Sustained release tablet of glibenclamide and preparation process thereof
A technology of glyburide and sustained-release tablets, applied in the field of medicine, can solve the problems of short half-life, difficult to maintain hypoglycemic effect, hypoglycemia, etc., and achieve the effects of reducing side effects, prolonging elimination half-life, and reducing the number of times of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
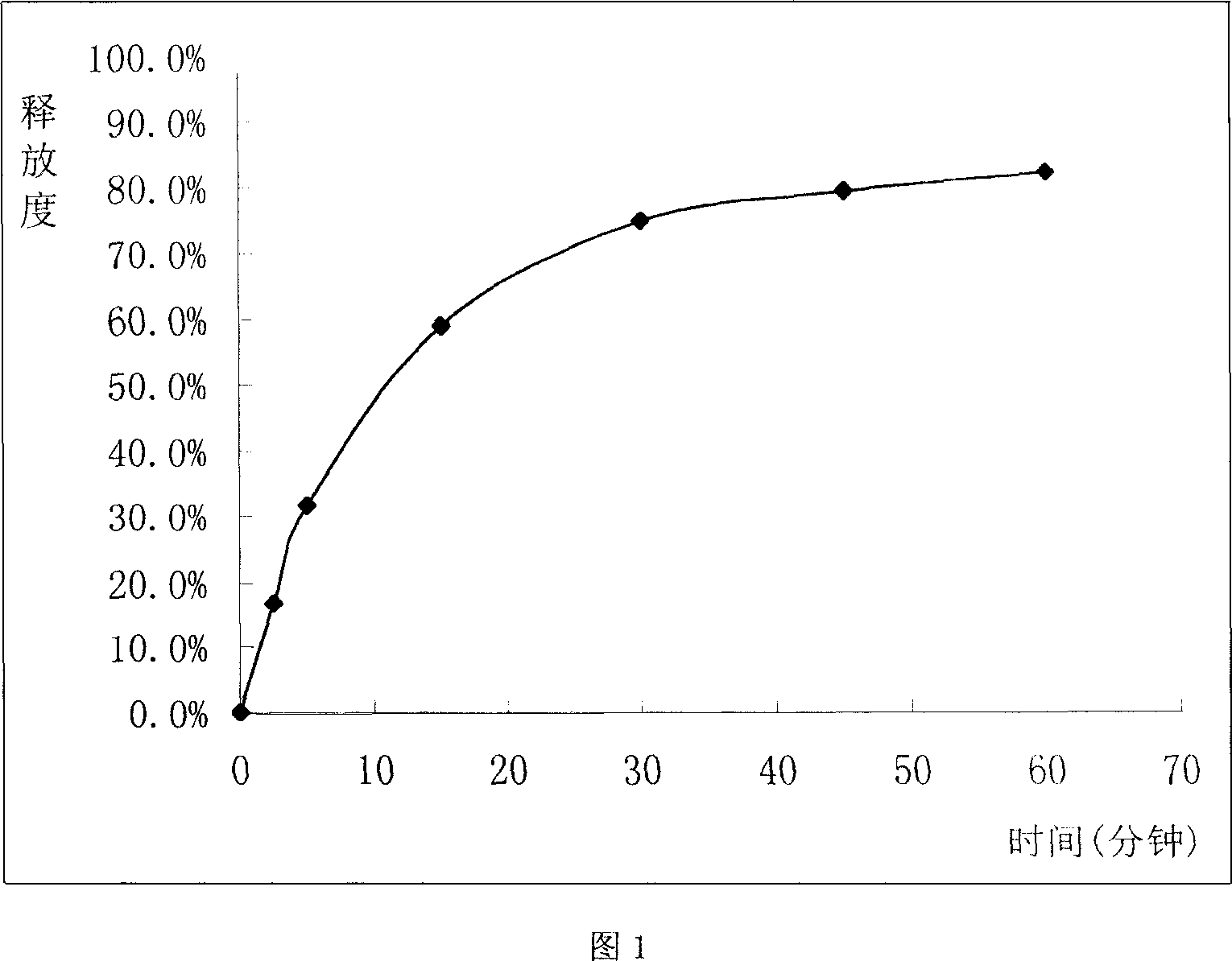
[0043] This example is a non-constant-rate-release glibenclamide ordinary sustained-release tablet, wherein each component and its weight ratio are as follows:
[0044] 1 part of glibenclamide, 125 parts of hydroxypropylmethylcellulose (K4M), 25 parts of ethyl cellulose, 40 parts of microcrystalline cellulose, 9 parts of talcum powder, appropriate amount of PVP (made into 10% ethanol solution for viscose) mixture).
[0045] The preparation method of this embodiment is as follows:
[0046] Weigh the glibenclamide, hydroxypropylmethylcellulose, ethylcellulose and microcrystalline cellulose in the stated amount, mix them evenly, and pass through an 80-120 mesh sieve after being evenly pulverized; add an appropriate amount of PVP10% ethanol solution, and use Wet granulation, use 18-mesh sieve to granulate, dry at 40-60°C for 1-3 hours, take out; add the stated amount of talcum powder, mix well, test the content of the main ingredient in the mixed powder, and then press it with a ...
Embodiment 2
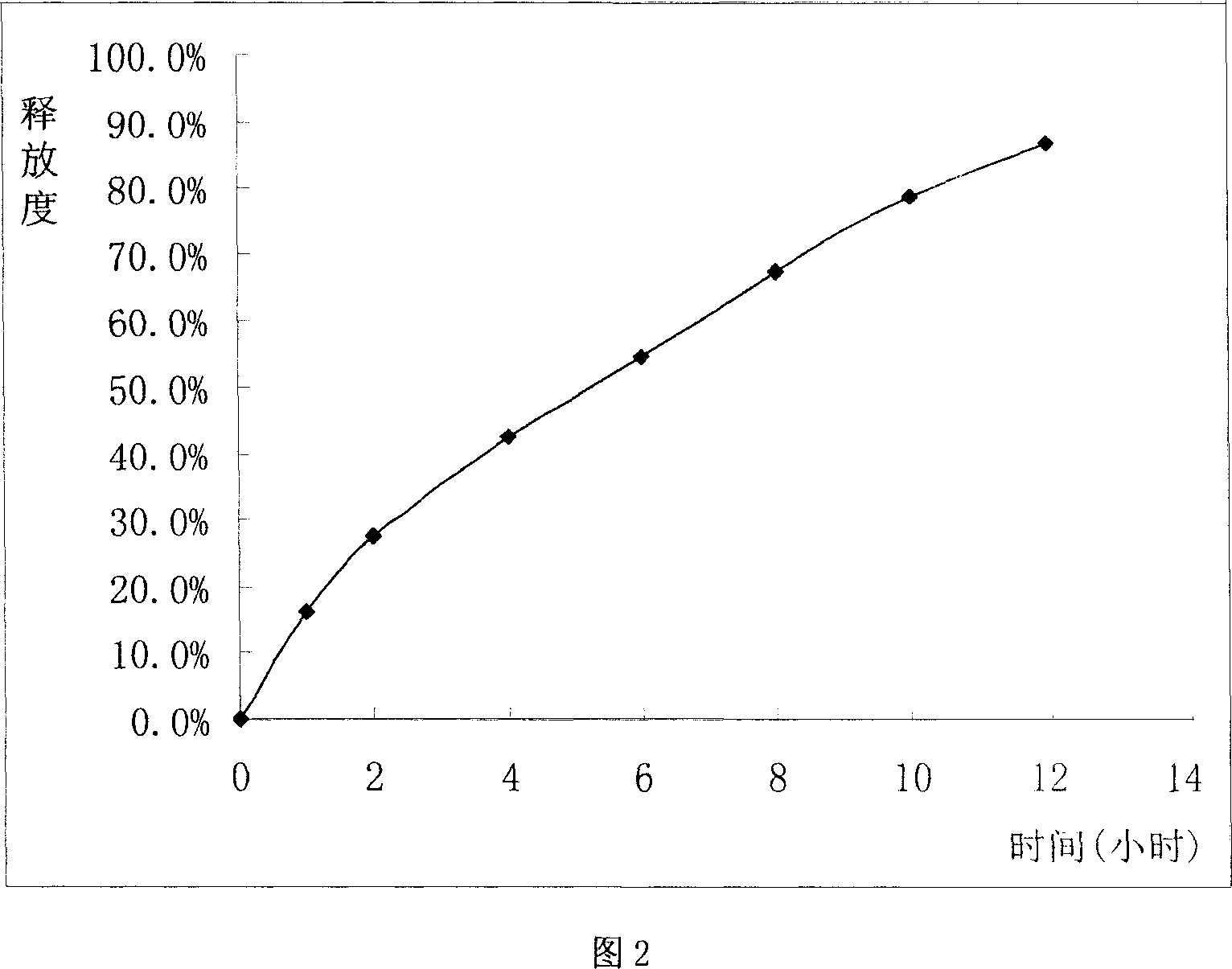
[0048] This example is a non-constant-rate-release glibenclamide ordinary sustained-release tablet, wherein each component and its weight ratio are as follows:
[0049] 2.5 parts of glibenclamide, 110 parts of hydroxypropylmethylcellulose (K4M), 35 parts of methylcellulose, 35 parts of microcrystalline cellulose, 5 parts of talcum powder, appropriate amount of hydroxypropylmethylcellulose (made into 5% aqueous solution as a binder).
[0050] The preparation method of this embodiment is as follows:
[0051] Weigh the glibenclamide, hypromellose (K4M), methylcellulose and microcrystalline cellulose in the stated amount, mix them evenly, and pass through a 80-120 mesh sieve after being evenly crushed; add an appropriate amount of hypromellose Base cellulose 5% aqueous solution to make wet granules, granulate with 18 mesh sieve, dry at 40-60°C for 2-4 hours and take out; add the stated amount of talcum powder, mix well, and test the content of the main ingredient in the mixed pow...
Embodiment 3
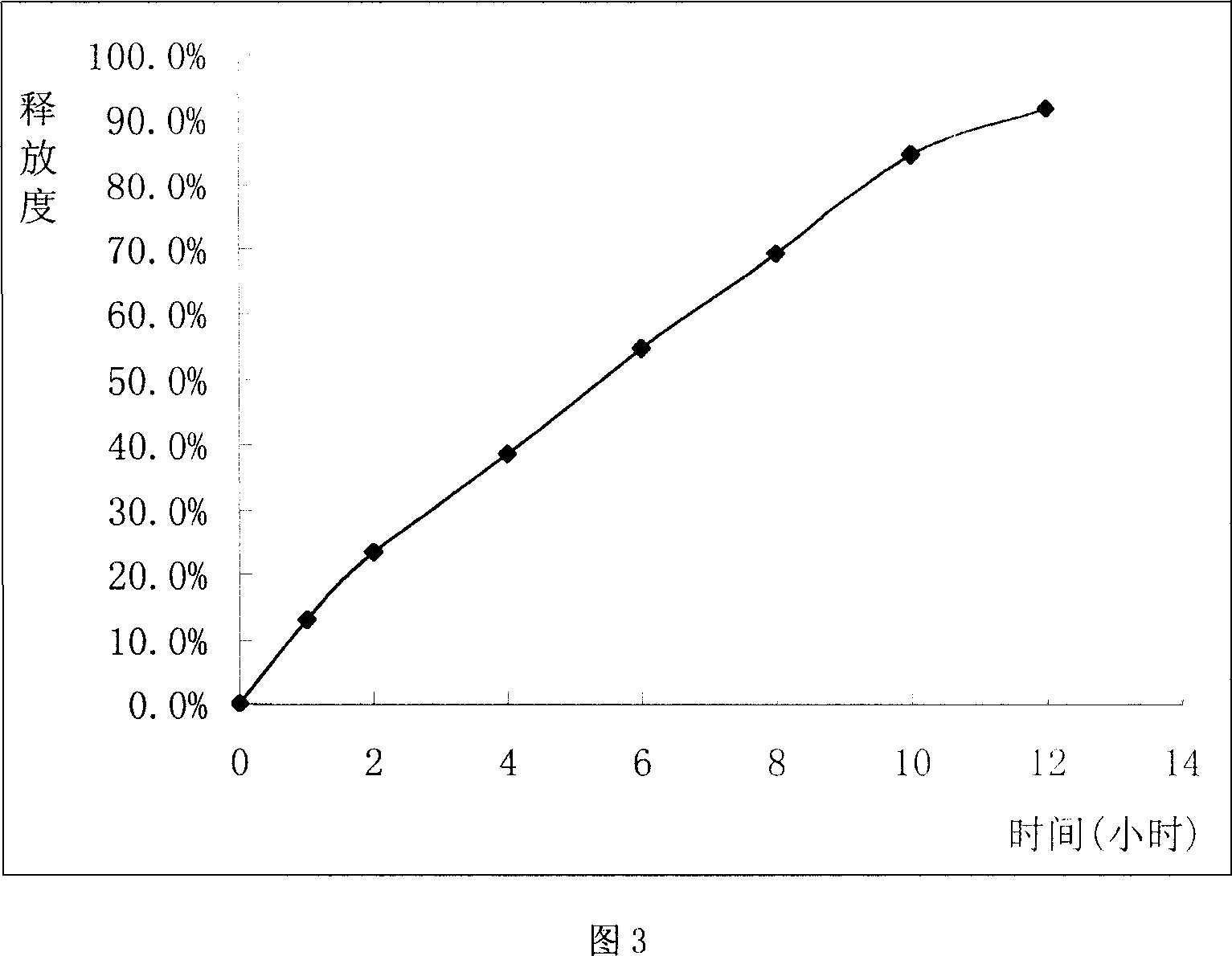
[0053] This example is a non-constant-rate-release glibenclamide ordinary sustained-release tablet, wherein each component and its weight ratio are as follows:
[0054] Glibenclamide 5 parts, carnauba wax 75 parts, hydrogenated vegetable oil 10 parts, calcium sulfate 20 parts, mannitol 12 parts, ethyl cellulose 50 parts, magnesium lauryl sulfate 12 parts, magnesium stearate 15 parts , an appropriate amount of sodium alginate (dubbed a 5% aqueous solution as a binder).
[0055] The preparation method of this embodiment is as follows:
[0056] Glibenclamide, carnauba wax, hydrogenated vegetable oil, calcium sulfate, mannitol, sodium alginate, ethyl cellulose, magnesium lauryl sulfate and magnesium stearate were weighed, and the glibenclamide Add it to the mixture of melted carnauba wax and hydrogenated castor oil, let it cool after stirring, grind it finely, and pass through an 80 mesh sieve; add calcium sulfate, mannitol, magnesium stearate, ethyl cellulose, mix well, and use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com