Medicine combination including high-concentration polydatin
A technology of polydatin and composition, which is applied in the field of pharmaceutical compositions containing high-concentration polydatin, and can solve the problems that it is not suitable for direct injection administration, the feasible preparation method has no further explanation, and the conventional aqueous solution is difficult to achieve.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
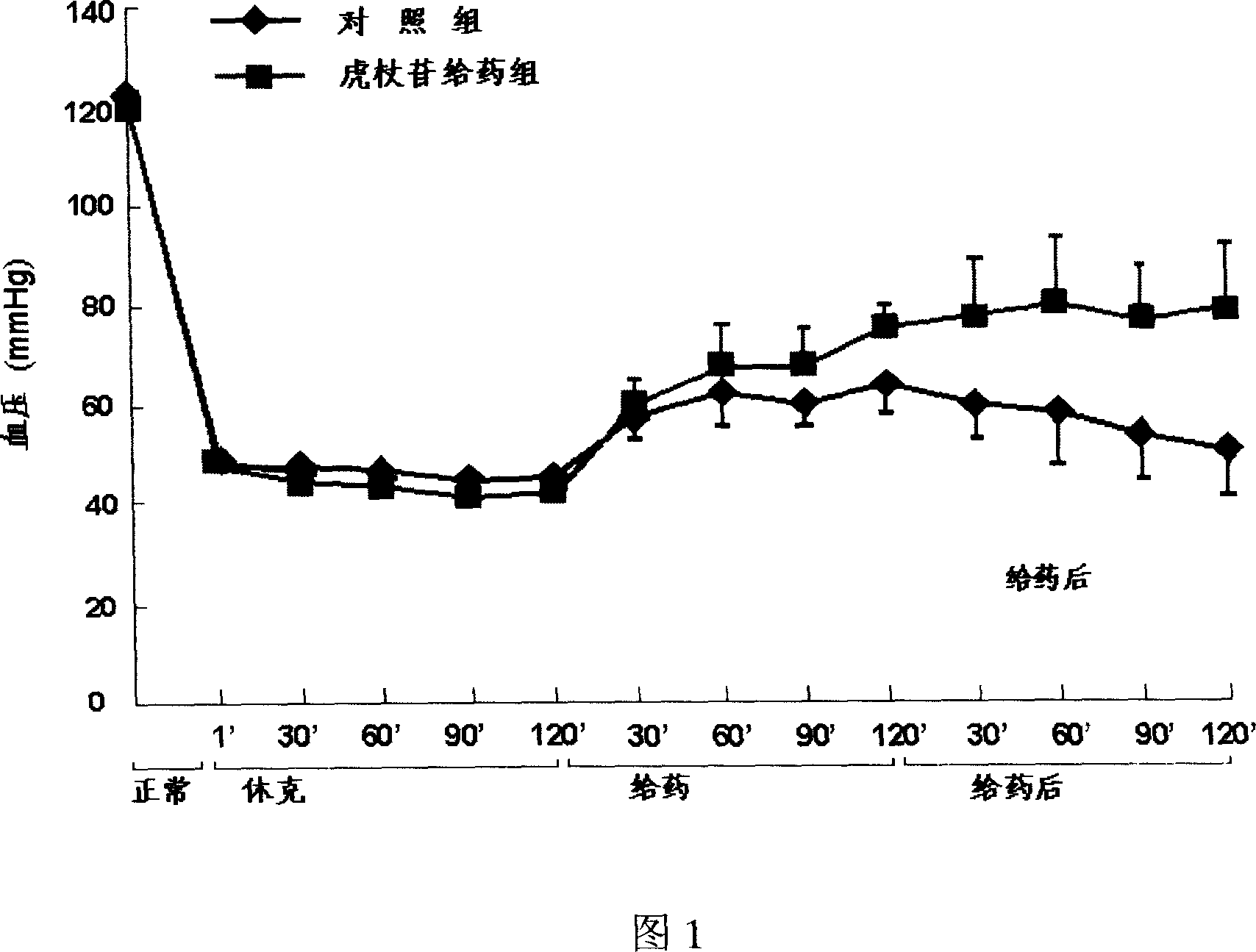
Image
Examples
Embodiment 1
[0055] 1. Prescription
[0056] Raw material dosage
[0057] Polydatin 25.0g
[0058] Meglumine 50.0g
[0059] pH 8.0 Na 2 HPO 4 -NaH 2 PO 4Buffer to 5.0L
[0060] Made 1000 pieces
[0061] 2. Preparation method Na 2 HPO 4 -NaH 2 PO 4 Buffer 4.8L, add polydatin 25.0g, meglumine 50.0g, ultrasonically induce dissolution at 40°C, add Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was adjusted to 5.0 L, ultrafiltered with a 0.2 μm microporous membrane, and filled in 1000 ampoules.
Embodiment 2
[0063] 1. Prescription
[0064] Raw material dosage
[0065] Polydatin 50.0g
[0066] β-cyclodextrin 250.0g
[0067] pH 9.0Na 2 HPO 4 -NaH 2 PO 4 Buffer to 5.0L
[0068] Made 1000 pieces
[0069] 2. Preparation method Na 2 HPO 4 -NaH 2 PO 4 Buffer 4.8L, add polydatin 50.0g, β-cyclodextrin 250.0g, sonicate at 40°C, add Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was adjusted to 5.0 L, ultrafiltered with a 0.2 μm microporous membrane, and filled in 1000 ampoules.
Embodiment 3
[0071] 1. Prescription
[0072] Raw material dosage
[0073] Polydatin 100.0g
[0074] Hydroxypropyl-β-cyclodextrin 600.0g
[0075] pH 7.5 Na 2 HPO 4 -NaH 2 PO 4 Buffer to 5.0L
[0076] Made 1000 pieces
[0077] 2. Preparation method Na 2 HPO 4 -NaH 2 PO 4 Buffer 4.8L, add polydatin 100.0g, hydroxypropyl-β-cyclodextrin 600.0g, sonicate at 40°C, add Na 2 HPO 4 -NaH 2 PO 4 The buffer solution was adjusted to 5.0 L, ultrafiltered with a 0.2 μm microporous membrane, and filled in 1000 ampoules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com