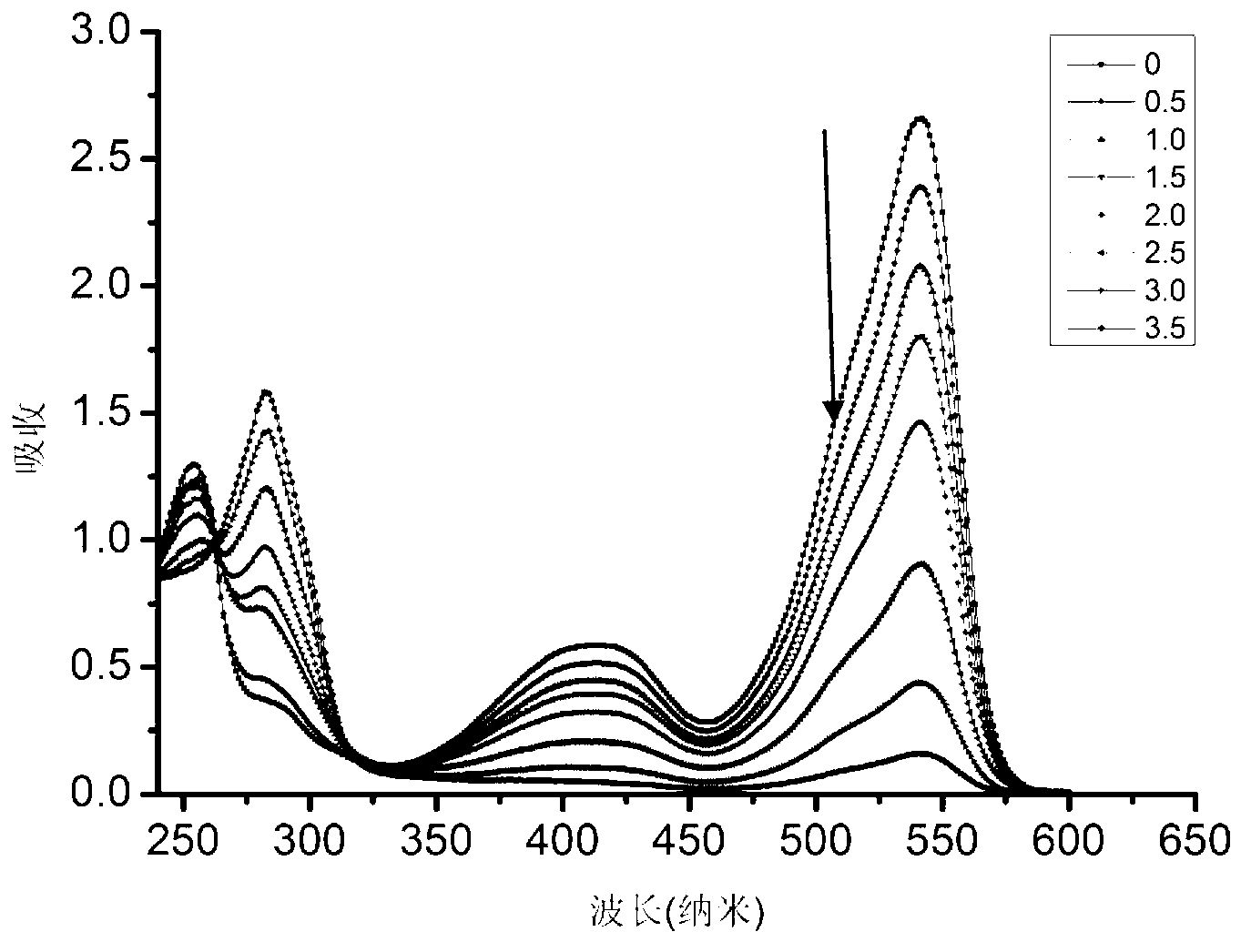
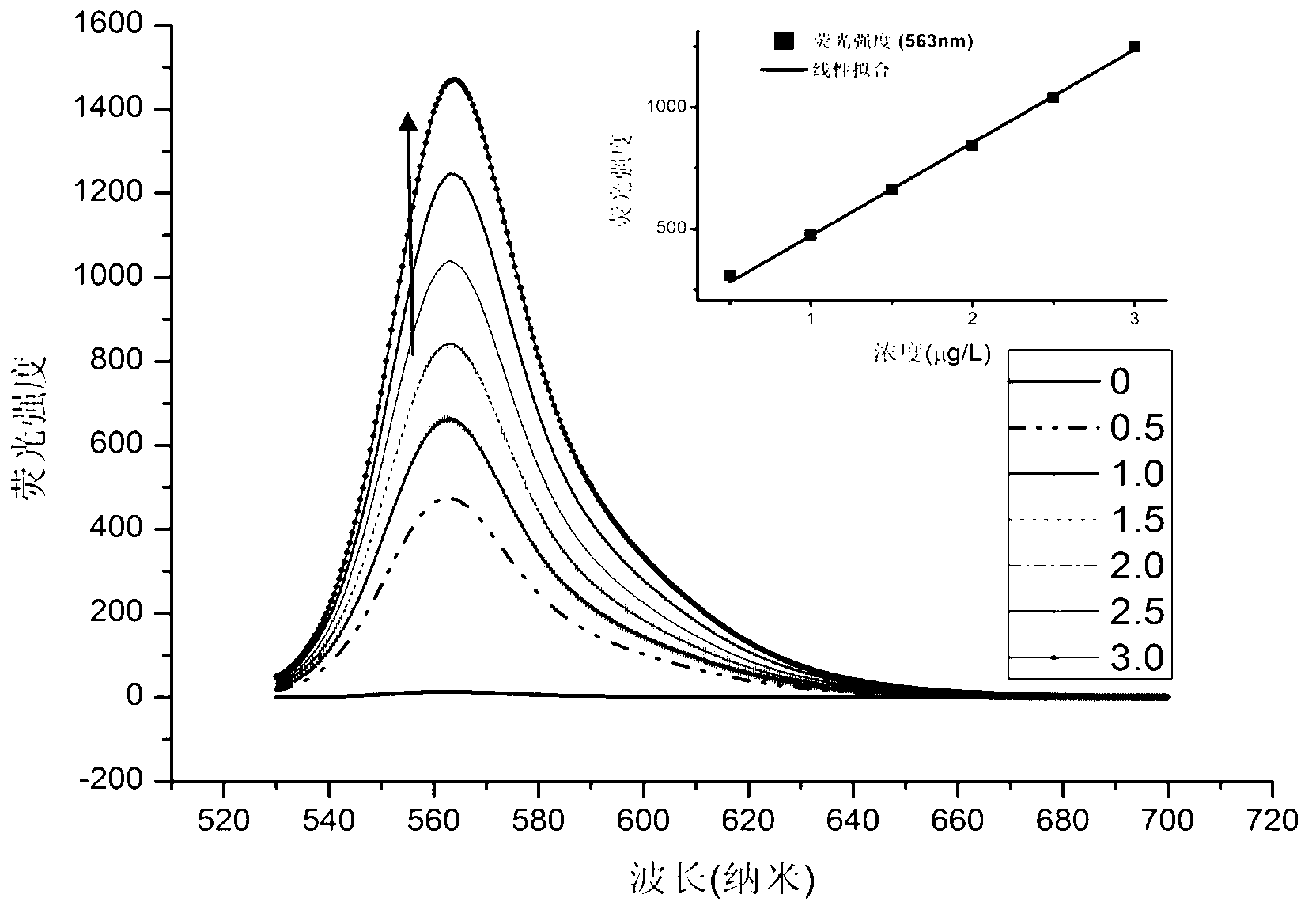
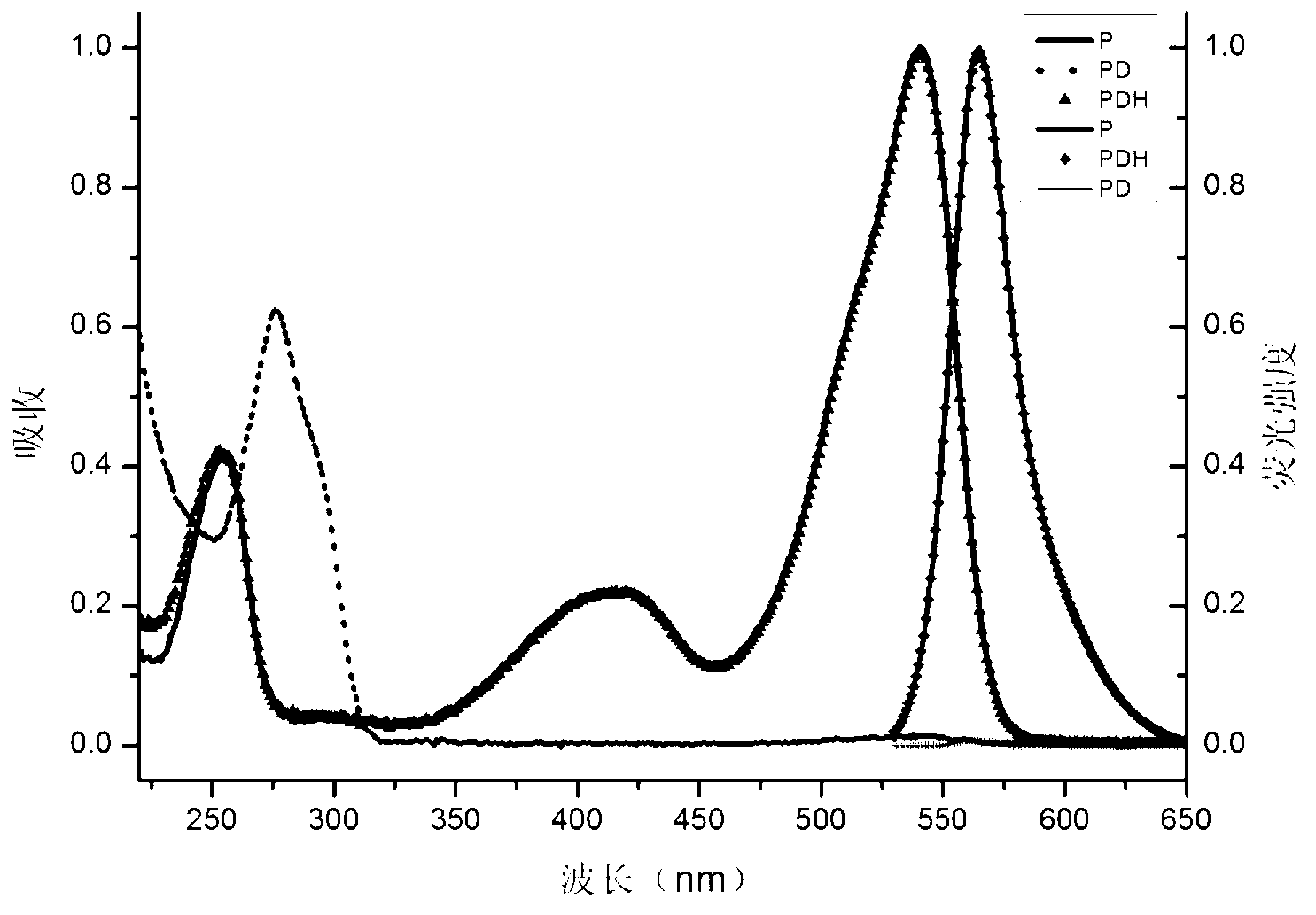
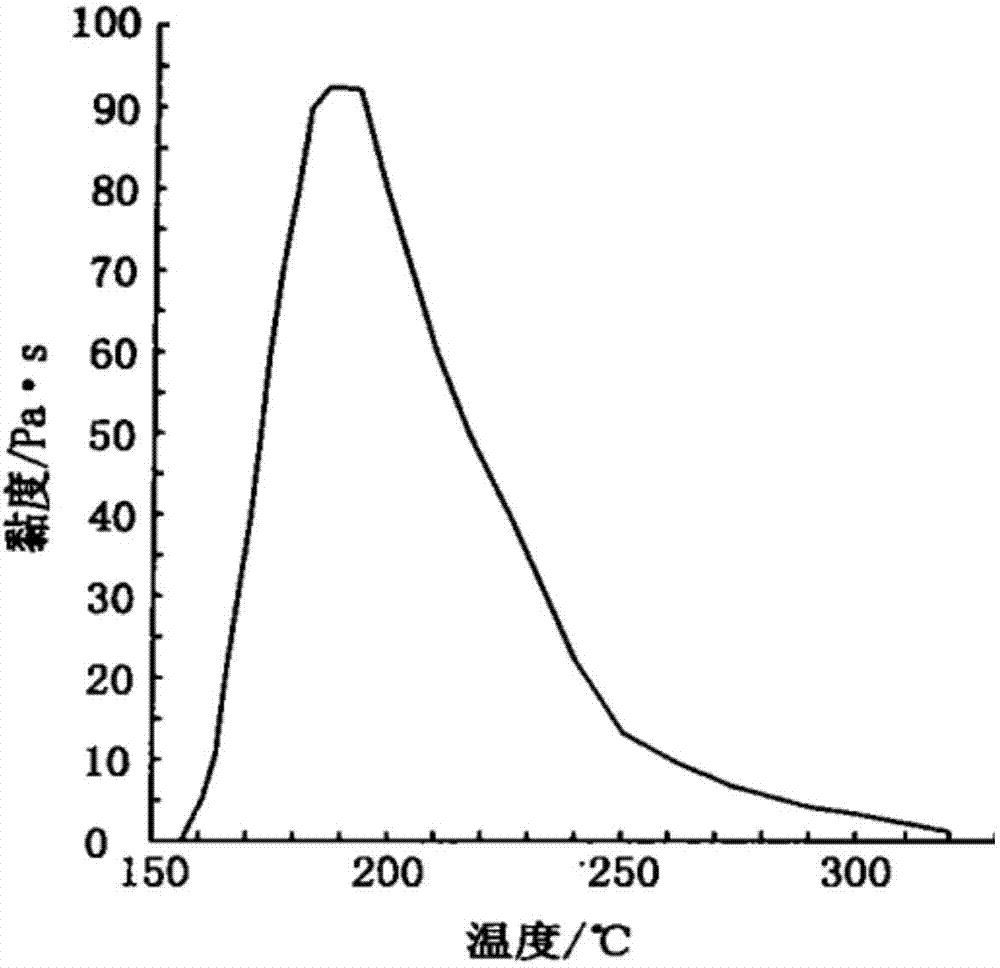
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
223 results about "Reversible reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A reversible reaction is a reaction where the reactants form products, which react together to give the reactants back. aA+bB<=>cC+dD A and B can react to form C and D or, in the reverse reaction, C and D can react to form A and B. This is distinct from reversible process in thermodynamics. Weak acids and bases undertake reversible reactions. For example, carbonic acid: H₂CO₃ ₍ₗ₎ + H₂O₍ₗ₎ ⇌ HCO₃⁻(aq) + H₃O⁺(aq).
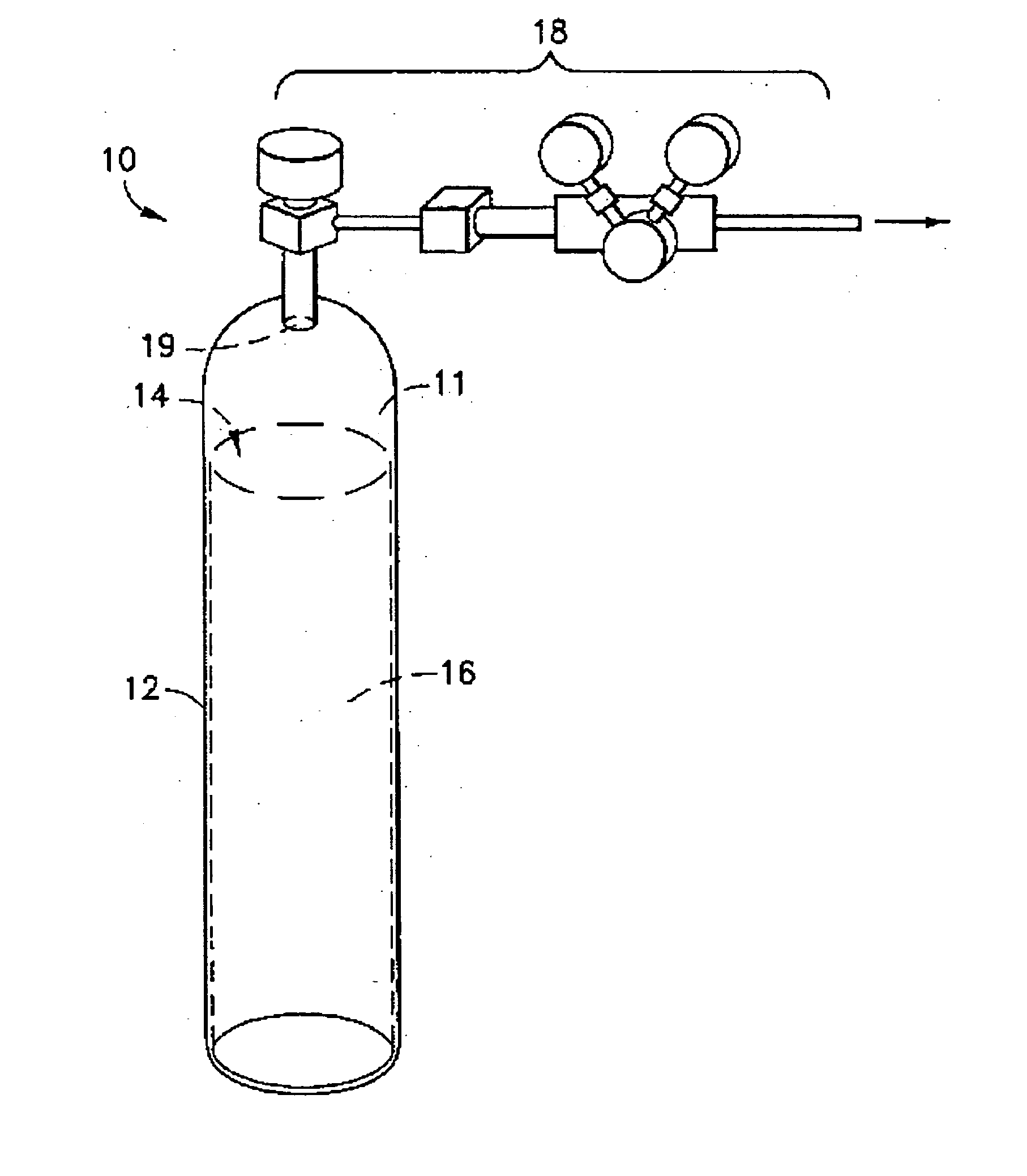
Reactive liquid based gas storage and delivery systems
ActiveUS7172646B2High gas (or working) capacityReliable sourceLiquid degasificationUsing liquid separation agentChemistryProduct gas
This invention relates generally to an improvement in low pressure storage and dispensing systems for the selective storing of gases having Lewis acidity or basicity, and the subsequent dispensing of said gases at pressures, e.g., generally below 5 psig and typically below atmospheric pressure, by modest heating, pressure reduction or both. The improvement resides in storing the gases in a reversibly reacted state within a reactive liquid having opposing Lewis basicity or acidity.
Owner:VERSUM MATERIALS US LLC
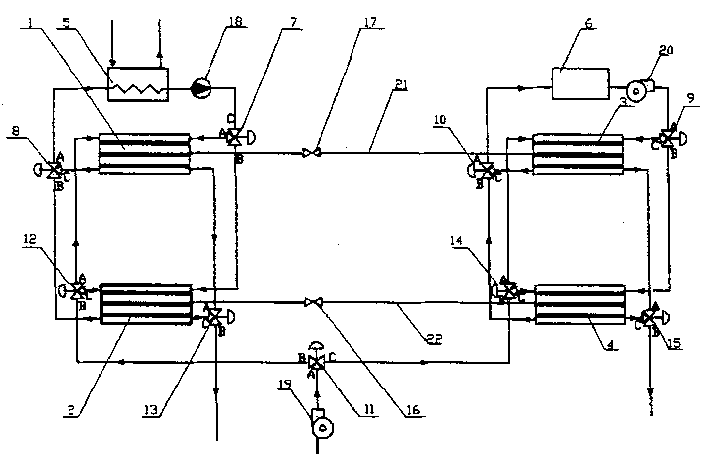
Vehicle air-condition with two-stage metal hydride
InactiveCN1482017AContinuous temperature adjustmentAir-treating devicesVehicle heating/cooling devicesAtmospheric airSolenoid valve
The two-stage metal hydride automobile air conditioner refrigerates via the heat effect of reversible reaction between metal hydride and hydrogen. Waste heat of tail gas as high temperature heat source and peripheral atmosphere as medium temperature heat source are utilized to drive the refrigerating circulation of metal hydride, and two pairs of metal hydride reactors are adopted for alternate refrigeration to continuous regulation of temperature inside the automobile. The air conditioner system includes mainly high and low temperature metal hydride reactors, heat exchanger, solenoid valve, water pump, blower, etc. Inside both the high temperature and the low temperature reactors, are arranged fine pipes with filled metal hydride and hydrogen penetrating film in the middle section of pipes and hydrogen collecting general pipe. The air conditioner has no corrosion, no wear, less motion parts, high vibration resistance, no damage to atmosphere ozone layer and other advantages.
Owner:SHANGHAI JIAO TONG UNIV
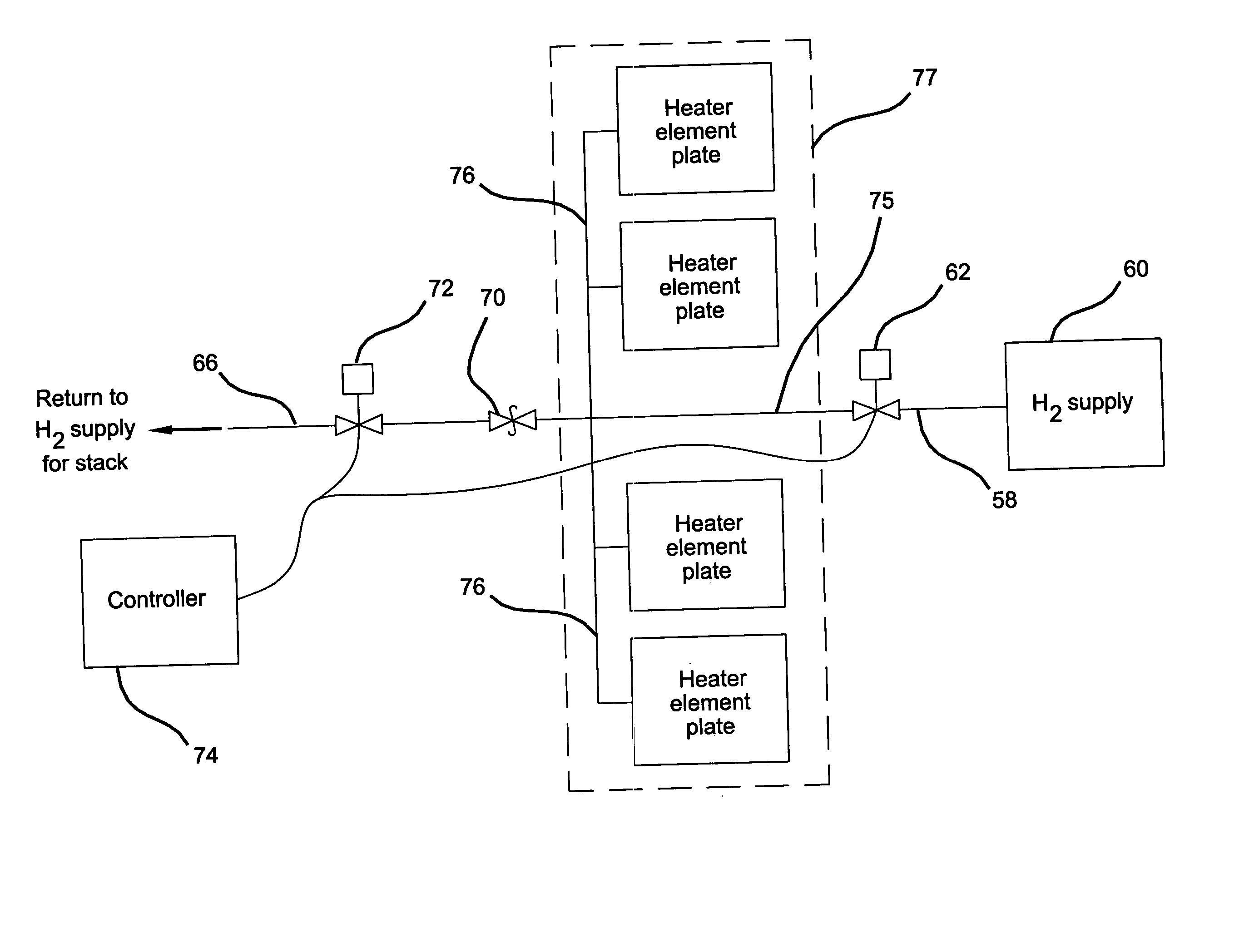
Metal hydride heating element
InactiveUS20050079397A1Fuel cell heat exchangeReactant parameters controlFuel cellsReversible reaction
A heating element for a fuel cell system comprising a body constructed of a thermally conductive material. The interior of the body has a plurality of fluid flow channels formed therein. A hydrogen absorption material capable of absorbing hydrogen in an exothermic reaction to form a metal hydride in a reversible reaction is disposed within the channels. A conduit provides fluid communication to and from the channels and the exterior of the body which is in the form of a storage vessel. Hydrogen is supplied via the conduit to the flow channels and is absorbed by the hydrogen absorption material which generates heat that is transferred through the thermally conductive material to regions surrounding the storage vessel. Methods of heating a fuel cell with a device storing material capable of an exothermic reaction that generates heat are also provided.
Owner:GENERAL MOTORS COMPANY
Supercapacitor using nickel aluminum hydrotalcite nanometer material as anode material
ActiveCN104779059ALarge specific surface areaIncrease contact surfaceHybrid capacitor electrodesReversible reactionNanomaterials
The invention discloses a supercapacitor using a nickel aluminum hydrotalcite nanometer material as an anode material. The supercapacitor comprises an anode current collector, an anode material, a battery diaphragm, an electrolyte, a cathode material and a cathode current collector, wherein the anode material adopts the nanometer flower-shaped nickel aluminum hydrotalcite material. The nanometer flower-shaped nickel aluminum hydrotalcite material is large in specific surface area, excellent in electrochemical performance, good in electrochemical reversible reaction and stable and reliable in performance; the prepared supercapacitor with the negative symmetrical structure can obtain the characteristics of high capacity density and high power density.
Owner:四川英能基科技有限公司
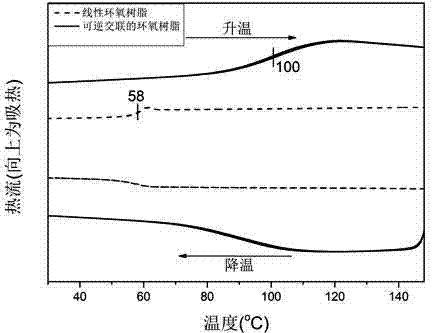
Reversible covalent cross-linked epoxy resin and preparation method thereof
The invention discloses a reversible covalent cross-linked epoxy resin. The reaction between diene monoamine and an epoxy resin monomer is adopted for obtaining a linear epoxy resin with a diene on a side chain, the linear epoxy resin and a dienophile cross-linking agent form a Diels-Alder reversible reaction cross-linked point on the side chain, the reversible covalent cross-linked bond of the cross-linked epoxy resin breaks at a temperature of 100 to 150 DEG C, and can be restored again when the temperature is lowered to below 90 DEG C, so that the crosslink reversibility can be achieved. The reversible covalent cross-linked epoxy resin and the preparation method, provided by the invention, have the advantages that: (1), the reversible covalent cross-linked point is introduced to the side chain of the linear epoxy resin, so that the thermostability, solvent corrosion resistance and mechanical property of the material are improved, and by changing the cross-linking density, the material performance can be adjusted within a larger range; (2), both structures of the diene and the dienophile in the cross-linked epoxy resin have reversible reaction properties, so that recycling and reprocessing under certain conditions can be conducted; (3), the preparation method is simple and convenient, and is easy to implement, the relative cost is low, and raw material sources are wide.
Owner:NANKAI UNIV
Reversible repair functional matrix resin for pultrusion and preparation method of matrix resin
InactiveCN104194269AMeet the requirements of pultrusion processImprove axial tensile strengthEpoxyDiphenylmethane
The invention discloses a reversible repair functional matrix resin for pultrusion and a preparation method of the matrix resin. The matrix resin is prepared from the following raw materials in parts by weight: 30-60 parts of 4,4'-diallylbisphenol A diglycidyl ether epoxy resin, 20-35 parts of furfuryl amine, 15-30 parts of 4,4'-bismaleimide diphenylmethane, 0-25 parts of liquid epoxy resin and 5-15 parts of curing agent. The preparation method comprises the following steps: dissolving the 4,4'-diallylbisphenol A diglycidyl ether epoxy resin in a formula amount into a solvent, slowly adding the furfuryl amine into a reaction container for reacting, adding the 4,4'-bismaleimide diphenylmethane, liquid epoxy resin and curing agent, reacting and mixing for a hour, performing reduced pressure distillation to remove the solvent, and cooling. The reversible repair functional matrix resin for pultrusion and the preparation method of the matrix resin disclosed by the invention have the beneficial effects that the matrix resin has good molding manufacturability, the molecular structure contains furan and imide structures, the heating and repairing functions of the prepared composite material can be realized by virtue of a Diels-Alder reversible reaction, and the matrix resin also has the advantages of high strength and high heat resistance.
Owner:HAIAN INST OF HIGH TECH RES NANJING UNIV
Fluidization calcium-based thermal-chemical high temperature energy storing/releasing system and working method thereof
InactiveCN105737658AEasy accessLow priceHeat storage plantsEnergy storageThermal energyDecomposition
The invention discloses a fluidization calcium-based thermal-chemical high temperature energy storing / releasing system and a working method thereof. The system is characterized in that the storing and releasing of energy are performed by means of a calcium-based thermal-chemical high-temperature reversible reaction through mutual transformation among solar energy, thermal energy and chemical energy. The system mainly comprises an energy input unit, an energy storing unit and an energy output unit. During storing of energy, calcium hydroxide is subjected to an endothermic decomposition reaction under a fluidization condition, and absorbed energy is stored in calcium oxide as a reaction product in a chemical energy form; during releasing of the energy, the calcium oxide and water vapor are subjected to an exothermic reaction under the fluidization condition, and released heat heats water to generate high-temperature high-pressure steam, so that a steam turbine is driven to generate power. The fluidization calcium-based thermal-chemical high temperature energy storing / releasing system has the characteristics of high unit mass energy-storing density, high energy quality, low energy-storing lost, no pollution and the like, can be modularly combined according to energy-storing capacity, and can be applied to fields such as large-scale solar thermal generation and peak-load regulation.
Owner:SOUTH CHINA UNIV OF TECH
Liquid media containing Lewis acidic reactive compounds for storage and delivery of Lewis basic gases
InactiveUS20050276733A1High binding affinityHigh gas (or working) capacityLiquid degasificationPhysical/chemical process catalystsAlkalinityLiquid medium
This invention relates to an improvement in a low-pressure storage and delivery system for gases having Lewis basicity, particularly hazardous specialty gases such as phosphine and arsine, which are utilized in the electronics industry. The improvement resides in storing the gases in a liquid incorporating a reactive compound having Lewis acidity capable of effecting a reversible reaction between a gas having Lewis basicity. The reactive compound comprises a reactive species that is dissolved, suspended, dispersed, or otherwise mixed with a nonvolatile liquid.
Owner:VERSUM MATERIALS US LLC
Dipyrrol borane (BODIPY) and preparation method and application thereof
ActiveCN103232483ARestore fluorescenceWide range of absorption spectraAnalysis by subjecting material to chemical reactionGroup 3/13 element organic compoundsQuantum efficiencyReversible reaction
The invention discloses a formaldehyde detection probe compound and a detection method. The compound is a high fluorescence quantum efficiency fluorescence dye based on dipyrrol borane BODIPY. The compound and alkali substances used in the invention generate a reversible reaction for forming a compound. The detection of formaldehyde is based on alkali reaction in the compound, and the reaction result causes the BODIPY to regenerate and develop colors, and simultaneously recover fluorescence. The detection method is a novel detection reagent which can be recycled. On the other hand, based on the high fluorescence quantum efficiency characteristics of the BODIPY compounds, the detection reagent has high sensitivity and lower detection limit.
Owner:INST OF CHEM CHINESE ACAD OF SCI
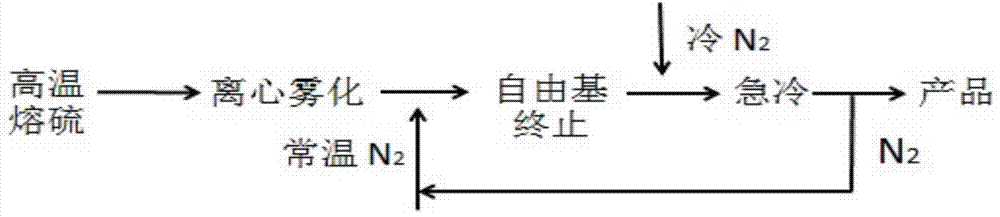
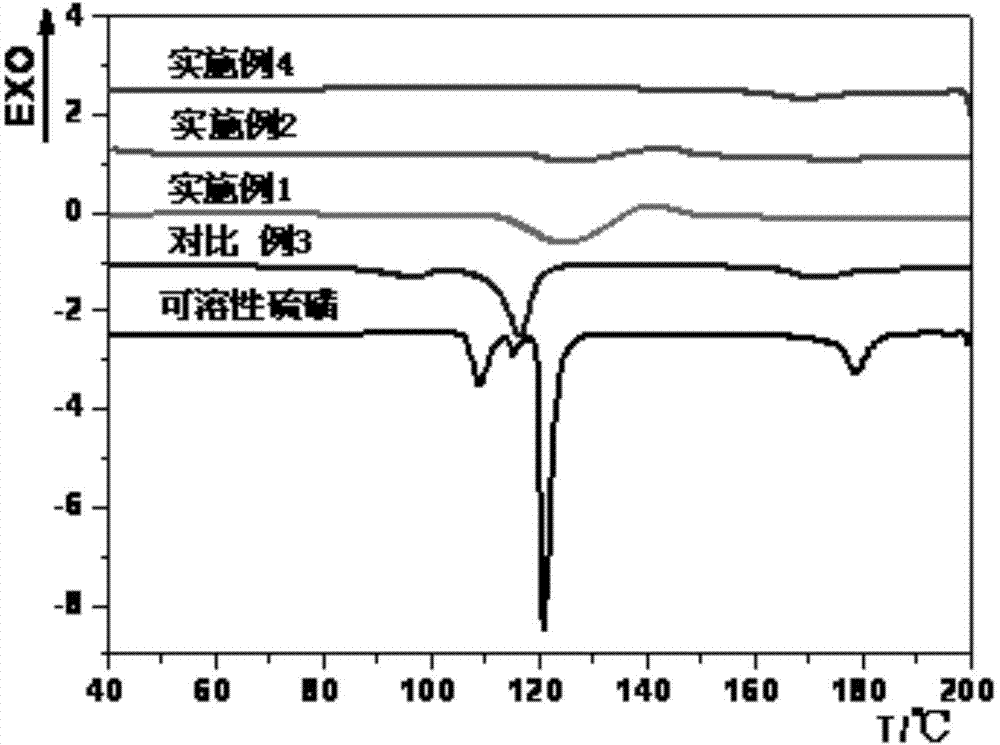
Method for preparation of insoluble sulfur
ActiveCN103539078AIncrease contentSmall particle sizeSulfur preparation/purificationSulfur productDirect effects
The invention relates to a method for preparation of insoluble sulfur. In a production process of insoluble sulfur, rapid cooling is one of very key process steps, realizes an effect of instantly stopping a reversible reaction and further directly affects the content of insoluble sulfur in a product. The method provided by the invention comprises the following steps of directly heating raw material sulfur to 280-400 DEG C, forming a large number of sulfur liquid droplets by centrifugal atomization through an atomizer, and increasing the specific surface area of sulfur; then cooling to 180-240 DEG C by circulating nitrogen at room temperature and keeping for 2-25 seconds; further cooling by circulating cold nitrogen to below 60 DEG C within 2-20 seconds; performing cyclone separation, collecting and packaging to obtain the insoluble sulfur product, wherein the average particle size of the product is 10 mu m-50 mu m; recycling the separated nitrogen. The method provided by the invention has the advantages of high safety in operation, low toxicity, high production efficiency, easiness in industrialization and high stability of prepared insoluble sulfur.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of tadalafil
The invention discloses a preparation method of tadalafil. The concrete preparation method is used for successfully synthesizing tadalafil by taking L-tryptophan methyl ester hydrochloride as an initial raw material and utilizing the characteristics that an ortho-position of an ester group has the chiral inversion property under an alkaline condition, the reaction between ester group and Pictet-Spengler is reversible reaction, and the ester group is easily converted into a cis-form product with relatively small solubility, therefore, the preparation method is a brand new technology. The price of L-tryptophan methyl ester hydrochloride is only less than 1 / 5 of that of D-tryptophan methyl ester hydrochloride, so that the cost of the overall route is greatly lower than that of the prior art, and then the preparation method is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
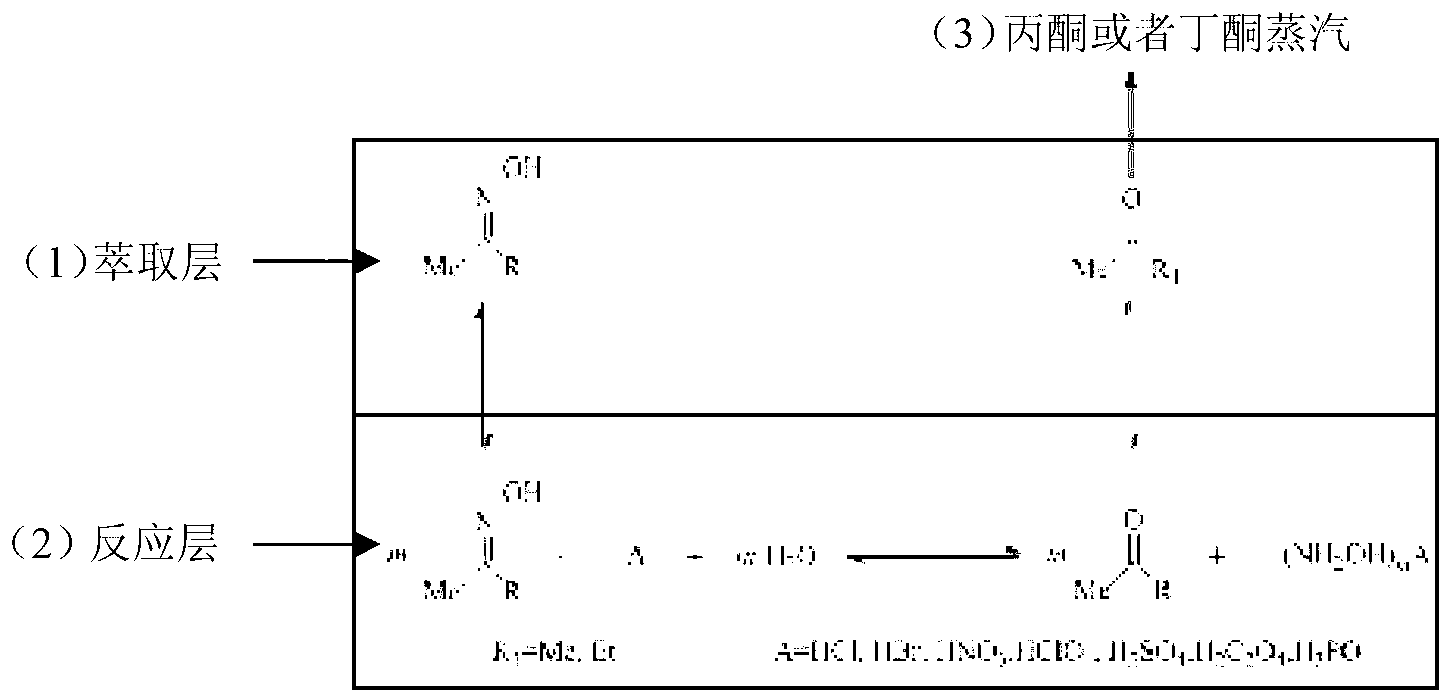
Method of preparing hydroxylamine salt by using reaction-extractive distillation coupling technology
ActiveCN103318858AHigh yieldReduce generationHydroxylamineCarboxylic acid salt preparationHydroxylamineExtractive distillation
The invention provides a method of preparing hydroxylamine salt by using reaction-extractive distillation coupling technology. The method comprises the steps of performing a hydrolysis reversible equilibrium reaction to acetoxime / butanone oxime; using reaction-extraction-distillation ternary coupling technology; extracting, distilling and separating acetone (or butanone) as one of reaction products continuously to make it leave the reactant, so as to break the limit of the reaction equilibrium to the reversible reaction, thereby the raw material conversion rate is greatly lifted, the target product hydroxylamine salt is constantly generated and collected in water phase, and the yield is high. The yield of hydroxylamine hydrochloride is 98%, and the yield of other hydroxylamine salts is over 90%; at the same time, reaction product acetone or butanone is separated as the synthesis raw material of acetoxime (or butanone oxime), and the cycle utilization technology is realized.
Owner:CHANGZHOU UNIV
Process for preparing nano ZnO
InactiveCN1884091ASimple methodLow costNanostructure manufactureZinc oxides/hydroxidesNanowireBand shape
The invention discloses a preparing method of ZnO nanometer structure etched by sulfourea solution; which comprises the following steps: synthesizing ZnO nanometer comb-shaped structure and nanometer bar through traditional heat-growth method; etching through sulfourea solution; preparing ZnO nanometer needle-tube structure and nanometer band shaped structure; dissolving sulfourea in the deionized water to proceed reversible reaction to produce weak hydrosulfuric acid. The reaction formula is that ZnO+H+-Zn++H2O to realize bottom-up and top-down ZnO nanometer structure. The invention is convenient to control diameter of nanometer line and thickness with low cost, which is compatible with micro-electronic technology.
Owner:EAST CHINA NORMAL UNIV
Liquid carbon dioxide absorbents, methods of using the same, and related systems
A carbon dioxide absorbent composition is described, including (i) a liquid, nonaqueous silicon-based material, functionalized with one or more groups that either reversibly react with CO2 or have a high-affinity for CO2; and (ii) a hydroxy-containing solvent that is capable of dissolving both the silicon-based material and a reaction product of the silicon-based material and CO2. The absorbent may be utilized in methods to reduce carbon dioxide in an exhaust gas, and finds particular utility in power plants.
Owner:AIR PROD & CHEM INC
Novel amino thermochemical energy storage system
ActiveCN104806311ARealize cascade utilizationEfficient use ofExothermal chemical reaction heat productionEnergy inputThermal energyChemical reaction
The invention discloses a novel amino thermochemical energy storage system. An amino thermochemical reversible reaction is adopted and the energy storage is performed through the mutual conversion among the electric energy, the thermal energy and the chemical energy, wherein the formula is as follows. The novel amino thermochemical energy storage system mainly comprises a storage unit, an energy storage unit, an energy release unit, a thermal storage tank and the like. During energy storage, a heat absorption decomposition reaction of the ammonia is produced under the action of a catalyst and the accepted energy is stored in the gaseous decomposition product nitrogen and hydrogen in forms of chemical energy and pressure energy through compression. During energy release, a reversible thermochemical reaction of the nitrogen and the hydrogen is produced under the action of a catalyst, the stored chemical energy is converted into the high grade thermal energy to be released for steam power generation, and the power generation is performed through reaction product gas expansion. The novel amino thermochemical energy storage system has the advantages of being high in energy storage density, high in efficiency, environmentally-friendly, reliable in application and suitable for various types of power stations comprising renewable energy power stations.
Owner:NANJING UNIV OF TECH
Hydrogen peroxide sensors based upon photo-induced electron transfer
ActiveUS20120183984A1Useful in detectionSufficient amountOrganic compound preparationMicrobiological testing/measurementAnalyteAbsorbed energy
The invention provides compounds of formula I F-L-Q (I) where F comprises a fluorophore capable of absorbing energy at an excitation wavelength and, in the absence of a quencher, emitting energy at an emission wavelength, which is different than the excitation wavelength; Q comprises a quencher; L comprises a linker moiety having two ends, one end being covalently bound to F and the other end being covalently bound to Q. The compounds are capable of undergoing a reversible reaction (1), provided below: (1) where Q+ is an oxidized form of Q representing the absence of a quencher, Ox comprises an oxidizing agent, which is capable of oxidizing Q to its oxidized form Q, and Red comprises a reducing agent, which is capable of converting Q back to its reduced form Q. The compounds can undergo photo-induced electron transfer when irradiated with energy and when Q exists in its oxidized form, Q+. The invention also provides methods of detecting and determining the presence of analytes and / or hydrogen peroxide in a sample, as well as a substrate that comprises the compound of formula I.
Owner:OPTI MEDICAL SYST
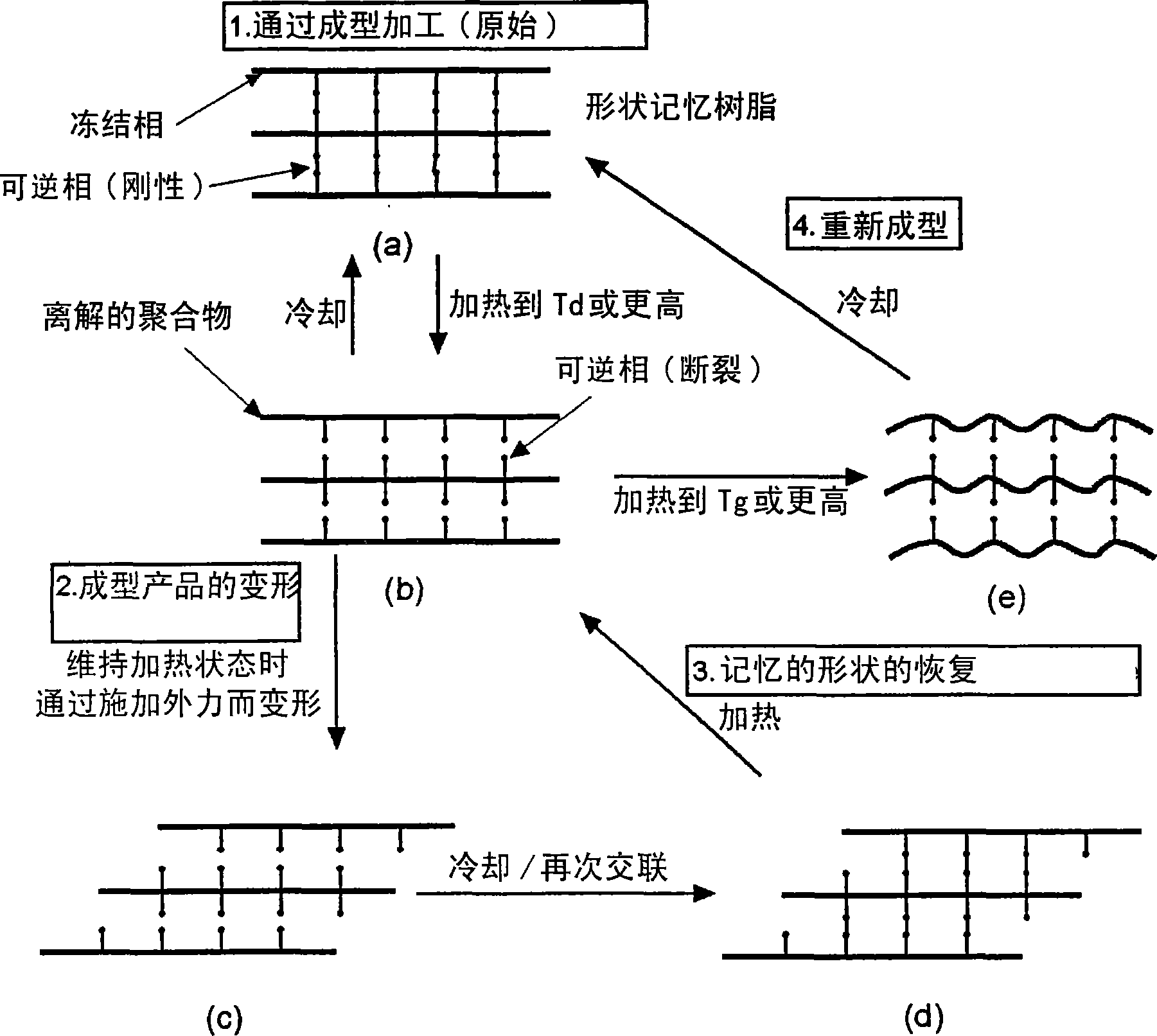
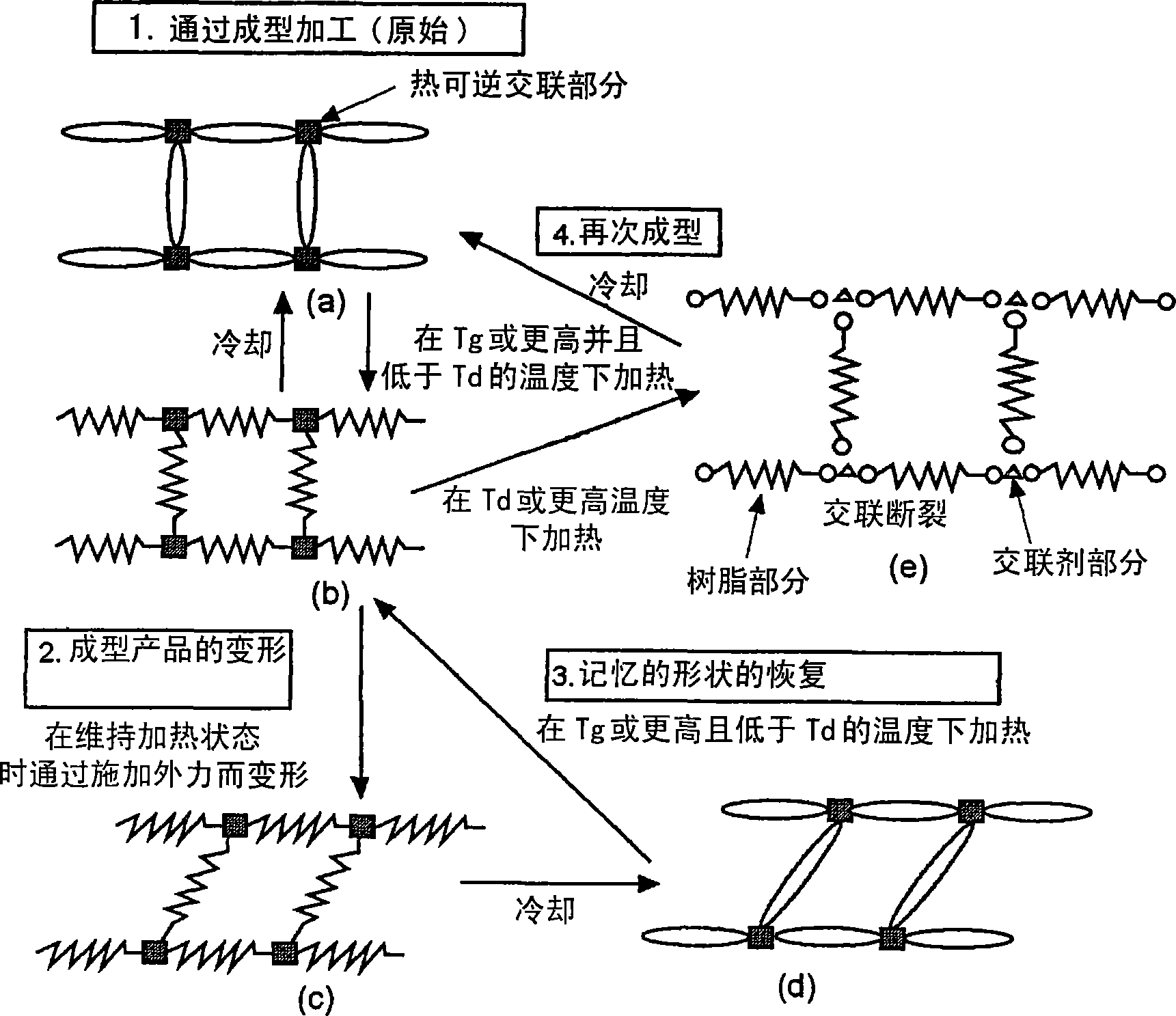
Reshapable shape-memory resin excelling in shape recovery capability and shaped item of the resin having been crosslinked
InactiveCN1894313AExcellent shape recovery performanceEasy to shapeProductsReagentsCross-linkReversible reaction
It is possible to provide a shape-memory molded product having excellent shape-memory properties and recycling efficiency by using a shape-memory resin having a glass transition temperature (Tg) within the range of 40 DEG C. to 200 DEG C. and a dissociation temperature (Td) of a thermo-reversible reaction within the range of 50 DEG C. to 300 DEG C. and satisfying the relationship: Tg+10 DEG C.<=Td, wherein the resin is deformed at a temperature of Tg to less than Td, and cross-linked through a thermo-reversible reaction in which a covalent bond is formed by cooling and dissociated by heating.
Owner:NEC CORP
Urea-formaldehyde resin additive, preparing method and application thereof
InactiveCN101585926AIncrease preloadImprove stabilityNon-macromolecular adhesive additivesMacromolecular adhesive additivesAmmonium compoundsReversible reaction
The present invention an urea-formaldehyde resin additive, a preparing method and an application thereof. The urea-formaldehyde resin additive comprises the following components: ammonium salt, polyhydroxy compound, pH buffering agent and filling agent. The urea-formaldehyde resin additive of the invention contains ammonium compound which reacts with the formaldehyde and generates urotropine. The reaction is reversible reaction and facilitates controlling the speed of polycondensation reaction so that the polycondensation reaction is executed uniformly for obtaining the urea-formaldehyde resin with homogeneous molecular weight distribution and the increase of the stability of the urea-formaldehyde resin is facilitated. The contained pH buffering agent increases the buffer capacity of the urea-formaldehyde resin and facilitates prolonging the pot life of the urea-formaldehyde resin. In the preparing process of urea-formaldehyde resin, the adding of the urea-formaldehyde resin additive can remarkably increase the prepressing performance of the adhesive and shorten the prepressing time. The prepared urea-formaldehyde resin adhesive has the advantages of high stability, greatly prolonged pot life, capacity for totally satisfying the production requirement of the veneer board and effectively increased production efficiency.
Owner:EVERFIRST WISEFUND TECH BEIJING
Dual-ion battery and preparation method thereof
ActiveCN108155408AIncrease capacityImprove cycle performanceFinal product manufactureCell electrodesUltrasound attenuationElectrical battery
The invention provides a dual-ion battery and a preparation method thereof, and relates to the field of batteries. The dual-ion battery comprises a positive electrode, a negative electrode, a diaphragm and electrolyte, wherein the diaphragm and the electrolyte are arranged between the positive electrode and the negative electrode. The negative electrode comprises negative electrode active materials which are organic materials capable of performing reversible reaction with positive ions in the electrolyte. The dual-ion battery can solve the technical problems of non-ideal electrochemical performance and low capacity of existing dual-ion batteries as well as cyclic stability attenuation caused by serious volume expansion or pulverization of aluminum negative electrodes in charging and discharging processes. The dual-ion battery is excellent in electrochemical performance, high in capacity and stable in cyclic performance.
Owner:SHENZHEN INST OF ADVANCED TECH
Reversible self-repair antibacterial acrylic coating and preparation and self-repair methods
ActiveCN106928797AReversible self-healingAntibacterialAntifouling/underwater paintsPaints with biocidesMethacrylateBetaine
The invention discloses a reversible self-repair antibacterial acrylic coating and preparation and self-repair methods. The preparation method comprises the steps of adding methyl methacrylate, butyl acrylate, sulfonyl betaine methacrylate and acetoacetoxy methacrylate gylcol ester into an alcohol solvent, and performing free radical polymerization under the initiation of azodiisobutyronitrile to obtain linear acrylic resin; and dissolving the linear acrylic resin and amino-containing hyperbranched polysiloxane into the alcohol solvent, coating the surface of a base material, and drying, thus obtaining the reversible self-repair antibacterial acrylic coating. Efficient and mild repair of the coating is realized by using the characteristic of reversible reaction of vinylogous urethane bonds at room temperature and amino rich at the terminal of the hyperbranched polysiloxane, and the acrylic coating is endowed with excellent antibacterial property by the safe, nontoxic and non-specific sulfonyl betaine. The product has good applicability and strong practicability; the preparation method has the characteristics of wide raw material source, simple process and environment friendliness.
Owner:SUZHOU UNIV
Negative active material for lithium secondary battery, method of preparing thereof, and lithium secondary battery including same
InactiveUS20080286656A1Improve high-speed performanceMaterial nanotechnologyNegative electrodesLithiumParticulates
A negative active material for a lithium secondary battery according to an embodiment of the present invention includes a core material including an inorganic particulate that is capable of forming a compound by a reversible reaction with lithium, and a surface-treatment layer disposed on the surface of the core material. The surface-treatment layer includes a metal having electronic conductivity of 103 S / cm or more. The negative active material can improve high-rate performance of a lithium secondary battery.
Owner:KUMOH NAT INST OF TECH IND ACADEMIC COOPERATION FOUND +1
Desulfurizer and its application
InactiveCN107353929AImprove desulfurization efficiencyGet rid of usabilityWater contaminantsGaseous fuelsSodium acetateSulfide
The invention discloses a desulfurizer, which is suitable for crude oil exploitation, gathering and transportation and treatment of sulfur-containing sewage. The desulfurizer is composed of the following components in mass percentage: 10-20% of N-methyl triethanolamine, 10-20% of ferric ammonium EDTA, 10-20% of hydroxyethyl hexahydro-s-triazine, and 1-2% of acetic acid , sodium acetate 0.1-0.2%, sodium nitrilotriacetate 0.1-0.2%, penetrant 1-2%, antifreeze agent 5-10%, defoamer 0.02-0.04%, and the balance is water. The desulfurizer provided by the invention uses ferric ammonium EDTA and hydroxyethyl hexahydro-s-triazine as main agents, and the s-triazine reacts with hydrogen sulfide to generate water-soluble sulfides to remove hydrogen sulfide through partial branched chain group substitution reaction. The reaction speed is fast, the reversible reaction is greatly reduced, the desulfurization efficiency is high at high temperature, and there is basically no reversible hydrogen sulfide release, thus getting rid of the use restriction and performance restriction of alcohol amine desulfurizers by temperature.
Owner:付增华
Preparation method and application for double-response bi-crosslinked injectable hydrogel used for fine-controlled release of insulin
InactiveCN105726463AMaintain biological activityReduce churnPeptide/protein ingredientsMetabolism disorderConcentrations glucoseBiocompatibility Testing
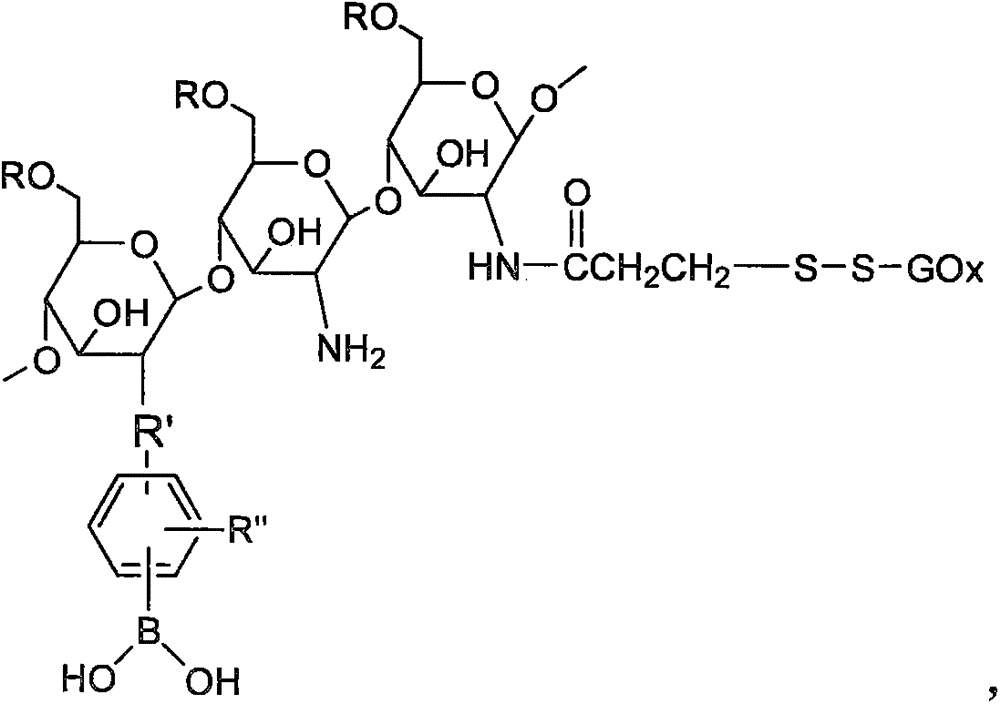
The invention relates to preparation and applications for double-response bi-crosslinked injectable hydrogel used for fine-controlled release of insulin. A chitosan derivative having excellent biocompatibility, biodegradability and antibacterial property is employed as a basic high molecule, and two dynamic bonds comprising imine bond and phenylboronate are employed for preparing pH / glucose bi-sensitive injectable hydrogel, and meanwhile bio-active molecules, such as glucose oxidase and the like, are introduced into the gel substrate through a disulfide bond and the like dynamic bonds to form a double glucose response system. Through the two glucose response factors, phenylboronate and the glucose oxidase, the concentration change of glucose in external environment can be sensed at the same time, thereby causing reversible reaction of the imine bond and phenylboronate in the system, so that the bi-crosslinked network structure of the hydrogel is changed greatly to release the medicines. High sensitivity and quick response to concentration change of glucose are achieved through double sensing units (the glucose oxidase and the phenylboronic acid group) and double accepting units (the imine and phenylboronate).
Owner:NINGBO UNIV
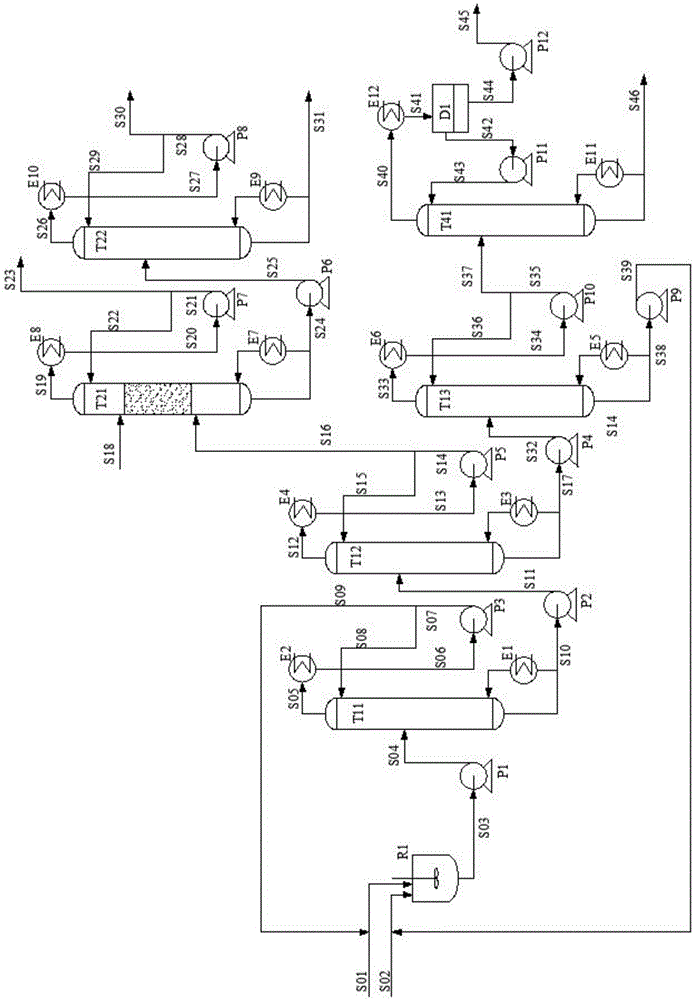
Method, process and apparatus for separation of ethylene glycol and 1,2-butanediol
ActiveCN105622338AHigh purityEfficient separationOrganic compound preparationHydroxy compound separation/purificationLiquid productBoiling point
The invention relates to a method, a process and an apparatus for separation of ethylene glycol and 1,2-butanediol. The method includes, (1) subjecting ethylene glycol and 1,2-butanediol to reaction through acetal / ketal to produce acetal / ketal liquid product mixture correspondingly, (2) separating the acetal / ketal liquid product mixture containing ethylene glycol and 1,2-butanediol by a series of rectifying columns, (3) separating different acetal / ketal products by rectifying according to difference of boiling points of acetal or ketal, (4) respectively hydrolyzing the acetal / ketal products to obtain an ethylene glycol primary product and a 1,2-butanediol primary product, and (5) purifying the ethylene glycol primary product and the 1,2-butanediol primary product respectively by rectifying to obtain an ethylene glycol product and a 1,2-butanediol product. The purity of the ethylene glycol product can be up to 99.9% and the recovery thereof can be up to 99.5%; the purity of the 1,2-butanediol product can be up to 98.5%. By a reversible-reaction conversion method, the difficulty in separating ethylene glycol and 1,2-butanediol which are of similar boiling points and low relative volatility and are azeotropic is changed into the problem about separation of acetal / ketal products which are easy to separate relatively.
Owner:TIANJIN UNIV
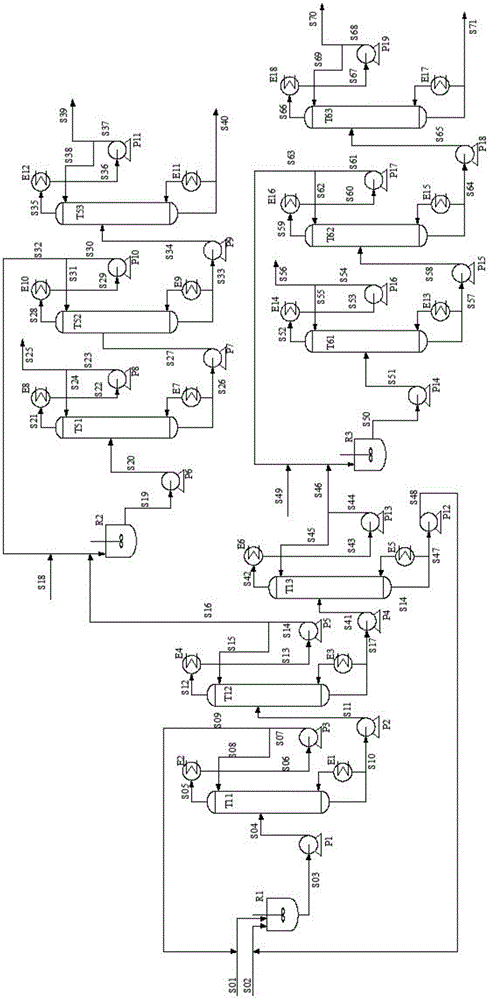
Reaction-rectification-separation-refinement novel method, technique and device of ethylene glycol and 1,2-butanediol
ActiveCN105541551AHigh purityEfficient separationOrganic compound preparationChemical industryKetoneReversible reaction
The invention relates to a separation method, technique and device of ethylene glycol and 1,2-butanediol. The method comprises the following steps: reacting a diol mixture and aldehyde or ketone in a reaction rectification tower by acetal or ketal reversible reaction to generate an acetal / ketone mixture, rectifying to separate the acetal / ketone mixture, and carrying out reaction, rectification and hydrolysis to obtain the high-purity ethylene glycol and 1,2-butanediol products. By using the technique for separation and purification, the purity of the main product ethylene glycol can reach 99.9% or above, the recovery rate can reach 99.5% or above, and the purity of the 1,2-butanediol product can reach 98.5% or above. By using the reaction rectification method, the difficulty in separating azeotropic ethylene glycol and 1,2-butanediol with approximate variable boiling point and low relative volatility becomes the problem of separation of the separable acetal / ketone product, thereby effectively implementing the high-efficiency separation on the ethylene glycol and 1,2-butanediol.
Owner:TIANJIN UNIV
Method for regulating and controlling stress relaxation and reprocessing molding temperature of vitrimer material by using content of dynamic bonds
The invention proposes a method for regulating and controlling stress relaxation and reprocessing molding temperature of a vitrimer material by using the content of dynamic bonds. The vitrimer is a novel crosslinked polymer material, and the material uses a reversible reaction of the exchangeable dynamic bonds in a crosslinked network to realize stress relaxation and reprocessing molding of the material and maintain the integrity of the crosslinked network. The method accelerates the stress relaxation of the polymer and reduces the reprocessing molding temperature of the polymer by improving the content of the exchangeable dynamic bonds in the cross-linked network of the vitrimer material, and the method slows down the stress relaxation of the polymer and improves the reprocessing moldingtemperature of the polymer by reducing the content of the exchangeable dynamic bonds in the cross-linking network, so that the method realizes the purpose of regulating and controlling the stress relaxation and reprocessing molding temperature of the vitrimer material by using the content of the dynamic bonds; and the method proposed by the invention provides a novel way for regulation and controlof properties such as stress relaxation and reprocessing molding of the vitrimer, and promotes a practical application of such materials.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Water electrolysis hydrogen producing process by cooling filtering method
The invention belongs to a water electrolysis hydrogen producing process, and relates to a water electrolysis hydrogen producing process by a cooling filtering method. The current hydrogen producing process has the defects that the H2 and O2 mixed gas can form reverse reaction easily under a suitable condition, the gas yield is reduced, the adjustability of gas is poor, and the use safety is low. In the process of the invention, the hydrogen and oxygen electrolyzed in an electrolytic bath (2) respectively enter a primary gas-water separator (3) and a primary gas-water separator (4) through respective pipes, and then enter a cooler (5), a secondary gas-water separator (6) and a secondary gas-water separator (7) for carrying out gas-water separation again; the hydrogen enters a washer (10); electrolyte separated at the lower part of the secondary gas-water separator enters an electrolyte storage tank (1); and a replenishment pump (9) pressurizes and replenishes the electrolyte in the storage tank (1) into the system. The invention has the advantages that the reversible reaction of gas is avoided, the gas production rate and the gas production efficiency are greatly improved, the gas can be conveniently adjusted in use, no auxiliary materials need to be added when the gas is used, the safety is improved, and the use cost is reduced.
Owner:SHANGHAI COCH ENERGY
Method for one-step preparation of insoluble sulfur
ActiveCN103539079AImprove production safetySave energySulfur preparation/purificationEnvironmental resistanceSulfur product
The invention relates to a method for one-step preparation of insoluble sulfur. In a production process of insoluble sulfur, rapid cooling is one of very key process steps, realizes an effect of instantly stopping a reversible reaction and further directly affects the content of insoluble sulfur in a product. The method provided by the invention comprises the following steps of directly heating raw material sulfur to 120-150 DEG C, forming a large number of sulfur liquid droplets by centrifugal atomization through an atomizer, and increasing the specific surface area of sulfur; then heating to 180-240 DEG C by circulating hot nitrogen for ring-opening polymerization, and keeping for 2-25 seconds; further cooling by circulating cold nitrogen, and cooling to below 60 DEG C at 2-20 seconds; performing cyclone separation, collecting and packaging to obtain the insoluble sulfur product, wherein the average particle size of the product is 10 mu m-50 mu m; recycling the separated nitrogen. The method provided by the invention has the advantages of simple process, safety, environmental friendliness and low cost.
Owner:BEIJING UNIV OF CHEM TECH
Stereo display device
The invention discloses a stereo display device. An electrolyte layer is utilized to provide ions for an electrochromatic sheet, and the electrochromatic sheet generates oxidation or reducing reversible reaction to generate color changes which comprise discoloration to become a transparent state or coloring to become an opaque state; when a format order that an image is displayed to be a two-dimensional planar display image is received, an electric field providing device cannot provides an electric field for an electrochromatic device, the electrochromatic sheet is discolored to become the transparent state, and the two-dimensional planar display image is obtained through light rays penetrating through a light-transmitting interval via a first conducting layer; when a format order that the image is displayed to be a three-dimensional stereo display image is received, the electric field providing device provides the electric field for the electrochromatic device, the electrochromatic sheet is colored to become the opaque state, a slit grating is formed through the light rays penetrating through the light-transmitting interval via the first conducting layer, the three-dimensional stereo display image capable of being watched by naked eyes is obtained, and the purpose that a viewer can enjoy the sight of the three-dimensional stereo display image through the naked eyes is achieved.
Owner:SHENZHEN MAGIC EYE TECH CO LTD
Hydrogen occluding material and method for use thereof
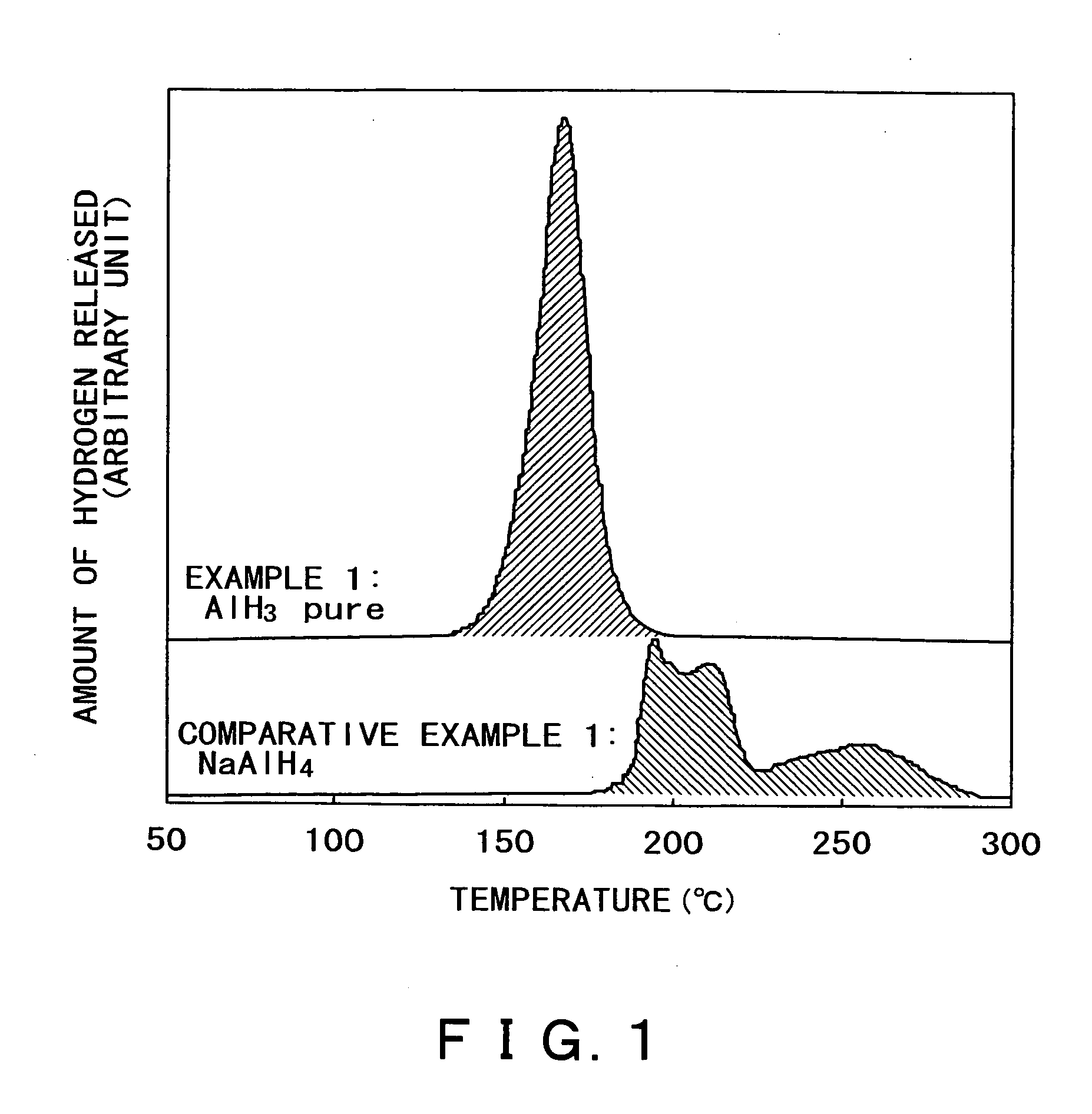
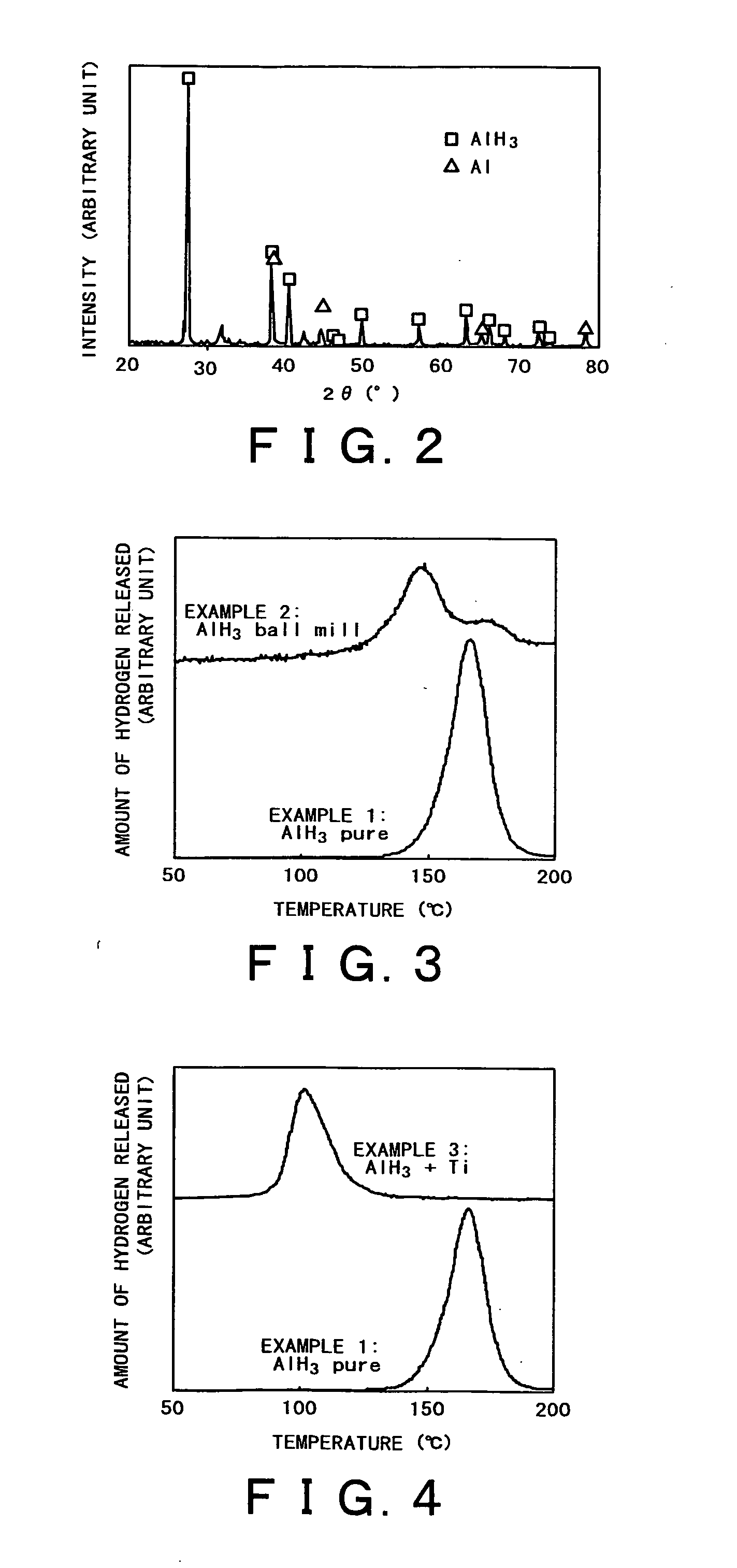
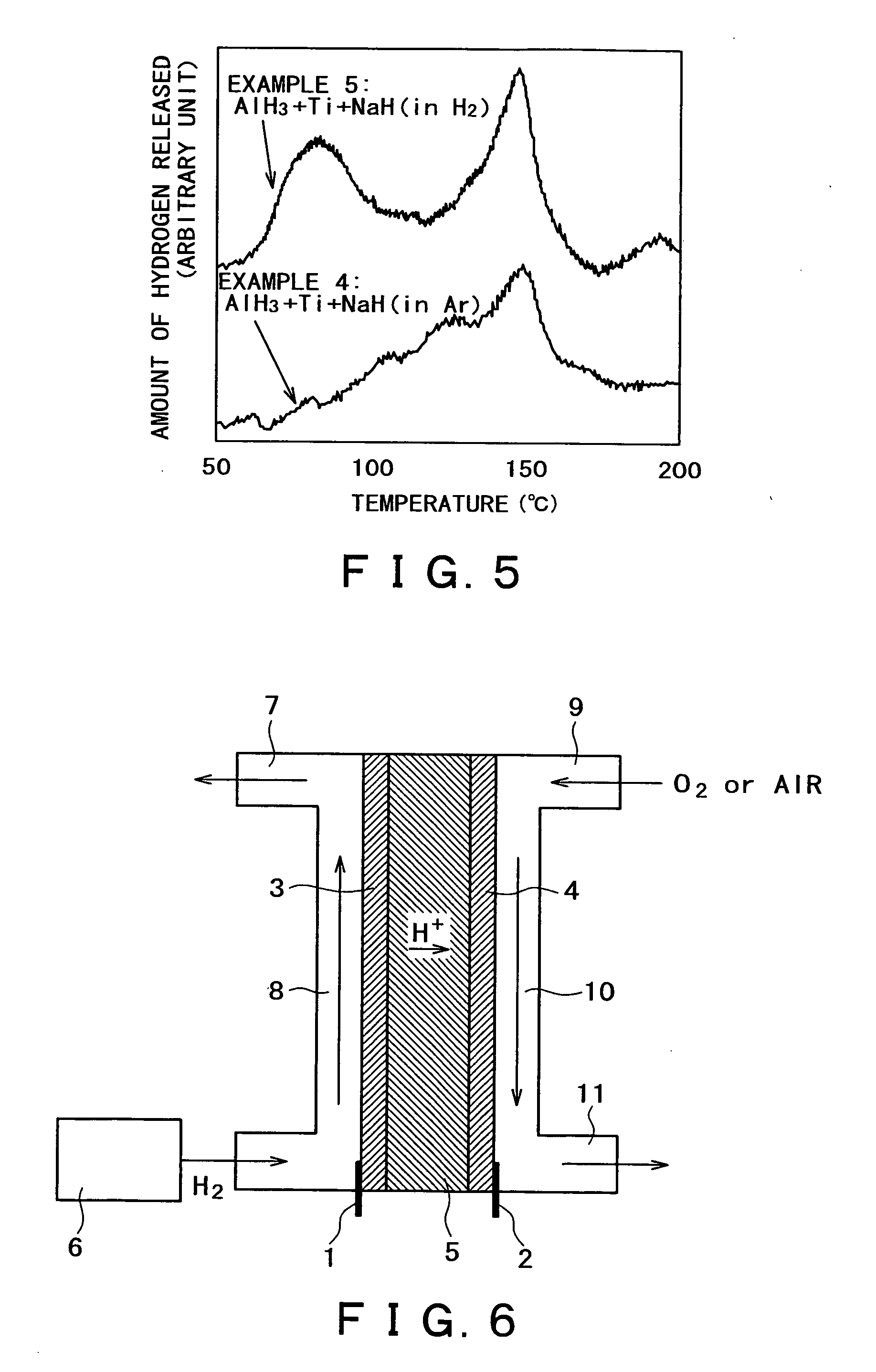
InactiveUS20050164878A1Hydrogen productionMetal/metal-oxides/metal-hydroxide catalystsReversible reactionALUMINUM HYDRIDE
A hydrogen occluding material which occludes much more hydrogen than conventional alkali metal hydride (such as NaAlH4) through reversible reactions and yet permits hydrogen occlusion and release in one stage at a lower operating temperature, and a method for using said hydrogen occluding material are provided. A hydrogen occluding material which comprises an aluminum hydride represented by the formula (1) below. AlHx (1) (where 0≦x≦3.) A method for using a hydrogen occluding material, said method comprising hydrogenating and / or dehydrogenating at 200° C. or below a hydrogen occluding material composed of an aluminum hydride represented by the formula (1) above.
Owner:SONY CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com