Lansoprazole for injecting and its preparation method
A technology for lansoprazole and injection, applied in the field of pharmaceutical dosage forms, can solve problems such as hidden safety hazards, local irritation, and short time to maintain stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] formulate a prescription
[0081] One, increase the solubility of lansoprazole and the technological research of stability
[0082] In this embodiment, two preparation process routes are designed and tested. One is to increase the solubility and stability of lansoprazole by adding different solubilizers; the other is to utilize the solubility of lansoprazole under alkaline conditions to increase and the more stable properties to prepare the alkali of lansoprazole neutral solution.
[0083] 1. Add solubilizer to increase solubility
[0084] The commonly used solubilizers for preparing injections have polyoxyethylene castor oil (EL), Tween 80, Prosolonic F-68 etc. at present, by adding surfactants in different proportions and different processing methods, to improve the lansoprazole in Solubility in water.
[0085] Test method: Weigh about 0.3 g of lansoprazole raw material, add 50 mL of aqueous solutions containing different concentrations of solubilizers, stir, and ...
Embodiment 2-4
[0142] * The above prescriptions are based on 1000 sticks
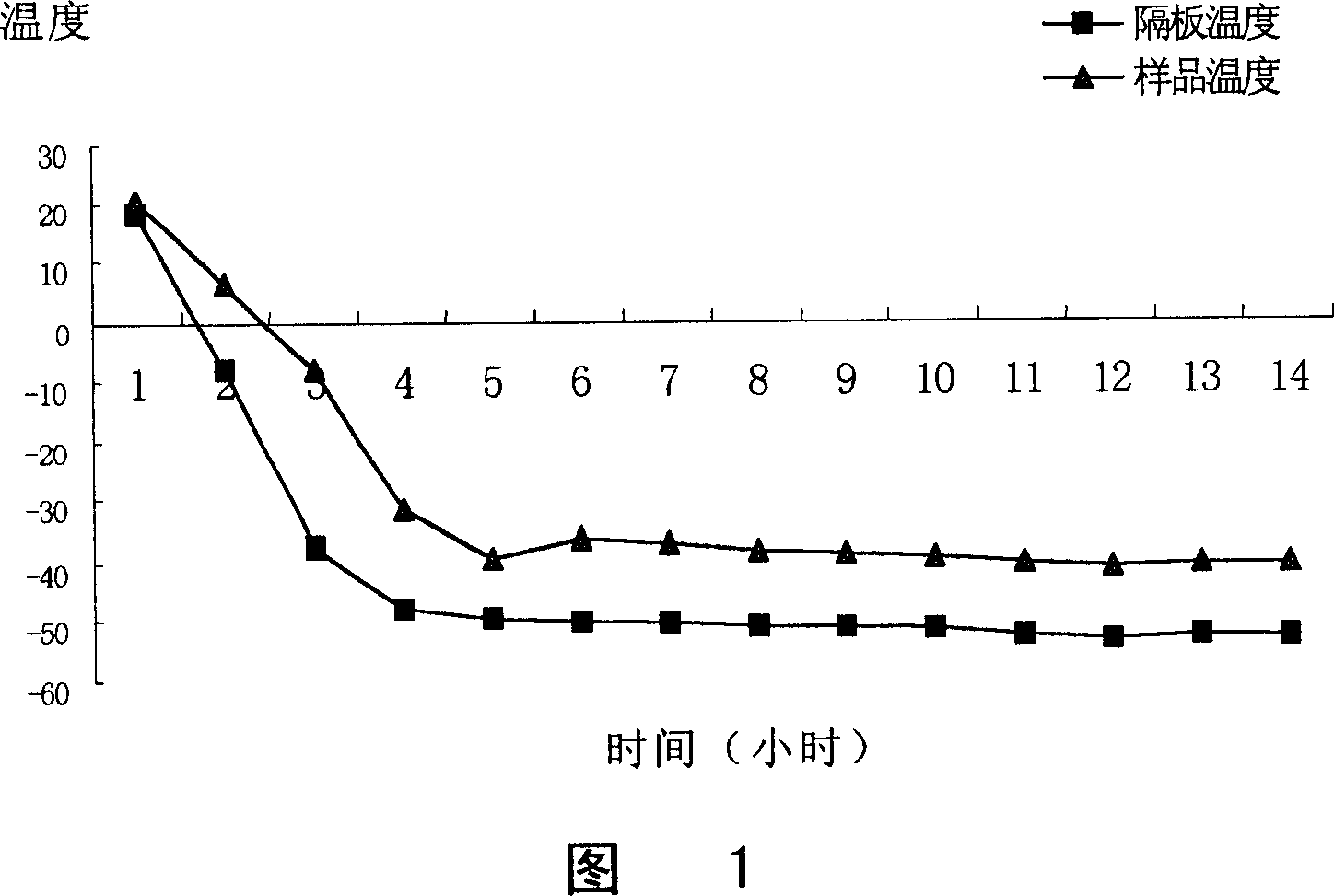
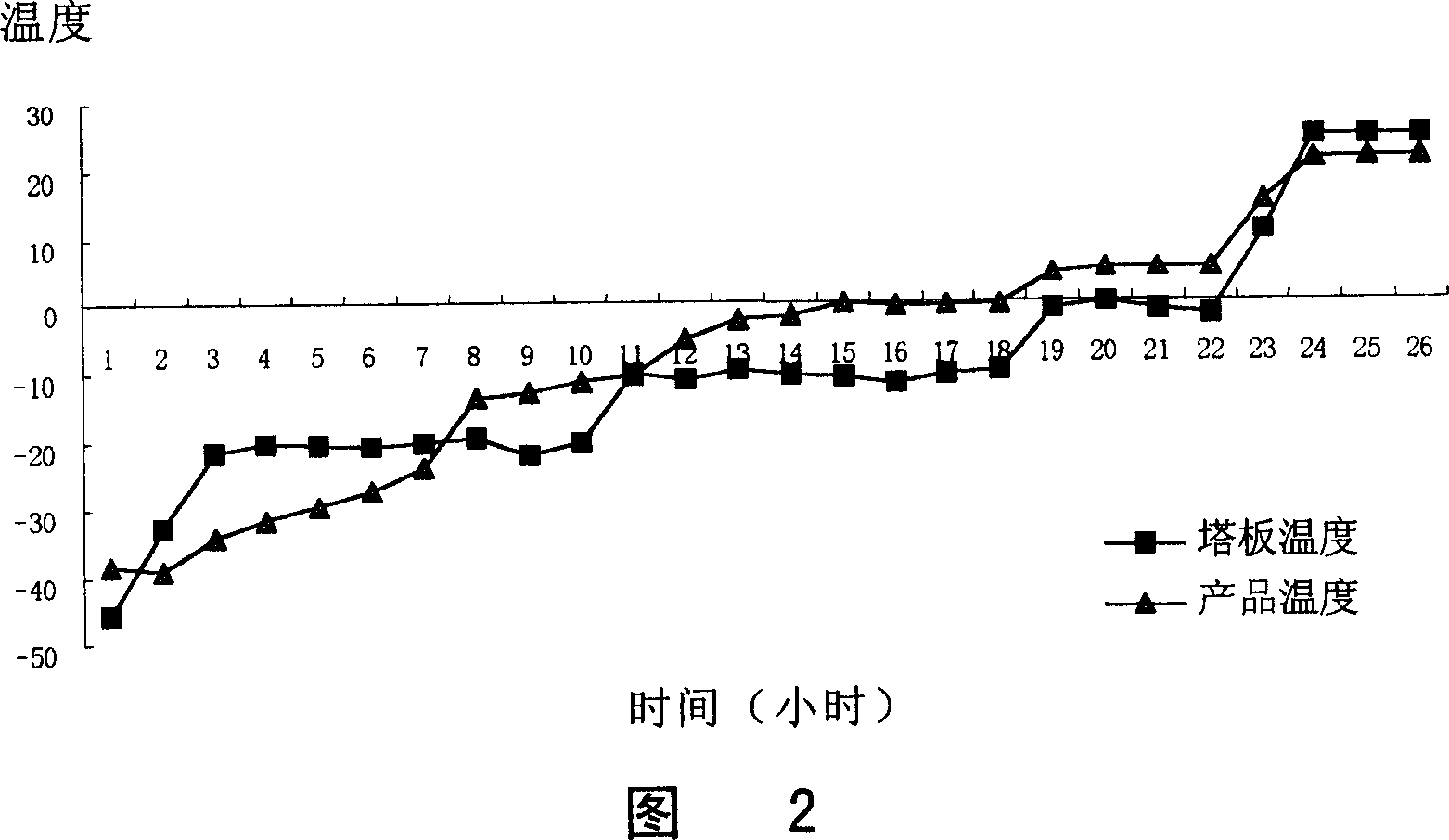
[0143] Weigh lansoprazole, add sodium hydroxide solution, stir, mix, add water for injection, add meglumine, stir to make the medicine dissolve completely, add mannitol, stir, dissolve and mix. Then add 0.2% activated carbon, stir for 20 minutes, filter and decarburize, then pass through 0.45 μm and 0.22 μm microporous membranes respectively, fill the filtrate into 7 mL vials, 2 mL per bottle, pre-freeze at -40 ° C for 6 h, and heat up to - Dry at 20°C for 8 hours, heat up to -10°C and dry for 4 hours, heat up to 0°C and dry for 2 hours, heat up to 25°C and dry for 2 hours, cap the treated vial stopper, crimp the cap, and seal it. Obtain the lansoprazole solid preparation for injection of the present invention.
[0144] The prepared lansoprazole solid preparation for injection is dissolved in water for injection to prepare the lansoprazole solution dosage form for injection.
Embodiment 5-7
[0146] Stability of solid dosage forms
[0147] According to the provisions of Appendix XIX C of the Chinese Pharmacopoeia in 2000, the investigation content of the stability study was selected. Accelerated tests and long-term tests were carried out on the solid preparations prepared in Examples 2-4, and the key investigation items included properties, identification, alkalinity, insoluble particles, related substances and their contents.
[0148] the result shows:
[0149] 1. Place the solid preparation prepared in Examples 2-4 for 6 months under the conditions of a temperature of 40°C ± 2°C and a relative humidity of 75% ± 5% according to the package to be marketed, and there is no significant change in each index.
[0150] 2. The solid preparations prepared in Examples 2-4 were placed in commercially available packages at a temperature of 25°C±2°C and a relative humidity of 60%±10% for 6 months, and all indicators had no significant changes.
[0151] 3. Place the solid pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com