Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Enalapril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enalapril is used to treat high blood pressure.
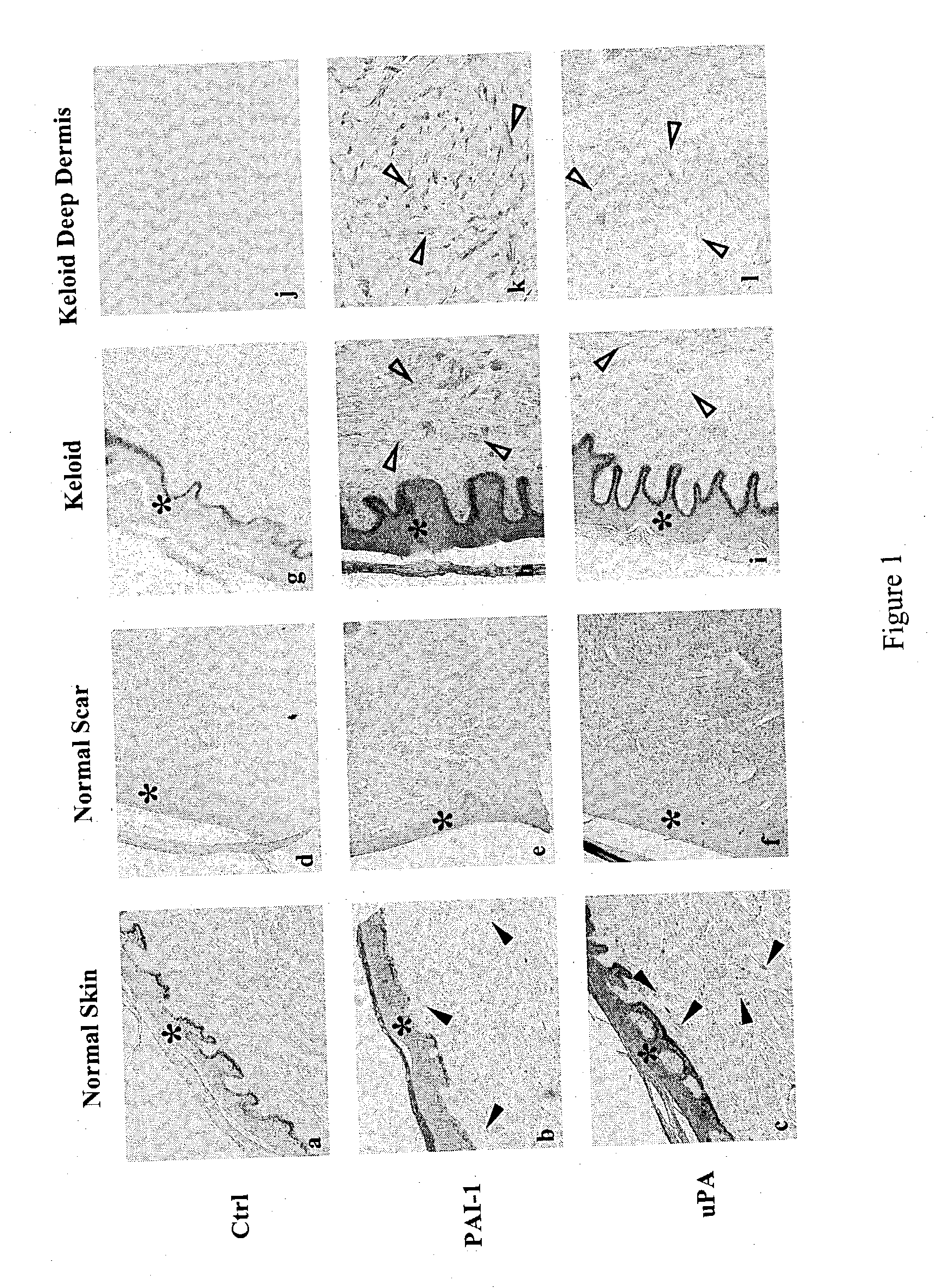
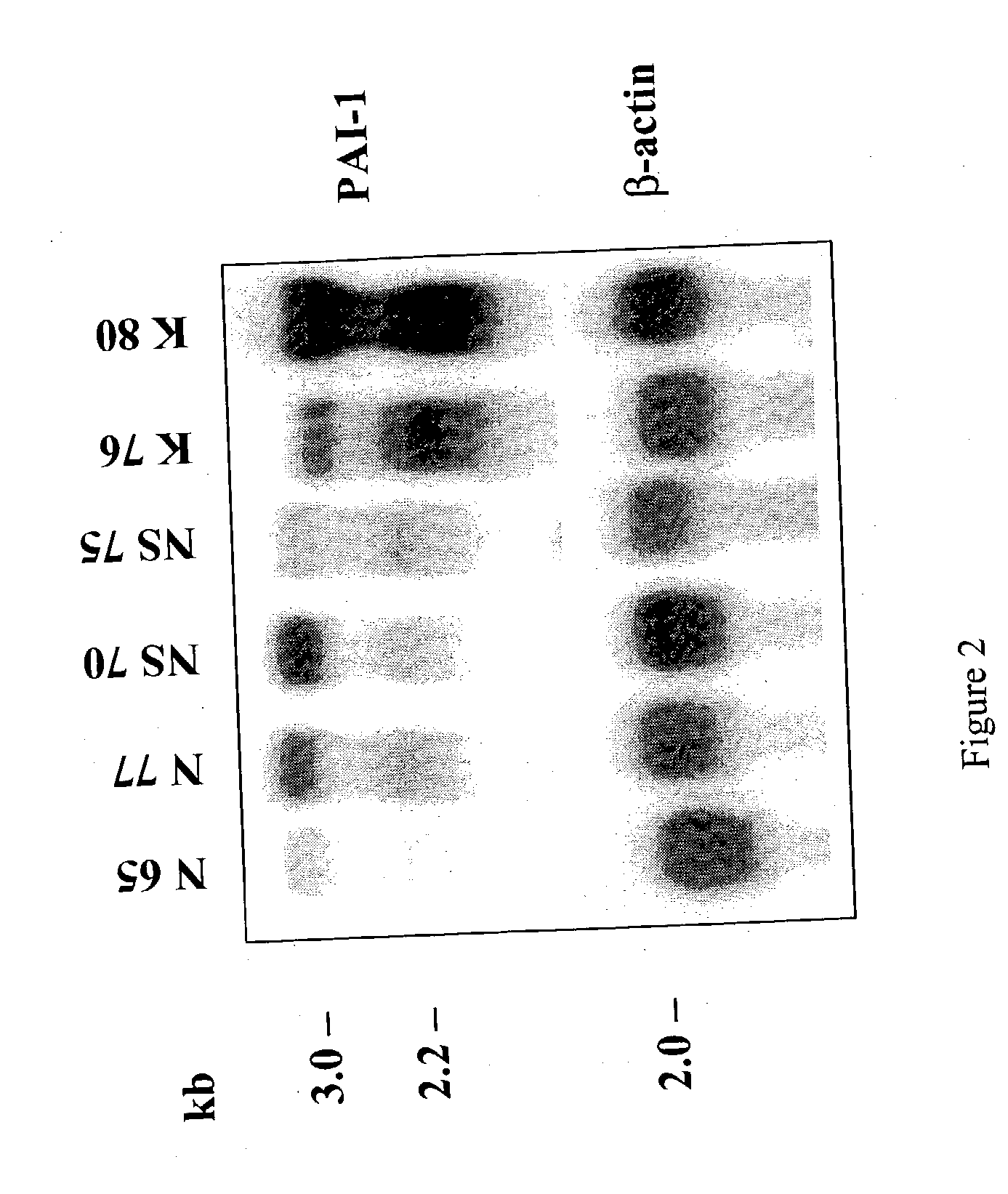
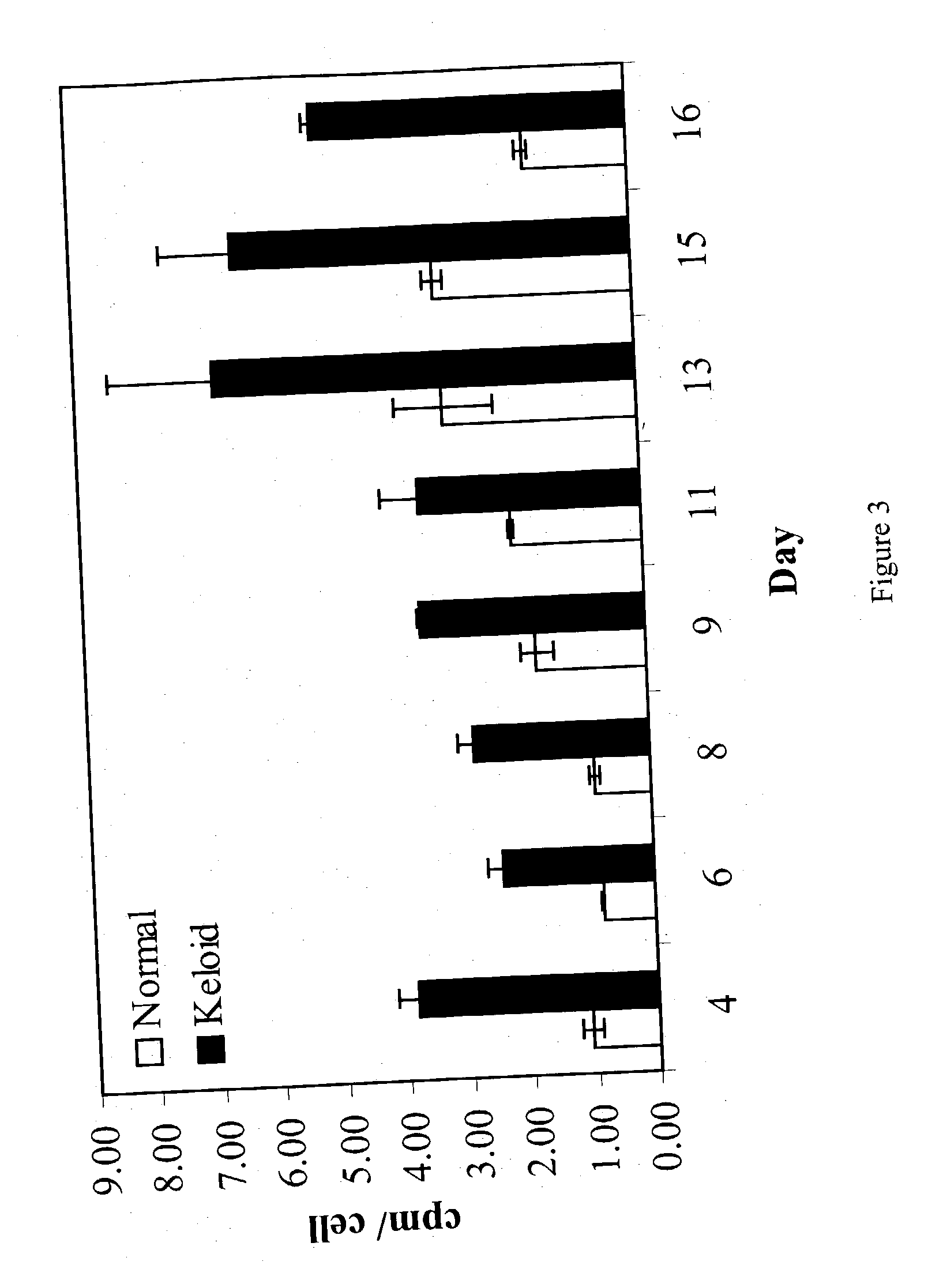
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
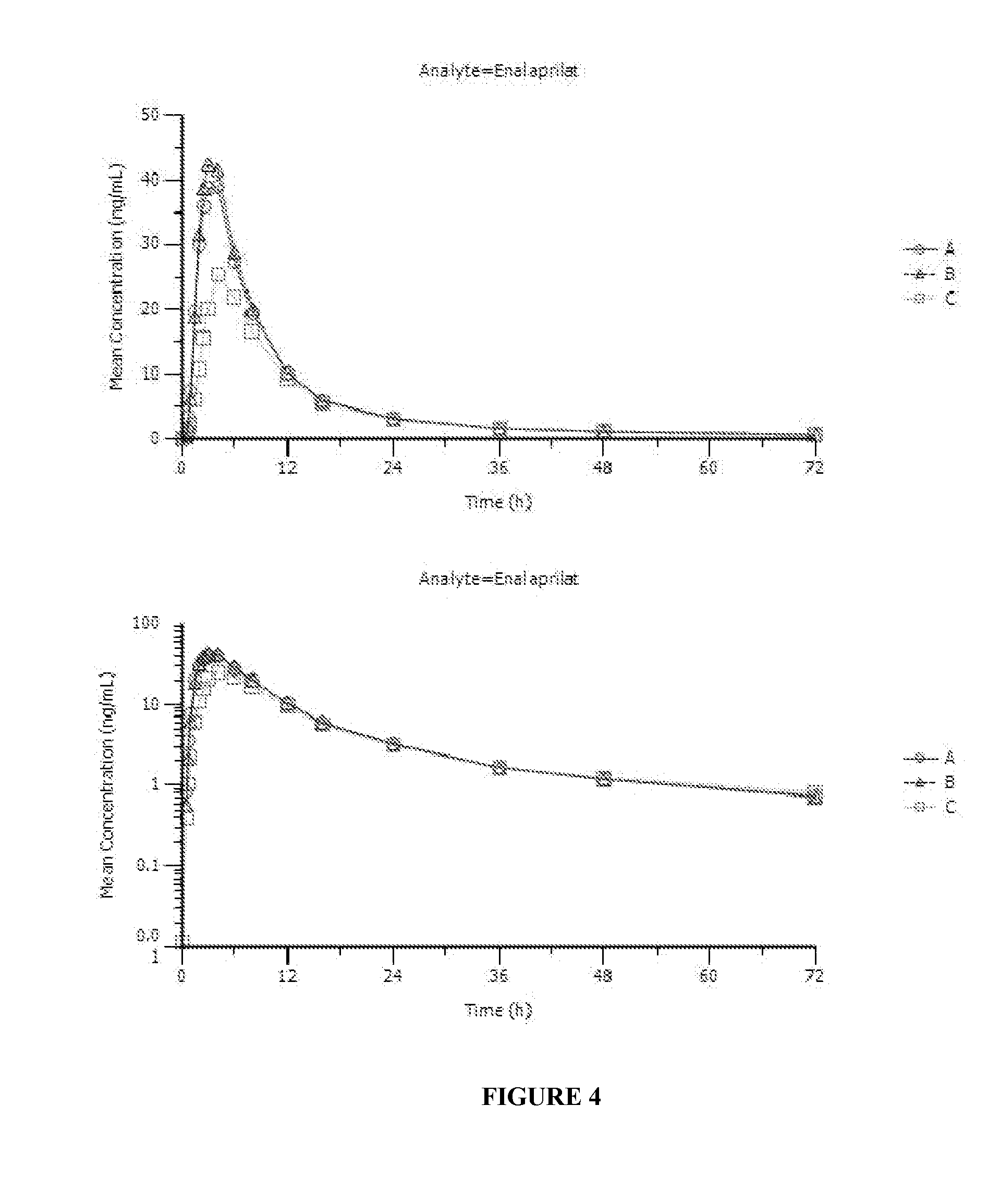
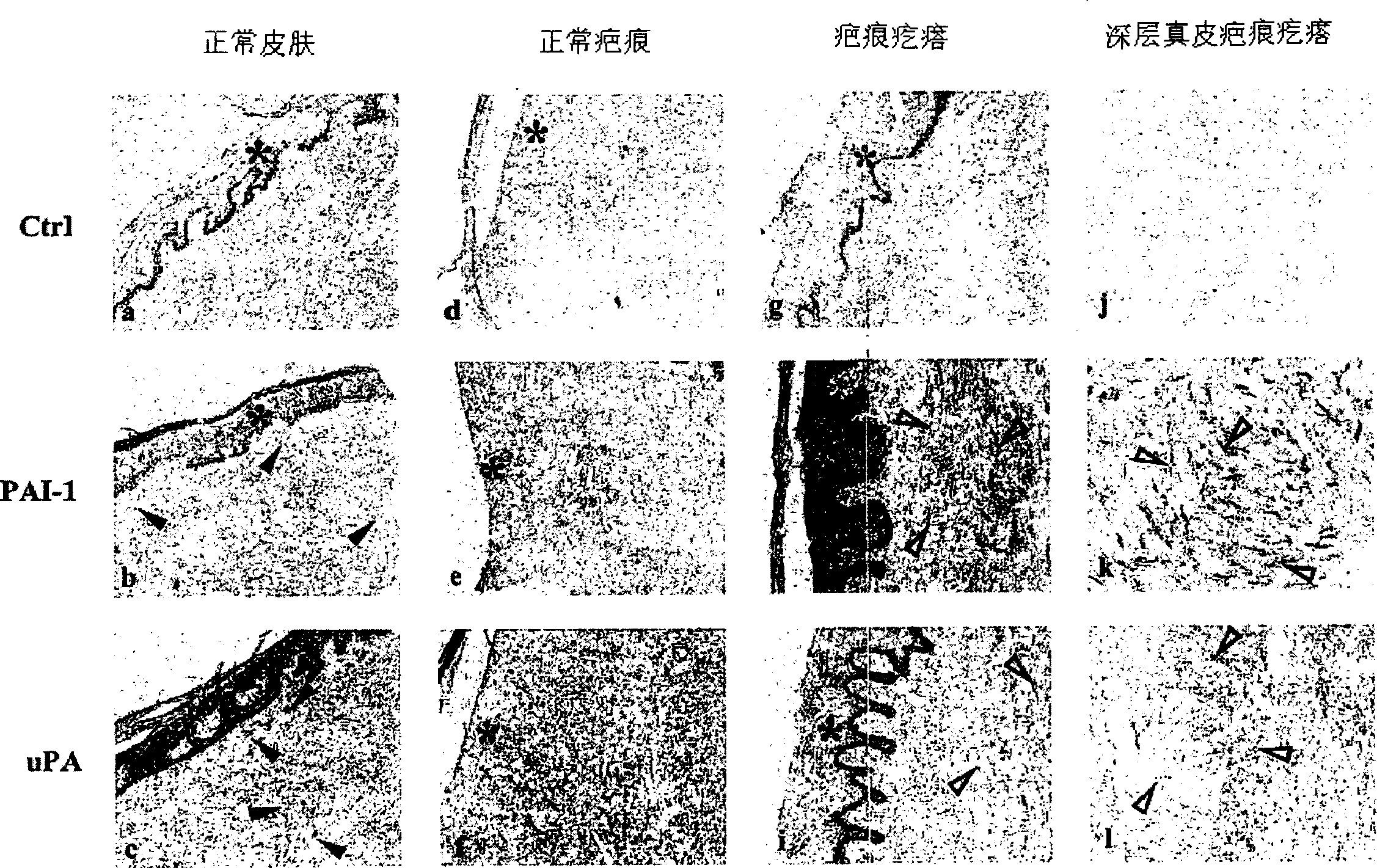
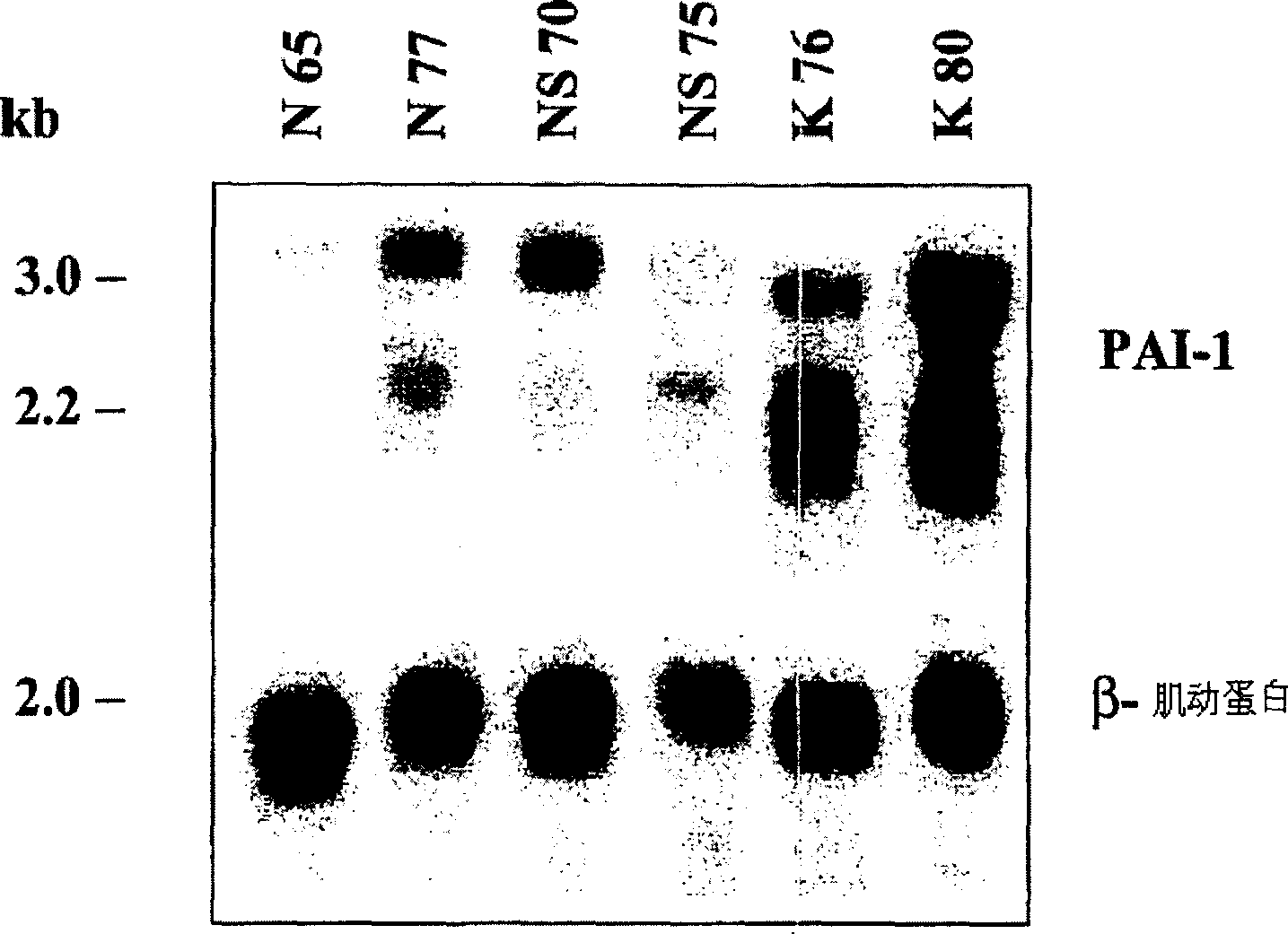
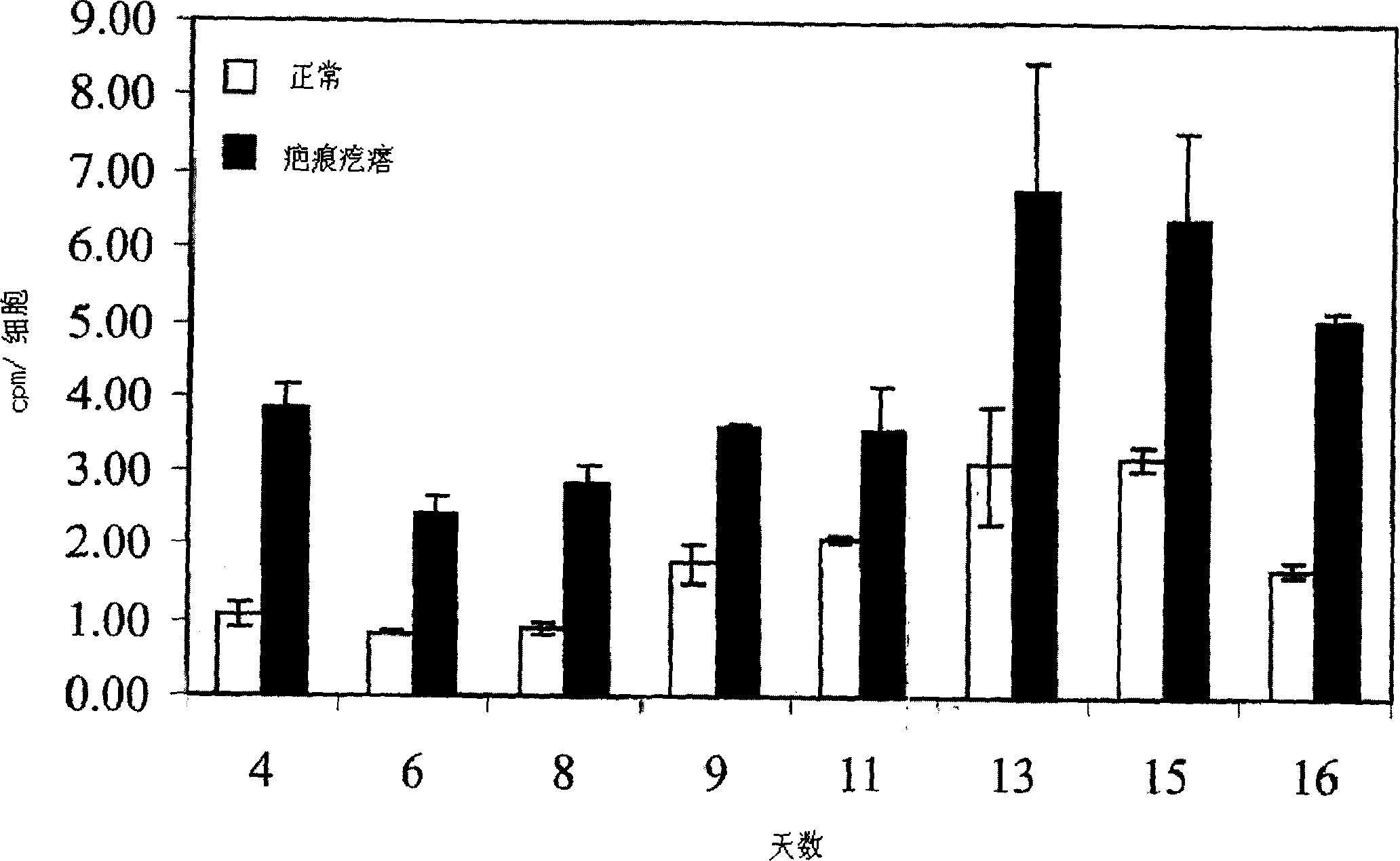
The present invention relates to findings that reducing the activity of Plasminogen Activator Inhibitor-1 (PAI-1) suppresses an excessive deposition of collagen which is known as a cause for the formation of abnormal scars. These abnormal scars include but are not limited to keloids, adhesions, hypertrophic scars, skin disfiguring conditions, fibrosis, fibrocystic conditions, contractures, and scleroderma, all of which are associated with or caused by an excessive deposit of collagen in a wound healing process. Accordingly, aspects of the present invention are directed to the reduction of PAI-1 activity to decrease an excessive accumulation of collagen, prevent the formation of an abnormal scar, and / or treat abnormal scars that result from an excessive accumulation of collagen. The PAI-1 activity can be reduced by PAI-1 inhibitors which include but are not limited to PAI-1 neutralizing antibodies, diketopiperazine based compounds, tetramic acid based compounds, hydroxyquinolinone based compounds, Enalapril, Eprosartan, Troglitazone, Vitamin C, Vitamin E, Mifepristone (RU486), and Spironolactone to name a few. Another aspect of the present invention is directed to methods of measuring PAI-1 activity in a wound healing process and determining the propensity of the formation of an abnormal scar.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Enalapril compositions
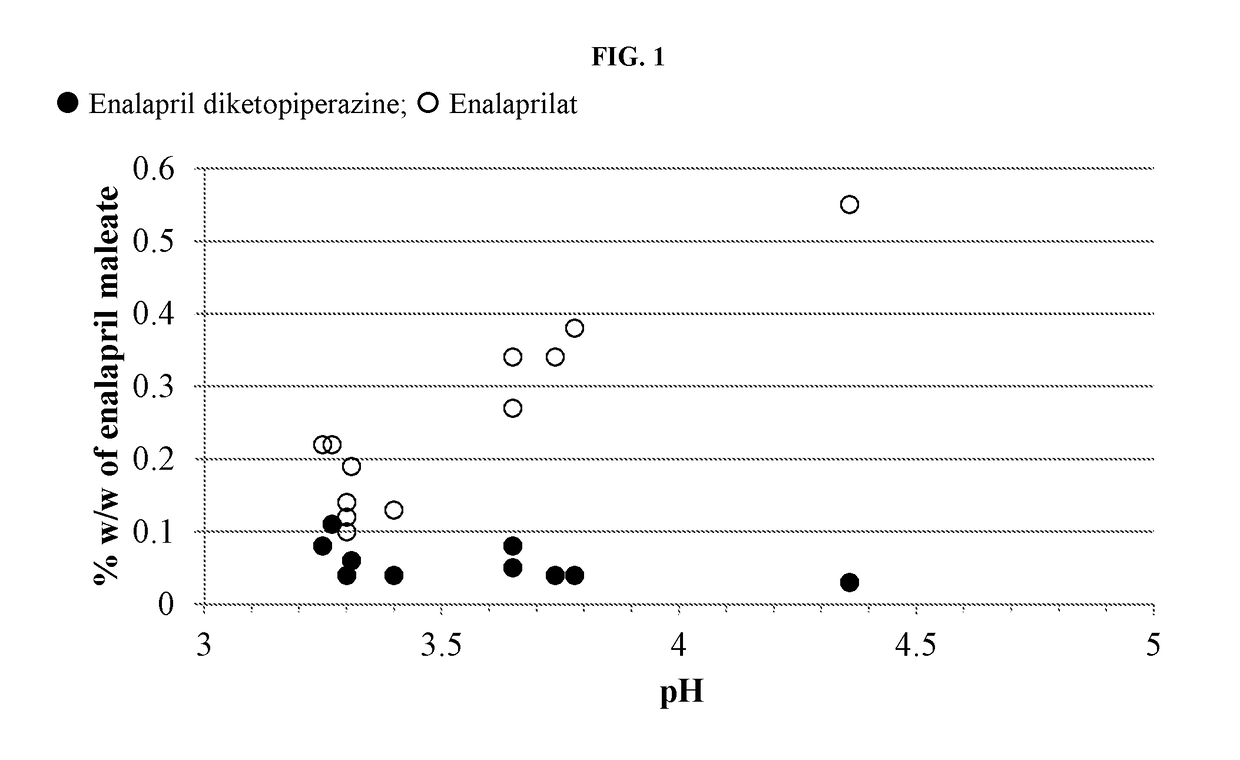
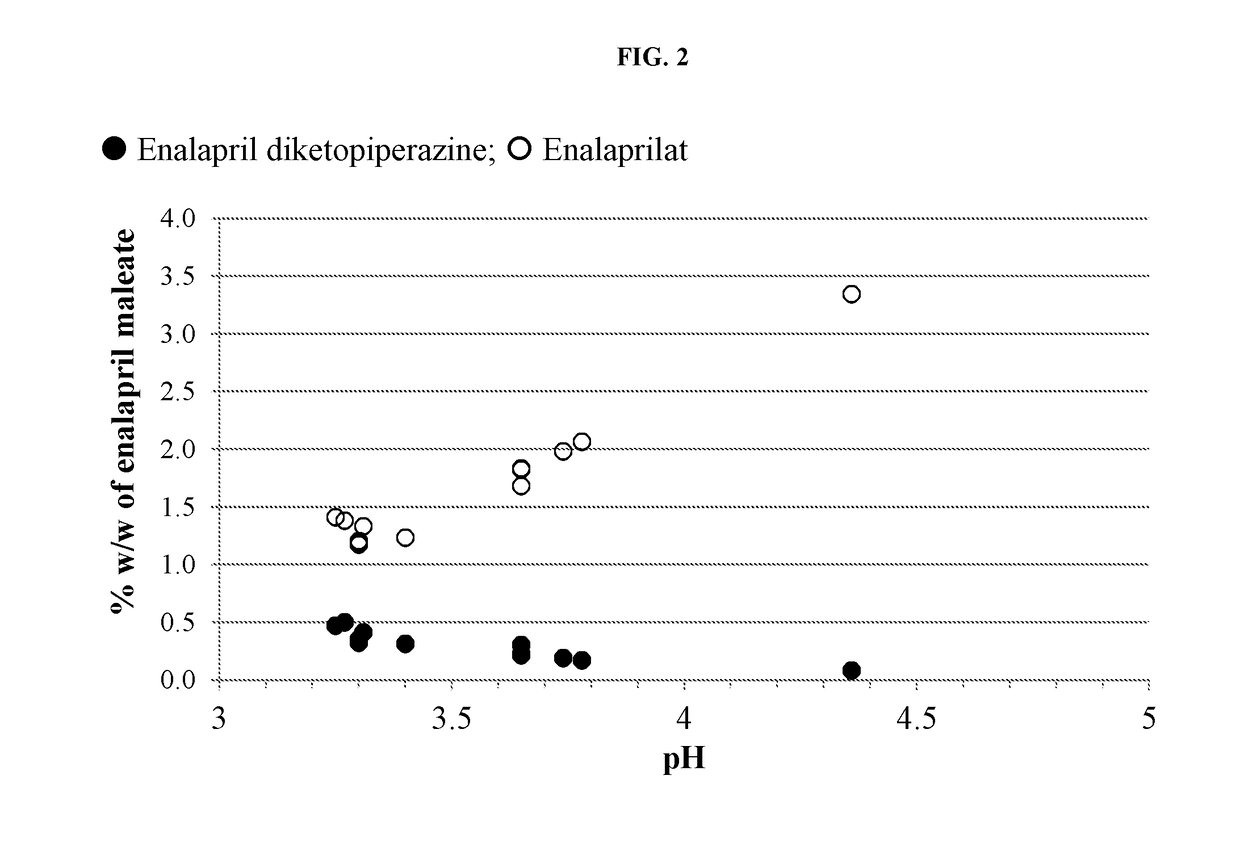
Provided herein are stable enalapril powder compositions for oral liquid formulation. Also provided herein are methods of using enalapril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and asymptomatic left ventricular dysfunction.
Owner:UNIVERSITY OF KANSAS +1
Enalapril Compositions
Owner:UNIVERSITY OF KANSAS +1
Treatment and prevention of abnormal scar formation in keloids and other cutaneous or internal wounds or lesions
InactiveCN1668312AReduced uPA activityImmunoglobulins against animals/humansMuscular disorderVitamin CFibrosis
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Enalapril formulations
Provided herein are stable enalapril oral liquid formulations. Also provided herein are methods of using enalapril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and asymptomatic left ventricular dysfunction.
Owner:AZURITY PHARMA INC
Enalapril Formulations
Provided herein are stable enalapril oral liquid formulations. Also provided herein are methods of using enalapril oral liquid formulations for the treatment of certain diseases including hypertension, heart failure and asymptomatic left ventricular dysfunction.
Owner:AZURITY PHARMA INC
Composition containing dihydropyridine calcium channel blocker and preparation method thereof
InactiveCN101548973APlay a sustained release roleLower blood pressure blood pressurePill deliveryHeterocyclic compound active ingredientsAngiotensin-converting enzymeAdditive ingredient
The invention discloses a composition containing a dihydropyridine calcium channel blocker and a preparation method thereof and relates to a sustained-release preparation of felodipine, which comprises felodipine which is dihydropyridine calcium channel blocker and function as an active ingredient, enalapril which is angiotensin converting enzyme inhibitor, as well as a slow release material, a filling agent and a lubricating agent which function as auxiliary materials; wherein the slow release material contains hydroxypropyl methylcellulose. As the enalapril which is angiotensin converting enzyme inhibitor is added in coating solution, the curative effect of the sustained-release preparation is improved.
Owner:YANGZHOU PHARM CO LTD +1
Compositions and Methods for Treatment and Prevention of Cardiovascular Disease
The present invention is in the fields of medicine, pharmaceuticals, neutraceuticals and cardiology. In one aspect, the invention provides compositions comprising enalapril and simvastatin for use in methods for the treatment and / or prevention of cardiovascular disease, and to the use of such compositions in the manufacture of products for such treatment and / or prevention. In another aspect, the invention provides methods for the treatment and / or prevention of cardiovascular disease using compositions comprising enalapril, simvastatin and acetylsalicylic acid. The compositions and methods of the invention are useful in the treatment and prevention of cardiovascular disease in a variety of animals, particularly humans.
Owner:NUCITEC DE C V
Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride
ActiveCN1611494AReduce dosageReduce manufacturing costOrganic chemistryChemical synthesisOrganic solvent
The invention relates to a kind of chemosynthesis method of N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine-N-hydroxy acid anhydride, which is the medicine midbody for synthesizing Pulitzer series medicine such as Enalapril, Ramipril, Lisinopril and so on. The invention uses couple (trichloromethyl) ester carbonate and N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine as row material, and get the product by reacting with catalyst action in organic solvent. This chemosynthesis method is a manufacturing method of N-[1(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine-N-hydroxy acid anhydride, which has acquirable raw material, low production cost, and no three wastes normally.
Owner:ZHEJIANG UNIV OF TECH
Carboxylesterase-1 Polymorphisms and Methods of Use Therefor
InactiveUS20110020801A1Improve throughputEfficient CatalysisMicrobiological testing/measurementBiological material analysisCaucasian populationNucleotide
Methods and kits are provided for detecting polymorphisms in carboxylesterase-1 (CES1). Several single nucleotide polymorphisms (SNPs) in CES1 in humans, and methods for detecting the same, are provided (e.g., Gly143Glu, 12754T>del). Results indicate that the Gly143Glu (9486G>A) polymorphism has an allelic frequency of 1.5% in the Caucasian population. Polymorphisms of the present invention may alter the function of the carboxylesterase-1 enzyme (hCES1). Thus, the methods and kits of the present invention may be used to personalize a therapy and / or avoid adverse consequences of altered metabolism of a therapeutic or compound (e.g., enalapril, methylphenidate, etc.) which may result due to a CES1 polymorphism. In addition, recombinant cells lines overexpressing wild-type CES1 or expressing CES1 mutants are provided. Such cell lines may be used to assess the effects of candidate compounds on CES1, and the action of CES1 on these candidate compounds.
Owner:MARKOWITZ JOHN S +1
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant
According to the conception of a novel drug design, the present invention provides improved decoration based on the existing ACEI and relates to a design comprising the following two types of conception: first, the toxic side-effect points of the existing ACEI are avoided to the greatest extent; secondly, the effects of the ACEI are improved to the greatest extent. Thus the present invention provides a novel ACEI compound. Based on the basic structure of enalapril, the present invention provides a synthetic route for improving decoration. The structure of propanamine-sulfonyl is changed into cattle sulphonyl; the praline is changed into timonacic acid and derivatives thereof, so as to prepare a series of compounds, namely, N-[2-(1-ethoxycarbonyl-3-phenylalanine)-cattle-sulfonyl]-timonacic acid and derivatives thereof. The drugs are screened to prepare novel high-efficiency and low-toxicity ACEI.
Owner:代斌
Orodispersible film composition comprising enalapril for the treatment of hypertension in a pediatric population
InactiveUS20170165315A1Reduce the amount requiredThe process is stable and efficientOrganic active ingredientsDipeptide ingredientsPharmacyPediatric population
The present invention relates to an oral applicable therapeutic dosage form, in particular an orodispersible film comprising Enalapril or pharmaceutically acceptable salts thereof for use in the treatment of hypertension in a pediatric population. The pediatric population is defined from 1 to 18 years of age. The present invention also provides a method of manufacturing of such a dosage form.
Owner:PHARMATHEN
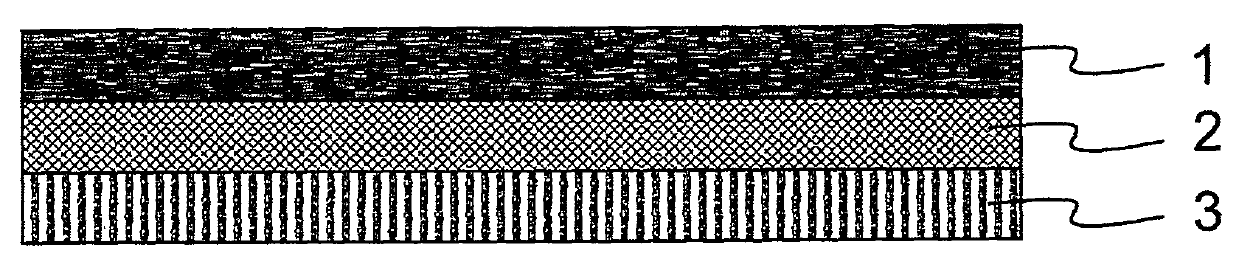
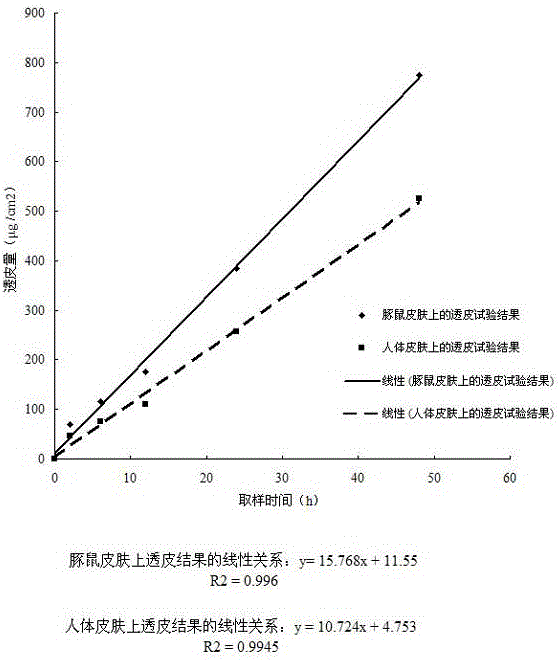
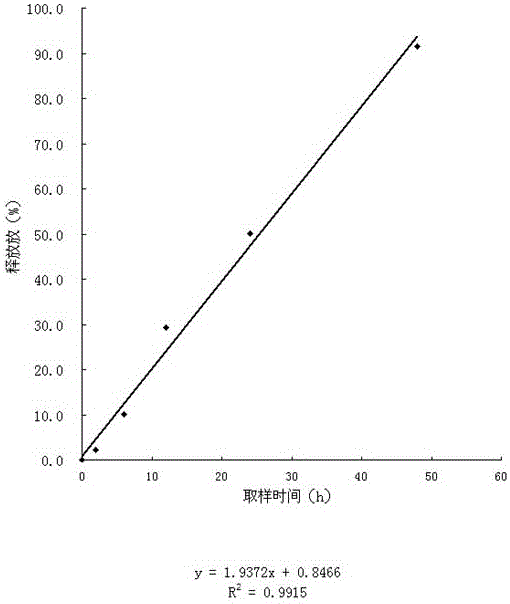
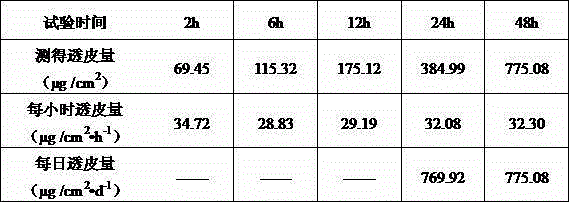
Formula composition and preparation system of enalapril (Enalapril) cutaneous penetration preparations
The invention relates to an enalapril (Enalapril) cutaneous penetration preparation which is used for delivering enalapril in a therapeutic dose to a human body through skin and serves as a new enalapril dosage form for treating hypertension. The enalapril (Enalapril) cutaneous penetration preparation comprises a three-layer structure, namely a backing layer, a drug storage layer and a release layer. The formula provided by the invention comprises a provided transdermal absorption enhancer. The enalapril (Enalapril) cutaneous penetration can be prepared.
Owner:鑫稳药泰医药科技(上海)有限公司
Orodispersible film composition comprising enalapril for the treatment of hypertension in a pediatric population
ActiveUS20200360461A1Reduce the amount requiredProcess stabilityOrganic active ingredientsDipeptide ingredientsPharmaceutical medicinePediatric population
The present invention relates to an oral applicable therapeutic dosage form, in particular an orodispersible film comprising Enalapril or pharmaceutically acceptable salts thereof for use in the treatment of hypertension in a pediatric population. The pediatric population is defined from 1 to 18 years of age. The present invention also provides a method of manufacturing of such a dosage form.
Owner:PHARMATHEN
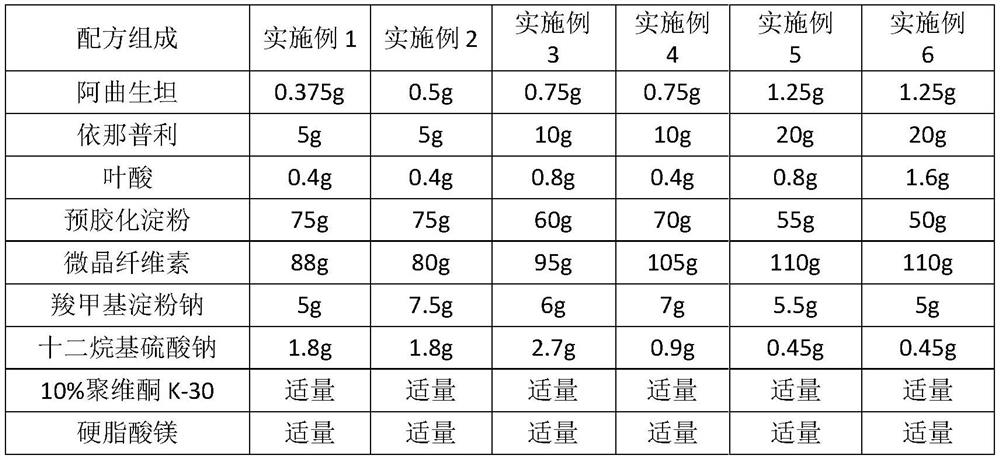
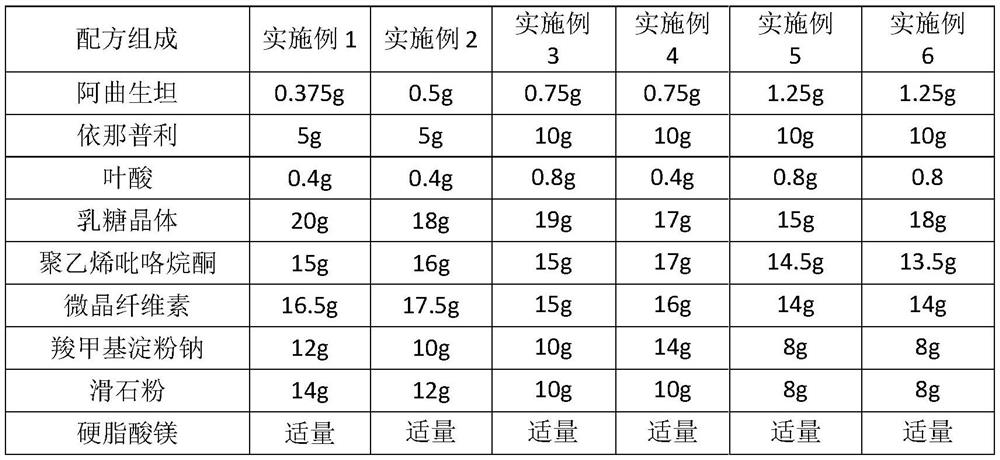
Tablet composition containing enalapril and folic acid and preparation method thereof
ActiveCN106310218ASlow down the dissolution rateProtection from destructionOrganic active ingredientsDipeptide ingredientsCurative effectTherapeutic effect
The invention relates to a tablet composition containing enalapril and folic acid and a preparation method thereof. The tablets prepared by the preparation method are capable of effectively reducing the degradation of active ingredients of a medicine while improving the stability and safety of a compound solid preparation as well as the disintegration rate and timeliness of the medicine in the stomach and intestines; the composition meets the dosage requirements for accurate absorption, and the therapeutic effect of the medicine is remarkably improved.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Medicament composition comprising Enalapril quick-releasing part and felodipine slow-releasing part
ActiveCN101843892AEvenly dispersedStabilize blood pressureOrganic active ingredientsDipeptide ingredientsChemical compositionActive component
The invention relates to a medicament composition preparation comprising an Enalapril quick-releasing part and a felodipine slow-releasing part. The medicament composition is a medical composition formed by mixing Enalapril and felodipine as medical active components with and pharmaceutically acceptable auxiliary materials. The composition preparation comprising comprises a slow-releasing part tablet core and a quick-releasing part coating, wherein the slow-releasing part tablet core is the felodipine, and the quick-releasing part coating is the Enalapril or Enalapril-acid addition salts. Thecomposition can rapidly stabilize blood pressure and permanently keep the blood pressure steady.
Owner:白云山威灵药业有限公司
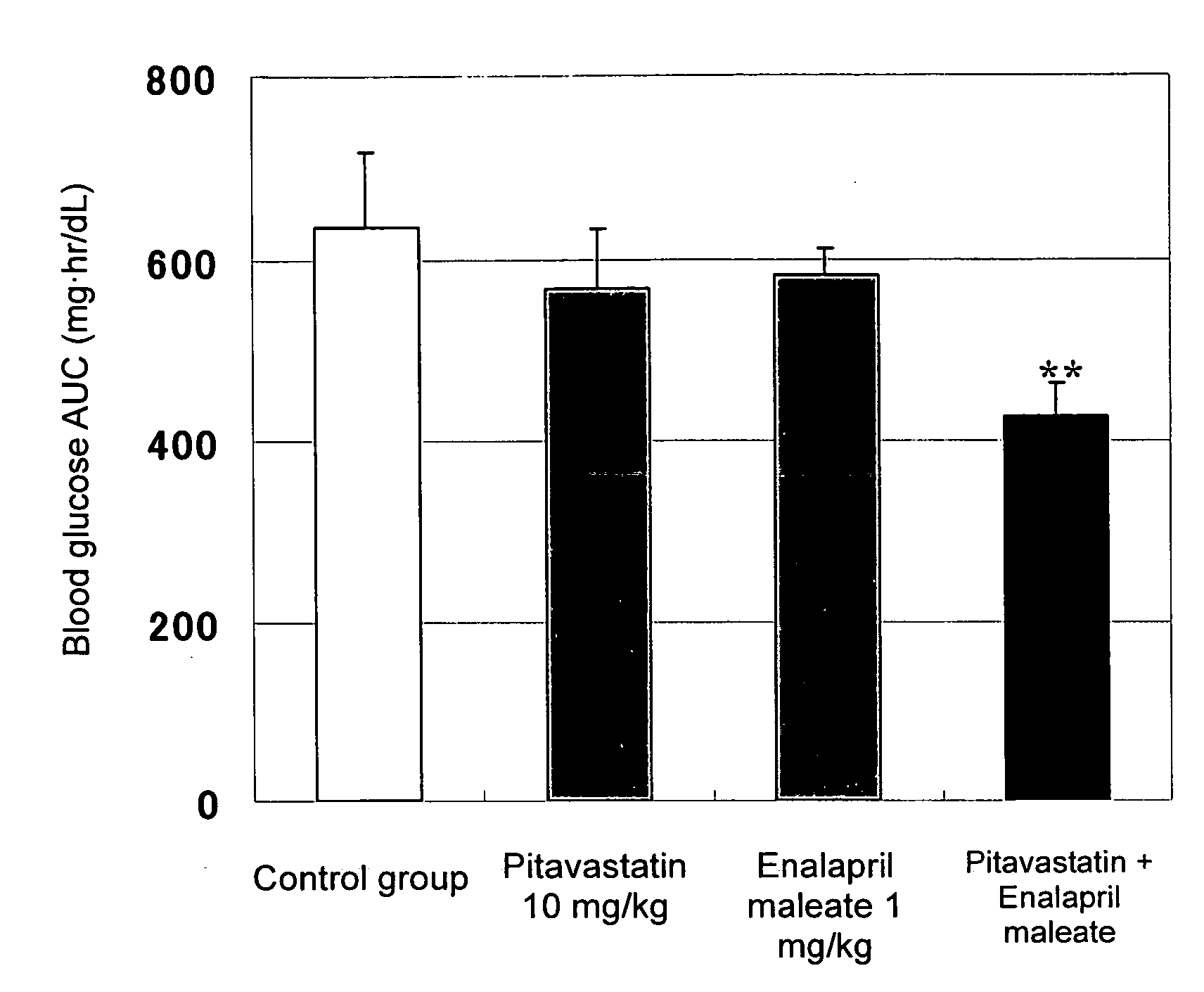
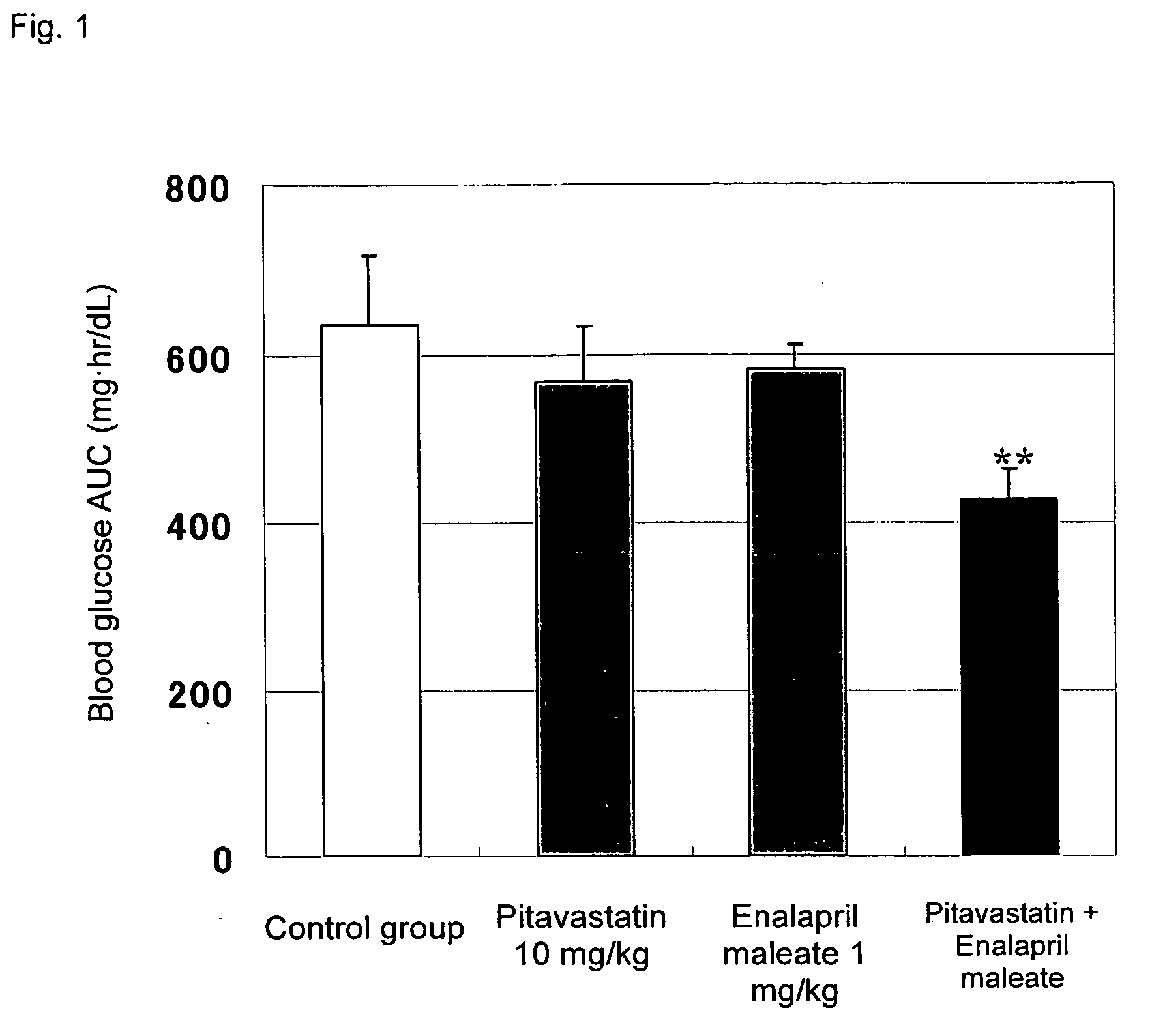
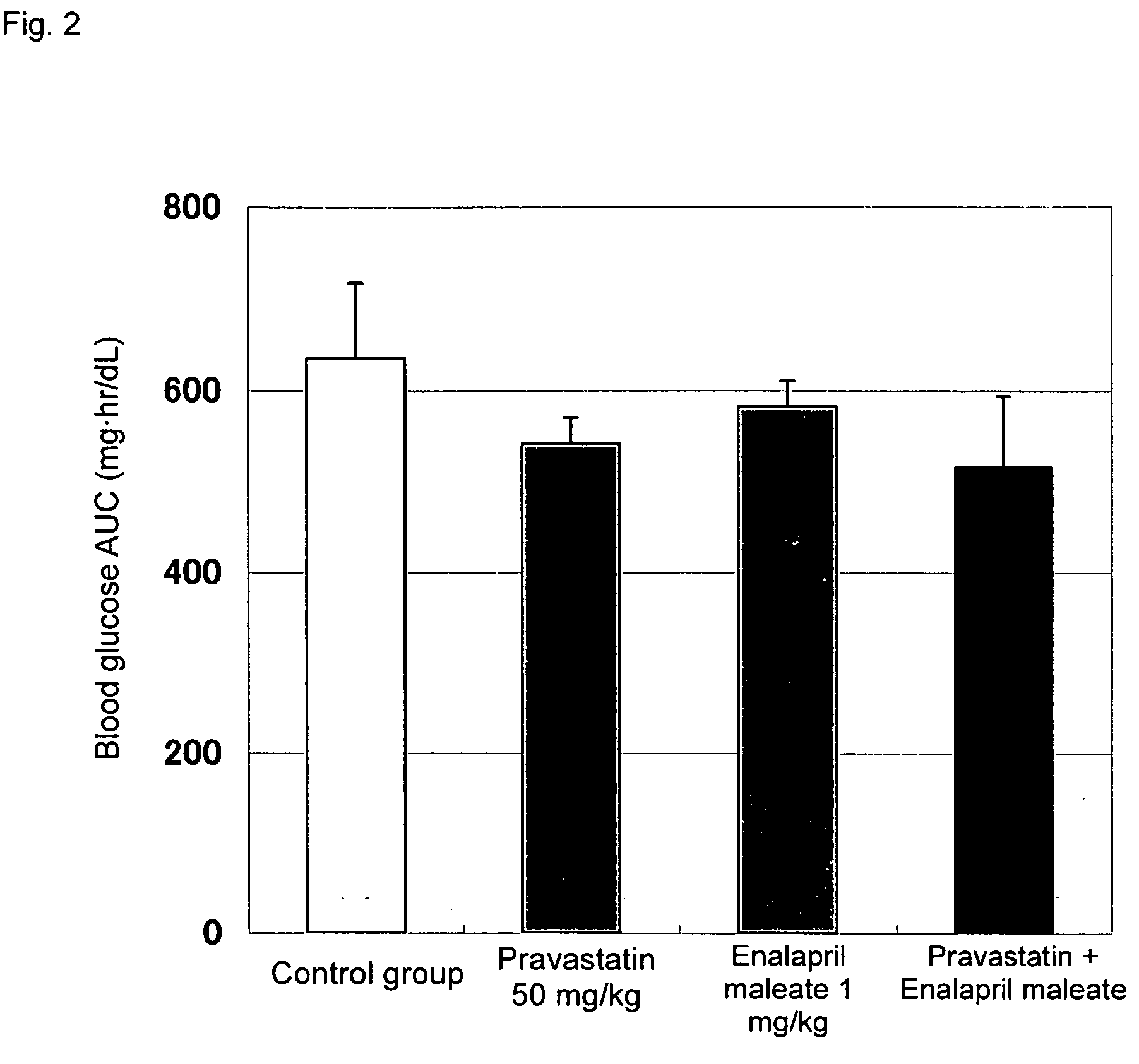
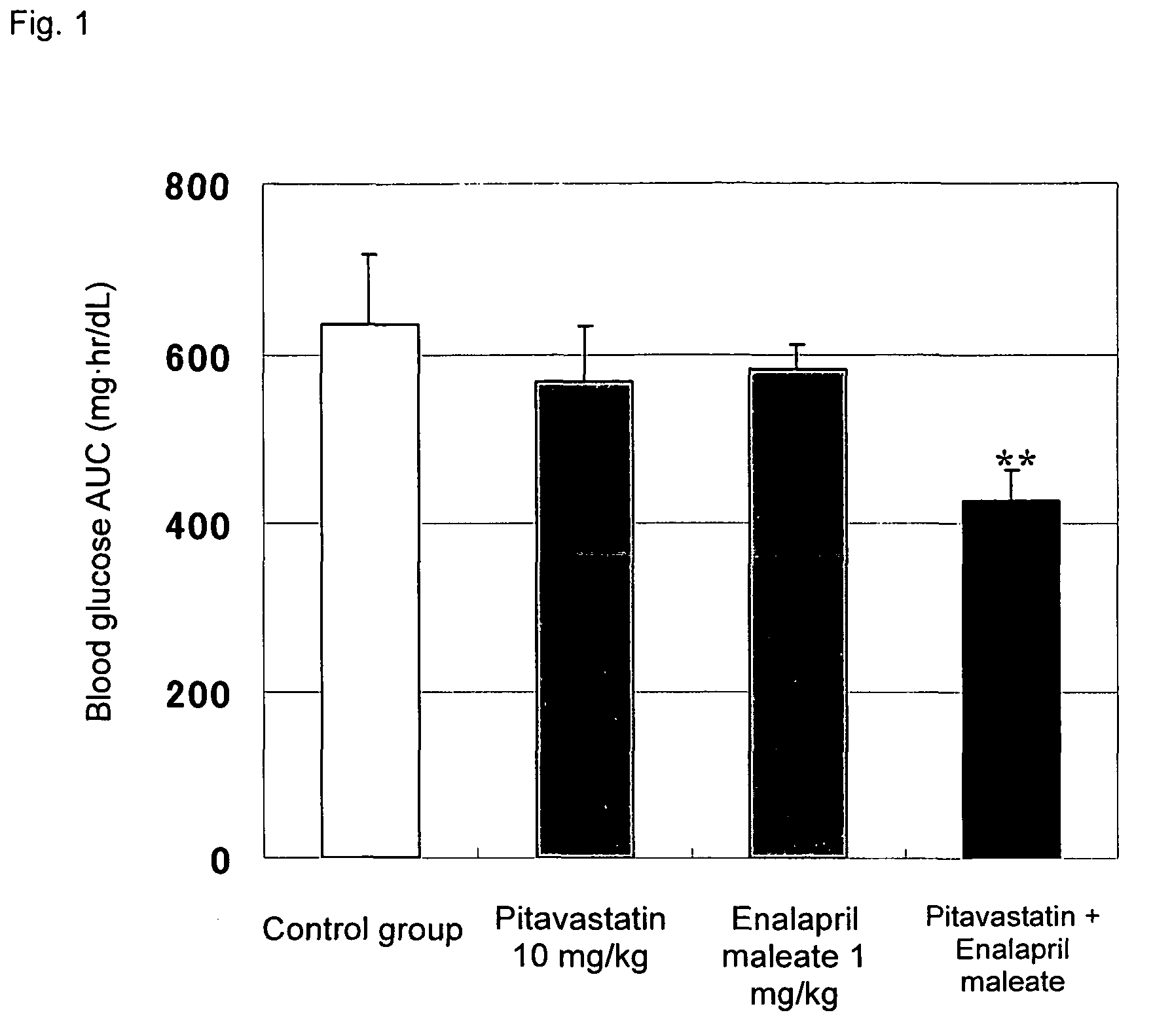
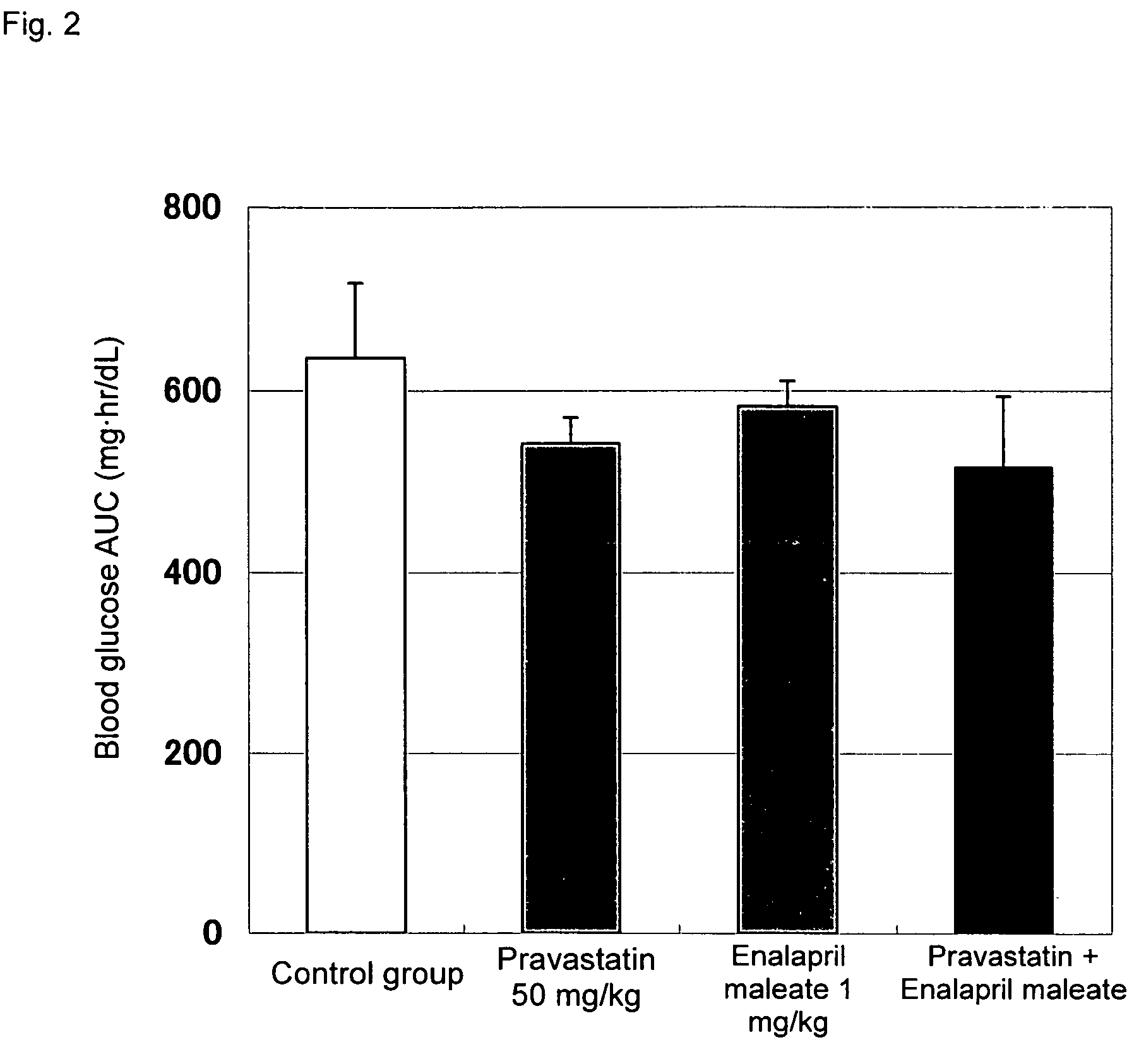
Method for the treatment of diabetes
InactiveUS20070179194A1Eliminate side effectsGood effectBiocideMetabolism disorderDiabetes mellitusPitavastatin
The present invention provides a method for treatment of diabetes, comprising administering a pitavastatin, and in combination therewith, enalapril or a salt thereof.
Owner:KOWA CO LTD +1
Oral sustained release hypotensive composition
ActiveCN101590038APlay a synergistic therapeutic effectStable blood pressure effectPill deliveryHeterocyclic compound active ingredientsEnalaprilCompounded preparations
The invention relates to a hypotensive compound preparation consisting of bevantolol or pharmaceutical salt thereof and enalapril or pharmaceutical salt thereof, wherein in the preparation, the enalapril or the pharmaceutical salt thereof is a fast release part, and the bevantolol or the pharmaceutical salt thereof is a sustained release part. The compound preparation, for moderate and severe hypertension patients, has strong hypotensive effect, sustained action, convenient administration for patients, and accurate dosage.
Owner:北京化药科创医药科技发展有限公司
External application patch containing enalapril and application thereof
InactiveCN104983723ASmall toxicityAvoid interferenceSheet deliveryHeterocyclic compound active ingredientsEnalaprilHigh Blood Pressures
The invention relates to an external application patch containing enalapril and a preparation method thereof. Enalapril is used as the main medicine of the external application patch and used for treating high blood pressure. According to the external application patch containing the enalapril, medicine release is even through transdermal absorption, the action is moderate and durable, the external application patch directly acts on the skin and will not be absorbed through the gastrointestinal tract, the first pass effect of the liver is avoided, adverse effects are reduced, and the occurrence rate of the adverse effects is reduced. The compliance of a patient is good, and the external application patch is convenient to use and carry.
Owner:徐静 +1
Compound preparation containing enalapril for treating hypertension
ActiveCN102008711AQuick resultsHigh blood pressureOrganic active ingredientsDipeptide ingredientsSide effectHydrochlorothiazide
The invention relates to a compound preparation containing enalapril for treating hypertension, comprising main compositions and a matched pharmaceutically acceptable vector, wherein the main compositions include L-amlodipine or a pharmaceutically acceptable salt of the L-amlodipine, hydrochlorothiazide and the enalapril or other pharmaceutically acceptable salt of the enalapril, and the L-amlodipine or the pharmaceutically acceptable salt of the L-amlodipine is contained in each preparation unit. According to an action mechanism that drug combination fully fulfills the function of drug complementarity, the invention increases the curative effect, rapidly reaches the standard with the blood pressure standard-reaching rate of 82 percent, reduces the adverse reaction relative to the increase of a certain dosage and keeps longer action time. The compound preparation has the advantages of fast effect, high blood pressure standard-reaching rate, little side effect and low cost.
Owner:YUANHE PHARMA CO LTD
Method for the treatment of diabetes
InactiveUS8685952B2Impaired glucose toleranceTo promote metabolismBiocideMetabolism disorderDiabetes mellitusPitavastatin
Owner:KOWA CO LTD +1
Medicinal composition containing enalapril or genazepril and sotalol
ActiveCN1891222AGood blood pressure effectImprove complianceSenses disorderDipeptide ingredientsPatient complianceCurative effect
The present invention relates to a medicine composition containing enalapril or benazepril and sotalol. It is a new type hypotensor medicine composition. The content of enalapril or benazepril is 5-40 mg and the sotalol content is 20-160mg.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Pharmaceutical composition for treating chronic heart failure
The invention relates to a pharmaceutical composition comprising an angiotensin converting enzyme inhibitor (ACEI), an enkephalinase inhibitor, a folic acid compound and a pharmaceutically acceptable carrier, wherein the ACEI is selected from the group consisting of enalapril, benazepril, ramipril, fosinopril, cilazapril, perindopril and the like, and the content is 1.25-75 mg; the enkephalinase inhibitor is sacubitril, and the content is 10-120 mg; and the folic acid compound is selected from the group consisting of folic acid, 5-methyltetrahydrofolate, calcium formyltetrahydrofolate, leucovorin, calcium levofolinate and the like, and the content is 0.1-5 mg. The invention provides the use of the pharmaceutical composition in the preparation of a medicament for the treatment of chronic heart failure and the prevention of stroke. By the implementation of the invention, the pharmaceutical composition can also improve the compliance of patients and improve the therapeutic effect by providing the pharmaceutical composition for a specific use to the patients.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Primary hypertension elastomer subcutaneous implant rod
InactiveCN108743955AProlong degradation timeGood biocompatibilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsEnalaprilSide effect
The invention provides a primary hypertension elastomer subcutaneous implant rod. The primary hypertension elastomer subcutaneous implant rod comprises, by weight, 100 to 200 parts of polycaprolactone, 70 to 200 parts of polyethylene glycol, 15 to 20 parts of polyvinylpyrrolidone, and 100 to 180 parts of bulk drug. The bulk drug is composed of enalapril, lacidipine, and irbesartan at a mass ratioof 1:1:1.6. The degradation time of the primary hypertension elastomer subcutaneous implant rod is long; drug effect lasts long; clinical treatment of hypertension can be realized; side effect causedby long term of eating of drugs is reduced; risk caused by missed doses of drugs is avoided; and the primary hypertension elastomer subcutaneous implant rod is mainly used for clinical treatment of primary hypertension.
Owner:吕鹏威
Pharmaceutical composition for treating hypertensive left ventricular hypertrophy and application thereof
InactiveCN103143020AAdvantages and Notable ImprovementsSignificant reversalOrganic active ingredientsDipeptide ingredientsCaptoprilLeft ventricular size
The invention discloses a pharmaceutical composition for treating hypertensive left ventricular hypertrophy; the pharmaceutical composition comprises total glucosides of paeony and at least one angiotensin converting enzyme inhibitor; the angiotensin converting enzyme inhibitor is captopril, enalapril, perindopril or fosinopril. The pharmaceutical composition has very significant reverse effect on hypertension induced ventricular hypertrophy; by adopting the pharmaceutical composition, the perfect synergy function on each index such as left ventricular index of myocardial hypertrophy is obtained.
Owner:海门市凤城旅游景点开发有限公司
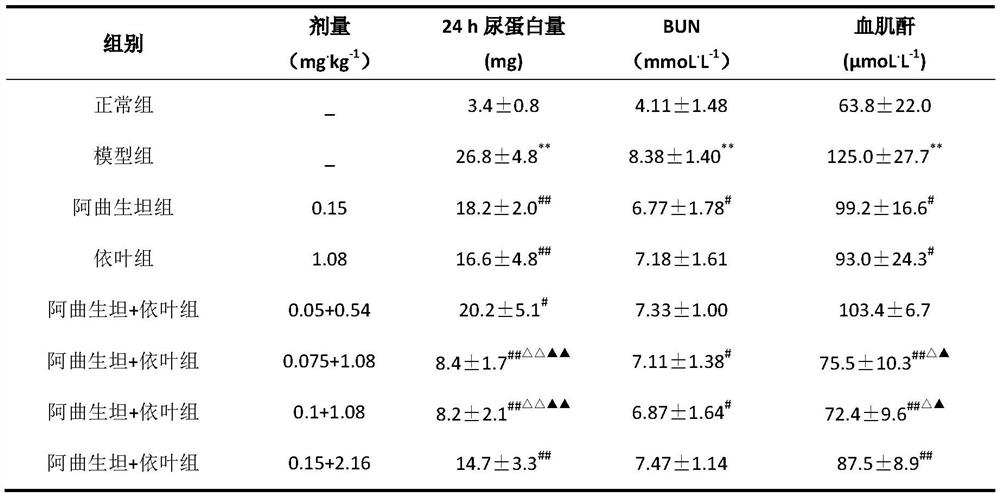
Pharmaceutical composition with kidney protection effect
ActiveCN112438976AImprove kidney functionLittle side effectsDipeptide ingredientsMetabolism disorderDiseaseAtrasentan
The invention relates to a pharmaceutical composition with a kidney protection effect. The pharmaceutical composition consists of an endothelin inhibitor, enalapril, folic acid substances and a pharmaceutically acceptable carrier, wherein the endothelin inhibitor comprises atrasentan. The invention provides application of the pharmaceutical composition to preparation of medicines for preventing, treating or delaying chronic kidney diseases and diabetic nephropathy. Through the implementation of the application, the pharmaceutical composition is provided for patients, the curative effect can beimproved, the treatment compliance is increased, and the medical cost is reduced.
Owner:SHENZHEN AUSA PHARM CO LTD +1
2-alkoxycarbonyl-3-phenylpropionate derivant-synthesis and uses thereof of novel ace inhibitor
An angiotensin converting enzyme (ACE) inhibitor is mainly used for treating diseases such as hypertension, congestive heart failure, myocardial infarction, renal dysfunction, etc. The existing medicines in the market comprise captopril, enalapril, benazepril, and the like, which have obvious adverse reactions such as severe dry cough, parageusia, proteinuria, hematuria, hypotension, and the like, and the medicines have some adverse reactions that threaten life such as acute thrombocytopenia, hyperkalemia, severe arrhythmia and renal failure. The invention relates to a technical scheme for chemosynthesis of a 2-alkoxycarbonyl-3-phenylpropionic acid ester derivative with the characteristic of the structural general formula I and provides a 2-alkoxycarbonyl-3-phenylpropionic acid ester derivative with the characteristic of the structural general formula I and the derivative is taken as an ACE inhibitor for treating diseases such as hypertension, congestive heart failure, myocardial infarction and renal dysfunction, and lowering blood sugar and blood lipid, etc. R1, R2, R3 and R4 are -H, C1-3-alkyl, C1-3-alkenylnyl, C1-3-alkoxy, aryloxy, arylalkoxyl, heterocycles, heteroarylalkyl, acyl, acyloxy, hydroxy C1-3-alkyl, hydroxy, amino, halogen, nitro, cyano, formyl, acylamino, C1-3-alkyamino, C1-3-alkoxycarbonylamino, C1-3-alkylcarbonylamino, C1-3-alkylsulfonyl and arylsulfonyl.
Owner:蔡小华 +1
Antihypertensive drug of enalapril and safflower oil nanoemulsion
InactiveCN102698246AEvenly distributedSystem transparencyDipeptide ingredientsEmulsion deliveryAdditive ingredientHalf-life
The invention discloses an oil-in-water type antihypertensive drug of enalapril and safflower oil nanoemulsion. The materials and the weight percentages of the materials are as follows: 1-20% of the enalapril, 15-35% of surfactant, 0-20% of cosurfactant, 1-25% of the safflower oil, and the balance of distilled water. The sum of the weight percentages of the materials is 100%. The nanoemulsion has the advantages of small emulsion droplet particles, uniform distribution, small viscosity and good flowability. With the adoption of the dosage form of the nanoemulsion, water-soluble enalapril and fat-soluble safflower oil are organically combined, so that the dissolving and penetrating abilities of the safflower oil are improved, and the stability and the pesticide effect of the enalapril are increased. The advantages of the enalapril and the safflower oil are combined after the enalapril and the safflower oil are prepared into nanoemulsion formulation, so that the antihypertensive effect is obviously increased, the half-life period of the drug is prolonged and the administration times are also reduced.
Owner:NORTHWEST A & F UNIV
Medicinal composition for lowering blood pressure
InactiveCN101612156ACardiovascular disorderHeterocyclic compound active ingredientsCaptoprilAdditive ingredient
The invention relates to a medicinal composition for lowering blood pressure, which consists of trichlormethiazide and angiotensin converting enzyme inhibitor which serve as active ingredients and a pharmaceutical carrier, wherein the angiotensin converting enzyme inhibitor may be enalapril, cilazapril, benazepril, captopril, ramipril, perindopril and fosinopril. The unit dosage of trichlormethiazide is 0.5 to 8 milligrams, preferably 2 to 4 milligrams. The unit dosage of angiotensin converting enzyme inhibitor is 1.5 to 50 milligrams. The medicinal composition can be made into various oral preparations including conventional tablets, capsules, chewable tablets, dispersible tablets and effervescent tablets.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C1.PNG)
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C2.PNG)
![Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride Method for chemical synthesis of N-[1 (1)-carbethoxy-3-hydro cinnamyl]-L-ulamine -N- carboxylic anhydride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0abb1c73-eb0a-49ec-bb28-93f2b69fc98d/A2003101082270002C3.PNG)



![Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a056192c-c6d5-4a05-85a3-cd1df6f9a071/a20071009120200101.PNG)
![Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a056192c-c6d5-4a05-85a3-cd1df6f9a071/a2007100912020002c1.PNG)
![Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant Method for preparing N-[2-(1-ethoxycarbonyl-3-phenylpropylamino)-tauryl]-thiazolidinecarboxylic acid and its derivant](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a056192c-c6d5-4a05-85a3-cd1df6f9a071/a2007100912020002c2.PNG)