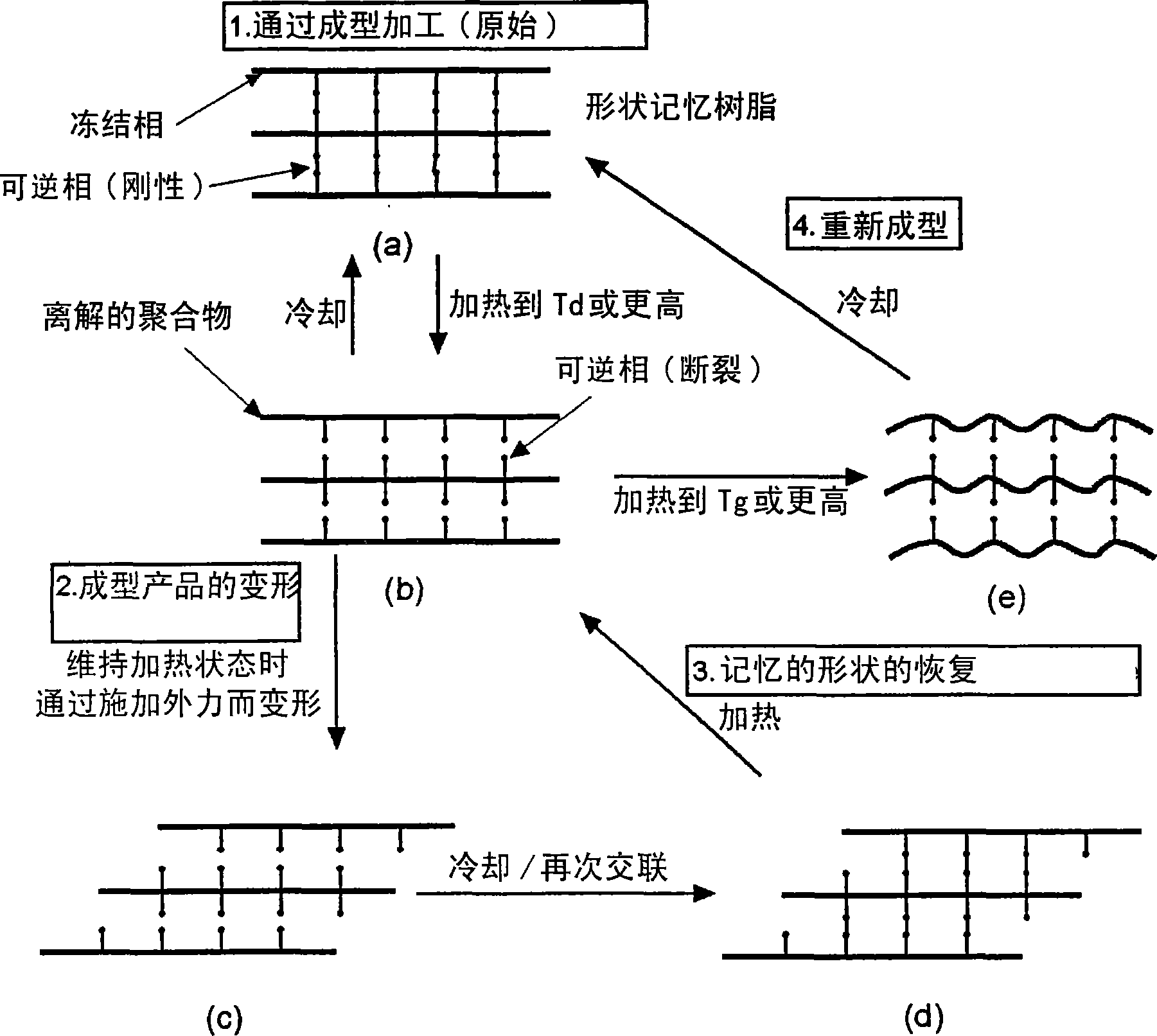
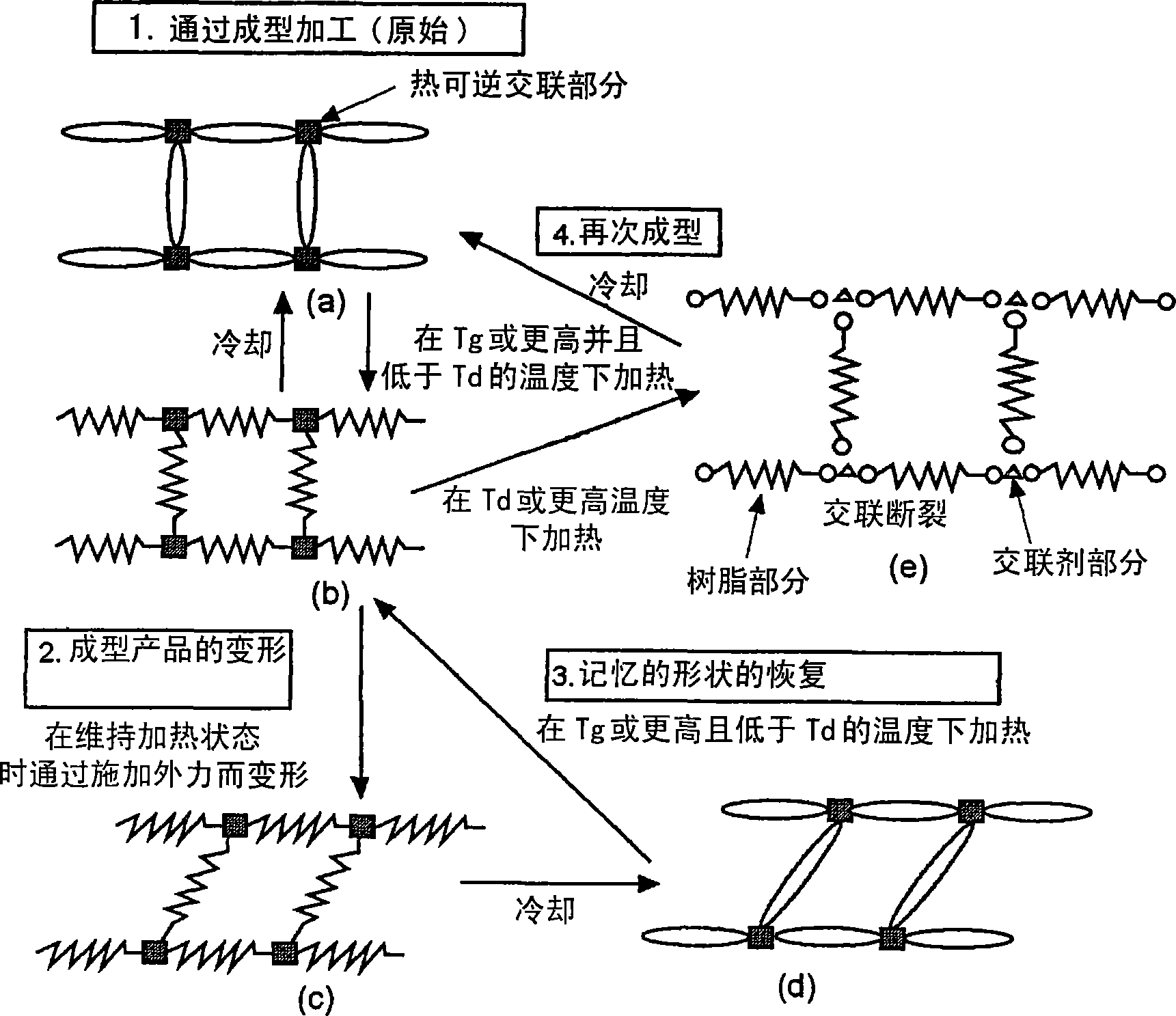
Reshapable shape-memory resin excelling in shape recovery capability and shaped item of the resin having been crosslinked
一种树脂、记忆的技术,应用在产品、运输和包装、塑料回收等方向,能够解决成型产品变形、没有提及生物降解性热固性或光固性树脂回收等问题,达到优异形状恢复性、优异成型和重新成型性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] First, 1000g of commercially available polylactic acid (trade name: Lacty (R) , produced by Shimadzu Corporation) and 75.6 g of pentaerythritol were melt-mixed at 200° C. for 3 hours. In this way, a transesterification reaction is carried out. The resulting mixture was dissolved in 1 L of chloroform and then poured into a large amount of methanol to precipitate hydroxyl-terminated polylactic acid [R1].
[0176] Next, 100 g of 2-furfuryl alcohol, 112 g of succinic anhydride, and 2 ml of pyridine were dissolved in 1 L of chloroform, and heated under reflux for 10 hours. The solution was washed with water, and the solvent was distilled off to synthesize a furan derivative [F1]. Add 31.4g of 1-ethyl-3-(3'-dimethylaminopropyl) carbodiimide hydrochloride (WSC), 13.0g of pyridine, 32.5g of [F1] and 100g of [R1] in 500ml of chloroform, Heat at reflux for 10 hours. The resulting solution was washed with water, dried over magnesium sulfate, and the solvent was distilled off t...
Embodiment 2
[0183] First, 2000 g of commercially available polylactic acid and 178 g of sorbitol were melt-mixed at 200° C. for 15 hours. In this way, a transesterification reaction is carried out. The resulting mixture was dissolved in 2 L of chloroform, and then poured into a large amount of methanol to precipitate polylactic acid [R5] with hydroxyl ends.
[0184] Next, 72 g of WSC, 30.0 ml of pyridine, 74.2 g of [F1] and 132 g of [R5] were added to 400 ml of chloroform, followed by heating under reflux for 43 hours. The solution was washed with water, dried over magnesium sulfate, and thereafter the solvent was distilled off to obtain furan-modified polylactic acid [R6] (molecular weight: 6940).
[0185] Then weigh 10.3g of [R6] and 1.20g of [R3] synthesized above, melt and mix them at 170°C for 3 minutes, and crosslink them by Diels-Alder reaction at 100°C for 1 hour to obtain the crosslinked product of polylactic acid [R7 ]. Tg is 65°C and Td is 155°C. The crosslink density was 0...
Embodiment 3
[0188] First, 2000 g of commercially available polylactic acid and 197 g of sorbitol were melt-mixed at 200° C. for 15 hours. In this way, a transesterification reaction is carried out. The resulting mixture was dissolved in 2 L of chloroform and then poured into a large amount of methanol to precipitate hydroxyl-terminated polylactic acid [R8].
[0189] Next, 72.3 g of WSC, 30.0 ml of pyridine, 74.5 g of [F1] and 120 g of [R8] were added to 500 ml of chloroform, followed by heating under reflux for 43 hours. The solution was washed with water, dried over magnesium sulfate, and thereafter the solvent was distilled off to obtain furan-modified polylactic acid [R9] (molecular weight: 6286).
[0190] Then weigh 10.0 g of [R9] and 1.23 g of [R3] synthesized above, melt and mix them at 170°C for 3 minutes, and crosslink them by Diels-Alder reaction at 100°C for 1 hour to obtain the crosslinked product of polylactic acid [R10 ]. Tg is 70°C and Td is 154°C. The crosslink density ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com