Pharmaceutical composition containing quinoline derivative or salt of quinoline derivative
A composition and drug technology, which is applied in the directions of drug combinations, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc., can solve the problems of large production difficulties of preparations, uneven mixing of materials, and injuries to the respiratory system of operators.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
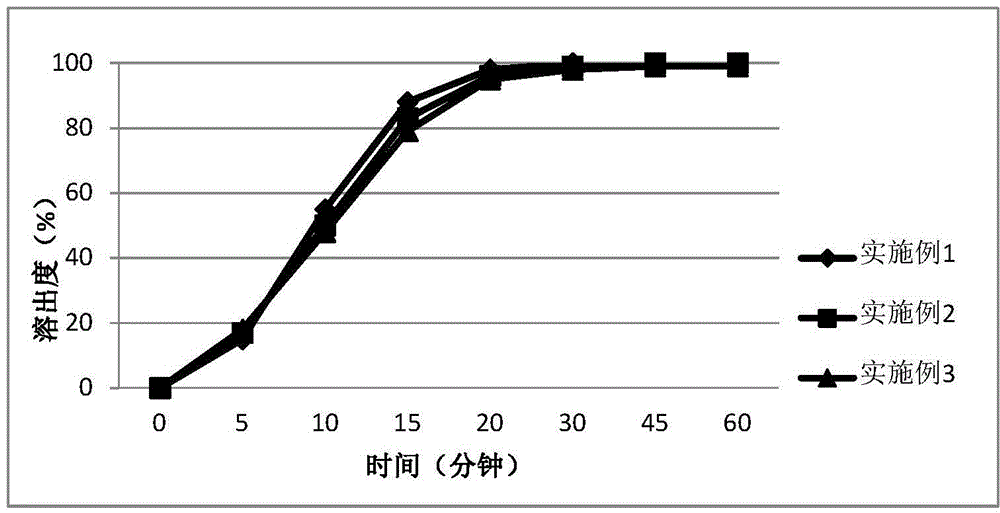
Embodiment 1~3
[0040] The methanesulfonate of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide (hereinafter referred to as compound A), arginine acid, D-mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and low-substituted hydroxypropyl cellulose, according to the proportions in Table 1, were wet-granulated using a high-speed shear granulator, and purified water was used as moistening agent. Wet agent, carry out wet granulation and drying treatment on wet and soft materials, then carry out dry granulation on dry granules (water content less than 2%), add prescription amount of talcum powder, and use rotary blender for mixing. The obtained blended granules are filled into capsules to prepare capsules.
[0041] Table 1
[0042] Element
[0043] Unit: mass%
Embodiment 4~6
[0045] The methanesulfonate of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide (hereinafter referred to as compound A), meglu Amine, D-mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and low-substituted hydroxypropyl cellulose, according to the proportions in Table 1, were wet-granulated using a high-speed shear granulator, and purified water was used as moistening agent. Wet agent, carry out wet granulation and drying treatment on wet and soft materials, then carry out dry granulation on dry granules (water content less than 2%), add prescription amount of talcum powder, and use rotary blender for mixing. The obtained blended granules are filled into capsules to prepare capsules.
[0046] Table 2
[0047] Element
[0048] Unit: mass%
Embodiment 7~16
[0049] Embodiment 7~16, comparative example 1~2
[0050]The mesylate of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolinecarboxamide (hereinafter referred to as compound A), arginine Acid or meglumine, potassium bicarbonate or potassium carbonate, D-mannitol, microcrystalline cellulose, hydroxypropyl cellulose, low-substituted hydroxypropyl cellulose, according to the ratio in Table 1, high-speed shear granulation The machine performs wet granulation, uses purified water as a wetting agent, performs wet granulation and drying treatment on wet and soft materials, and then performs dry granulation on dry granules (moisture is less than 2%), adds prescribed amount of talcum powder, and uses rotary blender for mixing. The obtained blended granules are filled into capsules to prepare capsules. And adopt the same method to prepare the capsules of Comparative Examples 1-2 that do not contain arginine, meglumine, potassium carbonate and potassium bicarbonat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com