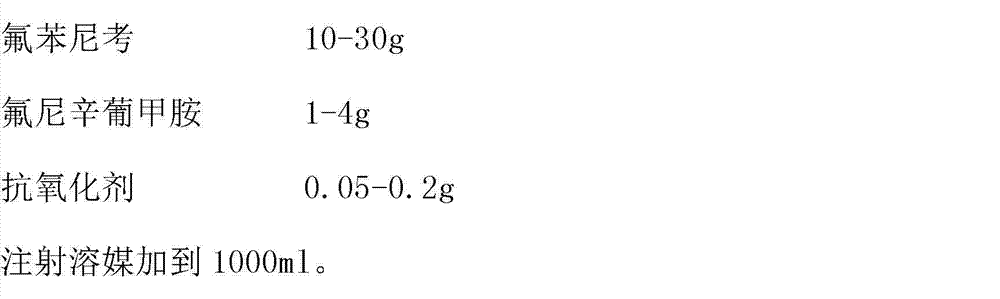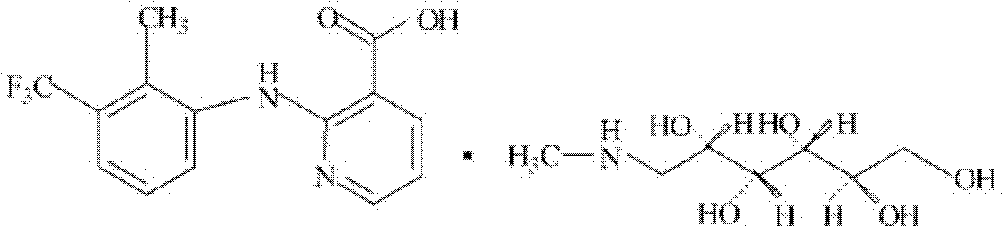Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "FLUNIXIN MEGLUMINE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
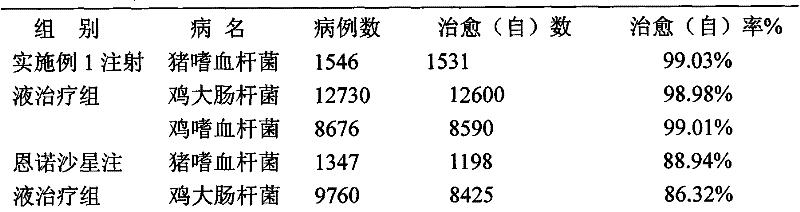
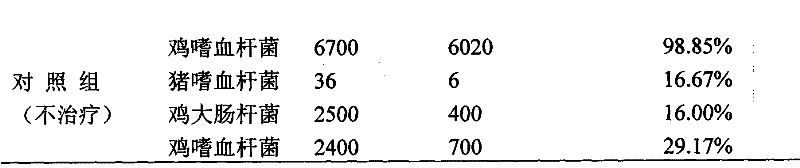
Flunixin meglumine is a non-steroidal anti-inflammatory medication used for treating inflammation and pain associated with musculoskeletal disease. Give the dose as soon as possible.
Long-acting flunixin meglumine injection and method for preparing same
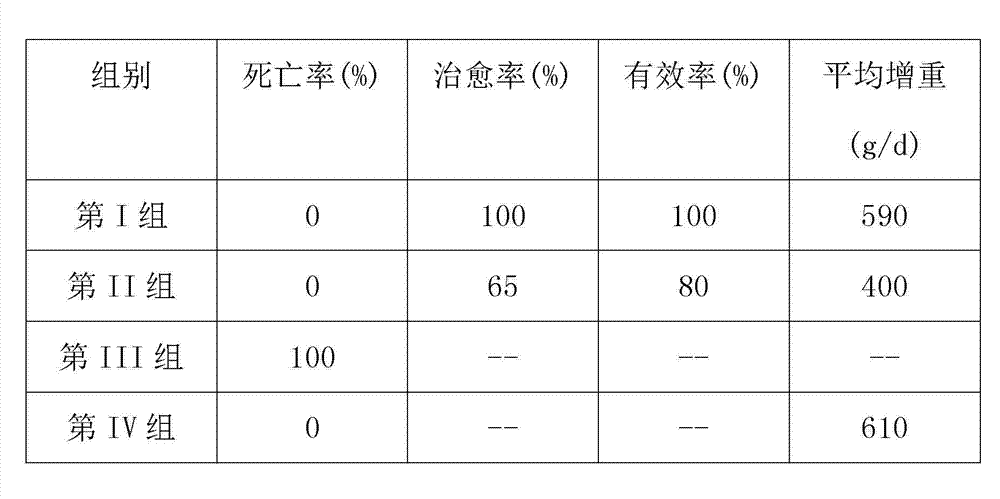
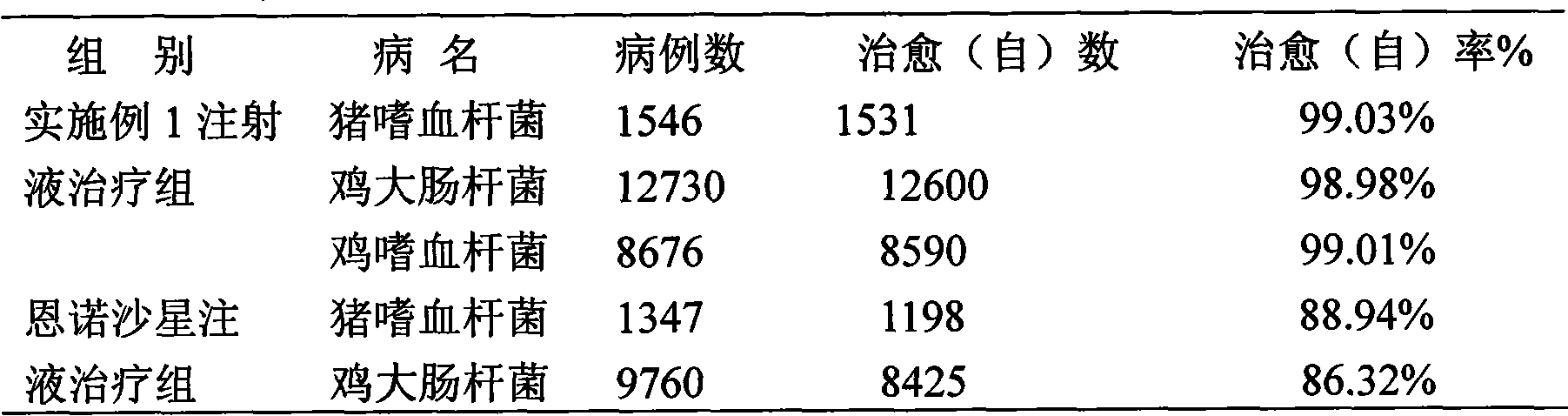
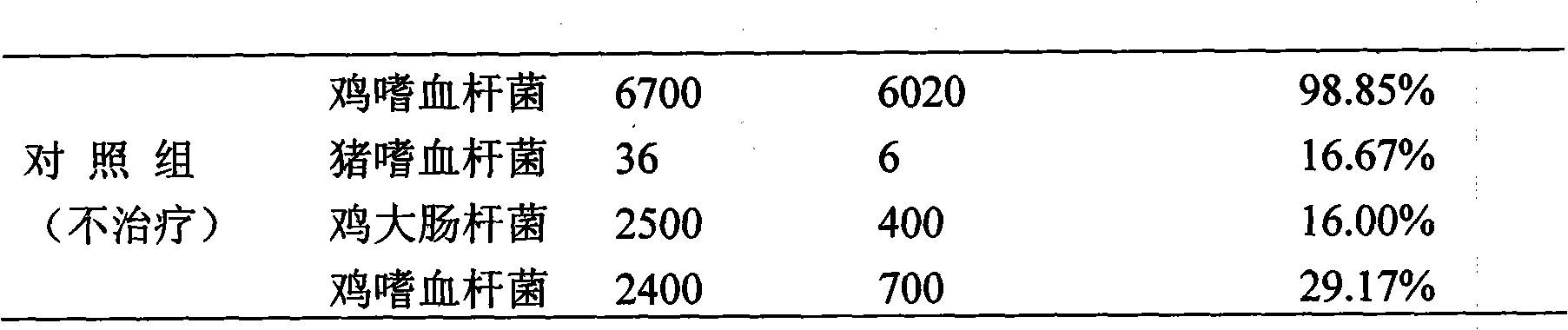
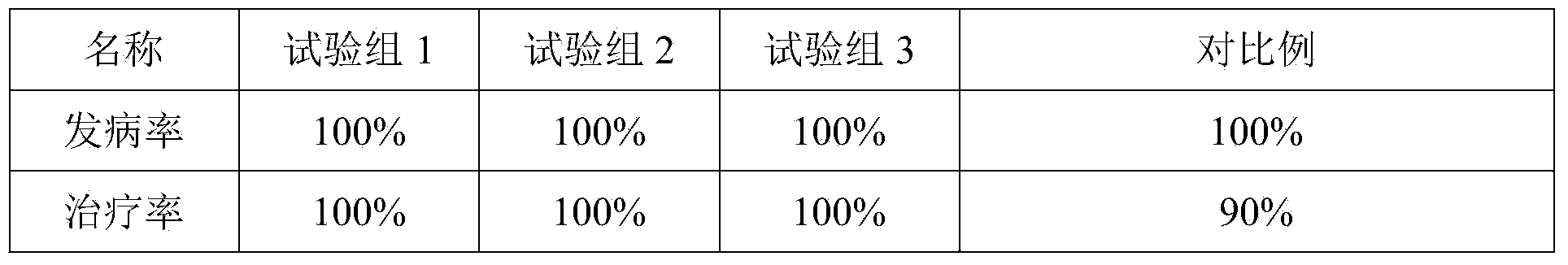
ActiveCN101548945ASolve the defect of short action timeReduce stress responseAntipyreticAnalgesicsFLUNIXIN MEGLUMINEClinical trial
The present invention relates to a long-acting flunixin meglumine injection for animals and method for preparing same. The invention provided flunixin meglumine injection delays medicine absorption so as to prolong medicine action time by adding proper polyvinylpyrrolidone and poloxamer high molecular compound in the preparation process. Approved by clinical experiments that action time of the invention is longer than common flunixin meglumine injection, the invention reduces labor strength and reduces stress response of animals with more obvious medicine effect, and capable of using with long-acting antibacterials to provide a synergistic effect. The invention provides a animals specific medicine of long-acting, effective antipyretic, antiphlogosis and analgesic for veterinary clinical.
Owner:SHAOSHAN DABEINONG ANIMAL MEDICINE +1
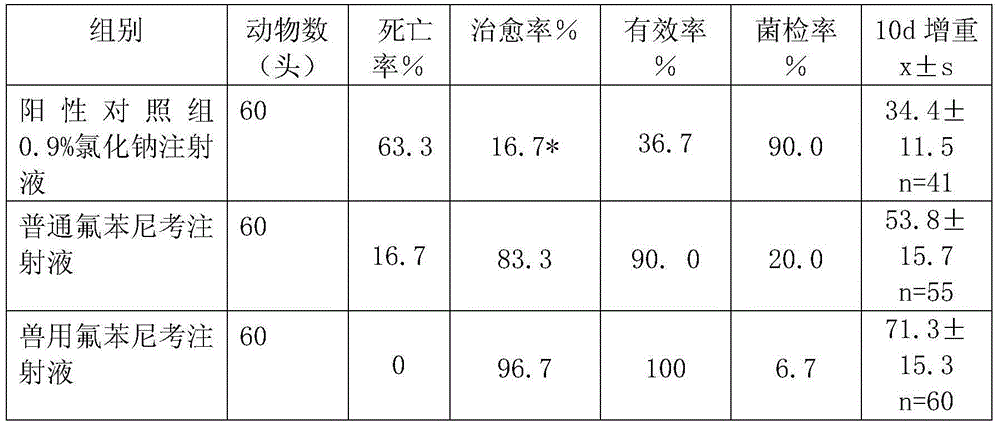
Compound florfenicol injection and preparation method and application thereof
ActiveCN102188422AReasonable compositionSignificant effect on infectionAntibacterial agentsHydroxy compound active ingredientsBovine respiratory diseaseFLUNIXIN MEGLUMINE
The invention discloses compound florfenicol injection and a preparation method and application thereof. The compound florfenicol injection comprises main medicaments, injection solvent and local anodyne; and the main medicaments are florfenicol and flunixin meglumine. The compound florfenicol injection is safe and convenient to use, controllable in quality, stable in preparation process, suitable for industrialized production and low in cost; and the compound florfenicol enhances the prevention and treatment effects of porcine or bovine respiratory diseases, improves the survival rate of pigs or cattle, and is suitable for popularization and application.
Owner:GUANGDONG WENS DAHUANONG BIOTECH
Method for preparing veterinary oxytetracycline-artemisinin injection and clinical application of veterinary oxytetracycline-artemisinin injection
InactiveCN102228462AReduce stimulationLeading technologyAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINECompound organic
The invention relates to a method for preparing a veterinary oxytetracycline-artemisinin injection and clinical application of the veterinary oxytetracycline-artemisinin injection. Raw materials of the injection mainly comprise oxytetracycline, artemisinin, trimethoprim, flunixin meglumine, magnesium oxide or magnesium chloride, an antioxidant, a pH regulator, a compound organic solvent and water. As a veterinary compound preparation, the veterinary oxytetracycline-artemisinin injection is mainly used for treating infectious diseases and secondary infection which are caused by sensitive gram positive bacteria and negative bacteria, eperythrozoon, rickettsia and mycoplasma. The method for preparing the veterinary compound oxytetracycline-artemisinin injection is simple, the production process is mild, the chelating temperature is low, the stability is high, and the injection is convenient to use and is suitable for industrialized production.
Owner:SHANDONG DEZHOU SHENNIU ANIMAL HEALTH PROD CO LTD
Perfusion medicament for treating milk cow mastitis and preparation method thereof
ActiveCN102488694ATreat both symptoms and root causesSolve the problem of easily inducing endotoxemia in dairy cowsAntibacterial agentsSexual disorderFLUNIXIN MEGLUMINEThird generation
The invention discloses a perfusion medicament for treating milk cow mastitis and a preparation method thereof. The perfusion medicament is prepared from the following raw materials by weight: 0.9-4.3g of cefquinome sulphate, 0.15-0.25g of prednisolone, 0.5-1.3g of flunixin meglumine, 1-1.5g of glycerin monostearate, 3-8g of Vaseline, 0.05g of ethylparaben and 65-70g of whiteruss. The preparationmethod comprises the following specific steps of: (1) putting one tenth of the liquid paraffin into a burdening tank in a certain weight ratio; (2) adding one third of the liquid paraffin into a heating tank; and (3) putting the rest of the liquid paraffin left after the steps (1) and (2) and auxiliary materials in the step (2) into an emulsifying tank, adding 0.9-4.3g of cefquinome sulfate in a certain weight ratio, and stirring till uniform distribution to obtain the perfusion medicament for treating milk cow mastitis. The perfusion medicament has the advantages of no induction of endotoxemia in a milk cow mastitis treating process, remarkable curative effect on mastitis and freeness from medicament tolerance.
Owner:QILU ANIMAL HEALTH PROD
In-situ gel for injecting flunixin meglumine and preparation method thereof
InactiveCN103230362ALess irritatingImprove complianceSenses disorderAntipyreticDiseaseFLUNIXIN MEGLUMINE
The invention belongs to the field of a special pharmaceutical preparation for animals, and relates to an in-situ gel preparation for injecting flunixin meglumine and a preparation method thereof. The in-situ gel preparation is prepared from water as a solvent, flunixin meglumine, in-situ gel matrix known in the field, and other pharmaceutical necessary auxiliary materials. The preparation method is clarified. The preparation disclosed by the invention is liquid which freely flows at room temperature; sold or semi-solid gel is formed after dosing by intramuscular injection or subcutaneous injection; drug release is delayed; the lasting time is long; just one-time dosing in a course of treatment is achieved; and the in-situ gel has the advantages that the in-situ gel is simple to prepare, stable in property, convenient to dose, strong in animal adaption, and the like. The preparation disclosed by the invention has the effects of relieving fever, easing pain, diminishing inflammation and the like, and is used for preventing and treating diseases of animals such as pigs, sheep, dogs, cats, cattle and birds.
Owner:NANJING AGRICULTURAL UNIVERSITY
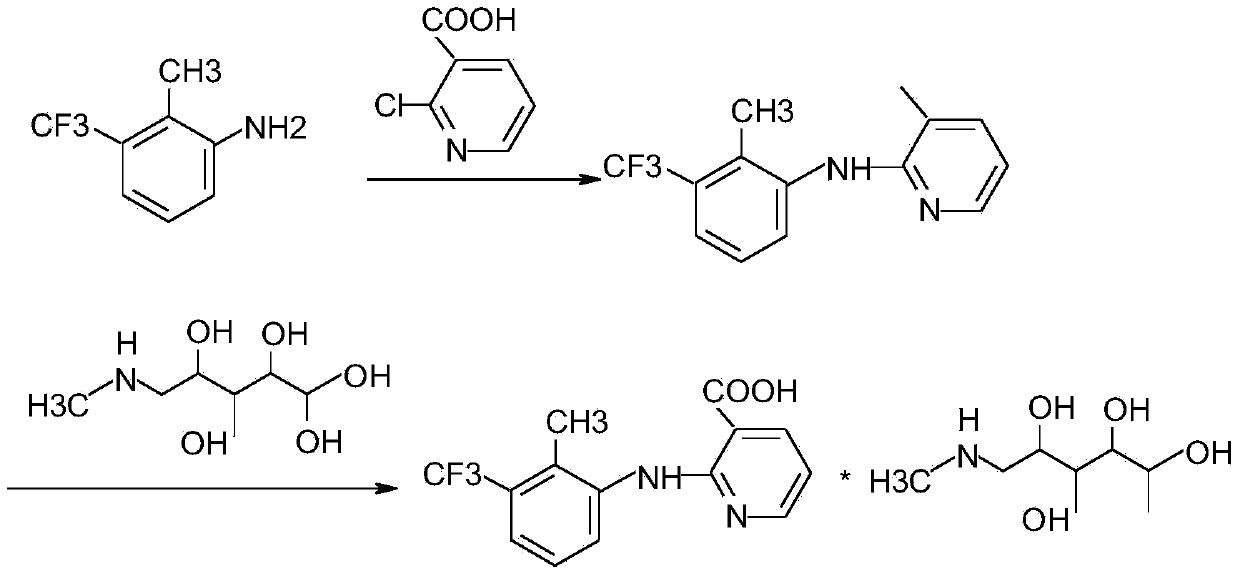
Method for synthesizing flunixin meglumine
InactiveCN103694167AHigh catalytic efficiencyEfficient responseOrganic compound preparationAmino-hyroxy compound preparationFLUNIXIN MEGLUMINEAcetonitrile
The invention provides a method for synthesizing flunixin meglumine. According to the method, reaction is carried out in a manner that 2-chloronicotinic acid and 2-methyl-3-trifluoromethylaniline serve as raw materials, water serves as a solvent and cupric oxide and para-toluenesulfonic acid serve as catalysts. According to the method, on the premise that the condition that para-toluenesulfonic acid serves as a catalyst is reported in the existing literature, cupric oxide is added, so that the catalysis efficiency is increased greatly, the reaction can be carried out efficiently, the reaction time is shortened, and the yield is increased; obtained flunixin and meglumine are salified in an acetonitrile solvent and are recrystallized, so as to obtain flunixin meglumine, and the total yield is about 90%; through synthesizing flunixin meglumine by the method, the operation is convenient and simple, and the requirements for equipment are low, so that the method is applicable to industrial large-scale production.
Owner:WEIHAI YARUI BIOLOGICAL SCI & TECH
Compound ceftiofur sodium freeze-dried power injection used for injection
ActiveCN102106857AHigh antibacterial activityDelay drug resistanceAntibacterial agentsPowder deliveryFLUNIXIN MEGLUMINECeftiofur sodium
The invention is a compound ceftiofur sodium freeze-dried power injection used for injection, and the compound ceftiofur sodium freeze-dried power injection provided by the invention is mainly composed of the following effective components in parts by weight: 2-4 parts of ceftiofur sodium, 1 parts of sulbactam sodium and 1 parts of flunixin meglumine; a vacuum freeze drying method is adopted to prepare the effective components into the compound ceftiofur sodium freeze-dried power injection used for injection. The compound ceftiofur sodium freeze-dried power injection has the advantages that the preparation cost can be lowered, and the drug resistance of bacteria on the ceftiofur sodium is relieved; and the broad antimicrobial spectrum is wide, the activity is strong, the drug administration times is less, and low stress and slight side effect are endured; and the negative effect of endotoxin can be effectively blocked.
Owner:QILU ANIMAL HEALTH PROD
Veterinary compound sulfadiazine sodium injection and preparation method thereof
InactiveCN101829124AImprove antibacterial propertiesLittle side effectsAntibacterial agentsPharmaceutical delivery mechanismFLUNIXIN MEGLUMINETrimethoprim
The invention relates to a veterinary compound sulfadiazine sodium injection and a preparation method thereof. The veterinary compound sulfadiazine sodium injection comprises sulfadiazine sodium, enrofloxacin, flunixin meglumine, trimethoprim, dexamethasone sodium phosphate, an organic solvent and water for injection. The injection serving as a veterinary special compound preparation has a special treating effect on various infectious diseases, mixed infections and systemic infections of the digestive system, the respiratory system, the urinary system and the skin soft tissue caused by sensitive bacteria and mycoplasmas of oxen, swines, poultries, canines, cats and aquatic animals The injection has the advantages of convenient use, short period of treatment, low medicament resistance and the like.
Owner:陈建波
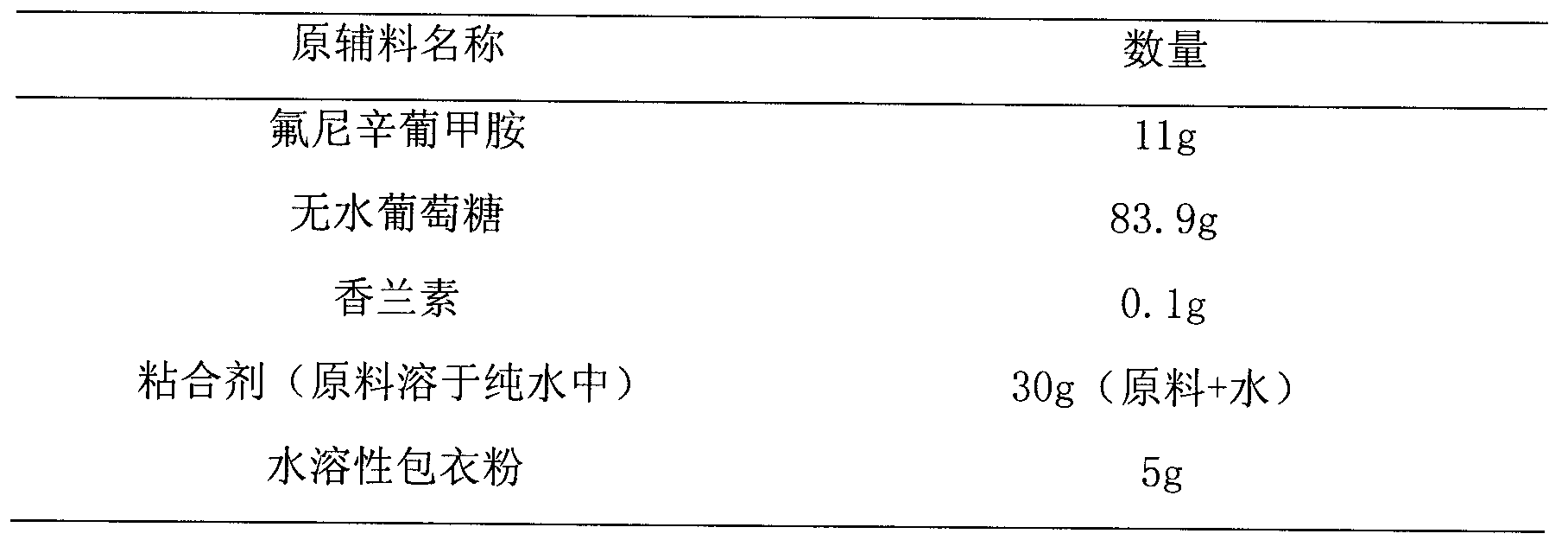
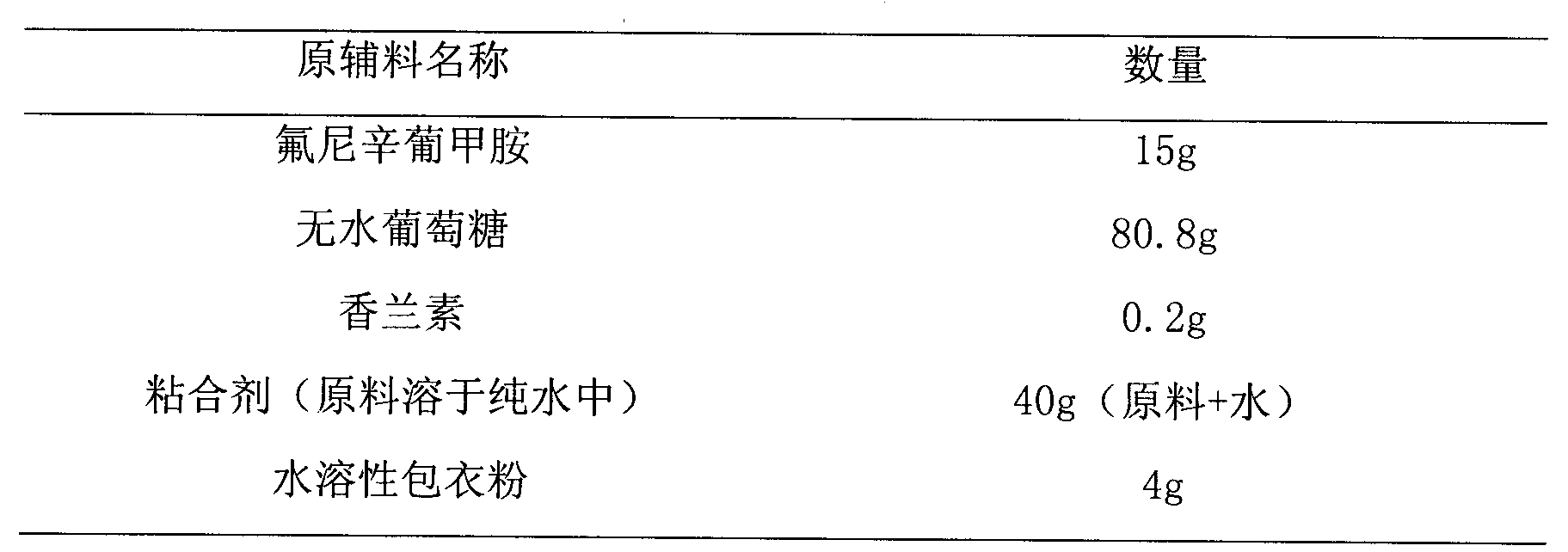
Flunixin meglumine coated granule and preparation method thereof
InactiveCN104257614AReduce chances of direct contactReduce irritabilityPharmaceutical non-active ingredientsGranular deliveryFLUNIXIN MEGLUMINEStiphra robusta
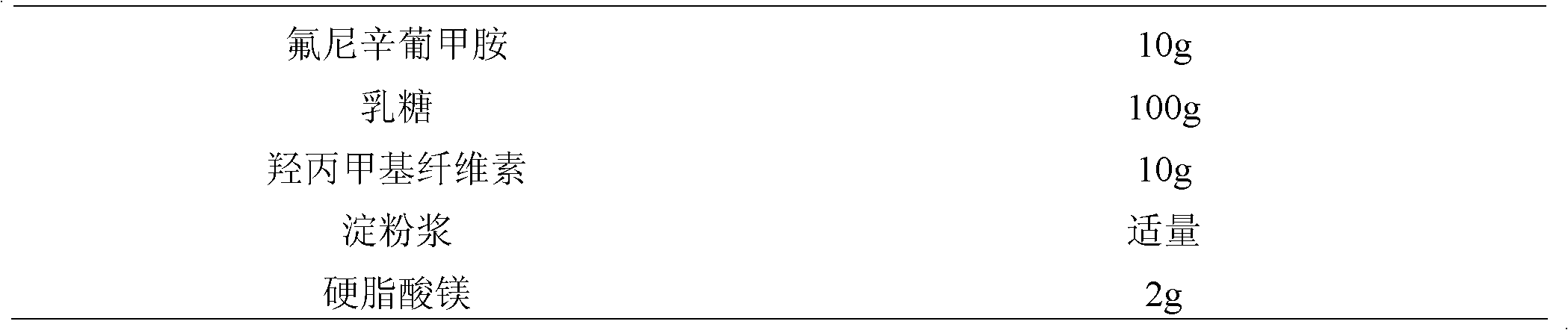
The invention relates to a flunixin meglumine coated granule and a preparation method thereof. The preparation method of the flunixin meglumine coated granule comprises the following steps: dissolving raw materials in a solvent (serving as an adhesive), spraying into auxiliary materials which are uniformly mixed in a fluidized bed to carry out granulation, and finally spraying into a water-soluble coating solution to prepare the flunixin meglumine coated granule. The flunixin meglumine has very strong pungent smell which causes abnormal discomfort to the respiratory tracts and eyes of people in the production, packaging and using processes; according to the flunixin meglumine coated granule, the flunixin meglumine is dissolved in the solvent, sprayed into the auxiliary materials of the fluidized bed and finally coated, so that the operators and farmers are prevented from the pungent smell in the whole production, packaging and using processes; and above all, the coated granule is capable of reducing the sensitivity of the animals and improving the compliance, so that the effect of the coated granule can come into play better.
Owner:ZHENGZHOU FUYUAN ANIMAL PHARMA
Synthesis and fine purification method of flunixin meglumine
The synthesis and extraction and refine method for meglumine flunixin comprises: with p-toluenesulfonic monohydrate as catalyst and 20% KOH as extract, selecting 2-methyl-3- (trifluoromethyl) aniline and 2-chloronicotioyl by mole ratio as 2:1 for reaction with end pH value more than 11.5; cooling and crystallizing the residual aniline to separate and recover; using methanol in extraction and purification for flunixin; static cooling and crystallizing, and separating and cleaning the crystal to obtain the refined product; finally, recrystallizing to prepare the flunixin methylglucamine in water. This invention has high yield.
Owner:SHANDONG LUKANG SHELILE PHARMA
Synthesis method of flunixin meglumine
ActiveCN104193674AThorough responseHigh yieldOrganic compound preparationAmino-hyroxy compound preparationFLUNIXIN MEGLUMINESynthesis methods
The invention discloses a synthesis method of flunixin meglumine, which comprises the following steps: adding 2-chloronicotinic acid and 2-methyl-3-trifluoromethyl aniline into a sodium hydroxide water solution, stirring, adding toluene and a phase-transfer catalyst, reacting at controlled temperature of 40-45 DEG C for 4-5 hours, regulating the pH value of the solution to 10-11, stirring, standing to stratify, regulating the pH value of the water layer to 5-6, stirring, filtering, washing the filter cake, and drying to obtain flunixin, reacting flunixin and N-methylglucosylamine in isopropanol, heating under reflux for 0.5-1.5 hours, filtering, cooling to 50-60 DEG C, and stirring to crystallize; and when the system temperature drops to 25 DEG C below, continuing stirring for 1 hour, carrying out vacuum filtration on the crystal, and washing with isopropanol to obtain the flunixin meglumine. The method lowers the reaction temperature, saves the energy, shortens the reaction time, and is simple for synthesis operation, low in facility requests and convenient for industrialized operation.
Owner:济南久隆医药科技有限公司 +1
Compound sulphamethoxazole injection and preparation method thereof
ActiveCN103222978ALong-lasting effectExtended half-lifeAntibacterial agentsAntipyreticEscherichia coliFLUNIXIN MEGLUMINE
The invention discloses a compound sulphamethoxazole injection and a preparation method thereof. The injection is prepared from 5-20 % of the main drug sulphamethoxazole, 2-10 % of flunixin meglumine, 1-4 % of trimethoprim, 0.2-0.4 % of an accessory anti-oxidant, 40-70 % of an organic solvent, 0.5-2 % of an adjusting reagent and the balance being water for injection. The injection prepared by the invention can provide effective treatment for secondary infections and mixed infections of streptococcus diseases, toxoplasmosis, eperythrozoonosis, E. coli diseases, asthma and other diseases, is obvious in long-term effects, with a drug effect being enhanced by several times to dozens of times, small in application amount, few in drug using times, little in irritation and fast in performance of drug effects, saves production cost, increases added value of products, and reduces toxic and side effects due to drug mismatch during treatment.
Owner:河南智盛优品生物科技有限公司
Compound florfenicol preparation for treating swine respiratory tract infection diseases and preparation method thereof
InactiveCN102920701ARapid relief of clinical symptomsGood antipyretic and analgesic effectAntibacterial agentsAntipyreticDiseaseCompound organic
The invention discloses a compound florfenicol preparation for treating swine respiratory tract infection diseases and a preparation method thereof. The compound florfenicol preparation comprises florfenicol, flunixin meglumine, a composite organic solvent and an antioxidant agent, and the florfenicol and the flunixin meglumine are used as main drugs. The compound florfenicol preparation has anti-infection, anti-inflammation, antipyresis and analgesia effects, is dosed through subcutaneous injection and has remarkable effects for preventing and treating the swine respiratory tract diseases. The compound florfenicol preparation is exquisite in preparing process, controllable in quality, safe, reliable, convenient to use, low in cost, and suitable for being popularized and applied to cultivation industry.
Owner:QINGDAO VLAND BIOTECH INC +1
Tablet containing flunixin meglumine and preparation method
The invention relates to a tablet containing flunixin meglumine and a preparation method, which belongs to the veterinary drug technical field. The tablet can prolong the action time and reduce the stimulation of drug on gastrointestinal tract, and the pesticide effect is obvious.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Veterinary compound enrofloxacin injection and preparation method thereof
InactiveCN101658526APromote absorptionEfficient drug deliveryAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINETrimethoprim
The invention relates to a veterinary compound enrofloxacin injection and a preparation method thereof. The veterinary compound enrofloxacin injection mainly comprises enrofloxacin, sulfamethoxazole,trimethoprim, flunixin meglumine, tylosin, organic solvent and water for injection. The veterinary compound enrofloxacin injection is used as a veterinary special compound preparation, used for treating various infectious diseases of a respiratory system, a digestive system and a skin soft tissue caused by livestock and poultry bacterial and mycoplasma infection, particularly has special effects on secondary hemophilus disease of pigs and poultries caused by hemophilus and has the advantages of convenient use, short course, low drug resistance and the like.
Owner:陈建波
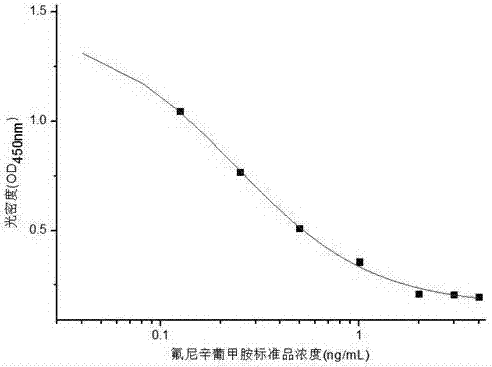
Flunixin meglumine monoclonal antibody hybridoma cell strain YY and application thereof
ActiveCN106867971AImprove featuresHigh detection sensitivityMicroorganism based processesTissue cultureFLUNIXIN MEGLUMINEMicroorganism
The invention discloses a flunixin meglumine monoclonal antibody hybridoma cell strain YY and application thereof and belongs to the field of food safety immunoassay. The flunixin meglumine monoclonal antibody hybridoma cell strain YY disclosed by the invention is preserved in China General Microbiological Culture Collection Center (CGMCC for short) with a preservation number of CGMCC No.13096. The monoclonal antibody secreted by the cell strain has better specificity and detection sensitivity (an IC50 value is 0.24ng / mL) to flunixin meglumine, provides raw materials for immunoassay of flunixin meglumine residues in food and has an actual application value.
Owner:JIANGNAN UNIV
High-concentration compound florfenicol injection, and preparation method and application thereof
InactiveCN104208062AReduce complicationsReduce recurrenceAntibacterial agentsAntipyreticBiotechnologyFLUNIXIN MEGLUMINE
The invention discloses a high-concentration compound florfenicol injection, and a preparation method and an application thereof. The method comprises the following steps: dissolving prescription doses of florfenicol and flunixin meglumine in a 75% single solvent, and carrying out heating stirring for dissolving; adding the solvent to a full capacity; adding 0.4% activated carbon, and adsorbing for 30min; and filtering, encapsulating, and carrying out 100DEG C flowing steam sterilization for 30minin order to obtain the high-concentration compound florfenicol injection. The compound florfenicol injection prepared through the preparation method has the advantages of safety, convenience, controllable quality, stable preparation process, suitableness for industrial production, and low cost; and the injection enhances the prevention effect on porcine and bovine infectious diseases caused by pathogenic microorganisms sensitive to florfenicol, improves the survival rate of pigs or cattle, and is suitable for popularization and application.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS +1
Compound terramycin injection and preparation method thereof
InactiveCN104161761AAntipyreticAnti-inflammatoryAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINETherapeutic effect
The invention discloses a compound terramycin injection and a preparation method thereof. The compound terramycin injection comprises, by weight, 20 parts of terramycin, 2 parts of flunixin meglumine, 30-40 parts of propylene glycol, 10-15 parts of dimethyl formamide, 3 parts of polyvinylpyrrolidone, 6-7 parts of magnesium oxide, 0.5 parts of an anti-oxidant, 6.1-6.5 parts of ethanolamine and 28 parts of injection water. The compound terramycin injection can be used for treating therapy-sensitive infectious diseases caused by gram-positive bacteria and gram-negative bacteria, rickettsia and mycoplasma and has good treatment effects. The compound terramycin injection has good stability, does not discolor after being heated or stored for a long time, does not product precipitates, solves the problem that under the acid or alkaline conditions, the existing terramycin injection discolors or precipitates after being heated or stored for a long time so that titer is reduced, and can slowly release drug active components in the human body so that long-acting effects are obtained.
Owner:ZHENGZHOU HOUYI PHARMA
Compound doxycycline hydrochloride injection for animals, and its preparation method
InactiveCN103816166ABroad chemical compatibilityRapid relief of acute infectious conditionsAntipyreticTetracycline active ingredientsFLUNIXIN MEGLUMINETreatment effect
The invention relates to a compound doxycycline hydrochloride injection for animals, and its preparation method. The above compound preparation is composed of doxycycline hydrochloride and fluoronicotinic acid. The method comprises the following steps: dissolving an antioxidant in injection water, adding the above obtained aqueous solution into an organic solvent, adding doxycycline hydrochloride and a complexing agent, adjusting the pH value, adding a metal ion chelating agent and parts of the antioxidant, adding flunixin dissolved in a proper amount of the organic solvent, uniformly mixing, filtering, filling nitrogen, and disinfecting to obtain the compound doxycycline hydrochloride injection for animals. The compound doxycycline hydrochloride injection prepared in the invention has the advantages of good stability, controllable quality, better treatment effect than single doxycycline hydrochloride injection and flunixin meglumine injection, reduction of the administration frequency, reduction of the labor intensity and the animal stress response, improvement of the treatment effect and efficiency, and wide application prospect in the veterinary clinical medicine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Veterinary florfenicol injection, and preparation method and application thereof
InactiveCN104127430ABroad spectrum antibacterialInhibit synthesisAntibacterial agentsAntipyreticFLUNIXIN MEGLUMINEBacterial disease
The invention discloses a veterinary florfenicol injection, and a preparation method and application thereof. Per 100 mL of the veterinary florfenicol injection comprises the following compositions by weight: 5-30 g of florfenicol, 15-80 g of an injection solvent, 0.5-3.5 g of a local analgesic, 0.1-1.0 g of an anti-oxidant, and 3-10 g of azithromycin. The injection is prepared by taking florfenicol and azithromycin as the main compositions of the antibacterial anti-inflammation medicine, combining flunixin meglumin and methscopolamine bromide, and employing an organic solvent propylene glycol, PEG-400, NMP, an anti-oxidant and the like. The florfenicol injection is safe and convenient to use, controllable in quality, stable in preparation technology, suitable for industrialized production and relatively low in cost. The florfenicol injection is capable of effectively treating and preventing livestock and poultry bacterial diseases, has special effects especially on respiratory system infection and intestinal infection, and is suitable for popularization and application.
Owner:PIZHOU ZHENGKANG BIOTECH
Flunixin meglumine taste masking orally-disintegrating preparation and preparing method thereof
ActiveCN105232486AGood taste masking effectMaintain the efficacy of the drugAntipyreticAnalgesicsSolubilityFLUNIXIN MEGLUMINE
Owner:GUANGXI UNIV
Long-acting compound florfenicol injection and preparation method thereof
ActiveCN108619142ALong duration of actionImprove bioavailabilityAntipyreticAnalgesicsFLUNIXIN MEGLUMINEDrug administration
The invention provides a long-acting compound florfenicol injection and a preparation method thereof, and belongs to the technical field of medicine preparation. Every 100mL of the injection comprises10-40g of florfenicol, 1-7g of flunixin meglumine, 1-10g of polyvinylpyrrolidone K30, 1-20g of poloxamer 407 and 60-85mL of solvents. The long-acting compound florfenicol injection has long-lasting drug effect which can last more than 40 hours or above, and the number of times for drug administration can be reduced effectively.
Owner:SHANDONG LUKANG SHELILE PHARMA
Long-acting compound tildipirosin injection and preparation method thereof
ActiveCN111603475AHigh antibacterial activityReduce usageAntibacterial agentsPharmaceutical delivery mechanismBiotechnologyFLUNIXIN MEGLUMINE
The invention discloses a long-acting compound tildipirosin injection and a preparation method thereof. The long-acting compound tildipirosin injection comprises the following main components: tildipirosin, flunixin meglumine and sinomenine. The invention provides a long-acting compound injection special for animals for veterinary clinic, wherein the injection not only can be used for treating respiratory system infectious diseases caused by sensitive bacteria, but also can help to treat or reduce the stress of sick animals; and combination of drugs can significantly improve the clinical symptoms, and can enhance the activity of antibacterial drugs, reduce the use amount of drugs, reduce the administration frequency, reduce animal stress, enhance animal compliance and reduce labor intensity, and the injection is convenient to use.
Owner:SOUTH CHINA AGRI UNIV +1
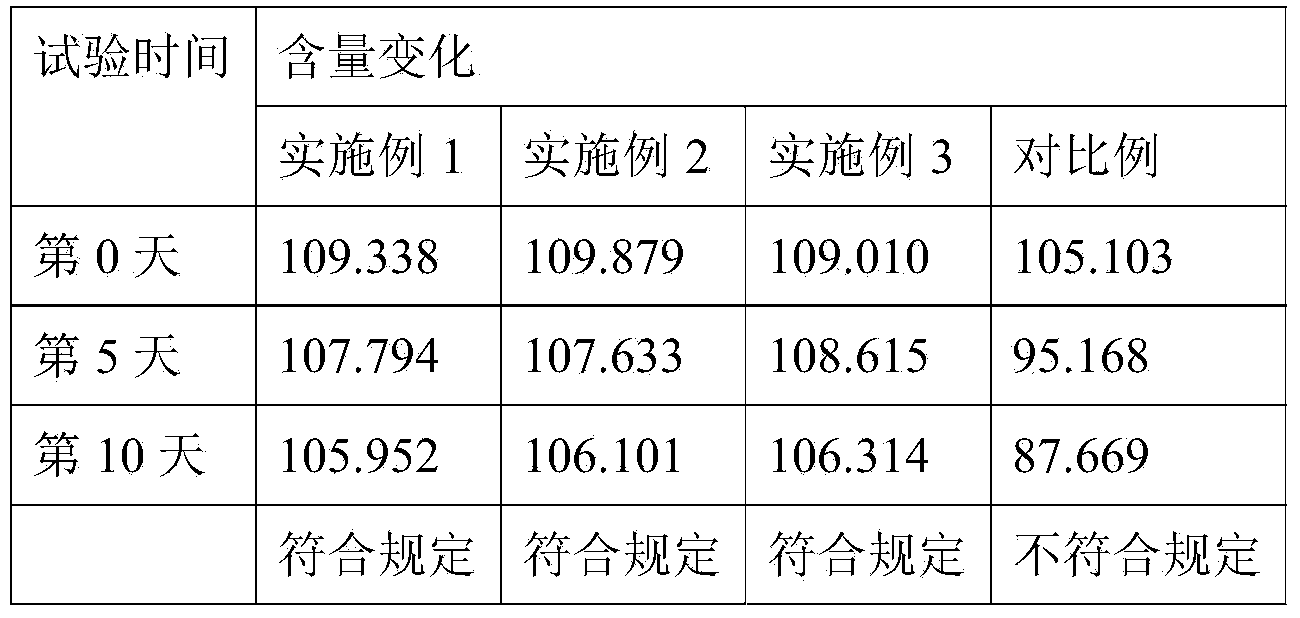
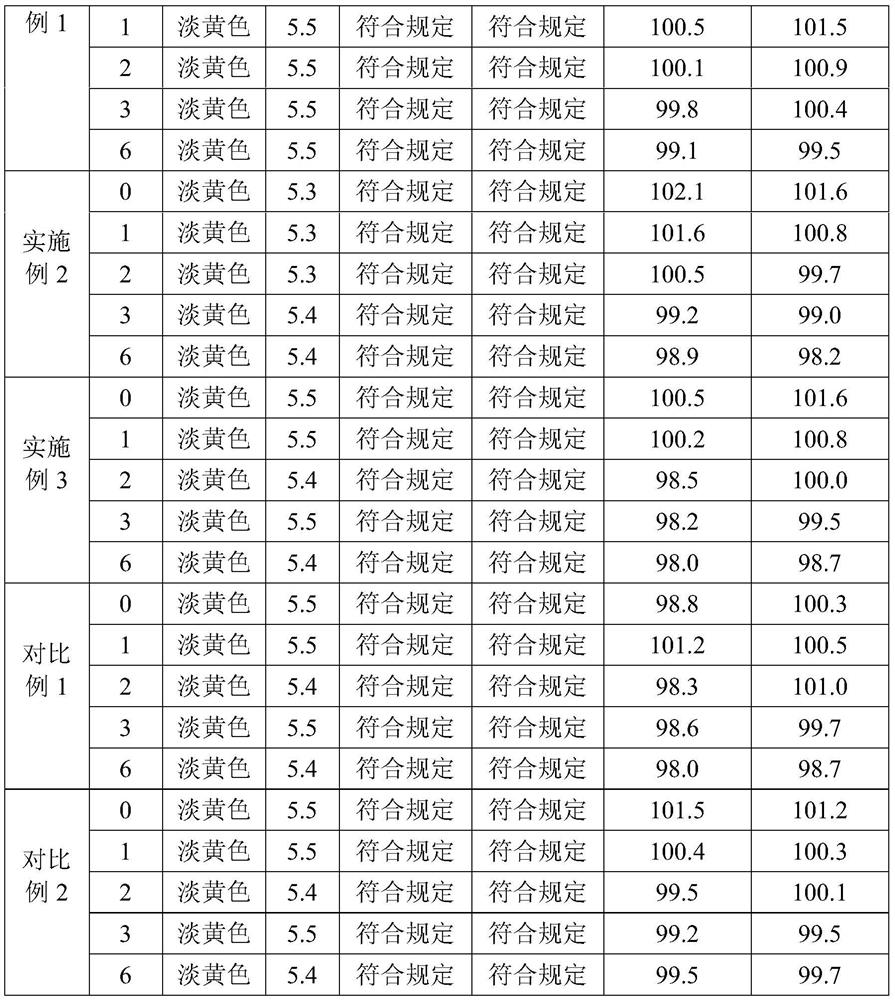
Preparation method of flunixin meglumine injection liquid
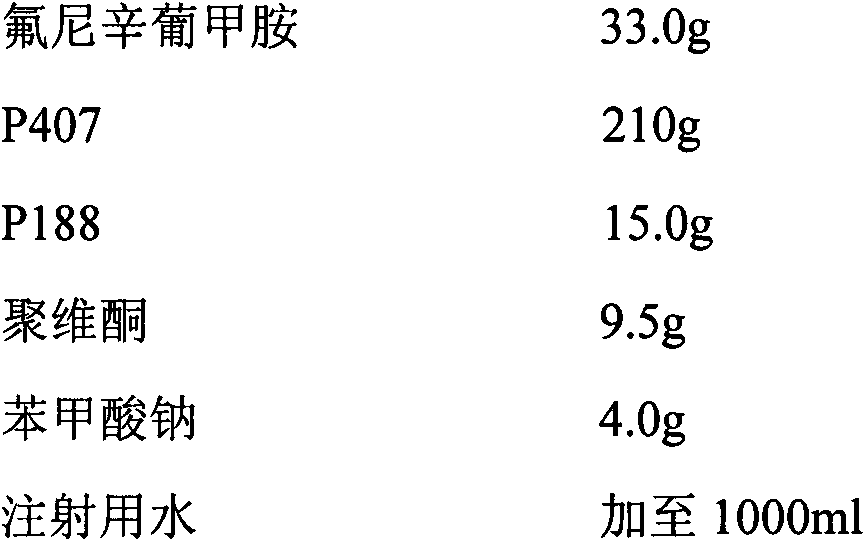
InactiveCN107233304AReduce precipitationEfficient removalAntipyreticAnalgesicsFLUNIXIN MEGLUMINEPEG 400
The invention belongs to the field of veterinary drugs, and particularly relates to a preparation method of flunixin meglumine injection liquid. The flunixin meglumine injection liquid comprises, by weight, 8.4 parts of flunixin meglumine, 15 parts of PEG (polyethylene glycol)-400, 0.2 part of anhydrous sodium sulfite, 0.02 part of edetate disodium and 100 parts of water for injection. According to the method, firstly, dissolving the flunixin meglumine by the aid of part of water to obtain solution A, and dissolving the anhydrous sodium sulfite and the edetate disodium by the aid of part of water to obtain solution B; secondly, adding the solution B into the solution A, adding the PEG-400, adding the balance water again, and uniformly mixing mixture; finally, absorbing suspended solid particles by the aid of drug active carbon, and removing carbon to obtain the flunixin meglumine injection liquid. The method is simple and easy to operate, and the possibility of sedimentation of the injection liquid is effectively reduced.
Owner:遂宁市中通实业集团动物药业有限公司
Preparation method of flunixin meglumine
ActiveCN109206365AHigh purityHigh yieldOrganic compound preparationAmino-hyroxy compound preparationFLUNIXIN MEGLUMINEReaction temperature
The invention discloses a preparation method of flunixin meglumine the preparation method, and the method specifically comprises the following steps: (1) adding 2-chloronicotinic acid and 2-methyl-3-trifluoromethyl aniline into a sodium hydroxide water solution for stirring, adding ethylene glycol as well as a phase transfer catalyst and p-toluenesulfonic acid and copper oxide, controlling the temperature and time, adjusting the pH value of the solution, stirring, standing, layering, stirring, filtering, washing filter cake, and drying to obtain flunixin; and (2) reacting the flunixin obtainedin the step (1) with N-methylglucosamine in isopropyl alcohol, and meanwhile, adding a filler; carrying out heating reflux, filtering, cooling, stirring and crystallizing, carrying out crystal suction filtration, and washing crystals with isopropanol to obtain the flunixin meglumine. The preparation method of the flunixin meglumine is convenient and simple in operation, the reflecting efficiencyis high, the reaction temperature is low, the reaction time is short, and the cost is low; moreover, the prepared flunixin meglumine is high in purity and high in yield.
Owner:龙岩台迈三略制药有限公司 +1
Compound Flunixin meglumine injection and preparation method thereof
InactiveCN105106935AExpanded antimicrobial spectrumNo incompatibilityAntibacterial agentsPeptide/protein ingredientsFLUNIXIN MEGLUMINEDisease
Owner:河南亚卫动物药业有限公司
Anti-stress feed additive and application method thereof
InactiveCN106721067AImprove anti-stress effectImprove stress resistanceAccessory food factorsAnti stressFLUNIXIN MEGLUMINE
The invention relates to an anti-stress feed additive which is composed of 1.9-2.1% of radix astragali polysaccharide extract, 24-26% of xylooligosaccharide, 39-41% of vitamin C, 0.9-1.1% of vitamin B1, 1.2-1.3% of vitamin B2, 0.7-0.8% of flunixin meglumine and 29-31% of glucose. The preparation method comprises the following steps: uniformly mixing the components, and drying to obtain the feed additive. The application method comprises the following steps: (1) the anti-stress feed additive is added into a livestock / poultry feed according to the proportion of 0.1-0.2%, and is uniformly mixed; (2) heat stress is continuously applied for 15 days, and the feed is used after 5 days; (3) in the immunity stress process, it is suggested to start using the feed five days before immunization, and the feed is continuously used for 20 days; and (3) the feed is added in the whole transport stress process, and is continuously used. The anti-stress feed additive can enhance multiple anti-stress capacities of livestock and poultry, and improves the anti-stress effect of animal organisms.
Owner:NINGXIA UNIVERSITY
Compound long-acting injection containing enrofloxacin and flunixin and preparation method of compound long-acting injection
ActiveCN110327294ASimple preparation processGood batch-to-batch repeatabilityAntibacterial agentsAntipyreticDiseaseFLUNIXIN MEGLUMINE
The invention discloses a compound long-acting injection containing enrofloxacin and flunixin. Each 1000mL of the injection is prepared from 50-300g of enrofloxacin or / and a salt thereof, 0-200g of flunixin or flunixin meglumine in a dispersed form, 0-150g of flunixin or flunixin meglumine inclusion compound, 5-20g of a suspending agent, 5-15g of a wetting agent and the balance of dispersion medium, and the flunixin and / or flunixin meglumine in the dispersed form, flunixin and / or flunixin meglumine inclusion compound cannot be zero at the same time. The injection can be used only for one injection, can address both symptoms and root causes, improves the compliance of animals, reduces the stress of the animals, and promotes and improves the outcome and prognosis of diseases. After long-termplacement, drug particles can be evenly dispersed; the injection does not need to be operated under aseptic conditions and can be sterilized only by sterilizing at 115 DEG C for 30 minutes after dispensing, indexes of the injection before and after sterilization are in compliance with the regulations, and the injection is suitable for industrial production.
Owner:NANJING AGRICULTURAL UNIVERSITY
Medicine formula for eliminating in-vivo enolotoxin
ActiveCN104800220AEffective treatmentInhibition of reproductionAntibacterial agentsAntipyreticEscherichia coliFLUNIXIN MEGLUMINE
The invention relates to a medicine formula for eliminating in-vivo enolotoxin. The medicine formula comprises a formula A and a formula B, wherein the formula A comprises raw materials in parts by weight as follows: 1-4 parts of flunixin meglumine and 1-6 parts of vitamin C; the formula B comprises raw materials in parts by weight as follows: 2-8 parts of ceftiofur sodium and 1.5-5 parts of enrofloxacin, wherein flunixin meglumine is an injection with a specification of 0.05g / ml, the vitamin C is an injection with a specification of 0.1g / ml, enrofloxacin is an injection with a specification of 0.05g / ml, and ceftiofur sodium is a freeze-dried powder injection. The medicine formula can effectively eliminate enolotoxin causing diseases to animals, has high sensitivity to pathogenic bacteria such as escherichia coli, infectious actinobacillus pleuropneumoniae, haemophilus parasuis and the like, can be used for effectively treating diseases such as hydropsy, infectious pleuropneumonia, fevering, loss of appetite and the like caused by the pathogenic bacteria and has remarkable curative effects.
Owner:SICHUAN CHENGKANG ANIMAL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com