A kind of repaglinide chitosan sustained and controlled release microspheres and preparation method thereof
A chitosan and microsphere technology, applied in the field of medicine and chemical industry, can solve the problems of inability to effectively control hyperglycemia, short gastrointestinal tract existence time, short half-life of repaglinide, etc., and achieve uniform quality, easy operation and control conditions. less demanding effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
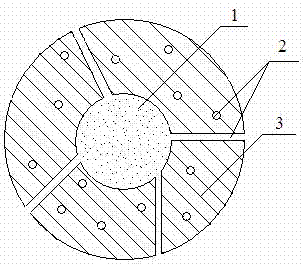
Image
Examples
Embodiment
[0043]
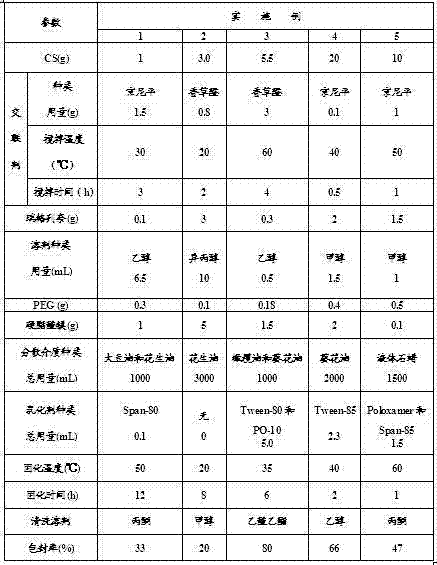
[0044] Embodiments 1-5 are respectively a kind of repaglinide chitosan sustained and controlled release microsphere preparation and preparation method thereof, and the raw material formula of making corresponding preparation is referred to above table, and their preparation method carries out according to the following sequence of steps respectively:
[0045] (1) Preparation of chitosan solution A
[0046] Dissolving chitosan in 2% acetic acid solution, ultrasonically eliminating air bubbles, and preparing chitosan solution A with a weight-to-volume ratio of chitosan and acetic acid solution of 1-6 grams: 100 ml;
[0047] (2) Preparation of Solution D
[0048] dissolving repaglinide in a solvent to prepare solution B of repaglinide;
[0049] Add solution B to chitosan solution A, then add PEG and magnesium stearate, and stir well to obtain mixture C;
[0050] Add the mixture C to the dispersion medium containing the emulsifier, and stir evenly to obtain the solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com