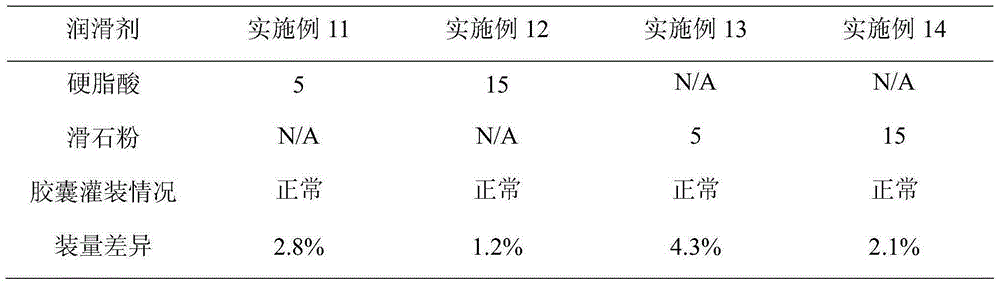
The invention belongs to the technical field of medicines and particularly relates to a metformin hydrochloride and glibenclamide capsule and a preparation method thereof. The metformin hydrochloride and glibenclamide capsule is prepared from the following raw materials in parts by weight: 200-300 parts of metformin hydrochloride, 1.0-1.5 parts of glibenclamide, 44-135 parts of filler, 2.5-7.5 parts of adhesive and 0.5-1.5 parts of lubricant. The process provided by the invention is simple and easy to implement, and the prepared metformin hydrochloride and glibenclamide capsule has the advantages of steady release and high dissolution rate.
Owner:REYOUNG PHARMA
Two-channel detection card for simultaneously detecting glibenclamide and gliclazide and detection method thereof
The invention relates to a two-channel detection card for simultaneously detecting glibenclamide and gliclazide and a detection method thereof, belonging to the technical field of detection of western medicine illegally added into Chinese medicine. The surface of a detection card casing is provided with a detection window opening and a sampling hole, a test strip is arranged in the casing, a nitrocellulose membrane is attached to the middle of a bearing back panel, a water sucking membrane is attached to one end of the bearing back panel, a sample pad is attached to the other end of the bearing back panel, two colloidal gold membranes are clamped and attached between the nitrocellulose membrane and the sample pad, wherein the colloidal gold membranes are glass fiber membranes which respectively contain an anti-glibenclamide colloidal gold labeled monoclonal antibody and anti-gliclazide colloidal gold labeled monoclonal antibody, three print display bands are intervally arranged on the nitrocellulose membrane along the traverse direction, one is a detection band containing a glibenclamide protein conjugate, another one is a detection band containing a gliclazide protein conjugate, and the third one is a quality control band containing an anti-rabbit antibody or an anti-mouse antibody, the sample pad is arranged just opposite to the sampling hole, and the nitrocellulose membrane is arranged just opposite to the detection window opening. The detection card can be used for simultaneously detecting two western medicine components illegally added into a glucose-lowering Chinese medicine sample, has the advantages of detection cost saving, rapid detection, high sensitivity, accurate result, and convenience for use.
Owner:无锡安迪生物工程有限公司
Quality control of compound preparation for treating diabetes
A quality control method for the Chinese medicine used for treating diabetes and prepared from glibenclamide and 7 Chinese-medicinal materials including pueraria root, rehmannia root, astragalus root, yam, etc features the differentiation test method and / or content measuring method for its multiple components.
Owner:BEIJING JIAHE LEKANG TECH
Glibenclamide, gliclazide and glipizide triple test card and test method thereof
InactiveCN102012427AEasy to manufactureLow detection costPreparing sample for investigationWestern medicineGlass fiber
The invention relates to a glibenclamide, gliclazide and glipizide triple test card and a test method thereof, belonging to the technical field of testing of western medicine components which are illegally added into traditional Chinese medicines. A test bar is arranged in a shell of the triple test card and comprises a sample pad, a plurality of sections of colloidal gold membranes, a nitrocellulose membrane and a water-absorbing membrane which are sequentially stuck on a supporting backboard, wherein the plurality of sections of the colloidal gold membranes are glass fiber membranes containing colloidal gold markers of an anti-glibenclamide antibody, an anti-gliclazide antibody and an anti-glipizide antibody sequentially, three test strips which respectively contain a glibenclamide protein conjugate, a gliclazide protein conjugate and a glipizide protein conjugate are arranged on the nitrocellulose membrane, and the triple test card additionally comprises a quality control strip containing an anti-rabbit antibody or an anti-mouse antibody. The triple test card has the advantages of being capable of simultaneously detecting glibenclamide, gliclazide and glipizide which are illegally added in a glucose-lowering traditional Chinese medicine. The test card is easy for preparation, convenient and fast for use and accurate in result, and can be used for saving the test cost.
Owner:长沙安迪生物科技有限公司
Colloidal gold chromatography test paper for detecting glibenclamide and derivatives of glibenclamide quickly and preparation method thereof
The invention discloses a colloidal gold chromatography test paper for detecting glibenclamide and derivatives of glibenclamide quickly, which consists of a substrate, a sample pad, a gold-marked combined pad, a covering film and an absorbent pad, wherein the gold-marked combined pad is polyester fibrofelt capable of absorbing the gold-marked antibody of the derivatives of glibenclamide; and a linear detection wire T coated with carrier protein solution for coupling the derivatives of glibenclamide and a linear reference wire C coated with goat anti-mouse IgG solution are arranged on the covering film, and the two wires are arranged parallelly. The method comprises the following steps: preparing artificial conjugated antigen; preparing monoclonal antibodies of the derivatives of glibenclamide; preparing solution of colloidal gold; and obtaining the colloidal gold marker of the monoclonal antibodies of the derivatives of glibenclamide and the gold-marked combined pad. The test paper has the advantage of high specificity, high sensitivity, simplicity, quickness, high timeliness, vivid result display and accuracy.
Owner:NANTONG EGENS BIOTECH
Dispersible tablet containing metformin and glibenclamide and preparation method thereof
InactiveCN101167731AShort peak timeLower blood sugar concentrationMetabolism disorderSulfonylurea active ingredientsGlibenclamideMetformin
The invention relates to dispersible tablets containing diabetosan and glibenclamide. The dissolving rate of glibenclamide is improved by making colloidal powder or solid dispersion powder of glibenclamide before making tablets. The dispersible tablets can disintegrate quickly, and the dissolving rates of diabetosan and glibenclamide of the dispersible tablets are significantly faster than that of common preparation.
Owner:林海平
Blood-glucose-lowering and pancreatic-islet-recovering plaster
InactiveCN102716361AAdjust the imbalanceImprove immunityAnthropod material medical ingredientsMetabolism disorderDiseaseDrug synergism
The invention discloses a blood-glucose-lowering and pancreatic-islet-recovering plaster, and belongs to externally-used medicine for curing diabetes mellitus. The blood-glucose-lowering and pancreatic-islet-recovering plaster comprises radix scrophulariae, radix rehmanniae, liriope, trichosanthin, astragalus membranaceus, salvia miltiorrhiza, codonopsis pilosula, angelica sinensis, white muscardine silkworms, caulis spatholobi, cortex lycii radicis, quasi rhizoma dioscoreae, poria cocos, kudzu roots, schisandra chinensis, periostracum cicadae, coptis chinensis, salt-fried dodder, raw anemarrhena, raw gypsum powder, dark plums, bitter oranges, radix saposhnikoviae, rhizoma typhoon, pericarpium citri reticulatae viride, cistanche, cassia seeds, rhubarb, white peony roots, fenugreek, radix glycyrrhizae preparata, round-shaped rice, phenformin, glibenclamide and sesame oil, wherein each medicines acts synergistically. The blood-glucose-lowering and pancreatic-islet-recovering plaster performs the functions of yin nourishment, body strengthening, adverse Qi descending, stagnation elimination, liver clearing, vision improvement, yin-yang adjustment, kidney essence nourishment, blood nourishment and cooling, liquid engenderinging, thirst allaying, blood circulation promotion, stasis removal, meridian and collateral dredging, blood glucose lowering, pancreatic islet recovery, internal insulin secretion increase and recovery, global balance adjustment, metabolism enhancement, microcirculation improvement, prevention of the occurrence of cardiovascular and cerebrovascular diseases, nephropathy, nervous lesions and ophthalmopathy, and prevention of ketone toxicosis of elderly patients, and the like, achieves a good curative effect when being used for curing the diabetes mellitus, take effect quickly, achieve an effect of treating both principal and secondary aspects of diseases, and can reach a goal that the diseases have low probability of relapsing after being cured.
Owner:庞保国
Rapid test card of glibenclamide and test method of glibenclamide
Owner:无锡安迪生物工程有限公司
Medicine for treating diabetes
InactiveCN101352483APrevent secondary failureAvoid failureMetabolism disorderSulfonylurea active ingredientsSalvia miltiorrhizaMedicinal herbs
The invention discloses a compound Chinese patent medicine which is prepared by combining Chinese medicines and western medicines, in particular to a medicine used for treating diabetes mellitus. The medicine takes the western medicines with the function of lowering blood sugar and the Chinese medicines used for preventing and treating chronic complicating disease as the main ingredients, and the ingredients particularly include: 14-18g of radix astragali paste, 16-20g of radix puerariae powder, 10-14g of angelica paste, 0.1-1g of salvia miltiorrhiza paste, 1-4g of rhizoma atractylodis paste, 0.05-0.5g of glibenclamide powder and 1.25-3.75g of phenformin powder; the preparation method is as follows: high-quality medicinal materials of radix astragali, angelica, salvia miltiorrhiza and rhizoma atractylodis are selected and decocted with water for 2 times, one hour per time; the dreg is removed, and the juice is taken and stands still for 8-12 hours; after being clarified, supernatant fluid is taken and is decocted into paste; radix puerariae is pulverized and sieved by a 120 meshes fine screen to remove fibers and prepare powder; then radix astragali paste and angelica paste as well as radix puerariae powder, salvia miltiorrhiza paste and glibenclamide powder, rhizoma atractylodis paste and phenformin powder are evenly stirred respectively and prepared into dried granules which are loaded into capsules after being evenly blended. By adopting a traditional processing technique, the product of the invention contains a variety of medicine ingredients for reducing blood sugar and preventing and treating various complicating diseases, has precise prescription, long lasting drug effect, remarkable curative effect and safe and reliable performance.
Owner:李官明 +1
Chinese-western composite medicine for treating diabetes and its preparing method
InactiveCN1554422ARelief of clinical symptomsSymptoms did not improve significantlyMetabolism disorderUnknown materialsWestern medicineSide effect
The composition Chinese-Western medicine for treating diabetes consists of 28 kinds of Chinese medicinal materials including tuckahoe, prepared rhizome of rehmannia, figwort root, trichosanthes root, astragalus root, etc. and Western medicine Glibenclamide. It has the toxic side effect of the Western medicine overcome and fast blood sugar reducing rate as well as simple preparation process, determined curative effect, lasting medicine effect and no bad reaction.
Owner:董瑞明
Glibenclamide-metformin combination for the treatment of diabetes mellitus of type II
Non-insulin dependent diabetes mellitus in cases of secondary failure is treated with a combination of glibenclamide and metformin.
Owner:ABIOGEN PHARMA SPA
Pharmaceutical compositions comprising metformine and glibenclamide for the treatment of type II diabetes mellitus
InactiveCN1596103AMetabolism disorderSulfonylurea active ingredientsGlibenclamideBULK ACTIVE INGREDIENT
Orally administrable pharmaceutical compositions in the form of tablets, comprising glibenclamide and metformin, or pharmaceutically acceptable salts thereof, as active ingredients, maintained separate from one another within the same composition, are described for the treatment of type-II diabetes mellitus.
Owner:MENARINI INT OPERATIN S LUXEMBORG SA
Glucose stimulated insulin secretion determination model
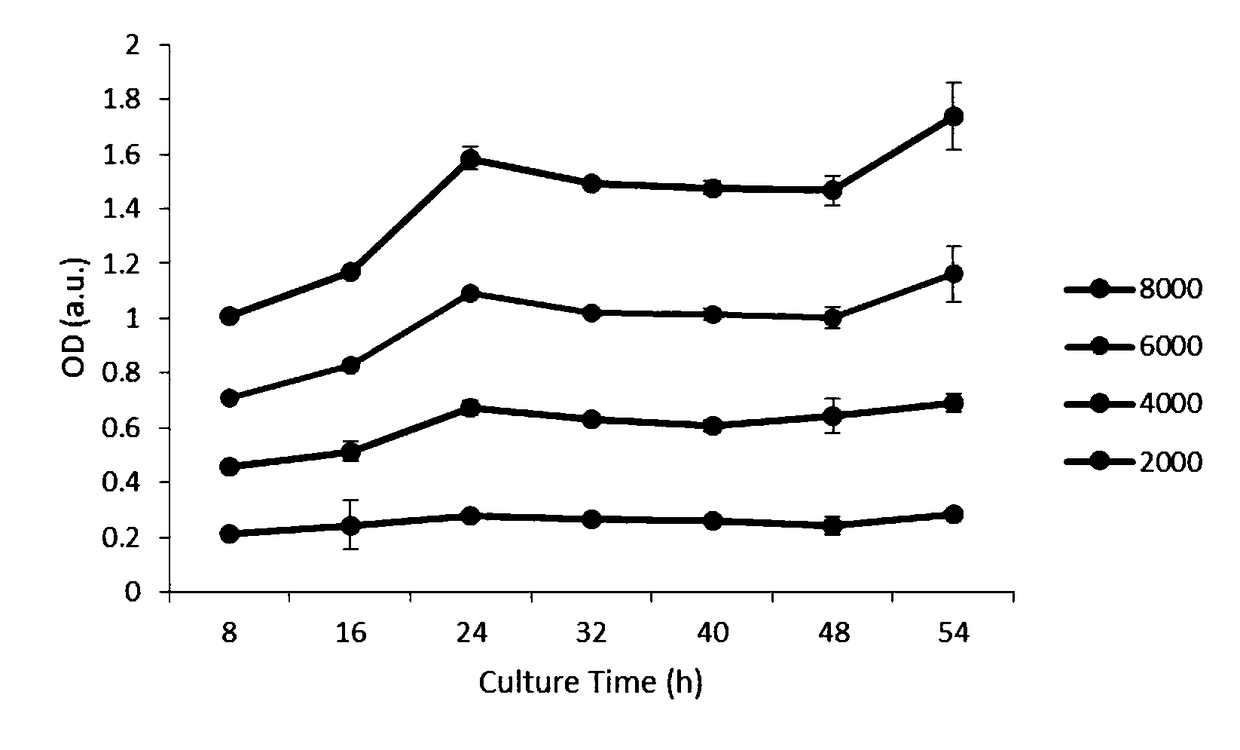
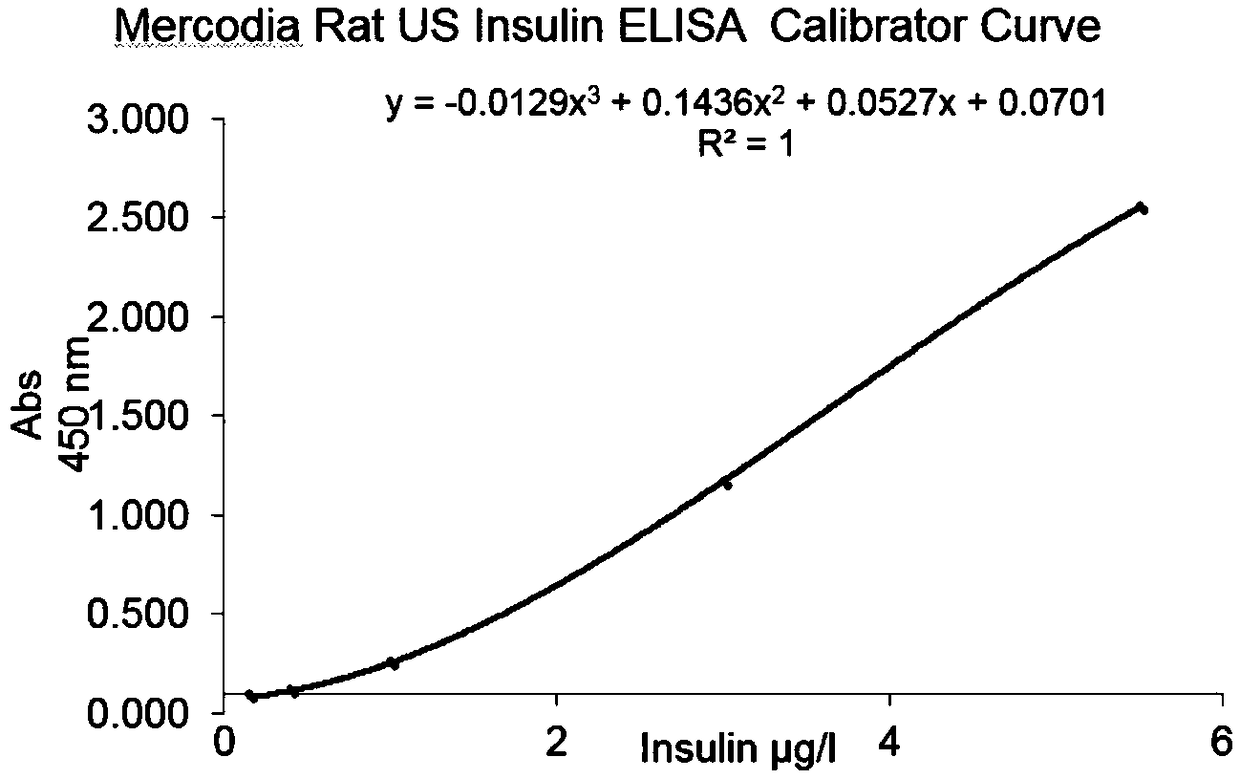
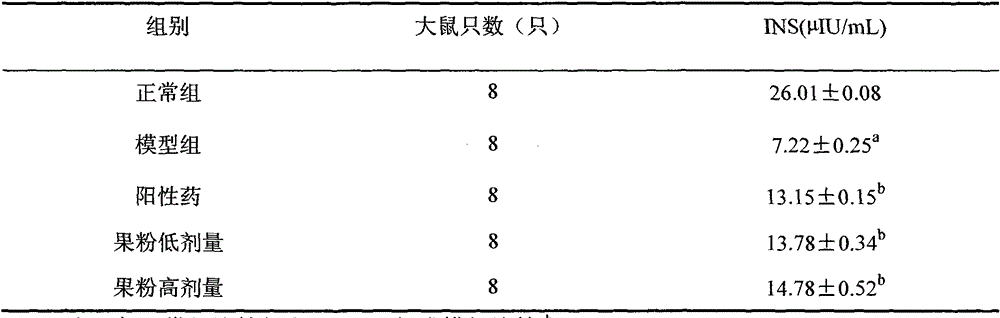
The invention discloses a glucose stimulated insulin secretion determination model. 24 hours are determined as culture and drug administration time of pancreatic beta cells; insulin concentration is determined by utilizing an ELISA method; an OD value is determined by virtue of a CCK8 method, thus quantity of the cells is indirectly reflected; HBSS solution is selected and used as later extracellular buffer solution; and an insulin secretion positive drug glibenclamide is selected for promoting insulin secretion of the pancreatic beta cells. The model built in the invention can reflect difference between different stimuli, namely high glucose and basic glucose, and can reflect insulin secretion stimulating effects of the positive drug glibenclamide under the high glucose and basic glucoseconditions.
Owner:QINGDAO UNIV
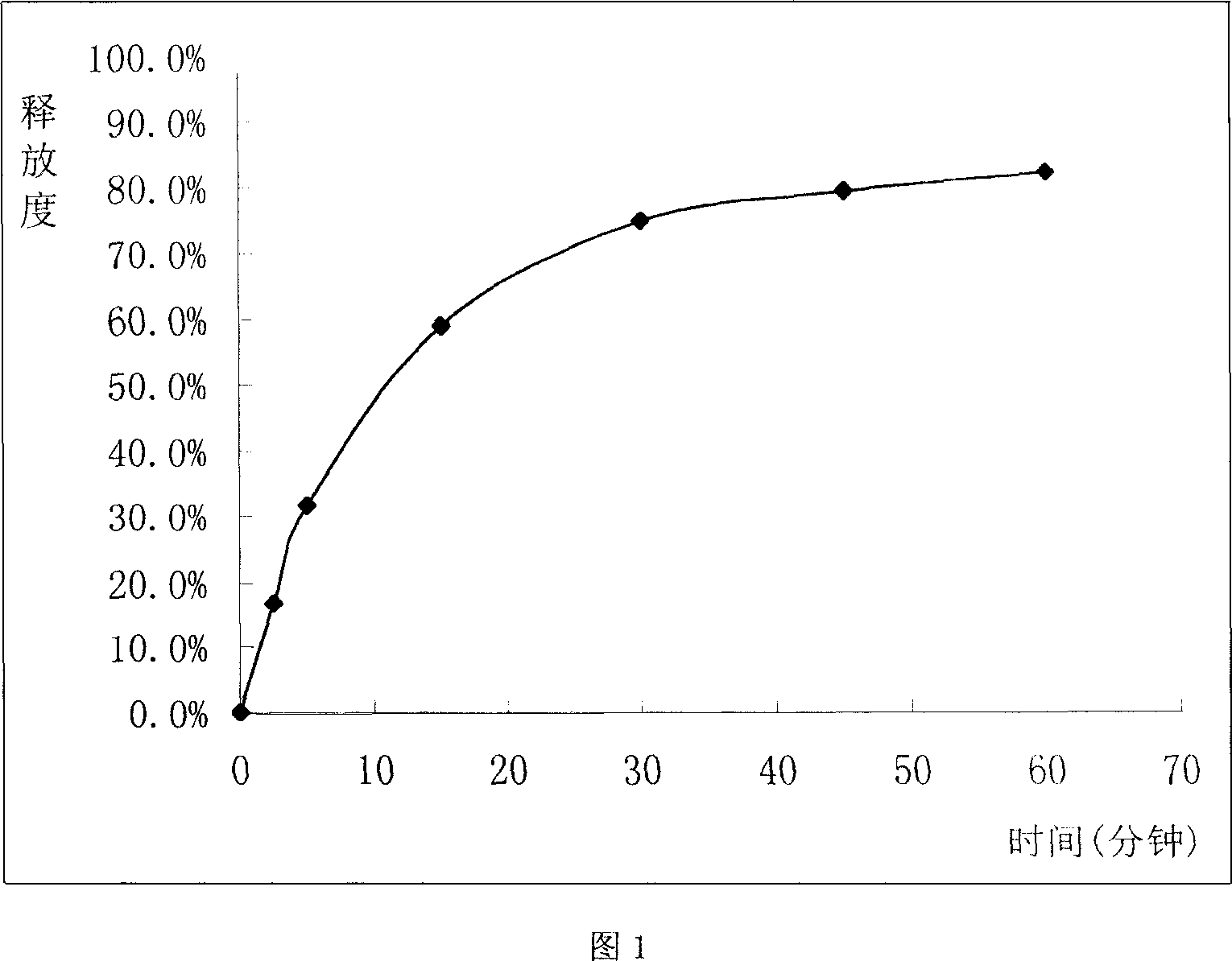
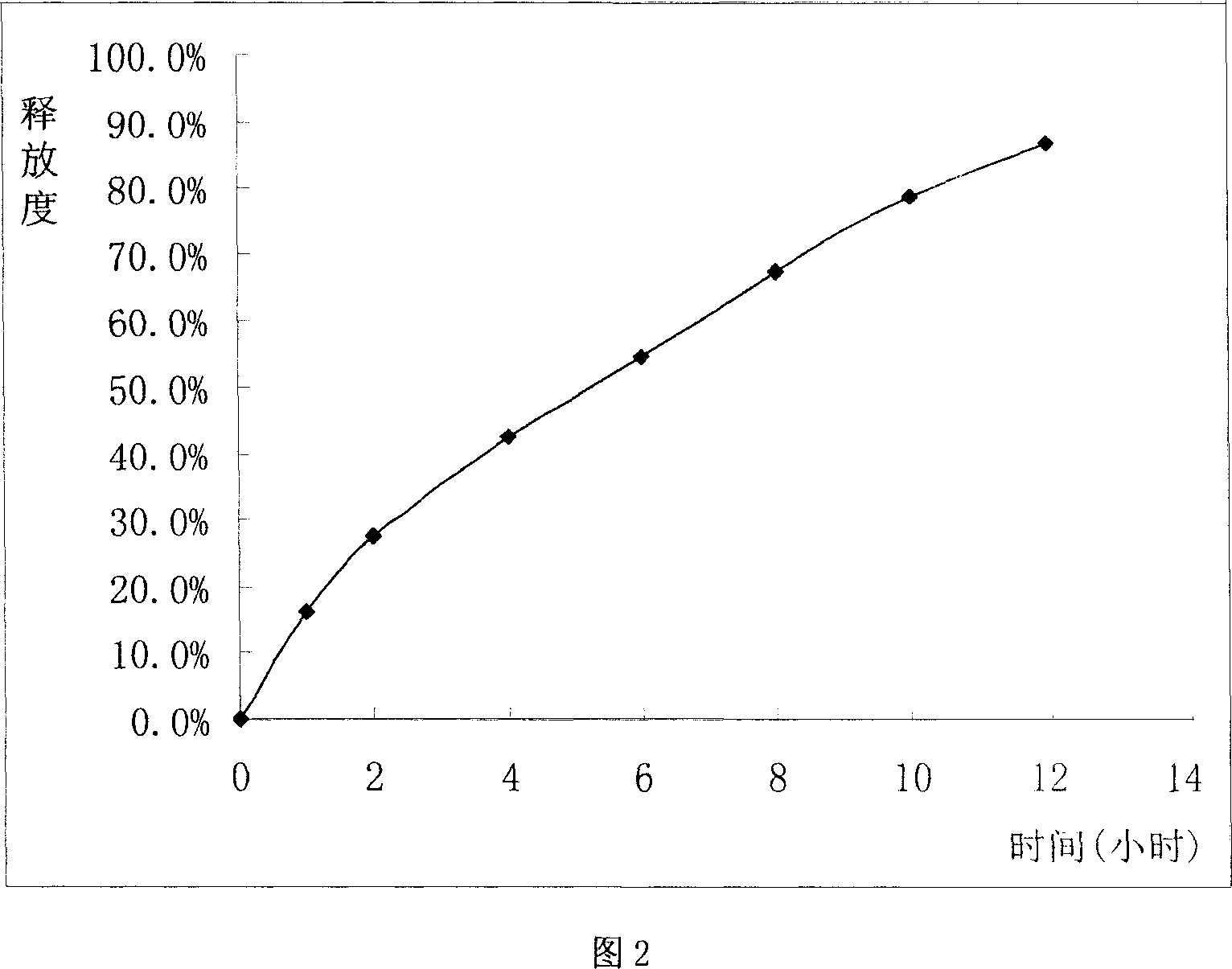
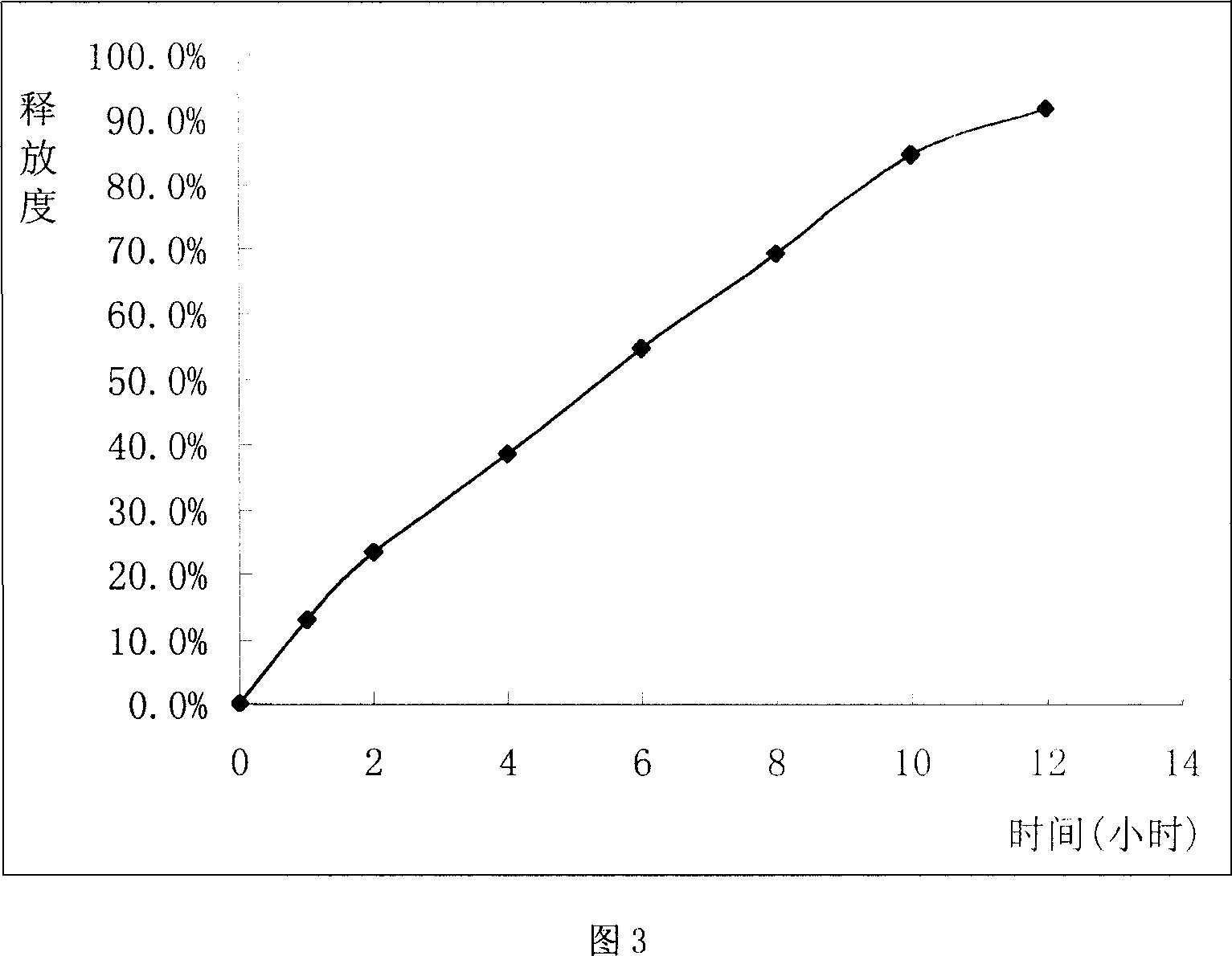
Sustained release tablet of glibenclamide and preparation process thereof
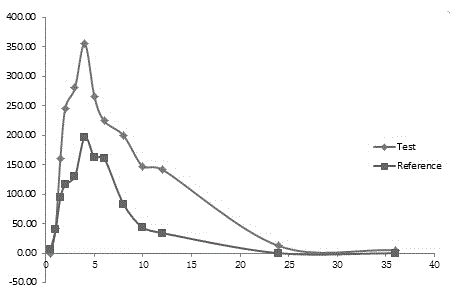
InactiveCN1965817AExtended time to peakElimination half-lifeMetabolism disorderPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention relates to a Glibenclamide slow release tablet preparation which comprises effective dose of main constituent of Glibenclamide and medicinal auxiliary materials, the constituents include (by weight portion) Glibenclamide 0.5-5.0 parts, slow release material 20.0-170.0 parts, filling agent 5-100 parts, lubricating agent 5.0-30.0 parts, and right amount of binding agent. The invention also discloses the process for preparing the tablet preparation.
Owner:SICHUAN UNIV
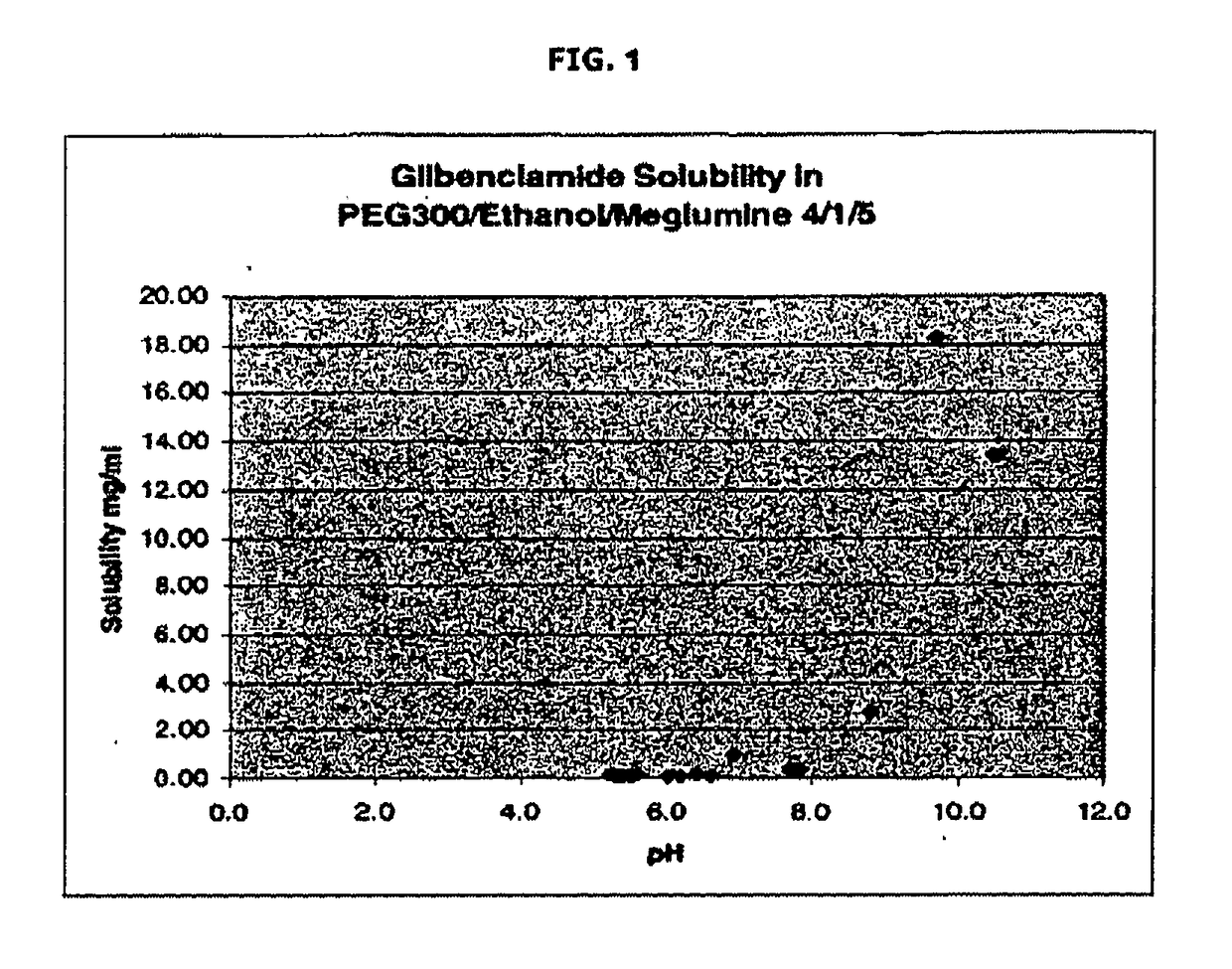
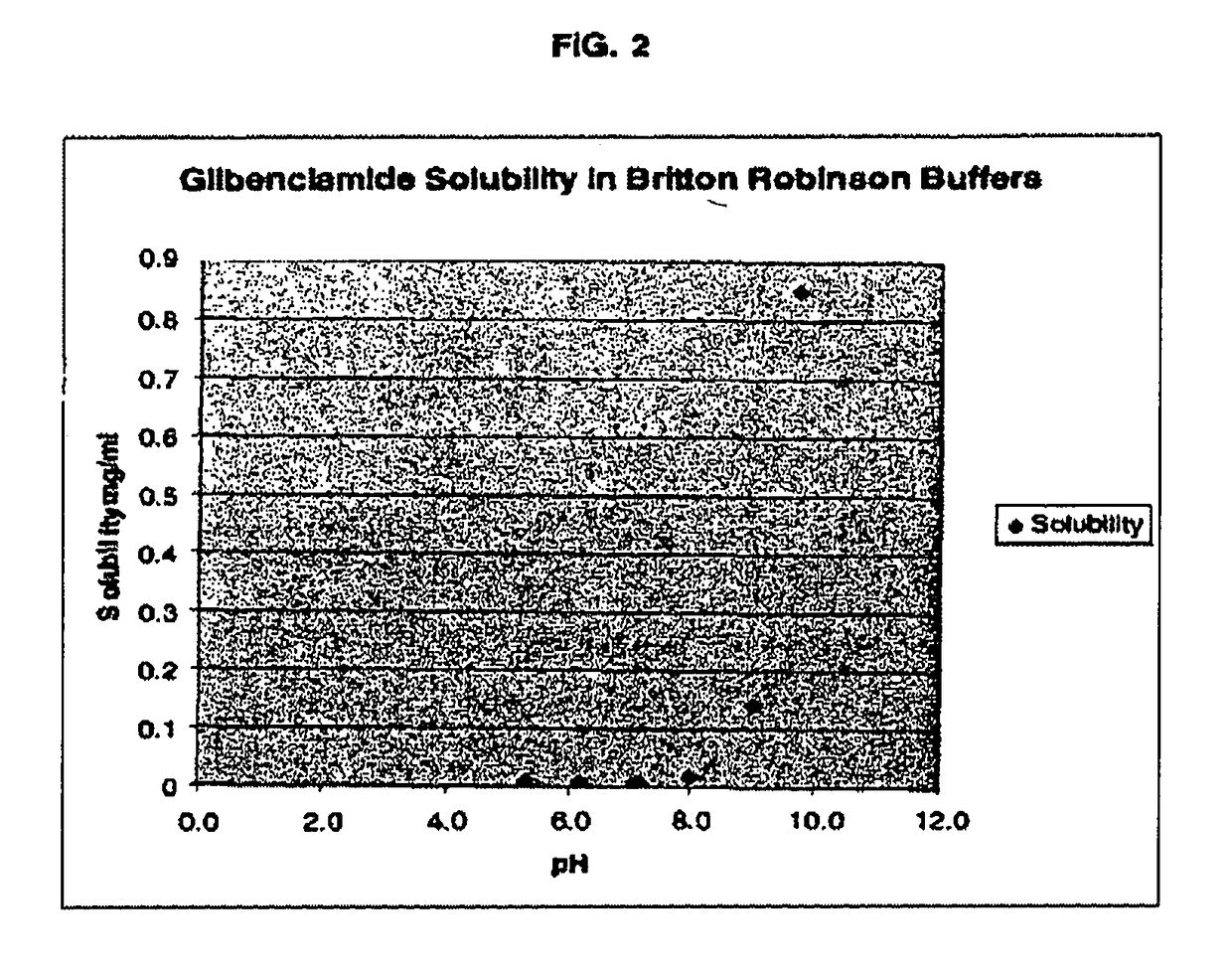
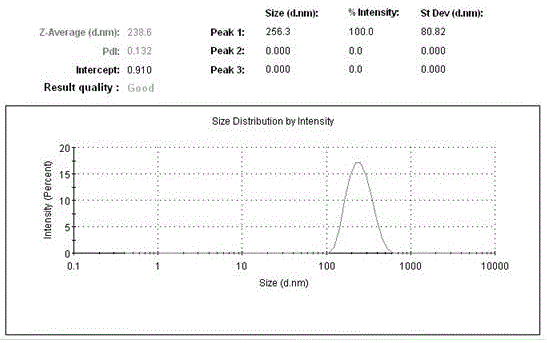
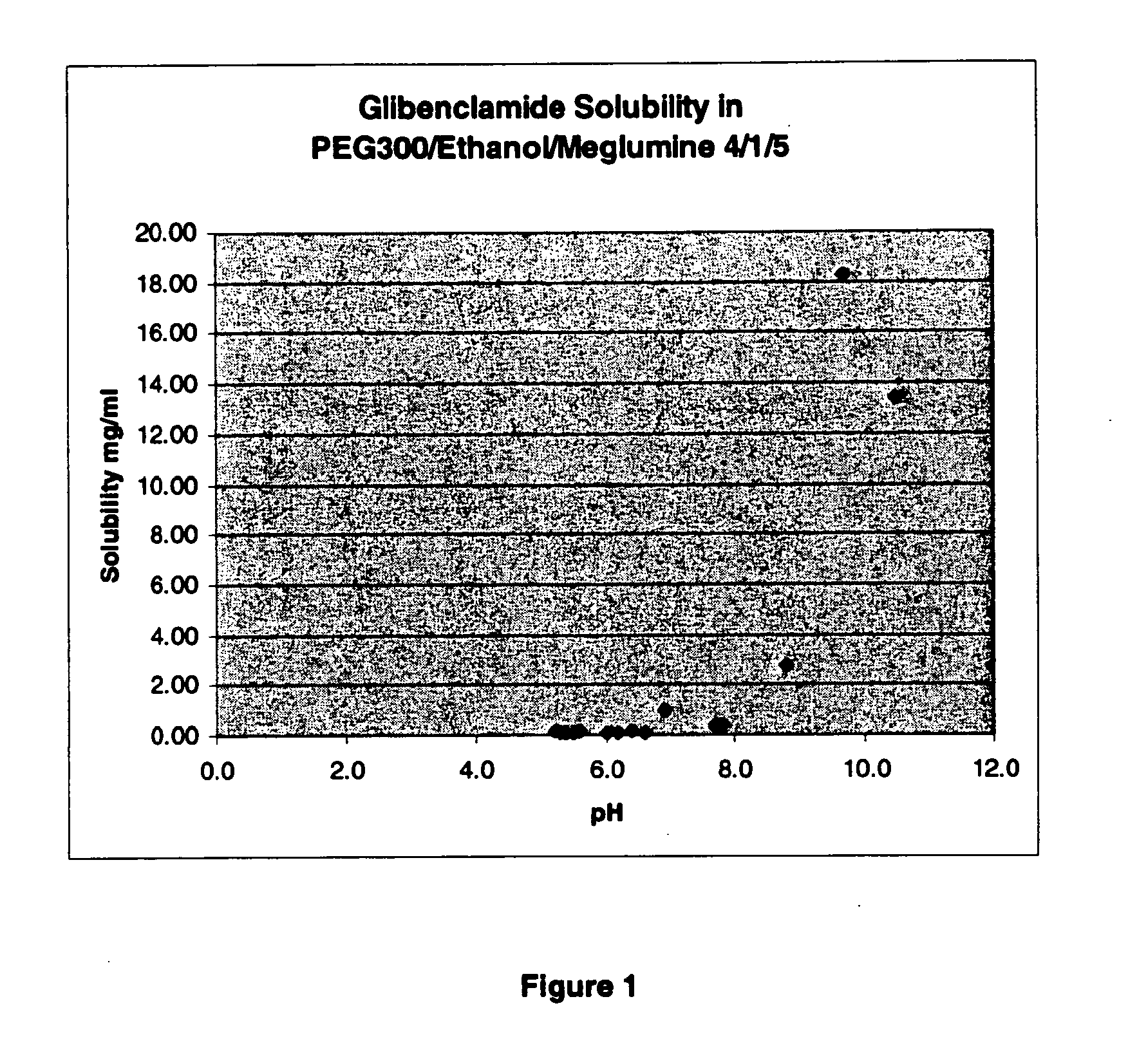
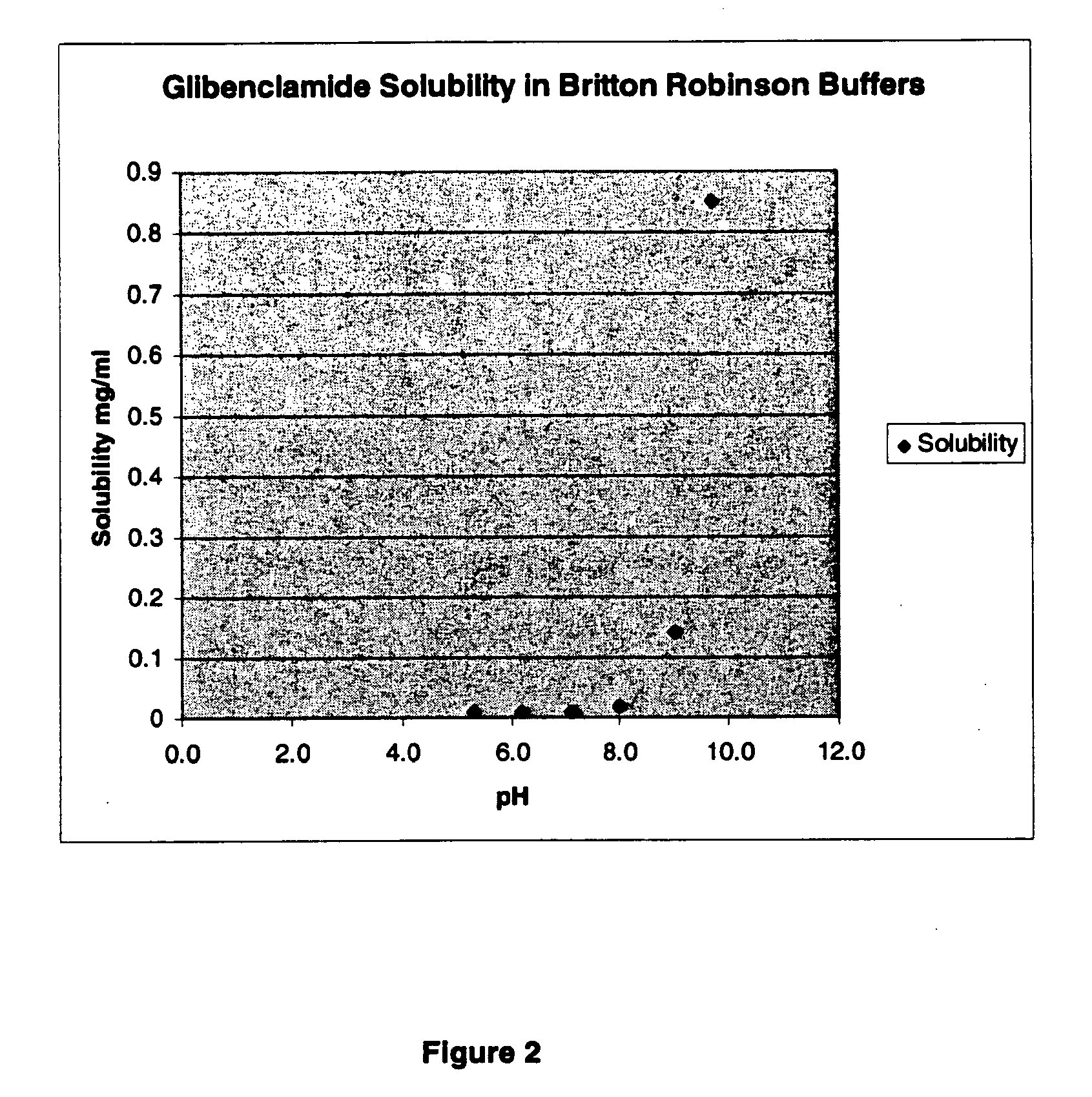
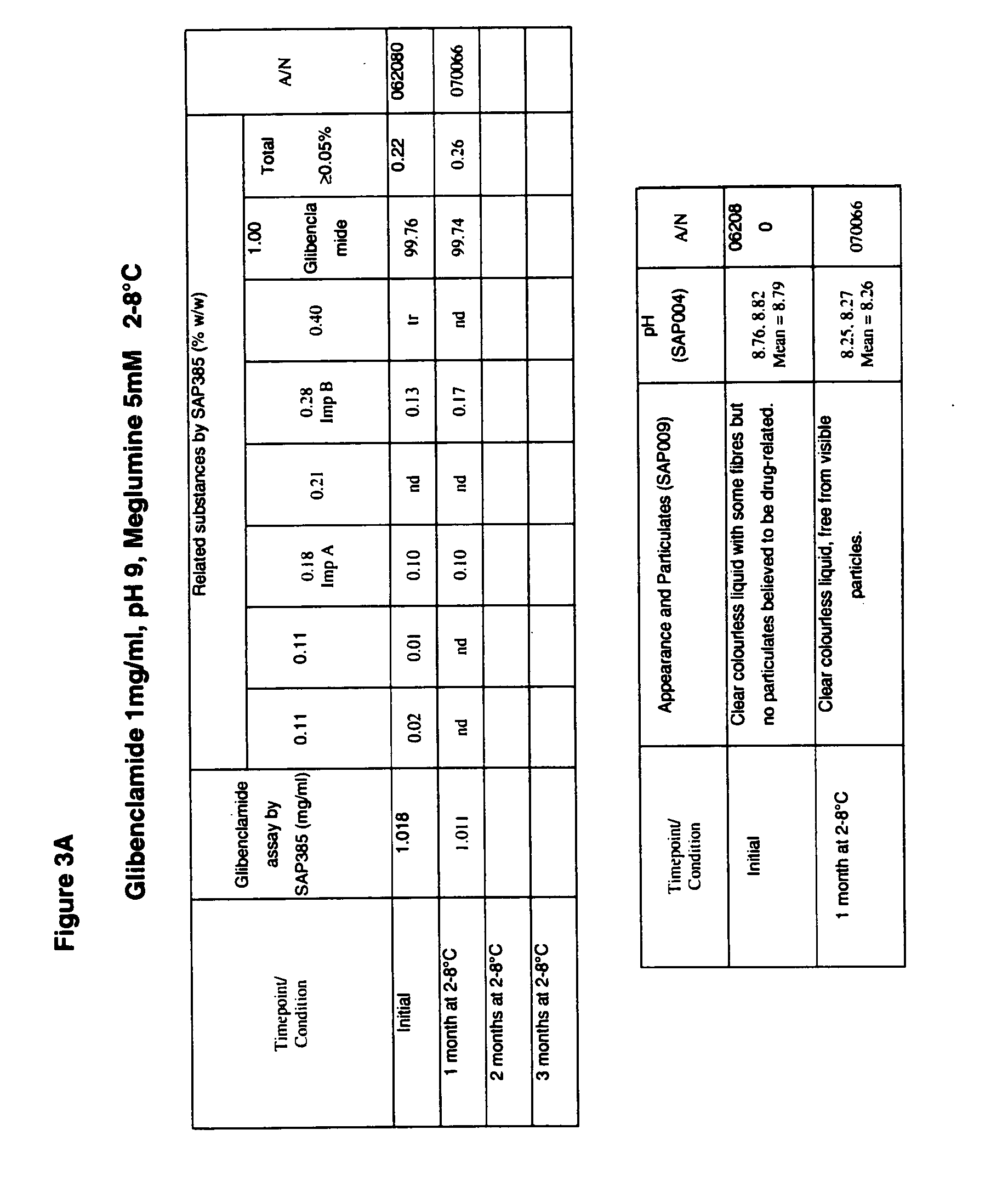
Liquid formulations of compounds active at sulfonylurea receptors
ActiveUS20170216321A1Rapid and readily controlled increase in circulating drug concentrationsQuick cureBiocideMetabolism disorderMeglitinideWater based
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intravenous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
Method for quantitatively detecting concentration of folic acid in rat plasma through HPLC-MS/MS (high-performance liquid chromatography-mass spectrometry/mass spectrometry)
ActiveCN106770819AMeet detectionHigh sensitivityComponent separationBlood plasmaHplc mass spectrometry
The invention discloses a method for quantitatively detecting concentration of folic acid in rat plasma through HPLC-MS / MS (high-performance liquid chromatography-mass spectrometry / mass spectrometry). Firstly, a rat plasma sample is treated through protein precipitation, and the content of the folic acid in rat plasma is detected with an HPLC-MS / MS method with glibenclamide as an interior standard. ESI positive ions and MRM (multiple reaction monitoring) are adopted in an MS acquisition method. With the adoption of the method, the linear relation of the folic acid at 10-1,000 ng / ml is good, and the recovery rate is in a range of 80.0%-95.4%; in-batch and batch-to-batch accuracy is 92.7%-111.4%, and the in-batch and batch-to-batch precision is 2.6%-8.3%. The method has good reproducibility, takes short analysis time, can meet the detection requirement of concentration of the folic acid in the rat plasma and can be used for pharmacokinetic study of folic acid tablets.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Glibenclamide nanocrystal preparation and preparation method thereof
InactiveCN105878194AImprove efficiencyHigh yieldPowder deliveryMetabolism disorderFreeze-dryingDissolution
The invention relates to a glibenclamide nanocrystal preparation and a preparation method and belongs to the technical field of pharmaceutical preparations. The glibenclamide nanocrystal preparation is prepared from, by weight, glibenclamide 35%-93%, a stabilizer 2%-7% and a freeze-drying protective agent 28-60%, wherein the ratio of the glibenclamide to the stabilizer is 30:1 to 15:1. The stabilizer is hydroxypropyl methyl cellulose, polyvinylpyrrolidone, poloxamer or a surface active agent. The glibenclamide nanocrystal preparation is controllable in particle size, good in stability and small in side and toxic effect and obviously improves the dissolution and bioavailability of the glibenclamide.
Owner:SHENYANG PHARMA UNIVERSITY
Targeting ncca-atp channel for organ protection following ischemic episode
ActiveUS20100143347A1Avoid depolarizationReduce and preventBiocidePeptide/protein ingredientsReperfusion injuryAngina
The present invention concerns protection of an organ or tissue following an ischemic episode In particular aspects, the invention concerns organ preservation for transplantation, angina pectoris, kidney reperfusion injury, and so forth In specific embodiments, the organ is subjected to an inhibitor of an NCCa-ATP channel that is regulated by SUR1 Exemplary inhibitors include sulfonylurea compounds, such as glibenclamide, for example
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Liquid formulations of compounds active at sulfonylurea receptors
InactiveUS20110034560A1Rapid and readily controlled increaseRapid adjustment and ready maintenance of circulating drug concentrationBiocideMetabolism disorderWater basedMeglitinide
The invention provides liquid formulations of compounds that act at sulfonylurea receptors that are suitable for intra-venous and intra-arterial infusion. Compounds active at a sulfonylurea receptor include glibenclamide, tolbutamide, repaglinide, nateglinide, meglitinide, midaglizole, LY397364, LY389382, glyclazide, and glimepiride. Liquid formulations may be concentrated solutions suitable for storage; may be diluted (e.g., dilution of 1:1 or 1:1.2) suitable for bolus injections, and may be further diluted (e.g., dilution of 1:10 or 1:20 or more) for intravenous and intra-arterial infusion over an extended period of time. For example, a liquid formulation may include at least about 0.05 mg / ml glibenclamide in a water-based solution including 40% polyethylene glycol 300, 10% Ethanol, 50% water, at about pH 9. The solution may include a buffer, and is suitable for storage in refrigerator or at room temperature. This solution may be diluted 1:1, or more (e.g., 1:20) without precipitation of the glibenclamide.
Owner:BIOGEN CHESAPEAKE LLC
Micronized glibenclamide and composition thereof
The invention relates to composition containing micronized glibenclamide. Particle sizes d0.5 of the micronized glibenclamide are in a range of 0.2 mu m-5 mu m, and the micronized glibenclamide is combined with a carrier to prepare a preparation with the proper dissolution rate. The invention further relates to a method for preparing the micronized glibenclamide solid preparation. By the aid of the method, the solubility of the glibenclamide is improved, the bioavailability is improved, and clinical effects of the pharmaceutical preparation are increased.
Owner:SHENYANG PHARMA UNIVERSITY +1
Metformin glibenclamide slow-releasing preparation
InactiveCN101057835AImprove securityImprove effectivenessMetabolism disorderInorganic non-active ingredientsBlood concentrationCurative effect
The invention discloses a slow release agent for dimethylbiguanide glibenclamide and the preparing method. The invention takes dimethylbiguanide glibenclamide as raw material, adds main slow-release material according to a certain proportion, produces solid dispersion agent with raw material, the medicine is release slowly according to requirement to keep effective blood concentration, and acts for a long time. The product is characterized by reduced medicine taking time, increased adjustment, reduced blood concentration peak-to-valley phenomenon, improved medicine effect and safety and etc.
Owner:刘凤鸣
Blood glucose reducing effervescent tablet and application thereof
ActiveCN104922178AMetabolism disorderPharmaceutical delivery mechanismSodium bicarbonateFood products
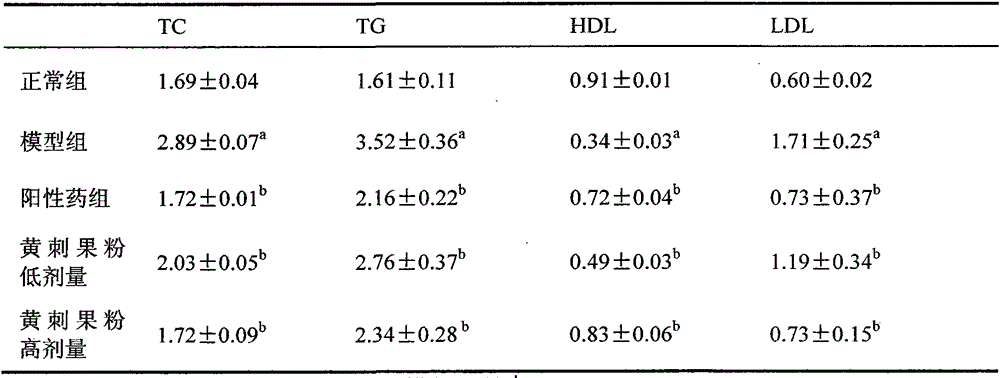
The invention provides a blood glucose reducing effervescent tablet. The blood glucose reducing effervescent tablet is prepared from, by weight, the original auxiliary materials of five to 85 parts of yellow bur powder, two to four parts of citric acid, three to five parts of fumaric acid, 12-15 parts of fructo-oligose, 15-35 parts of sorbitol and two to ten parts of sodium bicarbonate. The invention further provides a preparation method and application of the blood glucose reducing effervescent tablet. Researches find that the yellow bur powder has the good blood glucose reducing function, the amount of insulin can be improved, the blood glucose reducing function of the effervescent tablet is equal to that of diabetes first chemical medicine glibenclamide, and the difference is that the yellow bur powder cannot cause liver injuries, safety is good, the application range is wider, and the blood glucose reducing effervescent tablet is more suitable for being adopted as food or health care products or medicine for treating and preventing diabetes.
Owner:DELINGHA LINSHENG BIOTECH DEV +1
Medicine composition for treating diabetes
InactiveCN1857368AGuaranteed the effect of blood sugar controlVulnerability to change usageOrganic active ingredientsMetabolism disorderPuerarinCurative effect
The present invention belongs to the field of medicine technology, and especially a kind of diabetes treating medicine composition and its preparation process and use. Specifically, the medicine composition is prepared with glibenclamide, puerarin, trichosanthes root and taurine. The medicine composition has high curative effect on diabetes and can lower the incidence rate of diabetes complication, including diabetic nephropathy, and its taurine can reduce the angle of repose of the medicine composition powder to smaller than 30 deg and improve the flowability of medicine composition powder.
Owner:JILIN SIHUAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com