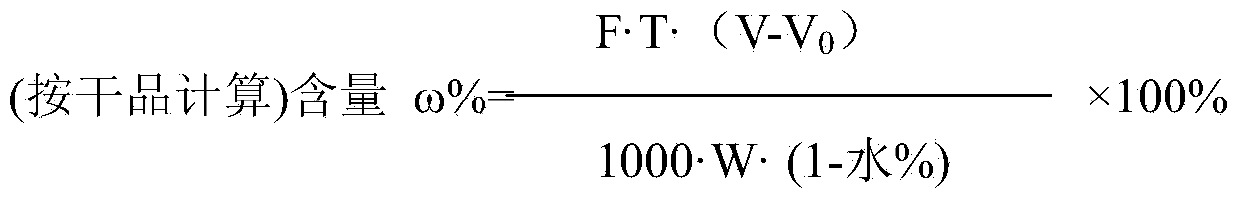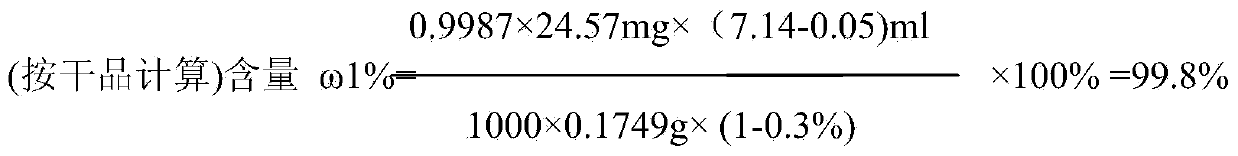Synthesis method of flunixin meglumine
A technology of flunixin meglumine and a synthesis method, applied in the field of drug synthesis, can solve problems such as increased production cost, high difficulty in purification and utilization, and achieve the effects of improved product yield, convenient industrial operation, and simple synthesis operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
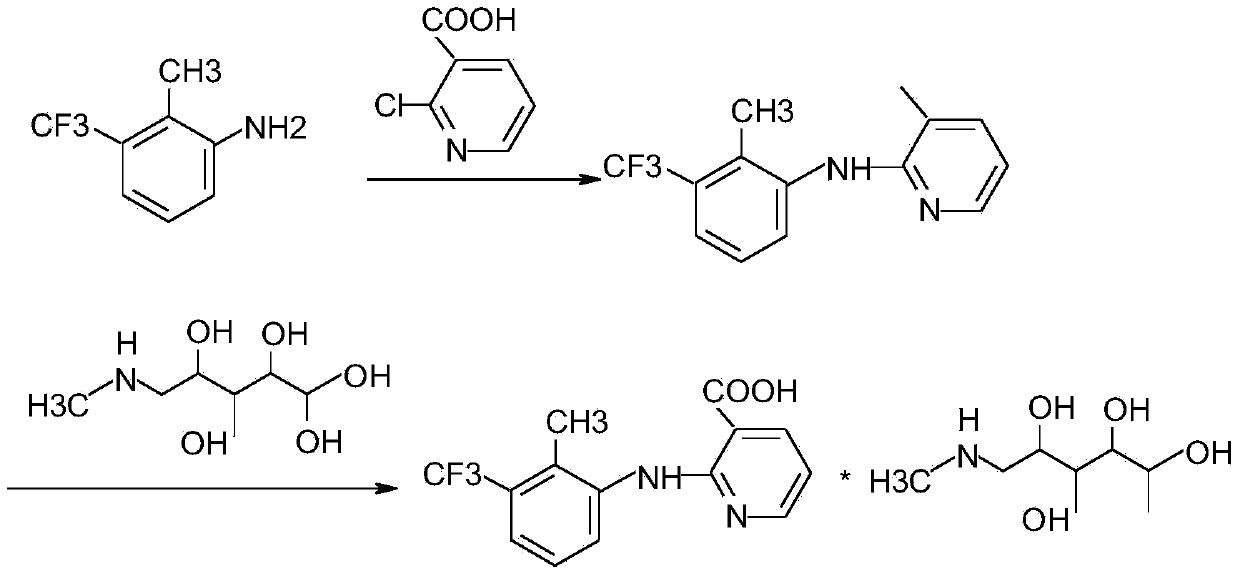
[0018] (1) Dissolve 4g of sodium hydroxide in 95ml of water. After the sodium hydroxide is completely dissolved and cooled, add 18.4g of 2-methyl-3-trifluoromethylaniline and 15.7g of 2-chloronicotinic acid into the sodium hydroxide solution After stirring and dissolving, add 95ml toluene and 0.157gTEBAC, control the temperature and stir at 40°C for 4 hours; adjust the pH to 10 with 3mol / L NaOH solution after the reaction solution drops to room temperature, stir for 10min, and remove the upper organic layer after standing for 20min. phase, and then adjust the obtained water phase to pH to 5 with 4mol / L sulfuric acid, after stirring for 1 hour, the filter cake was washed with purified water after suction filtration, and dried. 28.4 g of the product was obtained with a yield of 95.9%.
[0019] (2) Add 20.0 g of the product flunixin and 14.5 g of N-methylglucamine to 120 ml of isopropanol, heat to reflux for 1 hour, filter while hot to obtain the filtrate, and let the filtrate co...
Embodiment 2
[0033] (1) Dissolve 4g of sodium hydroxide in 95ml of water. After the sodium hydroxide is completely dissolved and cooled, add 18.5g of 2-methyl-3-trifluoromethylaniline and 15.7g of 2-chloronicotinic acid into the sodium hydroxide solution After stirring and dissolving, add 95ml toluene and 0.157g TEBAC, control the temperature at 45°C and stir for 4 hours; after the reaction solution drops to normal temperature, use 3mol / L NaOH solution to adjust the pH to 10, stir for 10min, and remove the upper organic layer after standing for 20min. phase, and then adjust the resulting aqueous phase to pH 6 with 4mol / L sulfuric acid, and after stirring for 1 hour, the filter cake was washed with purified water after suction filtration, and dried. Obtain product 28.2g, yield 95.3%
[0034] (2) Add 20.0 g of the product flunixin and 14.5 g of N-methylglucamine to 120 ml of isopropanol, heat to reflux for 1 hour, filter while hot to obtain the filtrate, and let the filtrate cool to 50 Stir...
Embodiment 3
[0036] (1) Dissolve 4g of sodium hydroxide in 106ml of water. After the sodium hydroxide is completely dissolved and cooled, add 18.4g of 2-methyl-3-trifluoromethylaniline and 15.7g of 2-chloronicotinic acid into the sodium hydroxide solution After stirring and dissolving, add 106ml toluene and 0.157g TEBAC, control the temperature and stir at 40°C for 4 hours; adjust the pH to 11 with 3mol / L NaOH solution after the reaction solution drops to room temperature, stir for 10min, and remove the upper organic layer after standing for 20min. layer, and then the resulting water layer was adjusted to pH to 5 with 4mol / L sulfuric acid, and after stirring for 1 hour, the filter cake was washed with purified water after suction filtration, and dried. 27.9 g of the product was obtained with a yield of 94.3%.
[0037](2) Add 20.0 g of the product flunixin and 14.5 g of N-methylglucamine to 130 ml of isopropanol, heat to reflux for 1 hour, filter while hot to obtain the filtrate, and let th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com