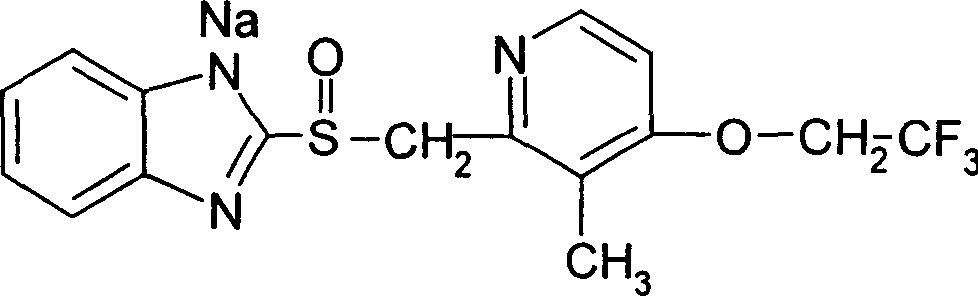
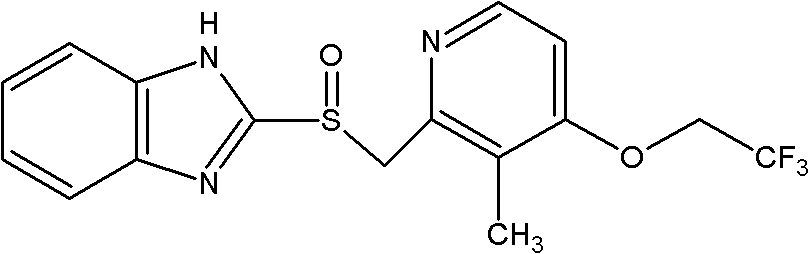
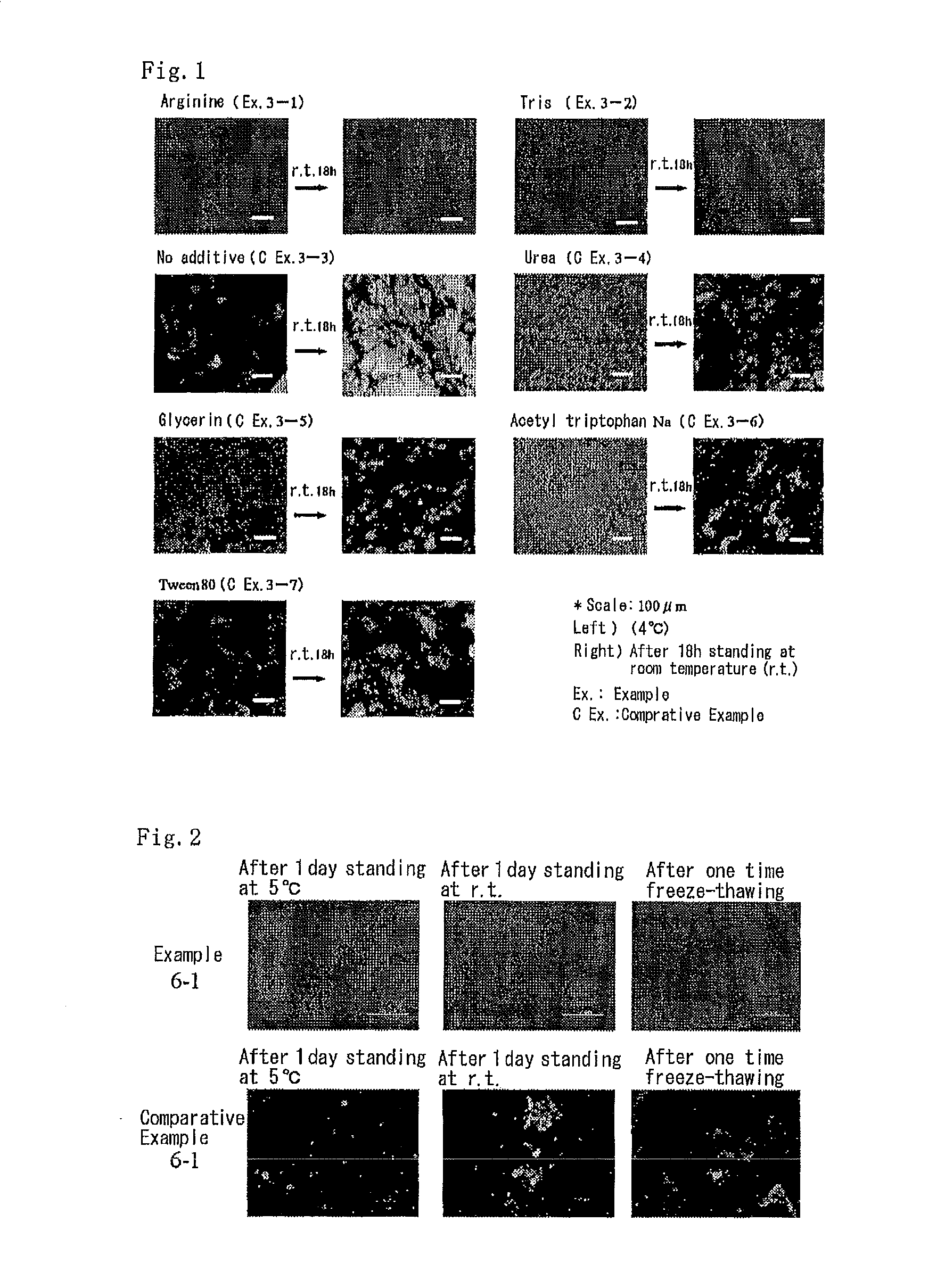
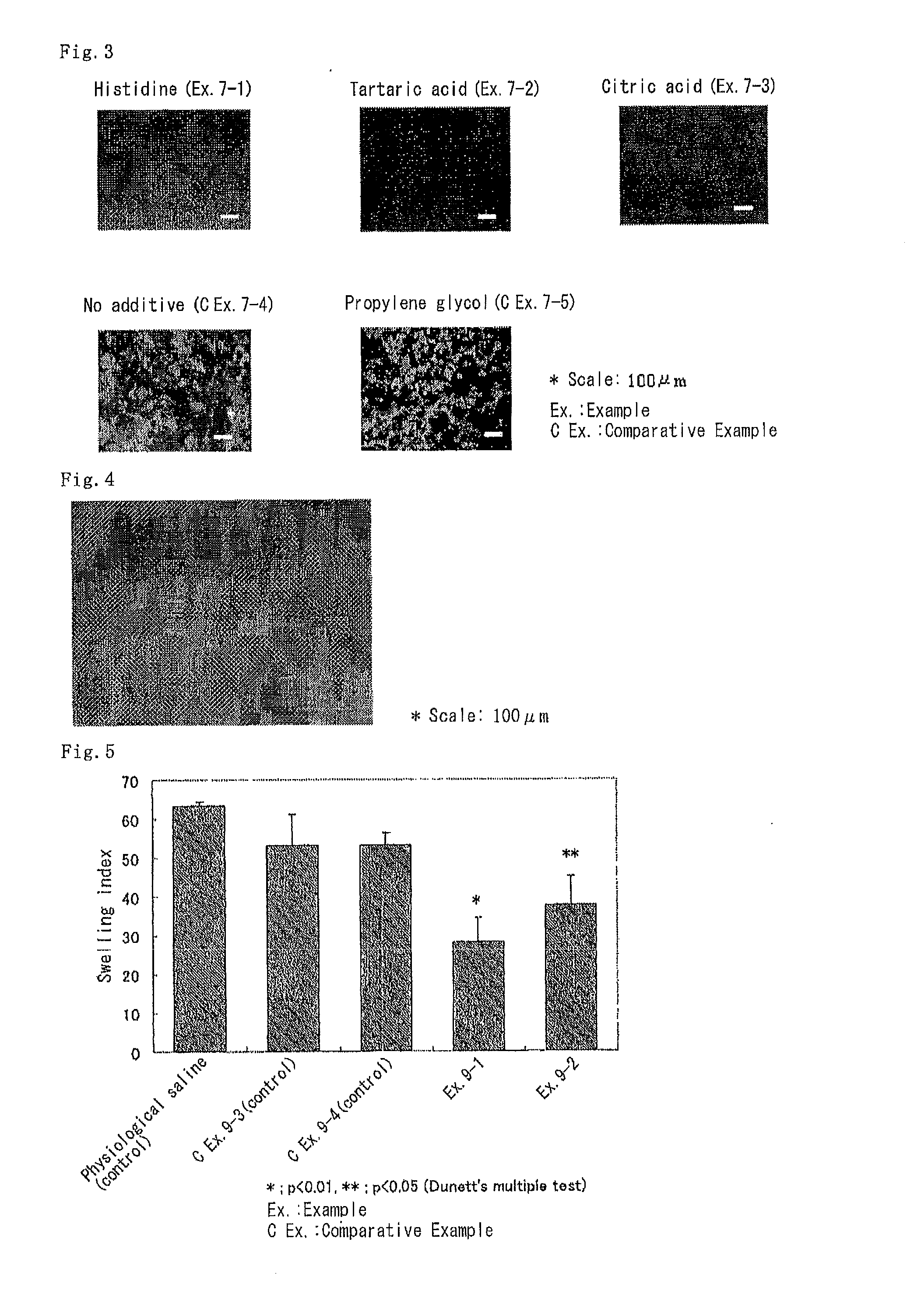
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
162 results about "Iothalamate Meglumine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
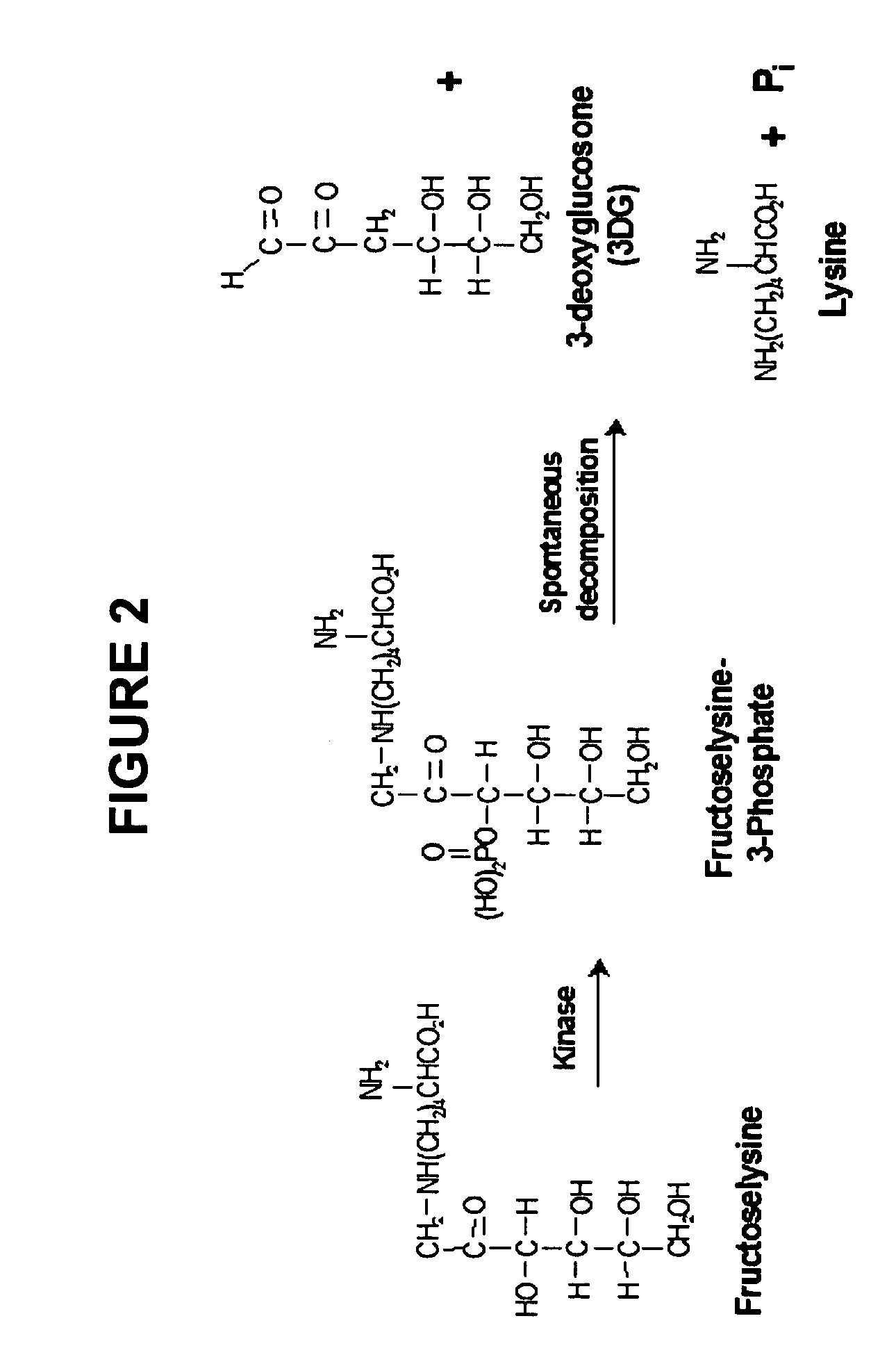
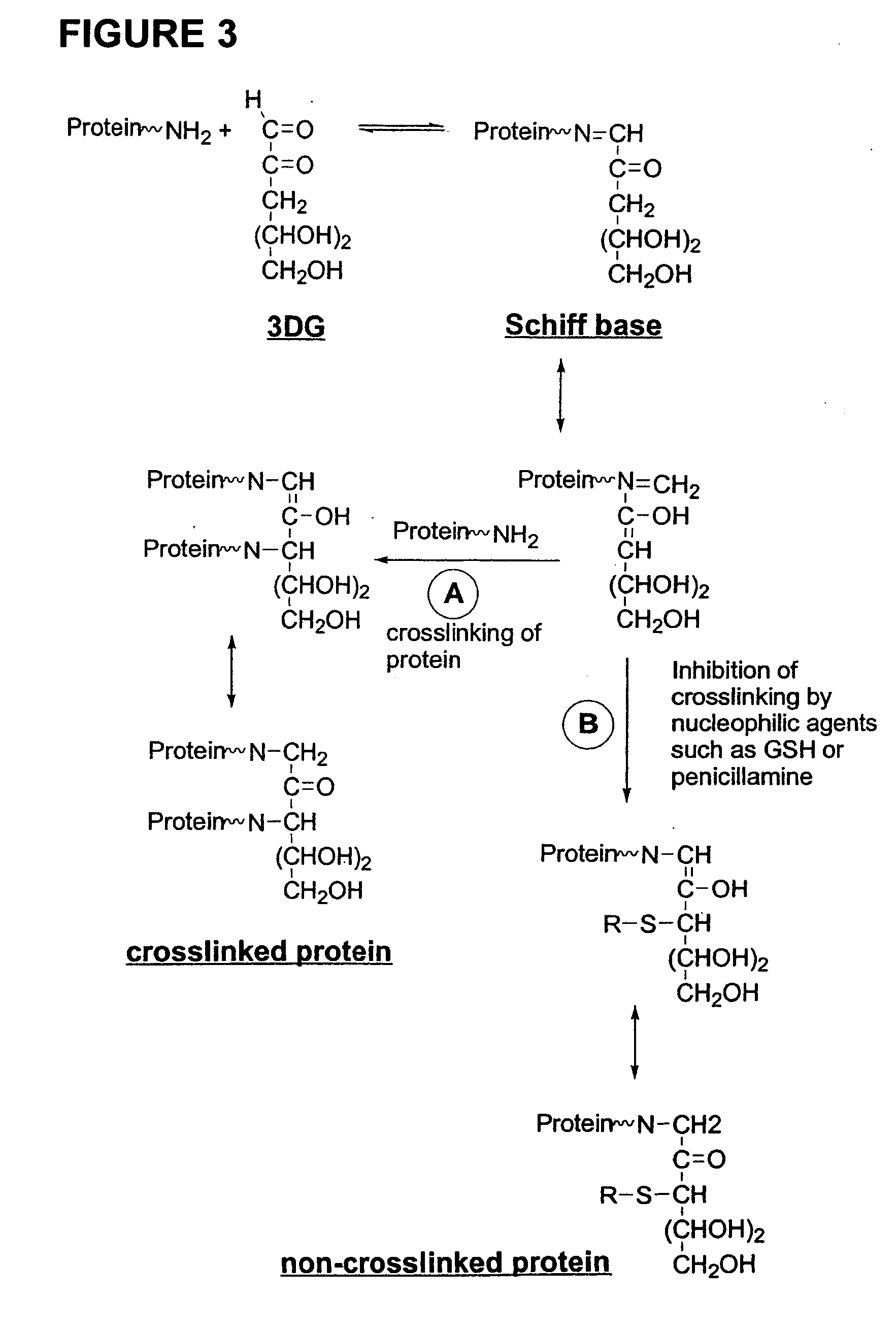
Meglumine is an amino sugar derived from glucose. It is often used as an excipient in pharmaceuticals and in conjunction with iodinated compounds in contrast media such as diatrizoate meglumine, iothalamate meglumine and iodipamide meglumine.
Aprepitant microemulsion for injection and preparation method thereof
InactiveCN102379845ARealize large-scale industrial productionLow costOrganic active ingredientsDigestive systemSocial benefitsOrganic solvent
The invention discloses an aprepitant microemulsion for injection. The aprepitant microemulsion consists of the following components in percentage by mass: 0.05 to 2 percent of aprepitant, 5 to 30 percent of oil for injection, 0.5 to 10 percent of emulsifier, 1 to 10 percent of co-emulsifier, 5 to 20 percent of protective agent and 60 to 80 percent of water for injection. Compared with the conventional oral aprepitant, the aprepitant microemulsion for the injection has the outstanding advantage that: the aprepitant is insoluble in the water and an organic solvent, in order to realize the aprepitant injection, the aprepitant microemulsion and aprepitant micro emulsion freeze-drying powder are prepared successfully and can be subjected to large-scale industrial production; and compared with a fosaprepitant dimethyl-meglumine injection, the aprepitant microemulsion has the advantages that: the cost is reduced greatly, the practicality is extremely high, and economic and social benefits are relatively good.
Owner:NANJING YOKO PHARMA GRP CO LTD
Dermal delivery of n-methyl-glucamine and n-methyl-glucamine compounds
InactiveUS20060110439A1Avoid crosslinkingCosmetic preparationsToilet preparationsIothalamate MeglumineLiposome
The present invention relates to methods and compositions for the treatment of skin-related conditions and disorders. In one aspect, the invention features methods and compositions for the transdermal delivery of compounds for the treatment of skin-related conditions and disorders, wherein the compositions include meglumine and a liposome component.
Owner:DYNAMIS THERAPEUTICS
Ginkgo leaf powder for injection and its prepn
InactiveCN1486715AImprove solubilityImprove uniformityPowder deliveryUnknown materialsSodium bicarbonateFreeze-drying
The ginkgo leaf powder for injection contains ginkgo extractive 1 weight portions, hydroxypropy1 betacyclodextrin 1-4 weight portions and pH regulator meglumine or sodium bicarbonate 0.02-0.08 weight portions. The preparation process includes mixing ginkgo extractive, hydroxypropy1 betacyclodextrin and pH regulator; filtering and ultrafiltering with 50000-150000 dalton ultrafiltering film; and direct bacteria-free freeze drying or spray drying of the ultrafiltered liquid to obtain the powder for injection. The said preparation process of the present invention can maintain fully the effective components while eliminating macro molecules, pyretogen, particle, microbe and other impurities to raise the safety and stability of the preparation obviously.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Gingko lactone powder injection and its preparation method
ActiveCN1557297AGood curative effectReduce lossesOrganic active ingredientsPowder deliveryCurative effectBilobalides
The present invention discloses one kind of bilobalide powder for injection and features its composition including bilobalide, meglumine and hydroxypropyl-beta-cyclodextrin in the weight ratio of 1 to 0.2-0.5 to 6-9. The present invention also discloses the preparation process of the bilobalide powder for injection. The bilobalide powder for injection has supplementary material capable of dissolving bilobalide completely without destroying its structure, and bilobalide as the main material is slightly soluble in water and easily soluble in acetone. The key point of the present invention is to select hydroxypropyl-beta-cyclodextrin as the solubilkizing supplementary material to raise the solubility of bilobalide greatly, and this raises the bioavailability of bilobalide and the curative effect of the injection greatly.
Owner:JIANGSU KANION PHARMA CO LTD
Lansoprazole for injecting and its preparation method
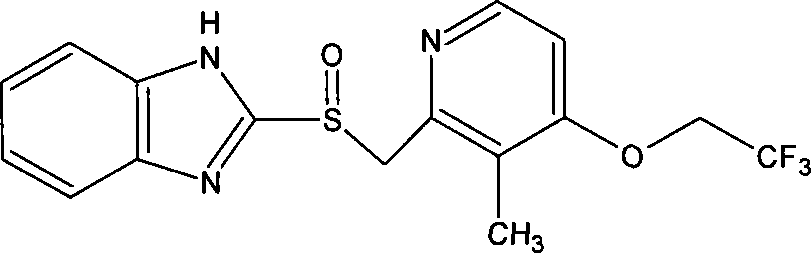
InactiveCN101057846AConvenient for clinical operationReduce dosageOrganic active ingredientsPowder deliveryLansoprazoleIothalamate Meglumine
The invention discloses a Lansoprazole preparation for injection comprising (1) Lansoprazole 10-50 weigh parts, (2) meglumine 2-20 weight parts, (3) sodium hydroxide 1-5 weight parts, (4) mannitol 20-100 weight parts. The invention also discloses the process for preparing the preparation.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Sacubitril derivatives and medicine compositions, preparation methods and application thereof
ActiveCN105693543AEasy to prepareCrystal form controllableAmino compound purification/separationOrganic compound preparationEthylenediamineArginine
The invention provides sacubitril derivatives and medicine compositions, preparation methods and application thereof and belongs to the fields of medicine compounds and preparation thereof. The sacubitril derivatives comprise sacubitril lithium salt, sacubitril kali salt, sacubitril magnesium salt, sacubitril calcium salt, sacubitril strontium salt, sacubitril zinc salt, sacubitril ferric salt, sacubitril ammonium salt, sacubitril diethylamine salt, sacubitril ethylenediamine salt, sacubitril piperazine salt, sacubitril N-(2-ethoxyl)-pyrrolidine salt, sacubitril choline salt, sacubitril cholamine salt, sacubitril diethanol amine salt, sacubitril triethanolamine salt, sacubitril tromethamine salt, sacubitril meglumine salt, sacubitril diisopropylamine salt, sacubitril tert-butylamine salt, sacubitril N, N'-bis-benzyl ethylenediamine salt, sacubitril L-lysine salt, sacubitril L-arginine salt or sacubitril L-histidine salt.
Owner:SICHUAN HAISCO PHARMA CO LTD
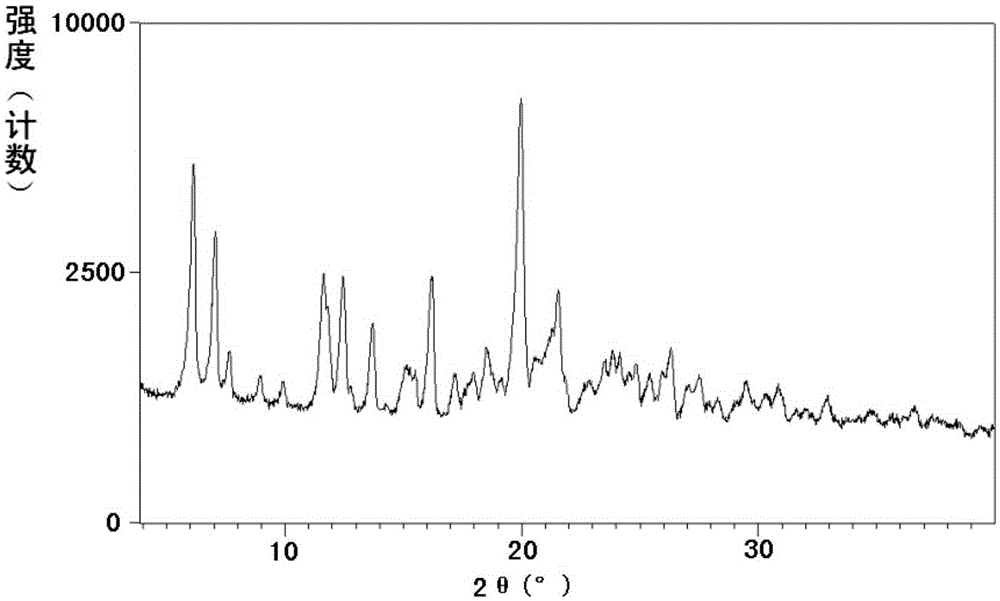
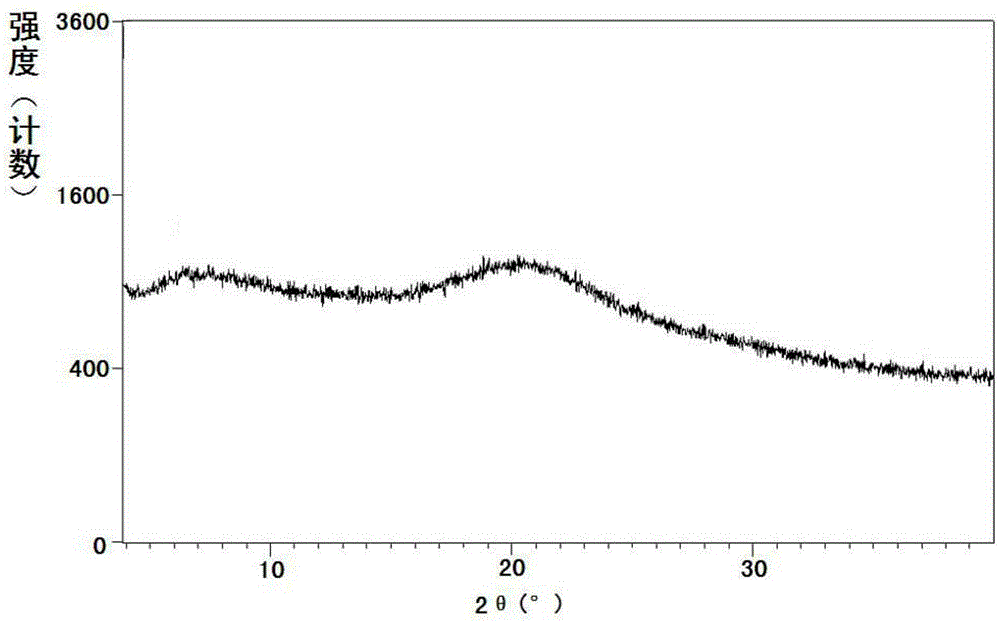
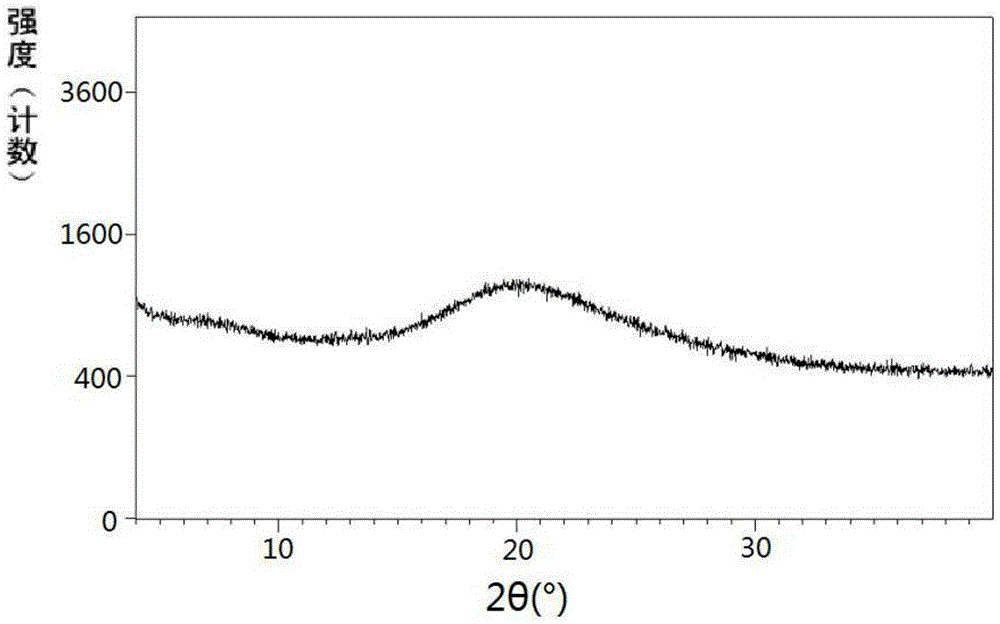
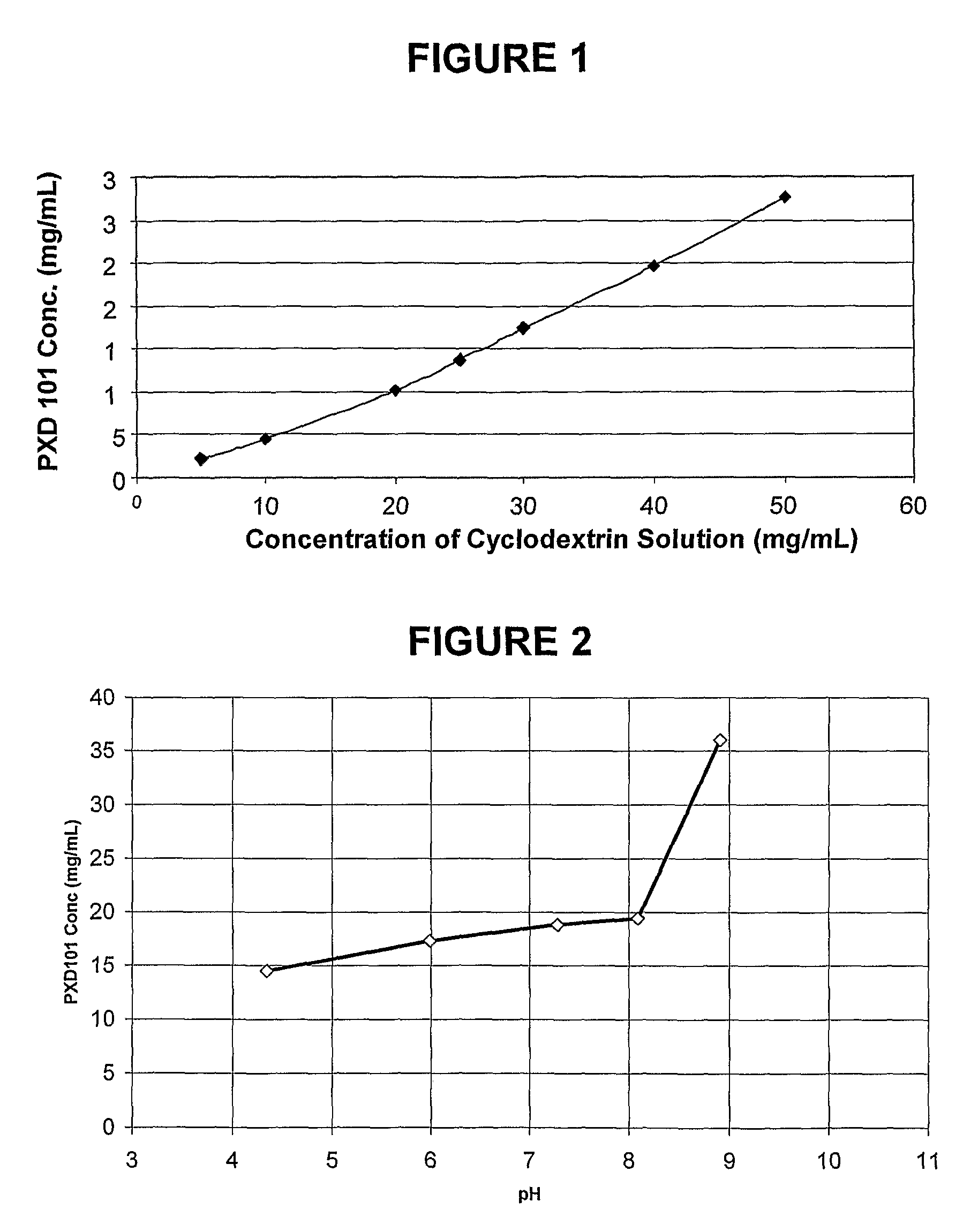
Pharmaceutical Formulations Of Hdac Inhibitors
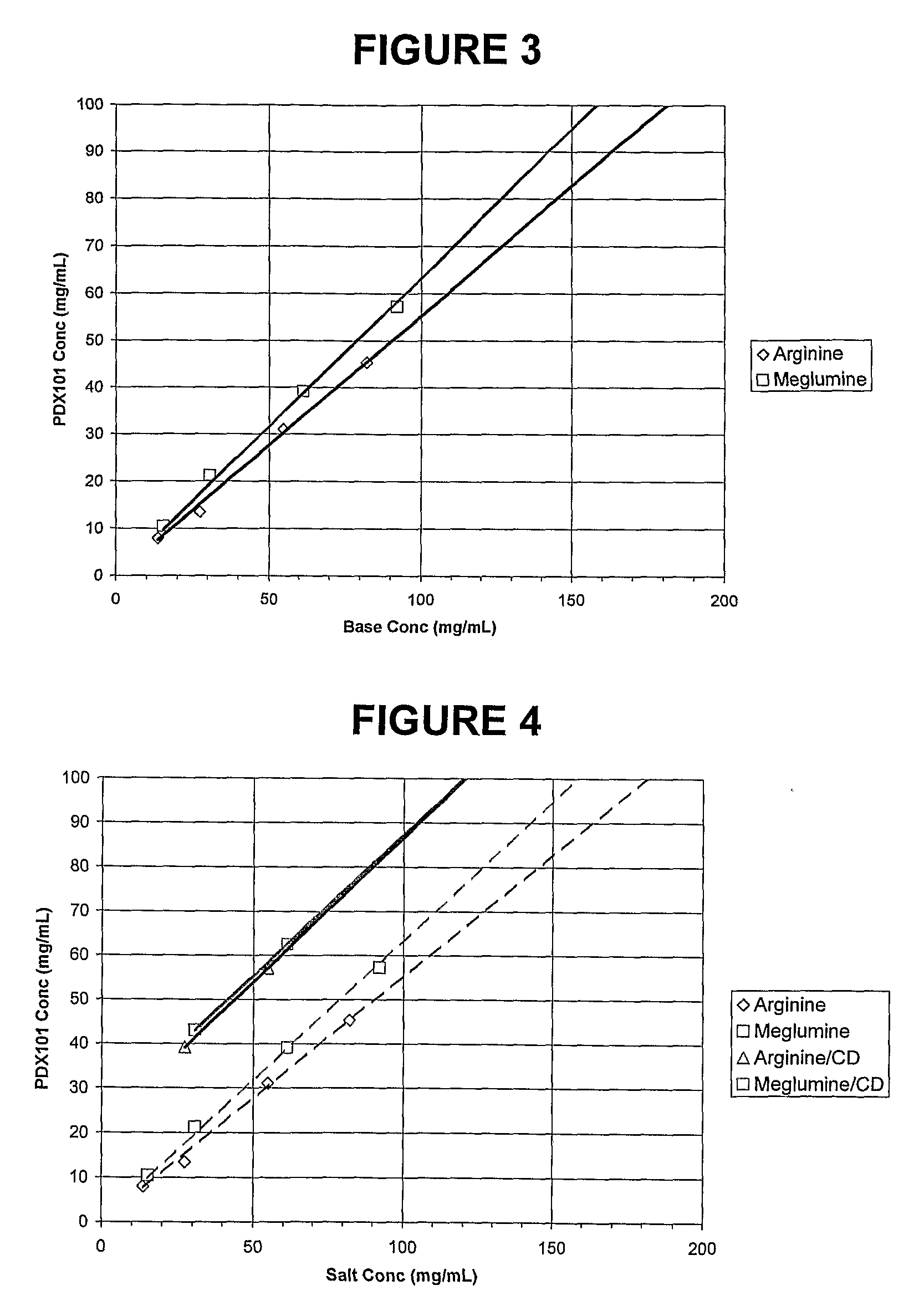
This invention pertains to pharmaceutical compositions comprising certain carbamic acid compounds (e.g., which inhibit HDAC (histone deacetylase) activity) (e.g., PXD-101, N hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide)) and one or more additional ingredients selected from cyclodextrin, arginine, and meglumine. The present invention also pertains to the use of such compositions, for example, in the inhibition of HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Lansoprazole freeze-dried powder for injection and preparing method thereof
ActiveCN101229136AFull appearanceImprove solubilityPowder deliveryOrganic active ingredientsLansoprazoleActivated carbon
The invention provides a freeze-dried powder of lansoprazole for injection and a preparation method of the freeze-dried powder which consists of lansoprazole, meglumine and mannitol with the weight ratio of 3:1:18 to 22. The invention provides the preparation method: dissolving raw and supplemental materials with water and adjusting the pH, and adding activated carbon to remove the color and filtering to remove the carbon; making fine filtration with a filtering film and loading separately and cooling rapidly to minus 50 to minus 46 DEG C with the speed of 1 to 1.2 DEG C per minute; preserving the heat and freezing for 3 hours and pumping the vacuum to 15Pa; heating uniformly to minus 22 to minus 18 DEG C in 7 to 9 hours and preserving the heat for 1 hour to 2 hours; heating rapidly to 3 to 7 DEG C in 4 to 6 hours and then heating to 40 DEG C in 4 hours; preserving the heat and drying for 3 hours and packaging and warehousing after the measuring there. Products prepared by the method are low in water content, beautiful in appearance and easy in storage and transportation.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
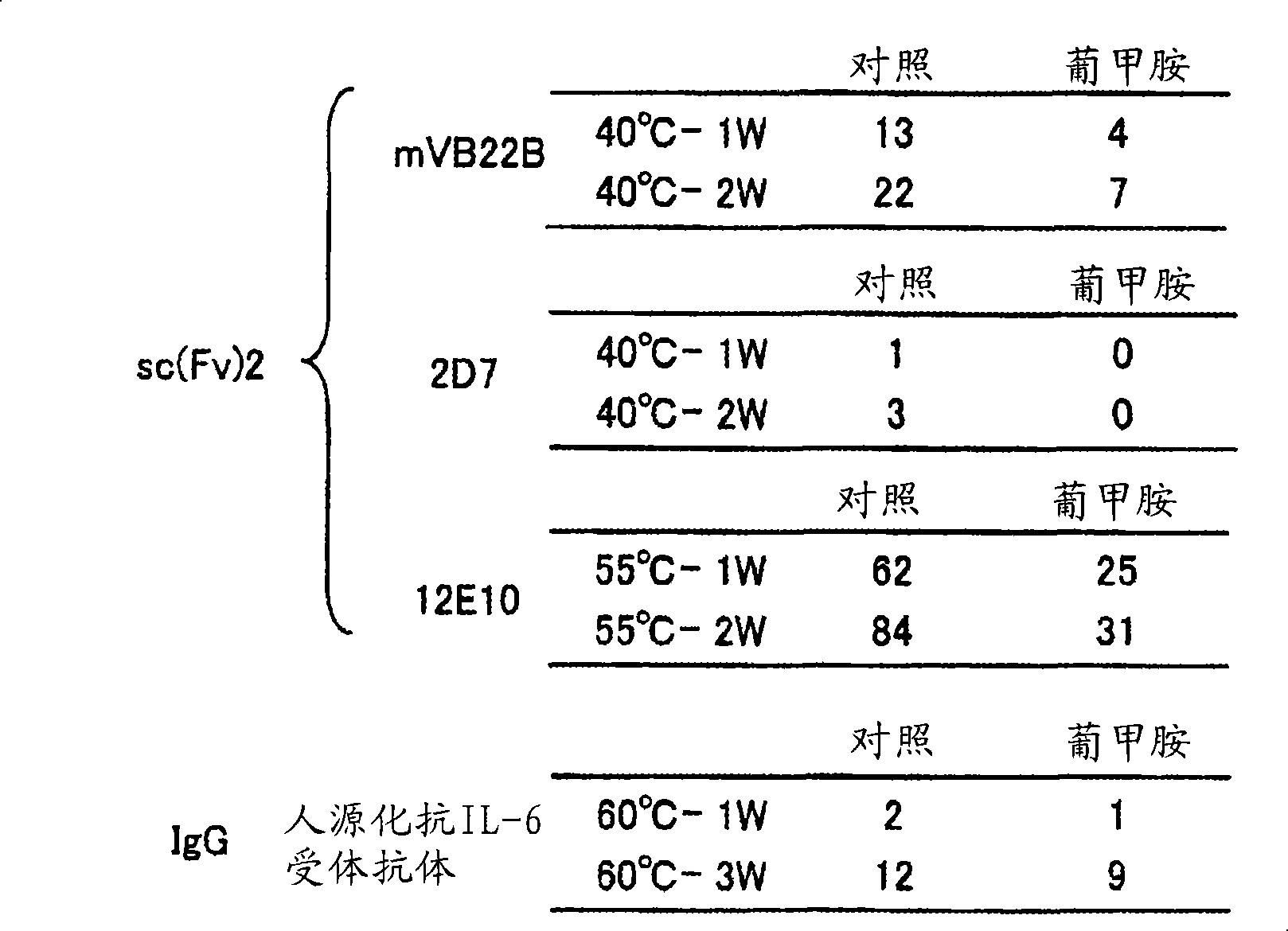
Stabilizer for protein preparation comprising meglumine and use thereof
An object of the present invention is to provide a method for stabilizing a protein or a method for inhibiting aggregation of a protein, these methods including the step of adding meglumine to the protein. The present invention also provides a drug containing meglumine, which stabilizes protein, or a drug which inhibits protein aggregation. Furthermore, the object of the present invention is to provide a pharmaceutical composition containing an antibody molecule stabilized by meglumine, a method for preparing the pharmaceutical composition, and a kit containing the pharmaceutical composition. In order to solve the above-mentioned problems, the present inventors studied the antibody stability effect of a kind of amino sugar, meglumine. It was found that meglumine can be used as a stabilizer for antibody molecules and also as an excipient for lyophilized formulations.
Owner:CHUGAI PHARMA CO LTD
Lansoprazole preparation for injection and preparation method thereof
Lansoprazole is a second generation proton pump inhibitor developed by Takeda in Japan, and is clinically and widely applied for treatment of acid related diseases and helicobacter pylori eradiation. The invention provides a preparation method of a Lansoprazole lyophilized powder injection preparation, creatively screens and tests the composition and dosage as well as the technology of the Lansoprazole lyophilized powder injection, obtains the optimal experiment condition, and gains good effect. The weight ratio of all the components of the Lansoprazole lyophilized powder injection satisfies the relation that Lansoprazole: meglumine: mannitol: sodium hydroxide equals to 30:10:60:4-4.1, the optimal weight ratio of all the components meets the relation that Lansoprazole: meglumine: mannitol: sodium hydroxide equals to 30:10:60:4.
Owner:JUMPCAN PHARMA GRP
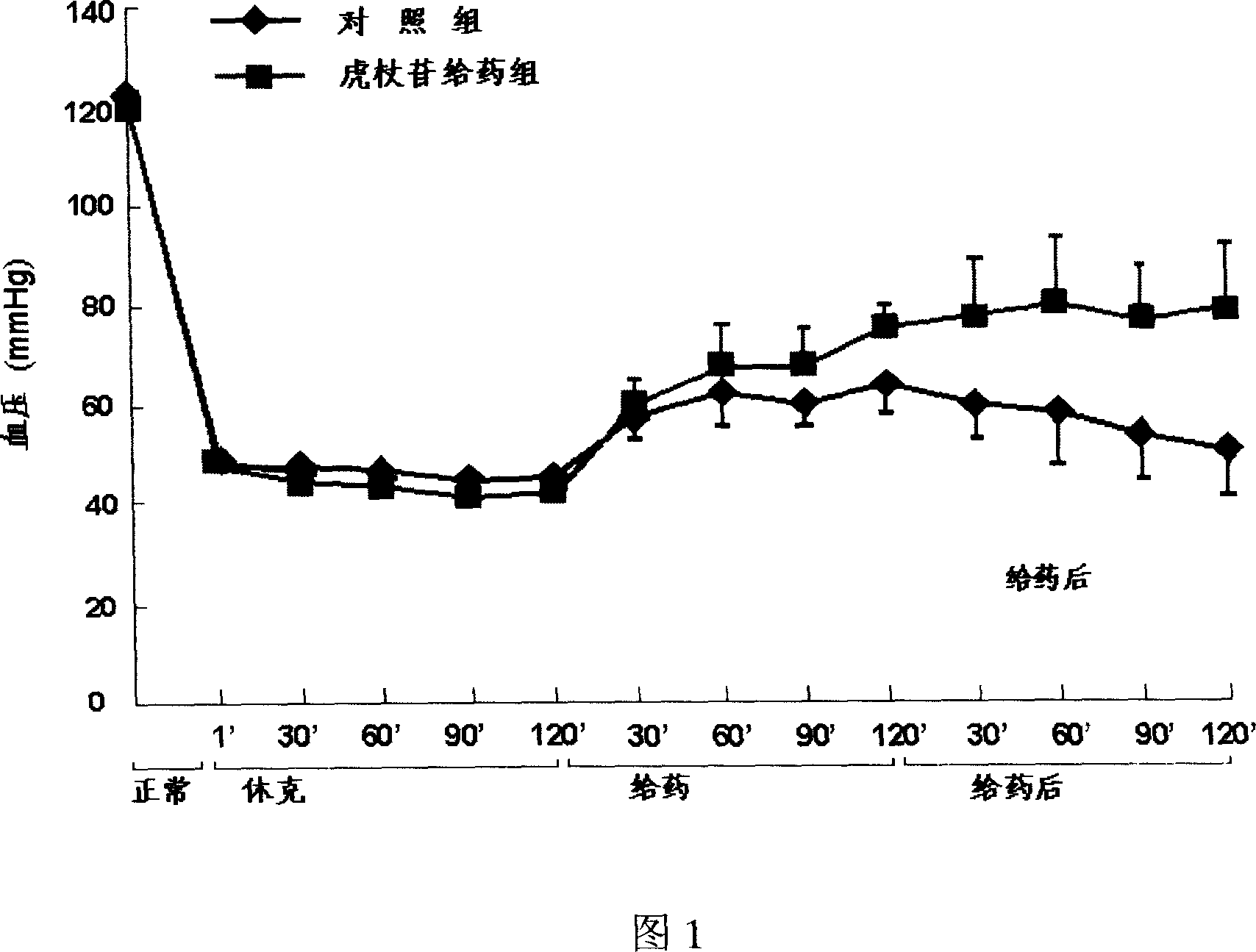
Medicine combination including high-concentration polydatin
ActiveCN101062044AMean arterial pressurePowder deliveryOrganic active ingredientsHigh concentrationCyclodextrin
The invention relates to a pharmaceutical composition containing high concentration of polydatin and process for preparation, wherein the composition comprises polydatin, meglumine and / or cyclodextrin, and other pharmaceutically acceptable auxiliary materials and carriers. The composition can be polydatin aqueous solution, or the lyophilized product prepared into aqueous solution before use. In the aqueous solution, the concentration of polydatin is not lower than 5mg / ml.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Freeze dried Lansoprazole sodium injection and its prepn process
InactiveCN1810244AAvoid destructionImprove efficacyOrganic active ingredientsPowder deliveryDiseaseFreeze-drying
The freeze dried Lansoprazole sodium sdinjection has Lansoprazole sodium as active component and mannitol or meglumine as excipient in the weight ratio of 15-30 g to 5-50 g. The preparation process of the freeze dried Lansoprazole sodium injection includes the following steps: dissolving Lansoprazole sodium in 15-30 g in injection water of 200-400 ml, dissolving mannitol or meglumine as excipient in 5-50 g in injection water of 100-300 ml, mixing the two kinds of obtained solutions and adding injection water to 2000-3000 ml, adding active carbon in 0.5-1.5 g, filtering with microporous filter membrane, packing in vial, freeze drying under bacteria-free condition, and pressing cap. The freeze dried Lansoprazole sodium injection is used clinically in intravenous drip or intramuscular injection to treat peptic ulcer and other diseases.
Owner:JINZHOU JIUTAI PHARML CHINA
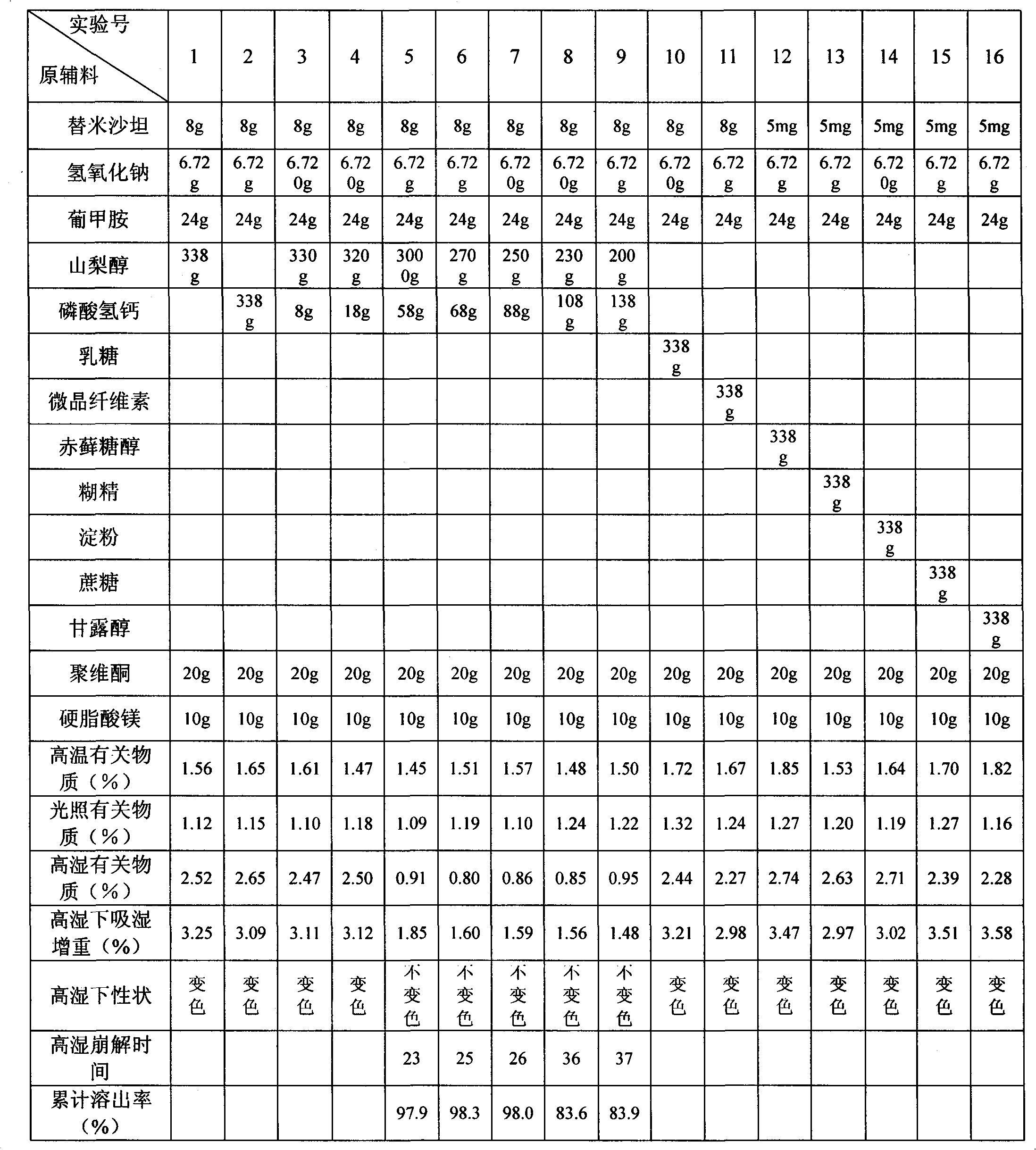
Telmisartan tablet composition
ActiveCN101897676ALow packaging requirementMeet stability requirementsOrganic active ingredientsPill deliveryMedicineMagnesium stearate
The invention belongs to the technical field of medicinal preparation, and in particular relates to a telmisartan tablet composition. The telmisartan tablet composition is characterized by being prepared from 80 parts of telmisartan, 6.72 parts of sodium hydroxide, 24 parts of meglumine, 250 to 280 parts of sorbierite, 58 to 88 parts of calcium hydrophosphate, 20 parts of polyvidone and 10 parts of magnesium stearate. By selecting and using the combination of sorbierite and calcium hydrophosphate as filler, the composition can obviously improve the stability of the preparation and is favorable for production and application of the preparation.
Owner:BEIJING JINGFENG PHARMA GRP
Meglumine cyclic adenosine injection and preparation technique thereof
InactiveCN101455631AAct directlyAct quicklyOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonIothalamate Meglumine
The present invention relates to an adenosine cyclophosphate injection and a preparing process thereof. According to the invention, acidum citricum is added into a magnetic stirring pot. Injection water with confect amount of 50% is added and dissolved. The adenosine cyclophosphate and meglumine are added into the solution. The obtained solution is mixed to be dissolved. The 0.02% of activated carbon with needle use in volume is added. The mixture is mixed for 30 minutes. The mixture is preliminarily filtered fro removing carbon. The adenosine cyclophosphate injection is prepared for standby. The injection water is added to the total amount. The pH value is adjusted to 6.0-6.5 by sodium hydroxide solution with concentration of 10%. The colorless transparent injection is prepared. The adenosine cyclophosphate injection of the invention not only has the advantages of straight function, high speed, etc., but also has the advantages of little adverse effect, normal temperature transportation and normal preserving, thereby increasing the stability and safety of the product.
Owner:湖北安邦医药有限公司
Lansoprazole composition freeze-dried powder for injection
ActiveCN101829065ASmall particle sizeHigh porosityPowder deliveryOrganic active ingredientsAcetic acidEthylene diamine
The invention relates to lansoprazole composition freeze-dried powder for injection. The lansoprazole composition freeze-dried powder is characterized by comprising lansoprazole used as a main material, meglumine, mannitol, sodium hydrogensulfite and ethylene diamine tetraacetic acid, wherein the proportion of the components is 3:(0.1-1):(1-20):(0.01-0.5):(0.01-0.5); preferably, the proportion is 3:(0.5-1):(10-20):(0.1-0.3):(0.05-0.3); and more preferably, the proportion is 3:1:20:0.2:0.2. The preparation method comprises the following steps of: dissolving the raw material and the auxiliary materials by adding water; regulating the pH value; adding active carbon for decolorizing; filtering for decarburizing; fine filtering by using a filter membrane; sub-packaging; cooling to -50 to -46 DEG C according to a speed of 1-1.2 DEG C / minute; preserving heat and freezing for 3 hours; vacuumizing to 15Pa; uniformly heating up to -22 to -18 DEG C within 7-9 hours and then preserving heat for 1-2 hours; uniformly heating up to 3-7 DEG C within 4-6 hours and then heating up to 40 DEG C within 4 hours; preserving heat and drying for 3 hours; and packaging and storing after inspection.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Stable formulations of ace inhibitors and methods for preparation thereof
Stabilized pharmaceutical solid composition of ACE inhibitor comprising an ACE inhibitor and a selective dosage formulation thereof comprising of meglumine. The ACE inhibitor selectively combined with a dosage form including essentially the meglumine is surprisingly found to avoid the degradation of ACE inhibitor by such dosage forms especially the commonly used pharmaceutical excepients. In particular, the presence of the meglumine in the dosage form for the active along with the active ACE inhibitor surprisingly avoid the degradation of the ACE inhibitor due to a) cyclization via internal nucleophilic attack to form substituted diketopiperazines, b) hydrolysis of the side chain ester group, and c) oxidation to form products having often unwanted coloration.
Owner:LUPIN LTD
Cyclic adenosine meglumine injection and preparation method thereof
InactiveCN102283804AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityFiltration
The invention relates to an adenosine monophosphate meglumine injection and a preparation method thereof. The preparation process is to take an appropriate amount of water for injection, add sodium chloride, cyclic adenosine monophosphate, and meglumine, stir to dissolve completely, add 0.05-0.2% (W / V) activated carbon for needles by volume, and stir for 15-30 minutes. Filter to remove carbon, add water for injection to nearly full amount, use phosphate buffer to adjust the pH value to between 6.0 and 6.5, add water for injection to the full amount, and check that the semi-finished product is qualified, filter, potting (full nitrogen gas in the whole process), and extinguish Bacteria, light inspection, packaging. The method has the advantages of selecting appropriate solvents and additives, improving the solubility and stability of meglumine cyclic adenosine monophosphate, and adopting a terminal sterilization preparation method to effectively ensure the sterility assurance level of the drug. The invention has the characteristics of formula composition, simple process, low production cost, strong drug stability and safety, and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Silibinin meglumine salt oral disintegration tablet preparation and its preparing method
InactiveCN1586471AQuality improvementEasy to takeOrganic active ingredientsMetabolism disorderOrally disintegrating tabletFibrillation
The present invention relates to one kind of orally disintegrated silibinin-meglumine salt tablet for protecting liver cell, stimulating the biological synthesis of protein inside liver cell, promoting recovery of damaged liver cell, resisting fibrillation, capturing free oxygen radical and inhibiting the deposition and infiltration of fat in liver, and its preparation. The orally disintegrated silibinin-meglumine salt tablet is prepared with silibinin-meglumine salt as main material, and through adding stuffing, disintegrating agent, corrective, flow assistant and other supplementary material, pressing into tablet and other steps. The present invention has the features of low production cost, fast disintegration, good taste, etc.
Owner:COSCI MED TECH CO LTD
Method for synthesizing flunixin meglumine
InactiveCN103694167AHigh catalytic efficiencyEfficient responseOrganic compound preparationAmino-hyroxy compound preparationFLUNIXIN MEGLUMINEAcetonitrile
The invention provides a method for synthesizing flunixin meglumine. According to the method, reaction is carried out in a manner that 2-chloronicotinic acid and 2-methyl-3-trifluoromethylaniline serve as raw materials, water serves as a solvent and cupric oxide and para-toluenesulfonic acid serve as catalysts. According to the method, on the premise that the condition that para-toluenesulfonic acid serves as a catalyst is reported in the existing literature, cupric oxide is added, so that the catalysis efficiency is increased greatly, the reaction can be carried out efficiently, the reaction time is shortened, and the yield is increased; obtained flunixin and meglumine are salified in an acetonitrile solvent and are recrystallized, so as to obtain flunixin meglumine, and the total yield is about 90%; through synthesizing flunixin meglumine by the method, the operation is convenient and simple, and the requirements for equipment are low, so that the method is applicable to industrial large-scale production.
Owner:WEIHAI YARUI BIOLOGICAL SCI & TECH
Garcinolic acid injection and its prepn
InactiveCN1452960AImprove anti-tumor effectOrganic active ingredientsAntineoplastic agentsFreeze-dryingArginine
The garcinolic acid injection as one kind of Chinese medicine preparation contains garcinolic acid as active component as well as cosolvent, excipient, and water for injection. Its preparation process includes weighing material garcinolic acid in 1-50 weight portions, adding water for injection in 0-4000 weight portions via stirring, adding cosolvent in 1-50 in weight portions before ultrasonic dissolving, adding excipient 1-100 in weight portions through stirring to dissolve, adding active carbon during stirring, eliminating carbon, filtering and packing. The injection may be freeze dried to prepare freeze dried injection. The cosolvent is selected from L-arginine, meglumine and lysine; and the excipient is selected from mannitol, dextran and sodium chloride.
Owner:戴建国
Pharmaceutical formulations of HDAC inhibitors
This invention pertains to pharmaceutical compositions comprising certain carbamic acid compounds (e.g., which inhibit HDAC (histone deacetylase) activity) (e.g., PXD-101, N hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide)) and one or more additional ingredients selected from cyclodextrin, arginine, and meglumine. The present invention also pertains to the use of such compositions, for example, in the inhibition of HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Fine particulate preparation comprising complex of nucleic acid molecule and collagen
The present invention relates an additive comprising at least one substance selected from arginine, trometamol, meglumine, lysine, histidine, monoethanolamine, diethanolamine, triethanolamine, succinic acid, citric acid, tartaric acid, lactic acid, and salts thereof, which is useful in the prevention of aggregation of fine particulate complexes of a nucleic acid and collagen, and the production of a preparation comprising particulate complexes of controlled size which is suited for transporting a nucleic acid into cells.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD +1
Pharmaceutical composition containing quinoline derivative or salt of quinoline derivative
ActiveCN106139156AImprove stabilityPromote dissolutionPharmaceutical non-active ingredientsCapsule deliveryIothalamate MegluminePotassium hydrocarbonate
The invention relates to pharmaceutical composition containing a quinoline derivative or salt of the quinoline derivative, in particular to pharmaceutical composition containing 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide or pharmacologically acceptable salt and basic amino acid or meglumine and / or at least one compound selected from potassium carbonate and potassium bicarbonate. The pharmaceutical composition has the characteristics of being high in dissolving-out rate and good in stability.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
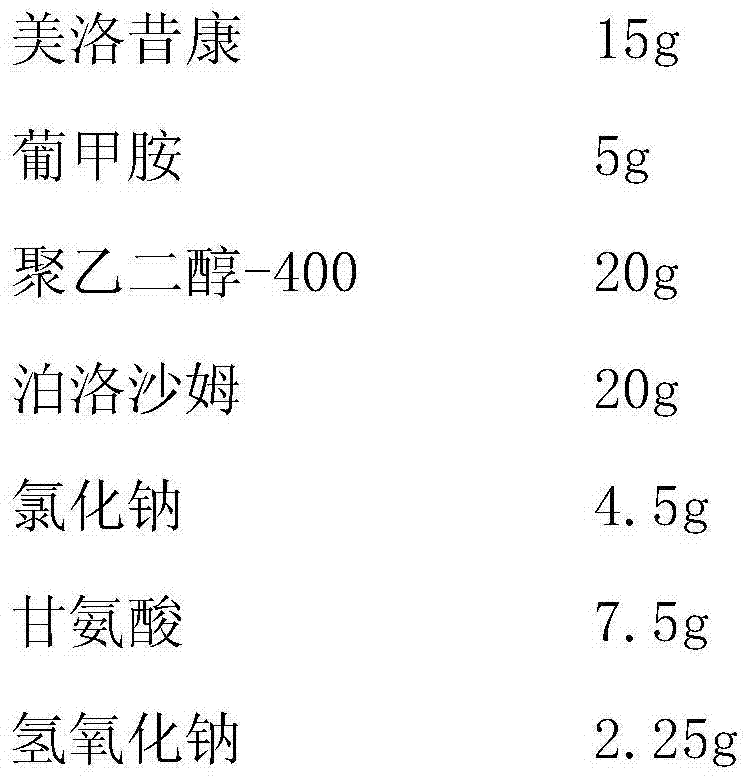
Meloxicam injection for treating rheumatoid arthritis and osteoarthritis and preparation method thereof
InactiveCN103690480AFast absorptionImprove bioavailabilityOrganic active ingredientsAntipyreticFiltrationPolyethylene glycol
The invention provides a meloxicam injection for treating rheumatoid arthritis and osteoarthritis and a preparation method thereof. Every 1,500ml of injection comprises the following components in: 7.0-20g of meloxicam, 5-9.45g of meglumine, 20-150g of polyethylene glycol-400, 20-75g of poloxamer, 3-9g of glycine, 1.5-5.25g of sodium chloride and 0.225-2.25g of sodium hydroxide. The preparation method comprises the following steps: adding 1,000ml of injection water in a preparation tank, adding meloxicam, meglumine, polyethylene glycol-400, poloxamer, sodium chloride, glycine and sodium chloride; dissolving, and adding meloxicam; heating to 80 DEG C, and stirring for dissolving the meloxicam; adding 0.05g of needle activated carbon; stirring and adsorbing, and performing coarse filtration to remove charcoal; adding injection water to 1,500ml, and filtering with a 0.45mu m filter membrane until the mixture is clear; pouring into an ampoule, and sterilizing. The meloxicam injection has the active effects of quick absorption and high bioavailability, is not affected by pH, enzyme, foods and the like in the digestive tract, and has greatly increased curative effect.
Owner:吉林修正药业新药开发有限公司
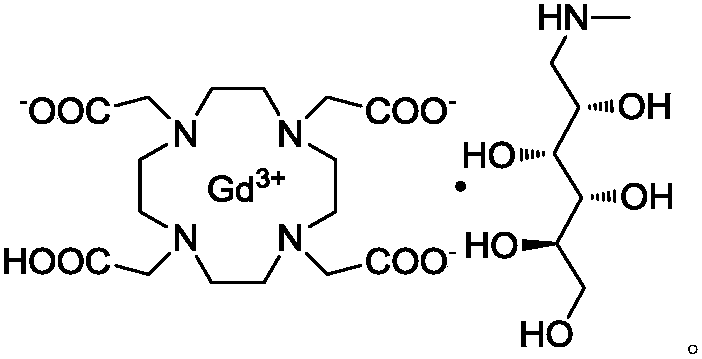
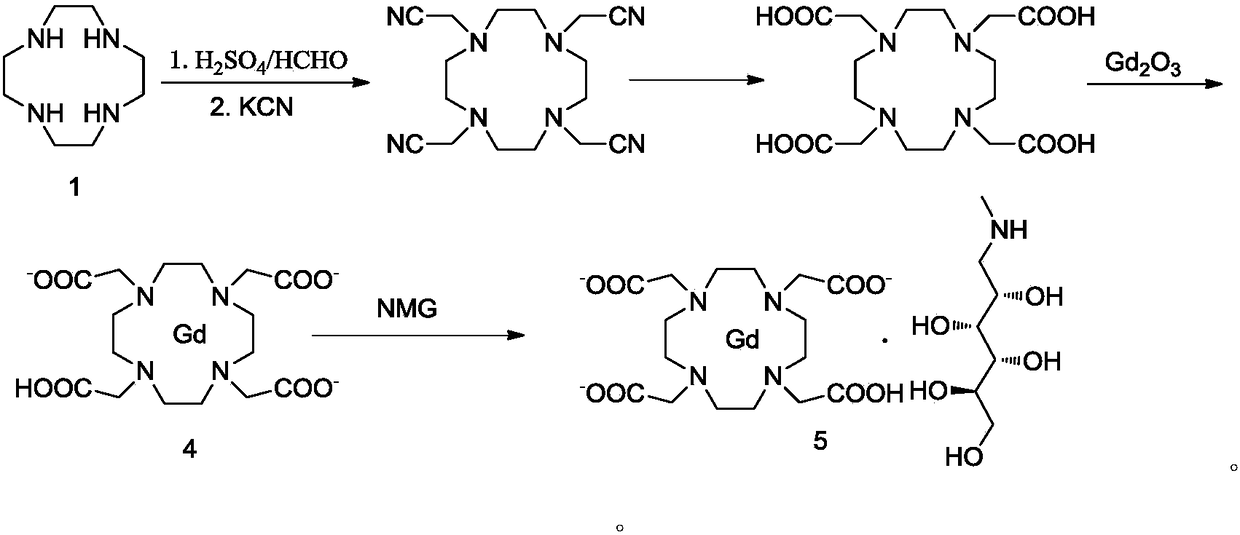
Preparation method for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
The invention relates to a preparation method for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid. Specifically, the method uses a recrystallization method to obtain a high-purity compound represented by a formula I shown in the description, and further, meglumine and Gd2O3 are complexed with the compound represented by the formula I to obtain the gadoteric acid meglumine. The method disclosed by the invention is simple to operate, has low costs, and is green, environmentally friendly and suitable for large-scale production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Meglumine adenosine cyclophosphate composition injection and preparation method thereof
InactiveCN102600070ASolve the crystallization problemFix stability issuesOrganic active ingredientsPharmaceutical delivery mechanismAdenosineAdditive ingredient
The invention relates to a preparation method of chemical medicine, in particular to meglumine adenosine cyclophosphate composition injection and a preparation method thereof. The meglumine adenosine cyclophosphate composition injection comprises the following ingredients in part by weight: 5 to 15 parts of adenosine cyclophosphate, 3 to 10 parts of meglumine, 2.0 to 2.2 parts of citric acid, 0.6 to 0.8 parts of sodium hydroxide, 2 to 4 parts of sodium chloride and 2 to 5 parts of water for injection. The preparation method comprises the following steps that the citric acid is mixed and dissolved in the water for injection, the pH value of the mixed solution is adjusted to 5.9 to 6.5 by the sodium hydroxide to form buffering solution, then the sodium chloride and latent solvent are added into the buffering solution to be stirred and dissolved, the water for injection is added, the adenosine cyclophosphate and meglumine are slowly added while the solution is stirred, active carbon is added and stirred in the solution after the adenosine cyclophosphate and the meglumine are completely dissolved, and the mixed solution is filtered and reflowed to prepare colorless clear and bright liquid. The clarity of the injection is good, the stability is good, and the injection is safe to use.
Owner:湖北安邦医药有限公司
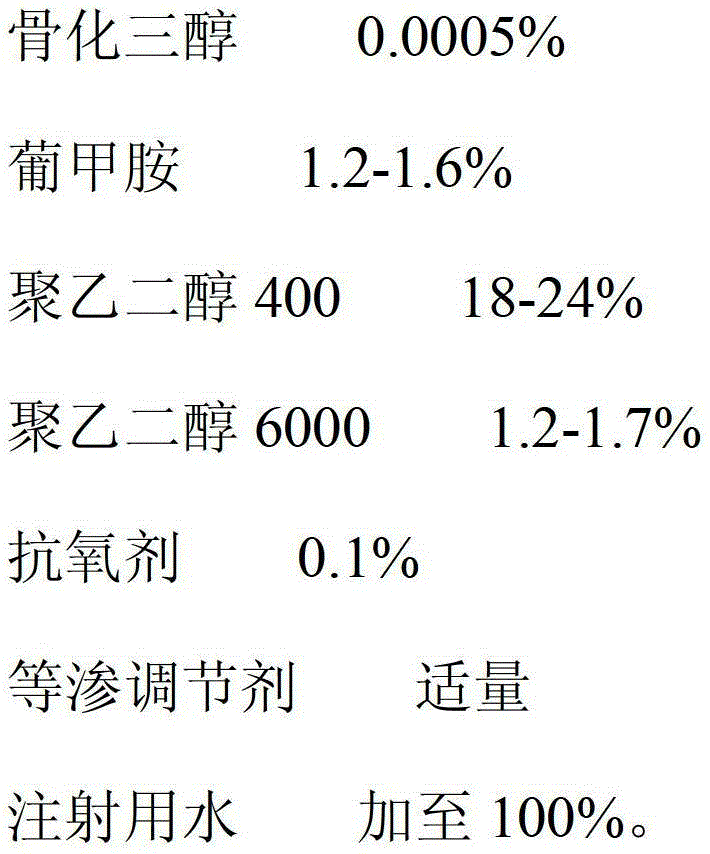
Calcitriol injection and preparation method thereof
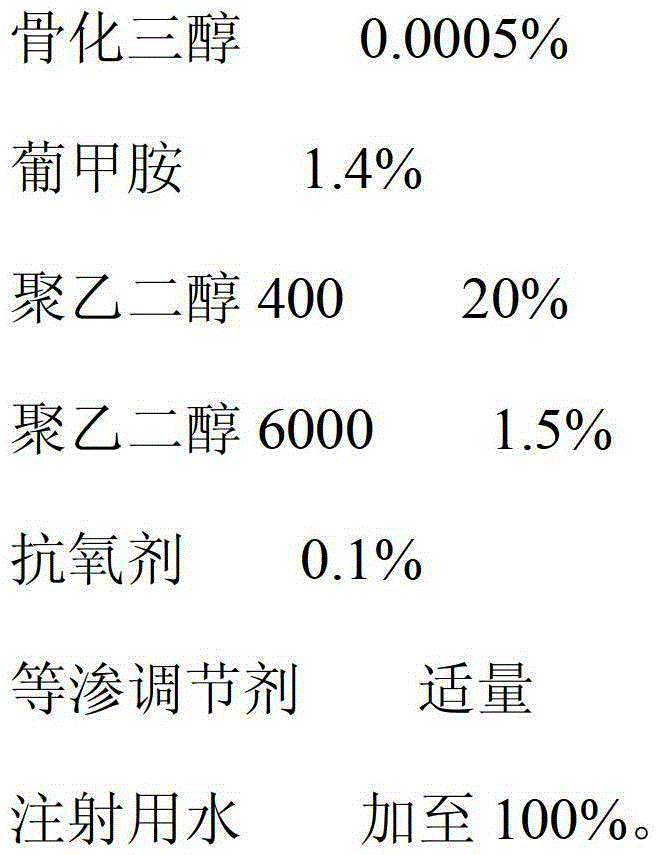
ActiveCN103142470AImprove stabilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderAdditive ingredientAntioxidant
The invention relates to a calcitriol injection and a preparation method thereof. The injection is prepared from the following ingredients in percentage by weight: 0.0005% of calcitriol, 1.2-1.6% of meglumine, 18-24% of polyethylene glycol 400, 1.2-1.7% of polyethylene glycol 6000, 0.1% of antioxidant, an appropriate amount of isotonicity regulator and the balance of water for injection. According to the injection, the content of calcitriol is remarkably increased, so that the drug dosage is reduced; and the stability of calcitriol to light and air is improved, and the bioavailability of calcitriol is also remarkably improved.
Owner:CP PHARMA QINGDAO CO LTD
Repaglinide-metformin hydrochloride tablet and preparing method thereof
InactiveCN104337811AUse less excipientsSolve the problem of insoluble in waterOrganic active ingredientsMetabolism disorderMetformin HydrochlorideSolvent
The invention discloses a prescription of a repaglinide-metformin hydrochloride tablet and a preparation technology thereof. In particular, a solid dispersion water solution technology is used; the problem that repaglinide is insoluble in water can be solved only through common wet granulations; dissolved objects and related matter are both superior to those of foreign objects. Microcrystalline celluloses and sorbitol serve as fillers; polyvinylpolypyrrolidone serves as disintegrating agents; PVPk30 serves as bonding agents; silicon dioxide serves as lubricating agents; meglumine serves as saltiness agents; and poloxamer 188 serves as solubilizers. The repaglinide-metformin hydrochloride tablet prepared with the technology is fewer in use auxiliary material; the high dissolving performance and the high stability can be kept; the preparation technology is simple; the technology cost is lower; and the repaglinide-metformin hydrochloride tablet is suitable for industrial production.
Owner:JIANGSU CAREFREE PHARM CO LTD
Water soluble silymarin and its prepn
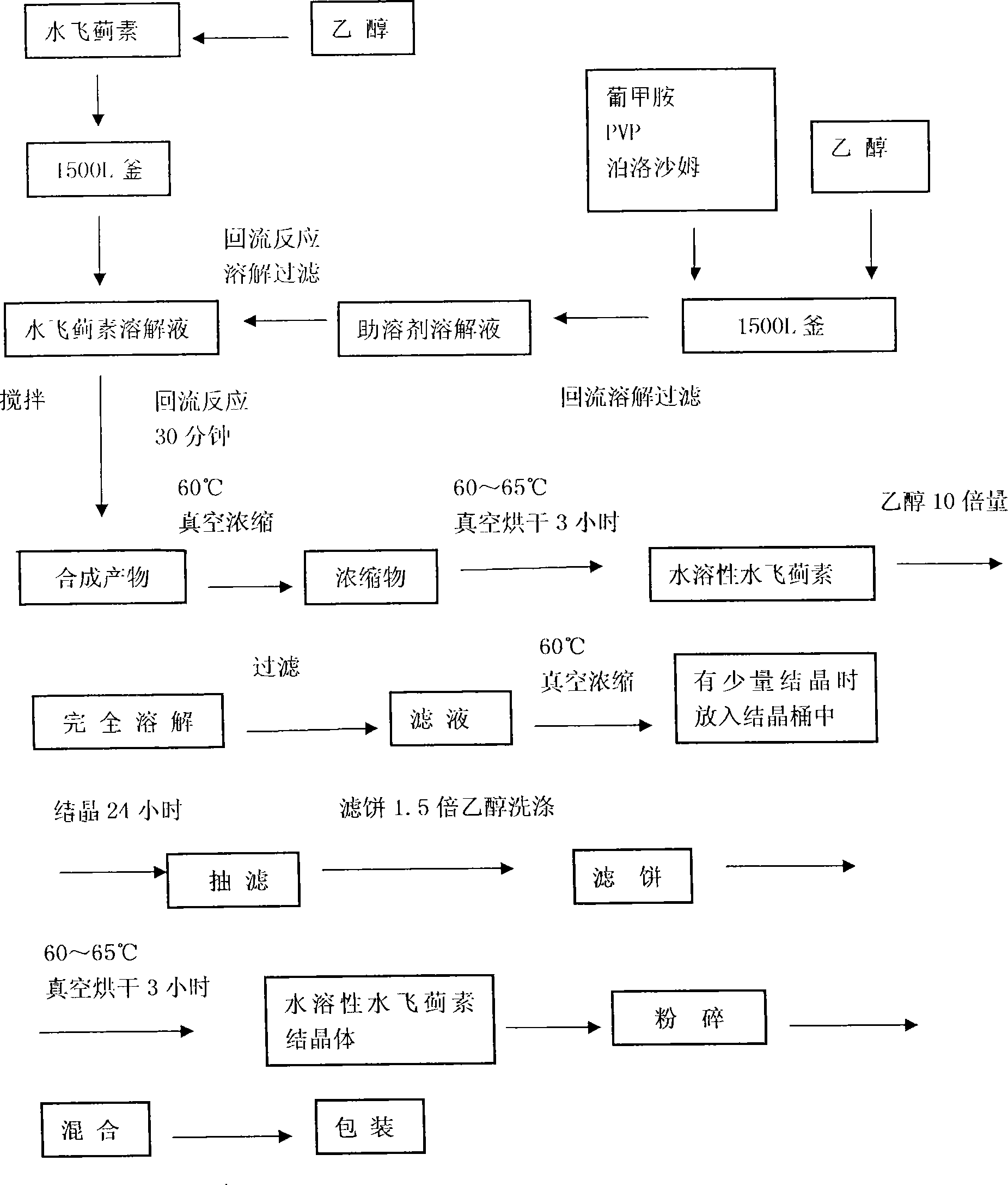
InactiveCN101019859AGood water solubilityImprove stabilityOrganic active ingredientsDigestive systemSolubilitySolvent
The water soluble silymarin preparation consists of silymarin, meglumine, PVP and poloxamer. It is prepared through dissolving silymarin and cosolvent in ethanol, filtering, reflux reaction and concentrating to obtain extractum; drying to obtain dry paste; redissolving, vacuum concentrating to crystallize, suction filtering to obtain crystal; ethanol washing, suction filtering, re-crystallizing and drying to obtain crystal; crushing, mixing and packing to obtain the water soluble silymarin preparation. The present invention features high water solubility, high stability and high bioavailability.
Owner:李宝
Synthesis and fine purification method of flunixin meglumine
The synthesis and extraction and refine method for meglumine flunixin comprises: with p-toluenesulfonic monohydrate as catalyst and 20% KOH as extract, selecting 2-methyl-3- (trifluoromethyl) aniline and 2-chloronicotioyl by mole ratio as 2:1 for reaction with end pH value more than 11.5; cooling and crystallizing the residual aniline to separate and recover; using methanol in extraction and purification for flunixin; static cooling and crystallizing, and separating and cleaning the crystal to obtain the refined product; finally, recrystallizing to prepare the flunixin methylglucamine in water. This invention has high yield.
Owner:SHANDONG LUKANG SHELILE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com