Medicinal preparation containing repaglinide and preparation of medicinal preparation
A pharmaceutical preparation and pharmacy technology, applied in the field of pharmaceutical preparations, can solve the problems of ensuring the dissolution stability of a small dose of insoluble drug repaglinide without any measures, and achieve the effects of good content uniformity, increased dissolution rate, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
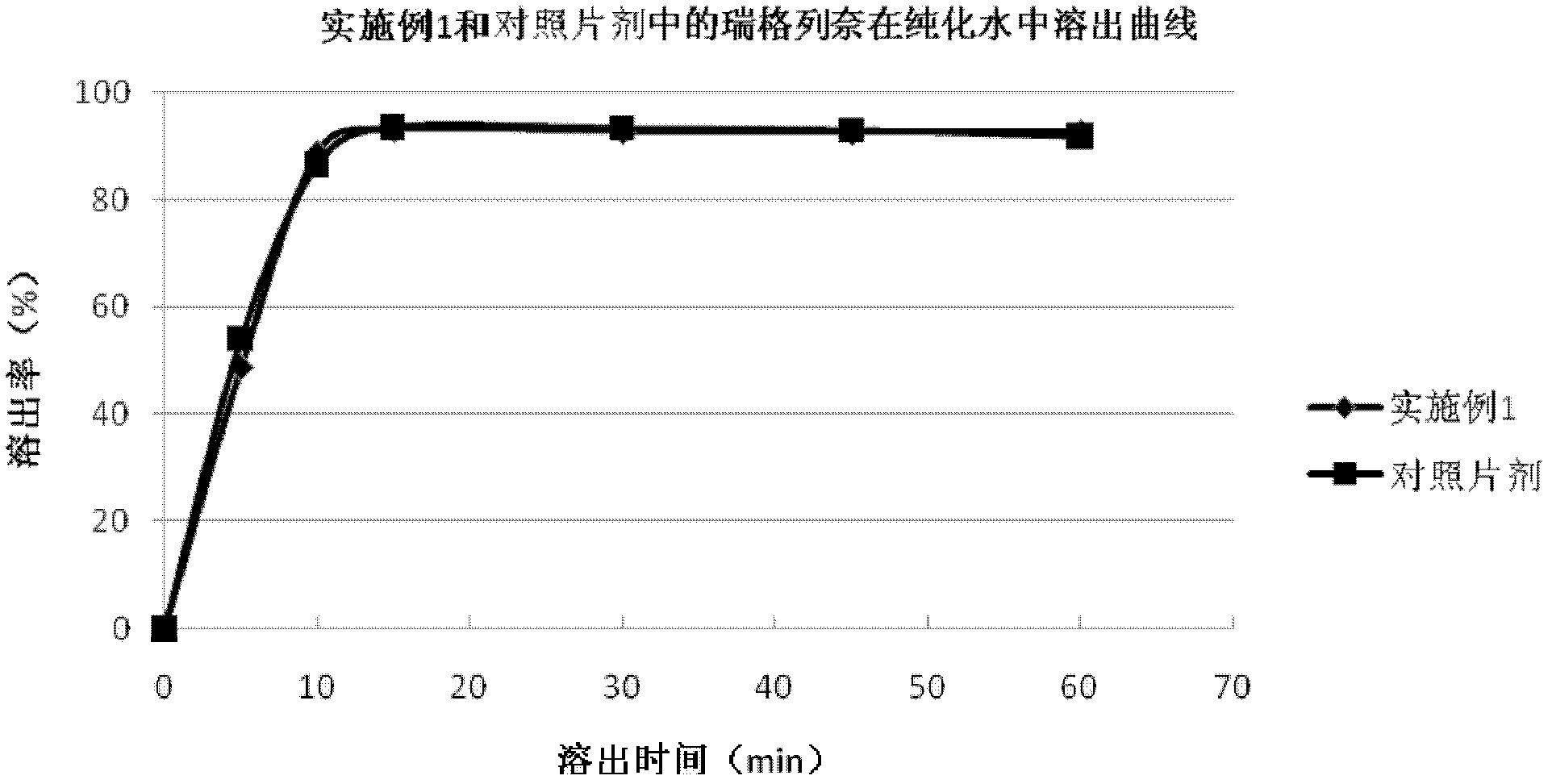
Embodiment 1
[0050]
[0051] Preparation:
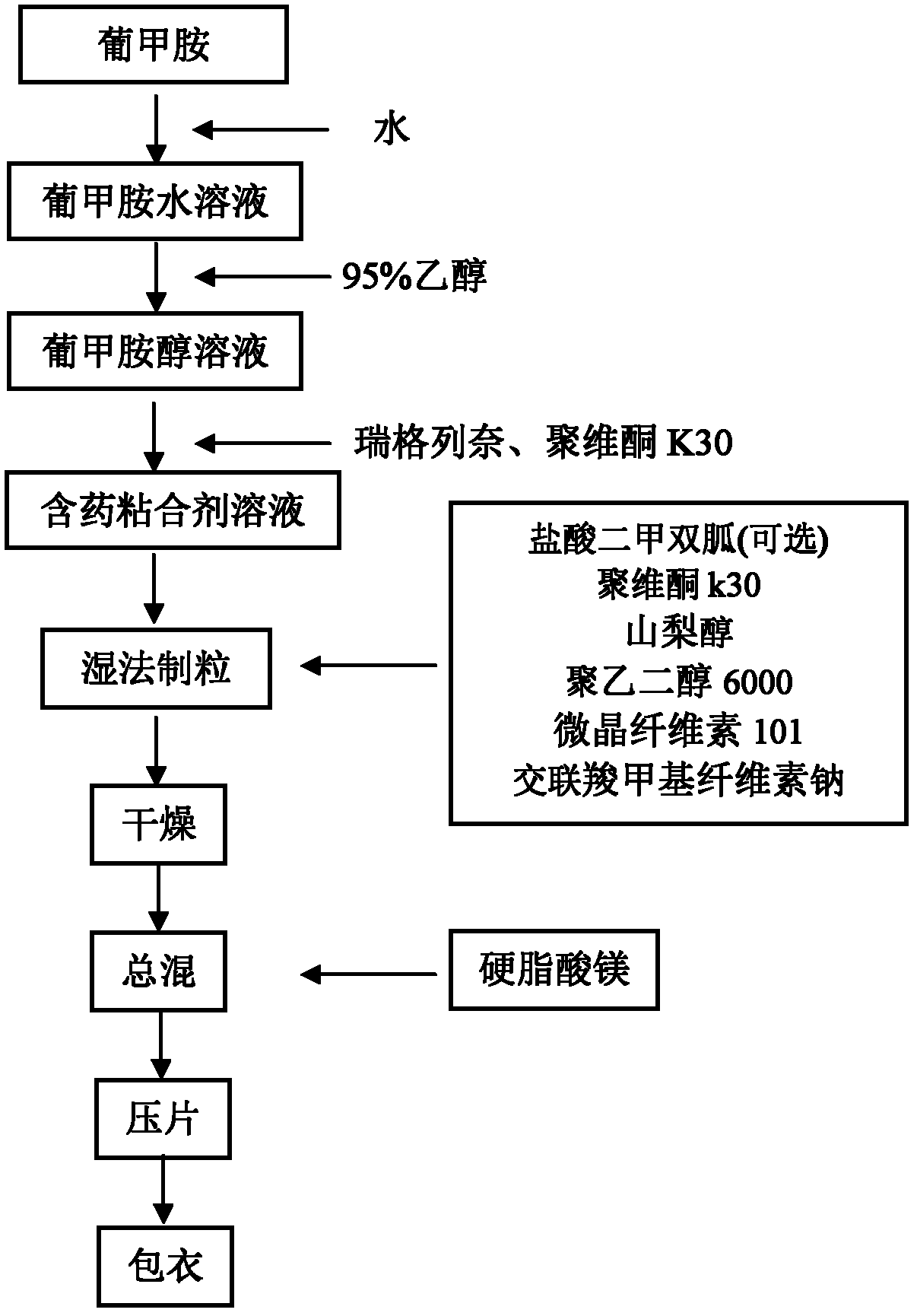
[0052] Weigh the prescribed amount of meglumine, dissolve in water and then add 95% ethanol, slowly add the prescribed amount of repaglinide, poloxamer 188 and povidone k30, stir to dissolve, and set aside. Mix the prescribed amount of metformin hydrochloride, povidone k30, sorbitol, polyethylene glycol 6000, microcrystalline cellulose 101 and croscarmellose sodium, add the drug-containing binder solution to make a soft material, 28 Mesh sieve for granulation.
[0053] Granulation conditions: temperature 18-26°C, relative humidity 45-65%; stirring speed: 130±30rpm; granulation time: 5 minutes.
[0054] After the granulation is completed, ventilate and dry at 40°C until the water content is 2-3%, add magnesium stearate and mix evenly, press into tablets, make Opadry 295F620019 into a 10% aqueous solution, and coat the product with a weight gain of 3%.
Embodiment 2
[0056]
[0057] Preparation:
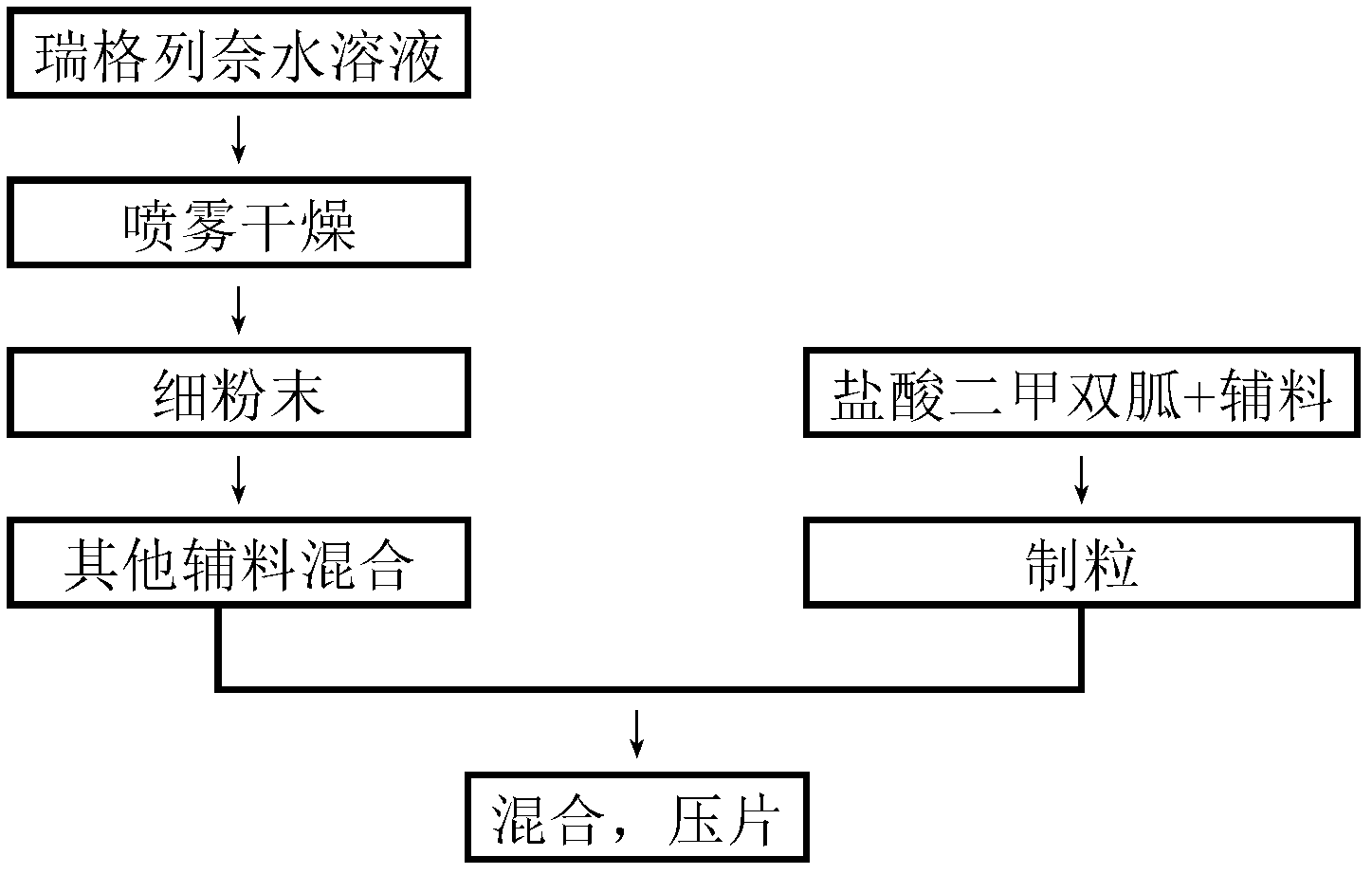
[0058] Take the prescription amount of meglumine, add 95% ethanol after dissolving in water, slowly add the prescription amount of repaglinide and povidone k30, stir to dissolve, set aside. Mix the prescribed amount of metformin hydrochloride, povidone k30, sorbitol, polyethylene glycol 6000, microcrystalline cellulose 101 and croscarmellose sodium, add the drug-containing binder solution to make a soft material, 28 Mesh sieve for granulation.
[0059] Granulation conditions: temperature 18-26°C, relative humidity 45-65%; stirring speed: 130±30rpm; granulation time: 5 minutes.
[0060] After the granulation is completed, ventilate and dry at 40°C until the water content is 2-3%, add magnesium stearate and mix evenly, press into tablets, make Opadry 295F620019 into a 10% aqueous solution, and coat the product with a weight gain of 3%.
Embodiment 3
[0062]
[0063] Preparation:
[0064] Take the prescription amount of meglumine, add 95% ethanol after dissolving in water, slowly add the prescription amount of repaglinide and povidone k30, stir to dissolve, set aside. Mix the prescribed amount of metformin hydrochloride, povidone k30, mannitol, microcrystalline cellulose 101 and sodium carboxymethyl starch evenly, add the drug-containing binder solution to make a soft material, and granulate with a 28-mesh sieve.
[0065] Granulation conditions: temperature 18-26°C, relative humidity 45-65%; stirring speed: 130±30rpm; granulation time: 5 minutes.
[0066] After the granulation is completed, ventilate and dry at 40°C until the water content is 2-3%, add magnesium stearate and mix evenly, press into tablets, make Opadry 295F620019 into a 10% aqueous solution, and coat the product with a weight gain of 3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com