Compound ceftiofur sodium freeze-dried power injection used for injection
A technology of ceftiofur sodium and freeze-dried powder injection, which is applied in the field of compound ceftiofur sodium freeze-dried preparations for injection, can solve the problems of bacterial drug resistance and high cost, and achieve the purpose of inhibiting the rise of body temperature and PGF-A concentration , reduce drug resistance, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Control the temperature at 10±5°C, add 1 part by weight of sulbactam sodium and 1 part by weight of flunixin meglumine into a sterile container, add water for injection to 85% of the total amount, and turn on the vacuum (to make the system slightly negative). pressure), stir to dissolve, then add 2 parts by weight of ceftiofur sodium to the above solution, turn on the vacuum, stir to dissolve, adjust the pH value to 4.5-6.5, add water for injection to the full amount, and stir evenly.
[0019] Sampling was taken to determine the pH value and the content of ceftiofur sodium, sulbactam sodium, and flunixin meglumine. After the test indicators were qualified, it was first filtered with a 0.45 μm cellulose filter, and then filtered with a 0.22 μm cellulose filter. Remove impurities.
[0020] The filtered filtrate is divided into control glass bottles, half-stopped, and vacuum freeze-dried to obtain the compound ceftiofur sodium freeze-dried powder injection of the present i...
Embodiment 2
[0022] Control the temperature at 10±5°C, add 4 parts by weight of ceftiofur sodium into a sterile container, add water for injection to 85% of the total amount, turn on the vacuum, stir to dissolve, and then add 1 part by weight of sulbactam sodium and 1 Add the flunixin meglumine in parts by weight to the above solution, turn on the vacuum, stir to dissolve, adjust the pH value to 4.5-6.5, add water for injection to the full amount, and stir evenly.
[0023] Sampling was taken to determine the pH value and the content of ceftiofur sodium, sulbactam sodium, and flunixin meglumine. After the test indicators were qualified, it was first filtered with a 0.45 μm cellulose filter, and then filtered with a 0.22 μm cellulose filter. Remove impurities.
[0024] The filtered filtrate was divided into control glass bottles, half-stopped, and vacuum freeze-dried to obtain the compound ceftiofur sodium / sulbactam sodium freeze-dried powder of the present invention.
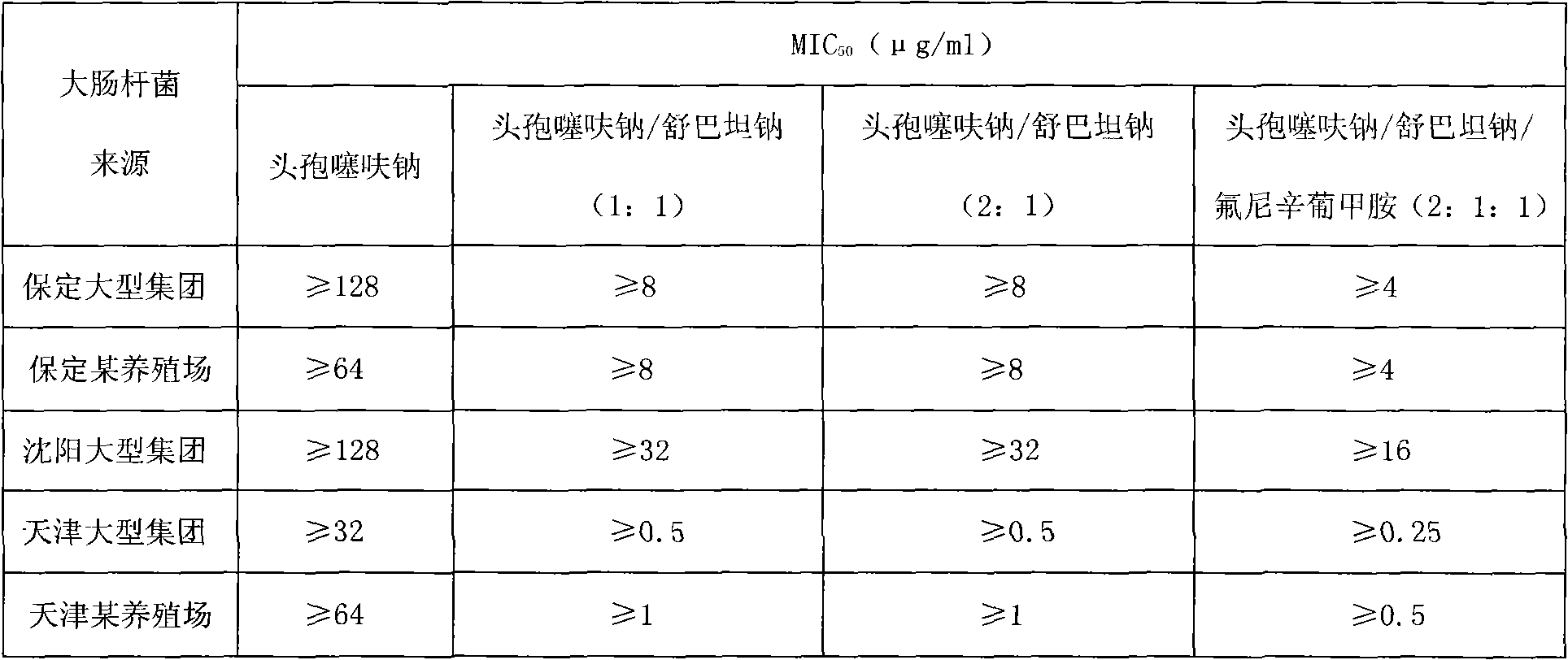
[0025] For the above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com