Telmisartan tablet composition
A technology of telmisartan and composition, which is applied in the field of telmisartan tablet composition, can solve problems such as poor dissolution rate, inability to obtain shaped solids, strong hygroscopicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
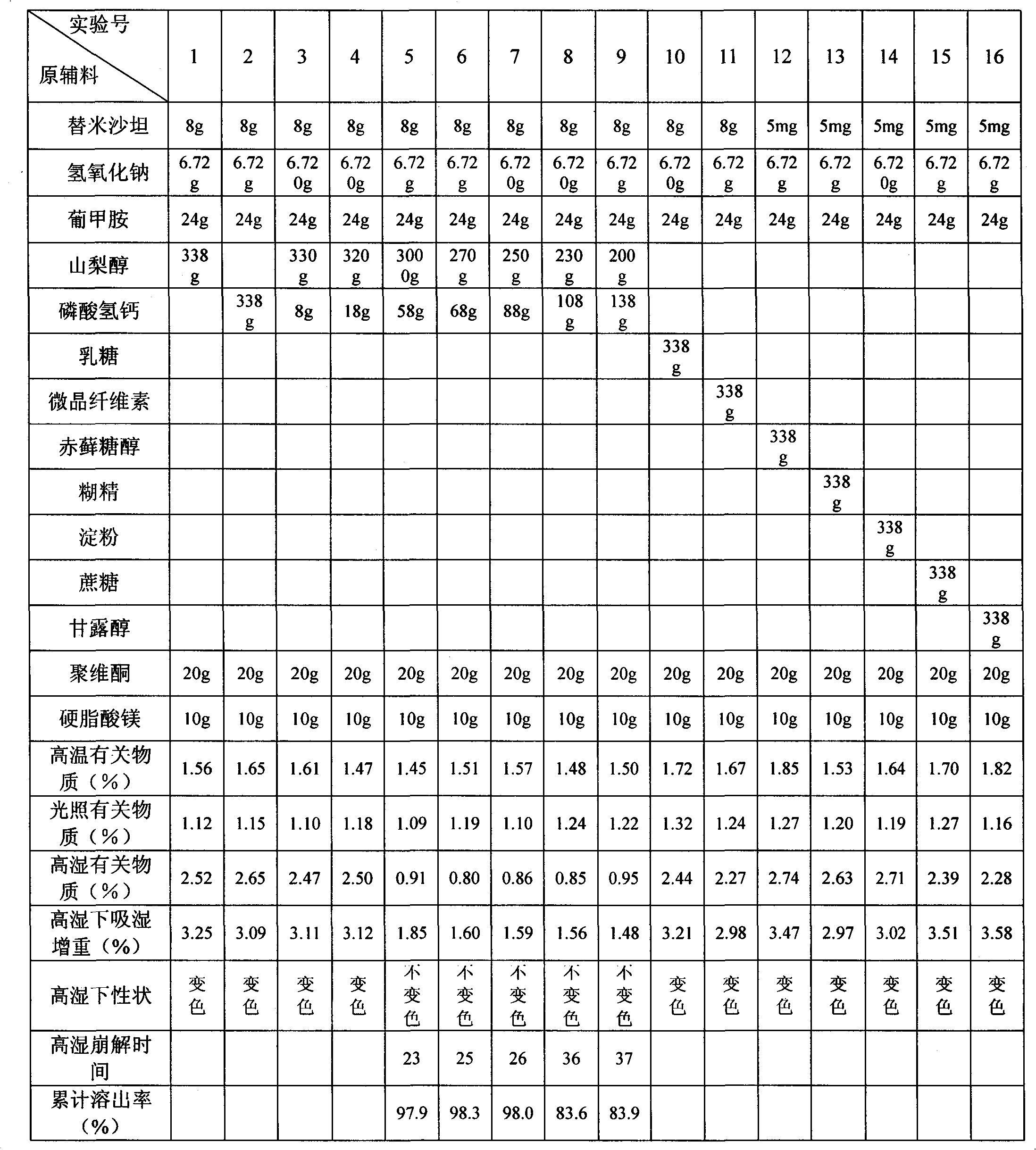
[0022] Embodiment 1 Telmisartan tablet
[0023] Name of raw material Amount (g)
[0024] Telmisartan 80
[0025] Sodium hydroxide 6.72
[0026] Meglumine 24
[0027] Sorbitol 270
[0028] Calcium hydrogen phosphate 68
[0029] Povidone 20
[0031]
[0032] A total of 2000 pieces
[0033] Preparation:
[0034] 1) Combine sodium hydroxide and meglumine, add 20ml of water to dissolve, then add telmisartan, and add 120ml of ethanol to dissolve, shake and dissolve telmisartan, dry under reduced pressure at 40°C to obtain a white puffy Solid, pulverized through 200 mesh drying, to obtain telmisartan salt powder;
[0035] 2) Sorbitol, calcium hydrogen phosphate, and povidone are pulverized through 100 mesh, and magnesium stearate is crushed through 40 mesh, weighed according to the prescription amount and mixed with telmisartan salt powder evenly;
[0036] 3) The mixed powder is directly compressed into tabl...
Embodiment 2
[0037] Embodiment 2 Telmisartan tablet
[0038] Name of raw material Amount (g)
[0039] Telmisartan 80
[0040] Sodium hydroxide 6.72
[0041] Meglumine 24
[0042] Sorbitol 250
[0043] Calcium hydrogen phosphate 88
[0044] Povidone 20
[0046]
[0047] A total of 2000 pieces
[0048] Preparation:
[0049]1) Take sodium hydroxide and meglumine, add 20ml of water to dissolve, then add telmisartan, and add 120ml of ethanol to dissolve, shake and dissolve telmisartan, spray dry to obtain telmisartan salt powder;
[0050] 2) Sorbitol, calcium hydrogen phosphate, and povidone are pulverized through 100 mesh, and magnesium stearate is crushed through 40 mesh, weighed according to the prescription amount and mixed with telmisartan salt powder evenly;
[0051] 3) The mixed powder is directly compressed into tablets to obtain the product.
Embodiment 3
[0052] Embodiment 3 Telmisartan tablet
[0053] Name of raw material Amount (g)
[0054] Telmisartan 80
[0055] Sodium hydroxide 6.72
[0056] Meglumine 24
[0057] Sorbitol 280
[0058] Calcium hydrogen phosphate 58
[0059] Povidone 20
[0061]
[0062] A total of 2000 pieces
[0063] Preparation method: refer to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com