Pharmaceutical composition with kidney protection effect
A composition and drug technology, applied in the field of pharmacy, can solve problems affecting the quality of life of patients, and achieve the effects of protecting the kidneys, lowering the dosage of drugs, and reducing the level of proteinuria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
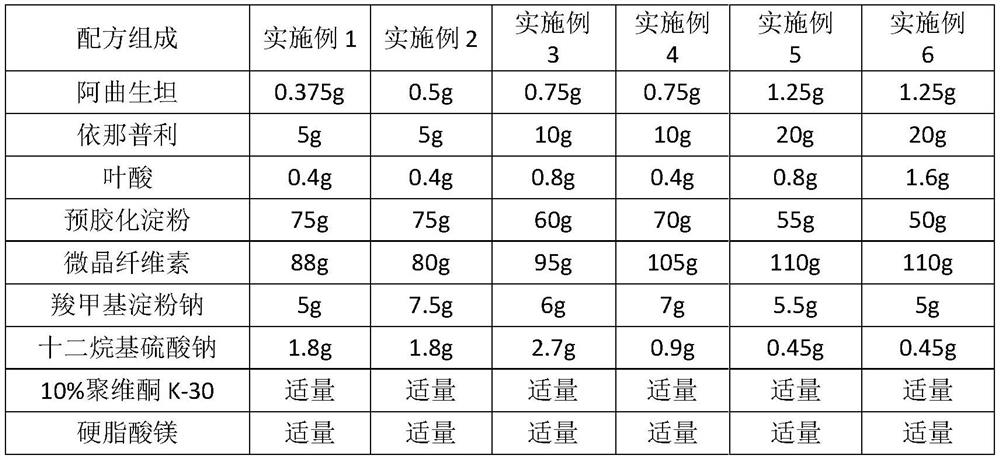
[0027] Embodiment 1-6. Atrasentan + enalapril + folic acid tablets (1000 pieces)
[0028]
[0029] Preparation Process:
[0030] Mix atrasentan, enalapril and folic acid, add carboxymethyl starch sodium and sodium lauryl sulfate, then add microcrystalline cellulose and pregelatinized starch and mix evenly, and use an appropriate amount of 10% povidone The ethanol solution is made into a soft material, granulated, dried, and granulated, and the granules with a water content of about 3% are evenly mixed with an appropriate amount of magnesium stearate, and compressed into 1,000 tablets.
Embodiment 7-12
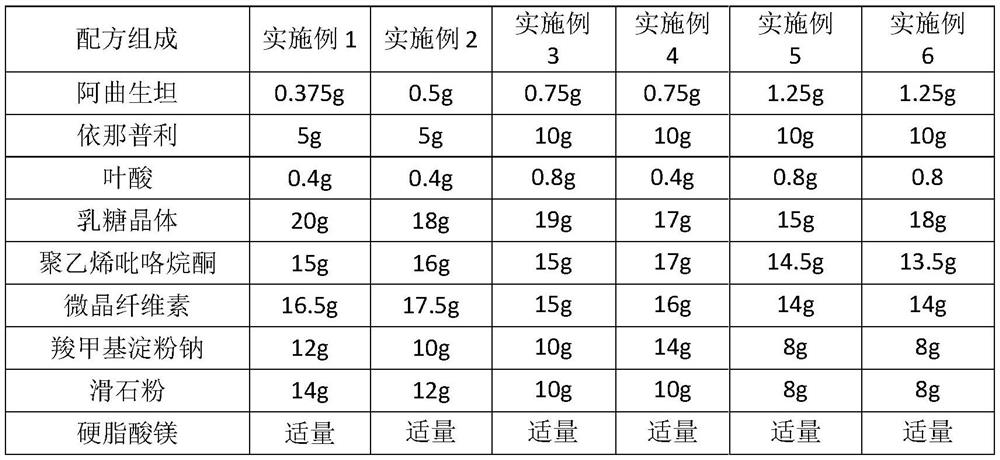
[0031] Example 7-12. Atrasentan + enalapril + folic acid capsules (1000 capsules)
[0032]
[0033]Preparation process: crush the raw and auxiliary materials through an 80-mesh sieve, and dry for later use. Mix atrasentan, enalapril, and folic acid evenly, add lactose crystals, polyvinylpyrrolidone, microcrystalline cellulose, and sodium carboxymethyl starch, mix evenly, make a soft material with micro-powder silica gel, and granulate with a 20-mesh sieve , dry at 60°C for about 2 hours, granulate with a 20-mesh sieve, control the water content of the granules to 2-3%, mix the dried granules with an appropriate amount of 1% magnesium stearate, test the semi-finished products, measure the content, and pack Empty capsules, that is, 1000 capsules. Protect from light during preparation. After the finished product passes the inspection, it is packed in aluminum-plastic blister and stored away from light.
Embodiment 13
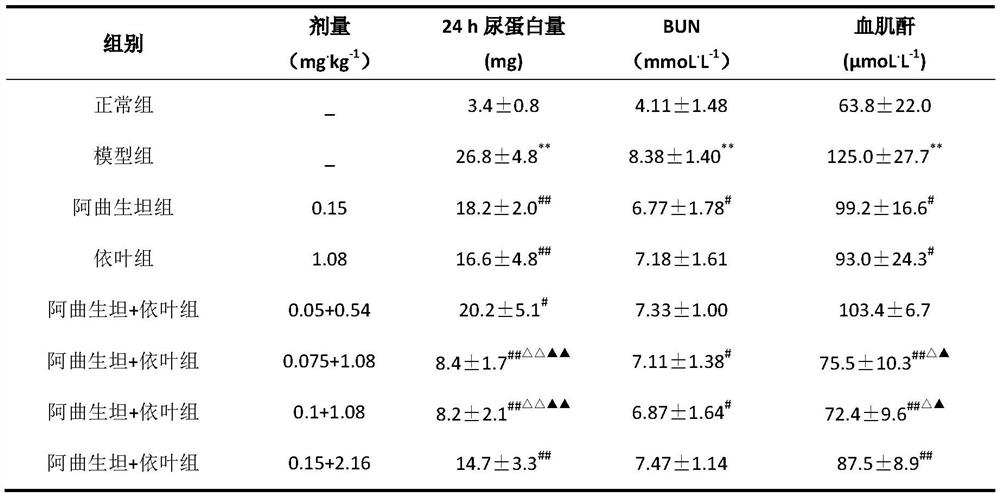
[0034] Example 13: The renoprotective effect of atrasentan + enalapril folic acid composition on rats with chronic kidney disease.
[0035] (1) Modeling:
[0036] Adult Wistar rats, male, weighing 200-220g, were fed with common feed and quarantined for 7 days. Animals whose body weight deviation exceeded 10% were excluded, and 10 rats were randomly selected as the normal control group, and fed with normal feed until the end of the experiment. Animals in the modeling group were fasted for 12 hours before modeling, and received a single tail vein injection of doxorubicin (7.9 mg / kg), and resumed normal diet and drinking water 2 hours after modeling. After 4 weeks, the 24-h urinary protein levels of all animals were detected, and model animals with 24-h urinary protein ≥ 20 mg were selected to enter the follow-up experiment.
[0037] (2) Grouping and administration:
[0038] There were 10 rats in the normal control group, and the successfully modeled animals were randomly divid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com