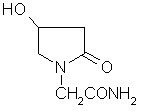
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
346results about How to "Low content of related substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
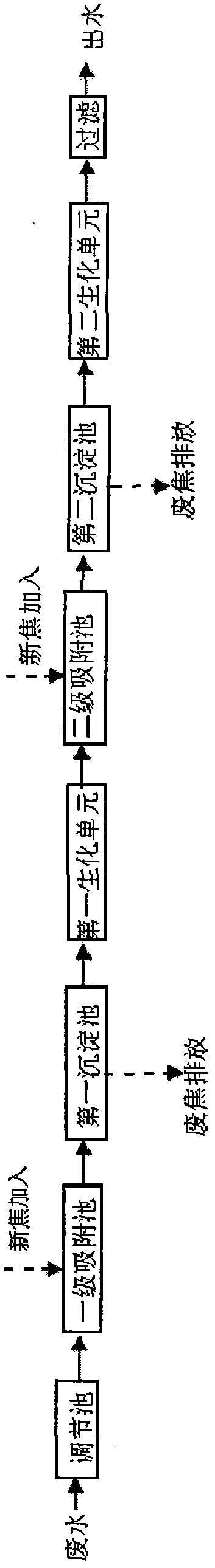
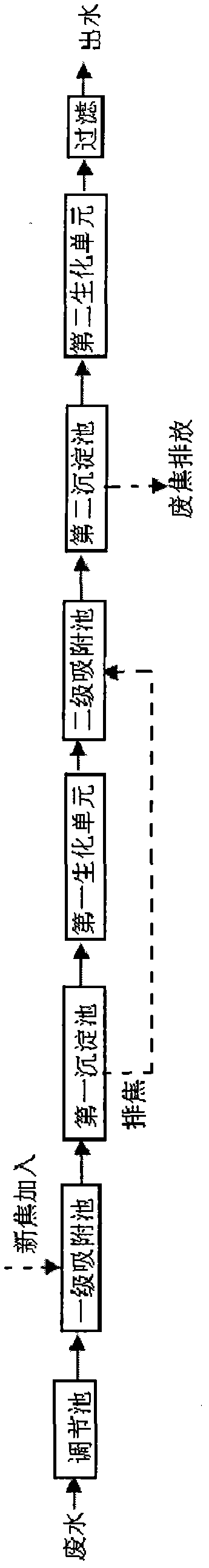
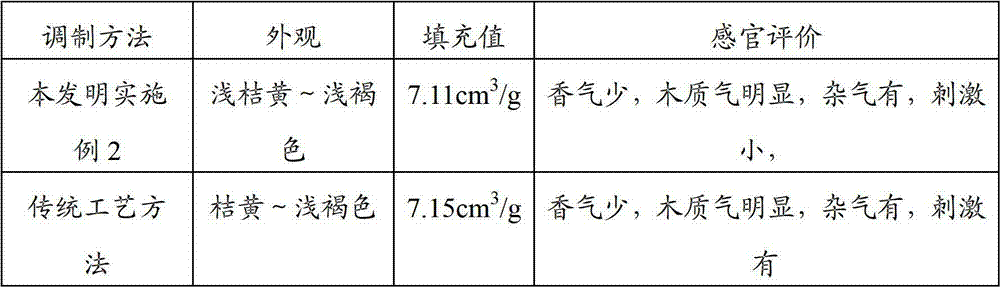
Coal gasification wastewater treatment process
InactiveCN102070277AImprove utilization efficiencyReduce dosageMultistage water/sewage treatmentCoal gasification wastewaterSalt content
The invention discloses a coal gasification wastewater treatment process, which comprises the following steps of: performing adsorption treatment in a primary adsorption tank; performing sedimentation treatment in a first sedimentation tank; performing biochemical treatment in a first biochemical unit; performing adsorption treatment in a secondary adsorption tank; performing sedimentation treatment in a second sedimentation tank; performing biochemical treatment in a second biochemical unit; and filtering and discharging. The coal gasification wastewater treatment process solves the technical problem that active carbon is added into an active sludge aeration tank to influence the effects of the biochemical treatment and the adsorption treatment in the conventional coal gasification wastewater treatment process, has the advantages of independent adsorption and biochemical treatment, combined arrangement, concise process, high treatment efficiency, no increase of salt content and stable operation, and is particularly suitable for treating coal gasification wastewater.
Owner:北京国能普华环保工程技术有限公司 +1
Gemcitabine hydrochloride lyophilized powder injection
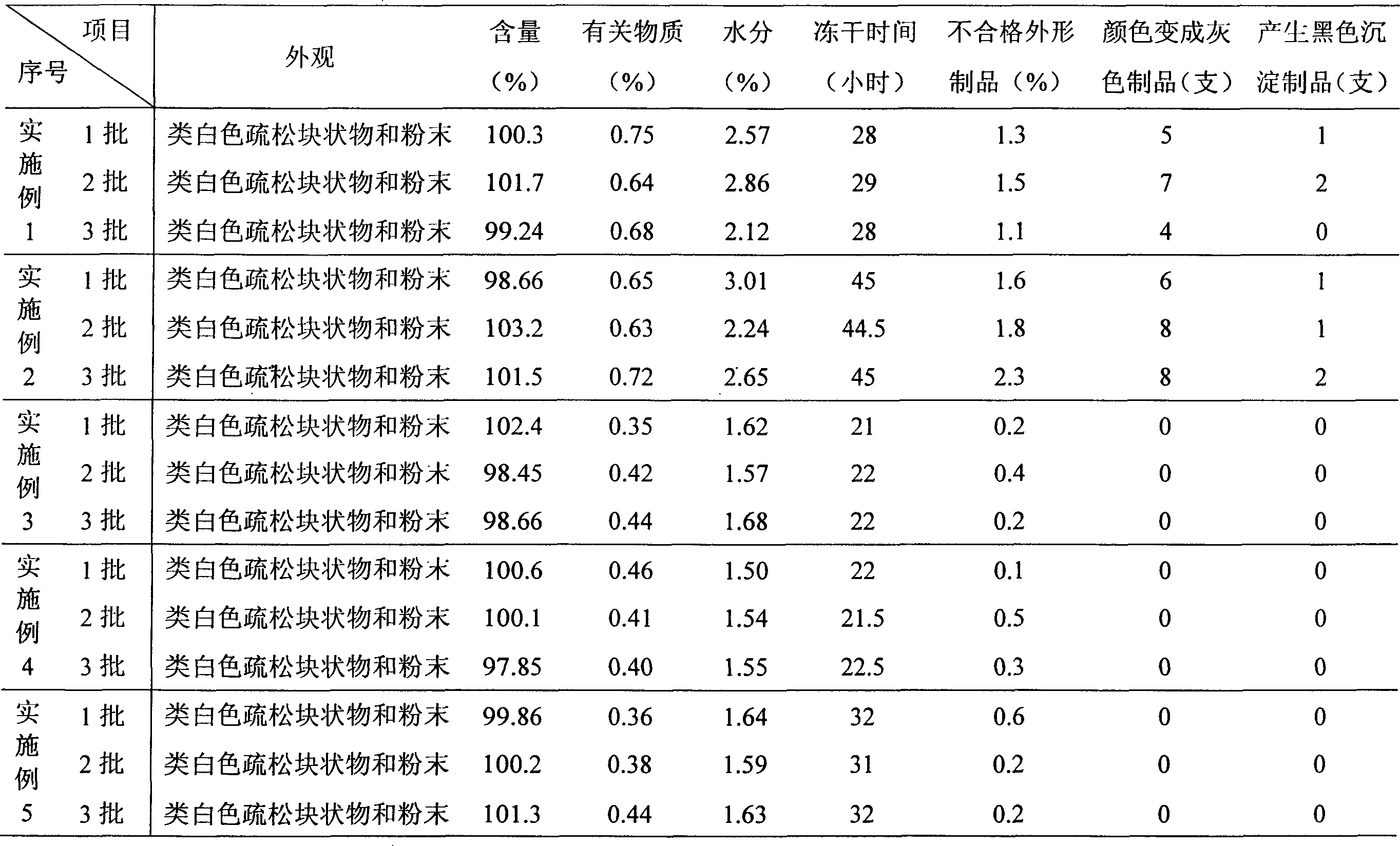
ActiveCN101564381AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsPancreas CarcinomaDrug
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The gemcitabine hydrochloride lyophilized powder injection prepared by the method can be used as a therapeutic medicament for treating middle and late non-small cell lung cancer, pancreatic cancer and the like. The gemcitabine hydrochloride lyophilized powder injection is characterized by consisting of gemcitabine hydrochloride, mannitol and sodium acetate, wherein the weight ratio of the gemcitabine hydrochloride to the mannitol is 1:0.5-5, and the weight ratio of the gemcitabine hydrochloride to the sodium acetate is 1:0.01-0.1. The preparation method comprises the following steps: taking the mannitol and the sodium acetate; dissolving the mannitol and the sodium acetate by adding injection water; adding the gemcitabine hydrochloride to the mixture, stirring and dissolving the mixture, and adjusting the pH to between 2.7 and 3.3; fixing the volume; filtering the product by a 0.22 mu m microporous membrane; filling, dishing up, lyophilizing, and compressing; taking the product out of a box, and tying the product with an aluminum-plastic composite cover; and inspecting the quality, and packaging the product after passing the quality inspection to obtain the gemcitabine hydrochloride lyophilized powder injection.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Refining method of low-odor and high-activity polyether polyol
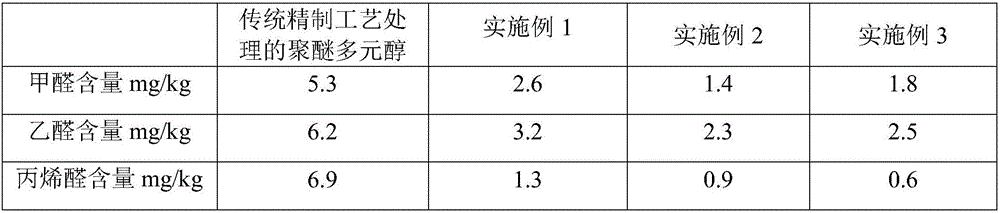
The invention relates to a refining method of low-odor and high-activity polyether polyol, and belongs to the technical field of polyether polyol. The refining method comprises the following steps of adding high-activity crude polyether polyol into a refining treatment kettle, and adding water to stir; under the condition of nitrogen pressure filling, adding a refining adsorbent A to stir, then adding a refining adsorbent B to stir, dewatering, drying and filtering, so as to obtain the low-odor and high-activity polyether polyol. Compared with the traditional refining technology of using phosphoric acid and water and adding one or two types of common adsorbent, the refining method has the advantages that the high-activity crude polyester is refined under the matching action of acid magnesium silicate, synthetic magnesium silicate and water, so that the alkaline metal ion can be removed like the conventional technology, and the polyester index and parameter meet the national standard; the two types of refining adsorbents are slightly weak acid, so that the new aldehyde and other impurities are not produced in the refining phase, the content of aldehyde matters is reduced, and the product odor is decreased.
Owner:SHANDONG INOV NEW MATERIALS CO LTD
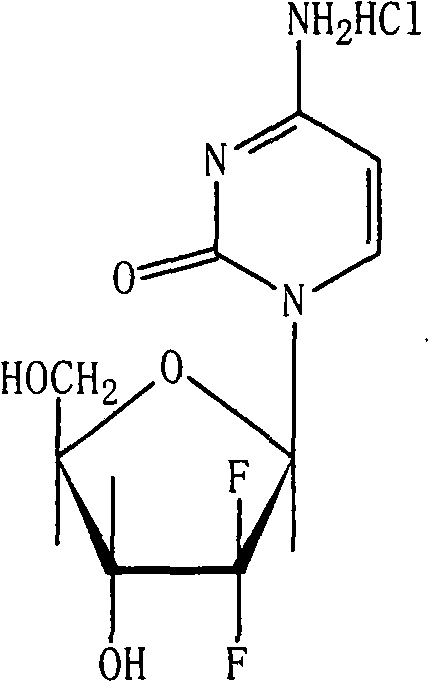
Decitabine freeze-dried powder injection
ActiveCN101584670AImprove stabilityLow content of related substancesOrganic active ingredientsPowder deliveryPhosphateFreeze-drying
The invention relates to a decitabine freeze-dried powder injection and a preparing method thereof. The prepared decitabine freeze-dried powder injection is used for treating myelodysplastic syndrome (MDS). The decitabine freeze-dried powder injection contains decitabine, utilizes the mixed solvent composed of the tert-butyl alcohol and the injection water in the preparation process, wherein the concentration of the decitabine in the mixed solvents is 2.5-5 mg / ml; and the volume ratio of the solvents is: 5-50% of tert-butyl alcohol and the balance of injection water. The potassium dihydrogen phosphate and the sodium hydroxide may be added for the pH regulator. The preparation process comprises the following steps: measuring tert-butyl alcohol, adding injection water, potassium dihydrogen phosphate and sodium hydroxide, stirring and mixing evenly, cooling to 2-15 DEG C, heat preserving, adding decitabine, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing plug, out box, tying and packing after quality test qualification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Processing technology of green tea with flower fragrance
InactiveCN102742677AReduce bitternessGuaranteed greennessPre-extraction tea treatmentChemistryQuality characteristics
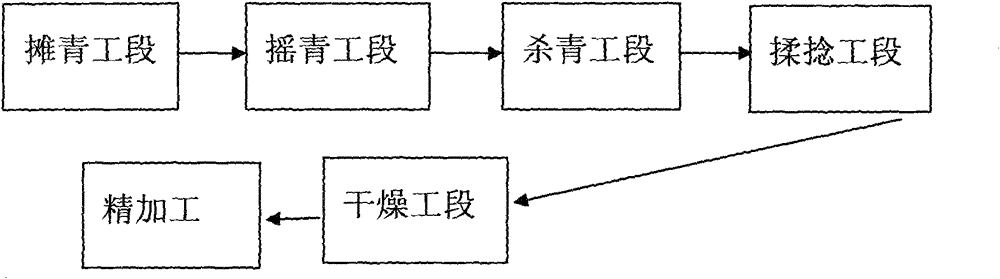
The invention discloses a processing technology of a green tea with flower fragrance. The processing technology comprises the sections of: fresh tea leaf spreading; tea leaf rocking; inactivating; rolling; drying; and finishing. The invention has the following beneficial effects: addition of technologies of tea leaf rocking and low temperature refrigeration and standing change aroma type of green tea, effectively reduce bitter and astringency taste of tea, effectively ensure degree of green of the tea, and reduce polyphenol content of the tea; on the premise of preventing the tea from turning red, time and frequency of tea leaf rocking, and time and temperature of refrigeration and standing are well controlled to prevent the leaf margin from turning red, as well as maintain quality characteristics of light soup and green leaf of the green tea, so that the rocked leaves produce a large amount of floral substances; and standing of the rocked leaves is carried out under condition of a constant temperature of 11 (+ / - 1 DEG C); meanwhile the standing time is prolonged correspondingly, so as to increase the content of the effective components in the leaves, change the proportion of primary effective components, and further expand and develop the flower fragrance.
Owner:杨秀波
Cefotiam hydrochloride medicament composition sterile powder injection and preparation method thereof
ActiveCN101584665AImprove stabilityHigh purityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideSodium carbonate anhydrous
The present invention discloses a cefotiam hydrochloride medicament composition sterile powder injection, including 500 to 600 weight shares of cefotiam hydrochloride and 110 to 150 weight shares of anhydrous sodium carbonate. The cefotiam hydrochloride medicament composition sterile powder injection has advantages of a good stability and a high purity. The invention also discloses a method for preparing the cefotiam hydrochloride medicament composition sterile powder injection, including steps of weighing the cefotiam hydrochloride and the anhydrous sodium carbonate according to the formula amount separately and mixing them in a sterile container uniformly. The method is simple, and the cefotiam hydrochloride prepared by the above method has a good stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
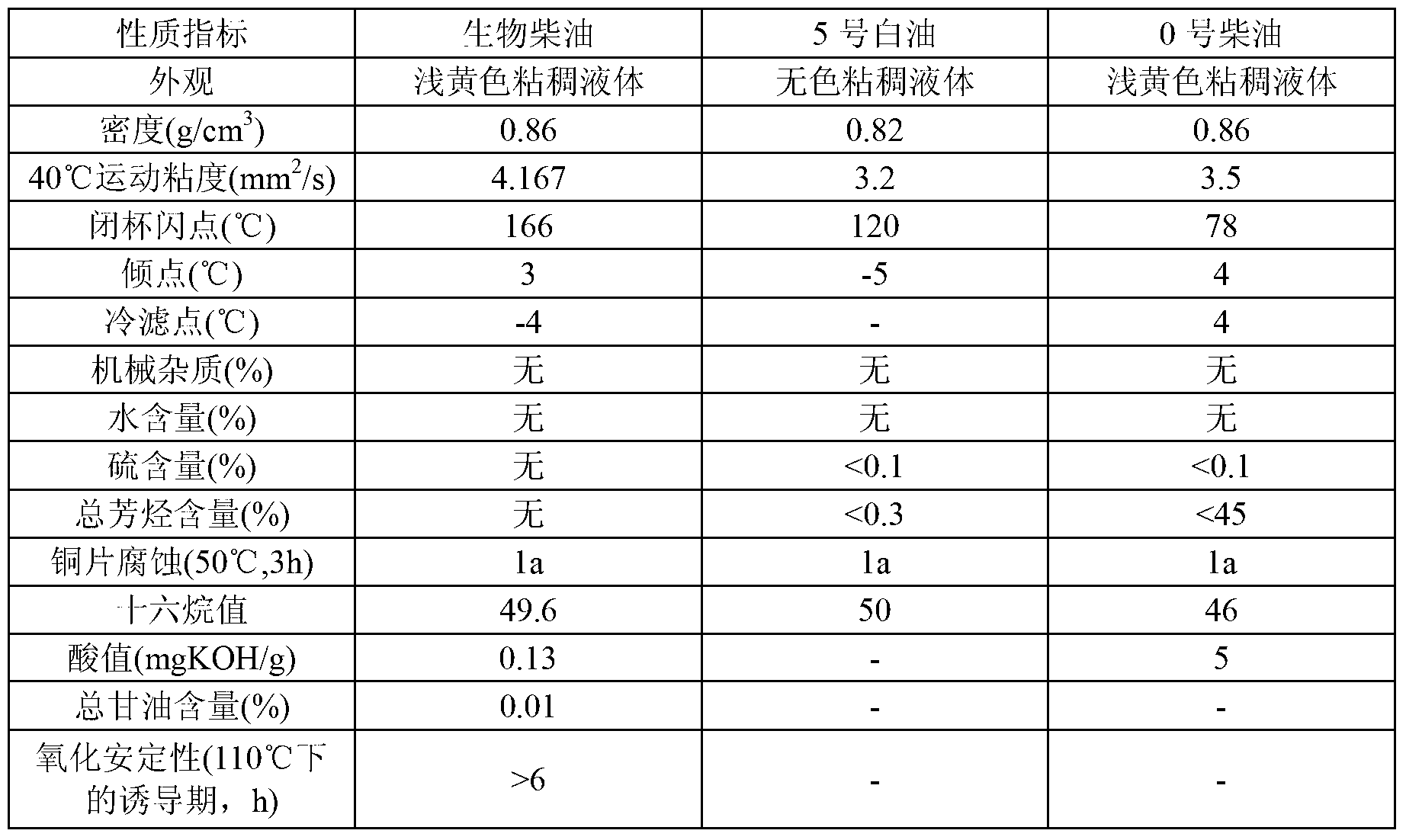
Water-in-oil-type biodiesel-based drilling fluid and preparation method thereof
ActiveCN103320104ASource requirements relaxedInhibit migrationDrilling compositionOil and greaseBiodiesel
The invention discloses a water-in-oil-type biodiesel-based drilling fluid and a preparation method thereof. The drilling fluid comprises the following components in parts by volume: 70 to 90 parts of biodiesel prepared by taking waste oils as a raw material, and 10 to 30 parts of calcium chloride aqueous solution. Based on the total volume of the biodiesel and the calcium chloride aqueous solution, the drilling fluid further comprises the following components in mass-volume ratio: 2% to 6% of calcium oxide, 1% to 6% of organic soil, 2% to 8% of emulsifier, 2% to 8% of wetting agent, 2% to 6% of filtrate reducer and 0% to 200% of weighting material. The drilling fluid disclosed by the invention has the characteristics of good operating performance, low cost, strong collapse-resisting inhibiting performance, excellent lubricating performance and excellent biological degradability.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
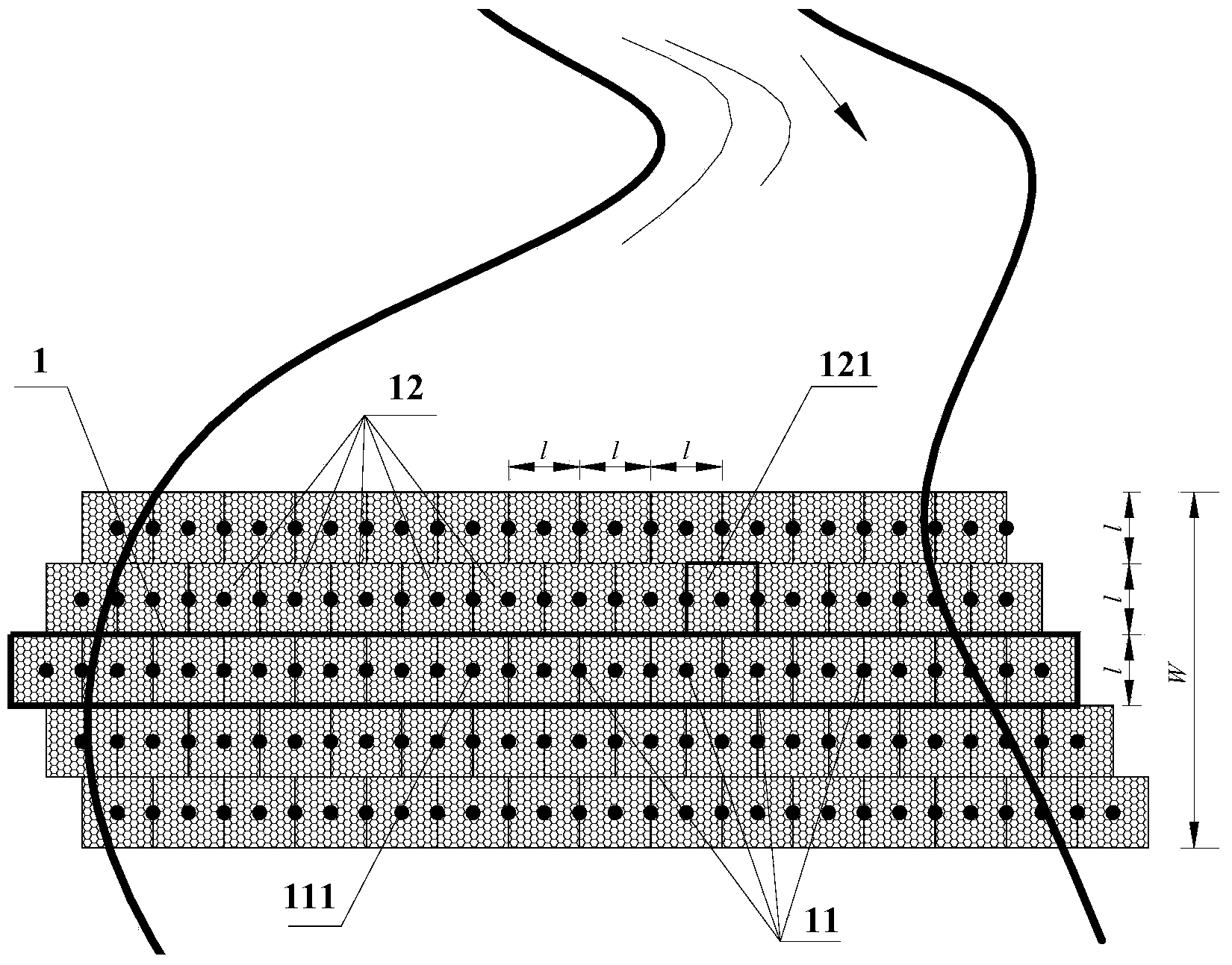
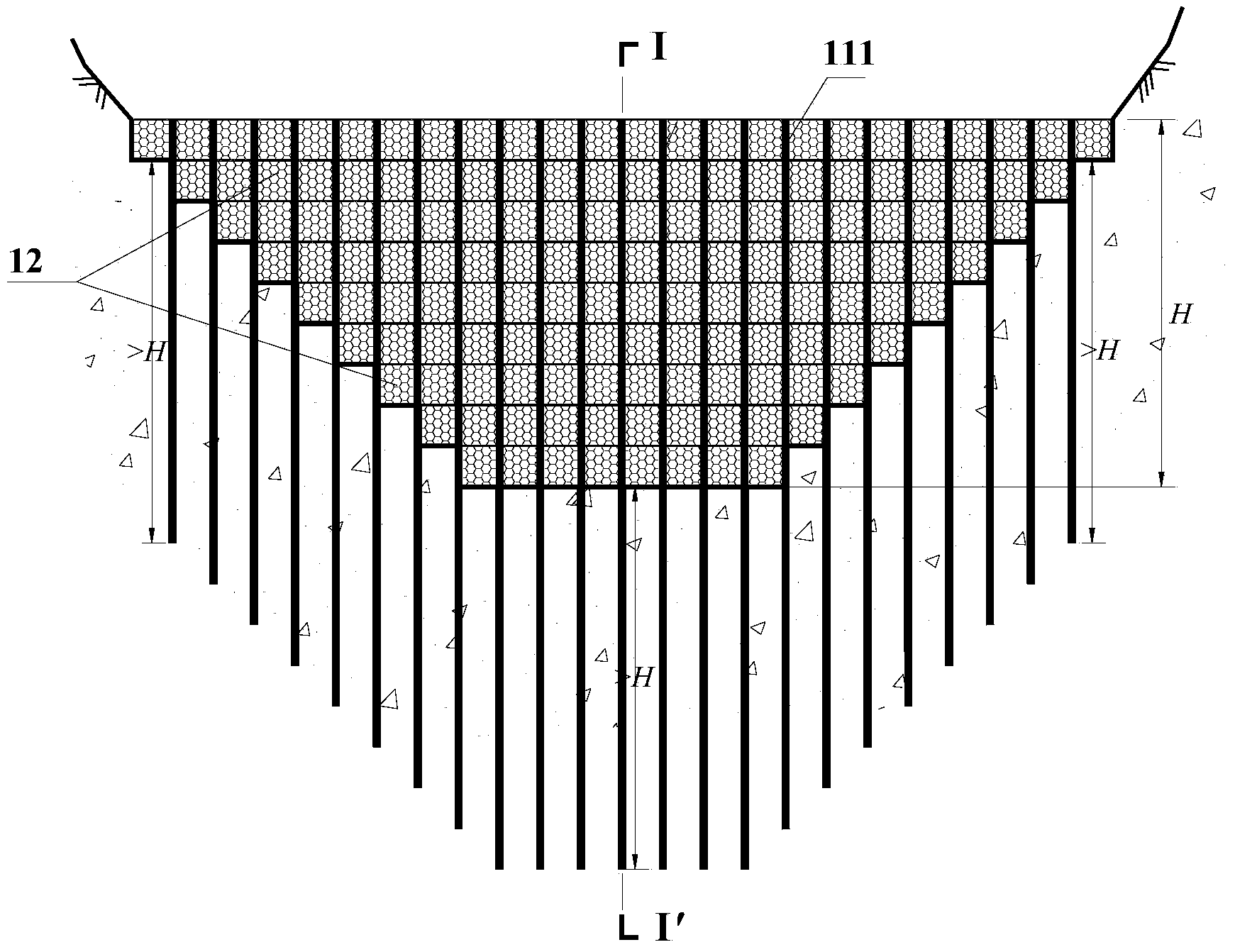
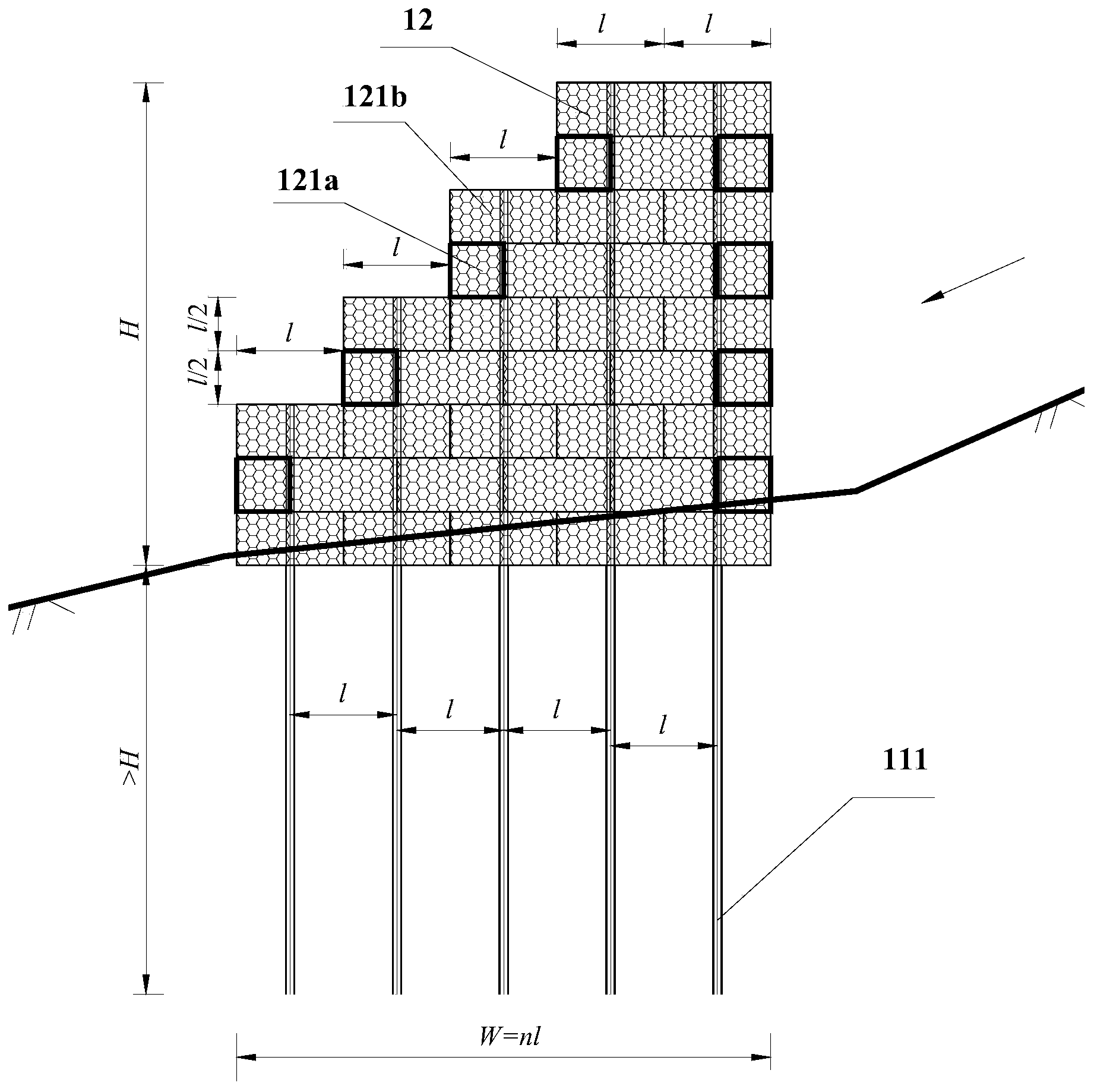
Debris flow gabion prevention and control structural body and design method thereof
InactiveCN103526722AImprove impact resistanceReduce operating and maintenance costsDamsClimate change adaptationEngineeringGabion
The invention discloses a debris flow gabion prevention and control structural body and a design method of the debris flow gabion prevention and control structural body. The flexible debris flow blocking structural body aims to overcome the defects that an existing permeable dam is limited in water permeability and is cracked and destroyed easily by over large instantaneous impact force. The debris flow gabion prevention and control structural body comprises at least one gabion dam prevention and control structural single body. Each single body comprises a lower steel pipe pile group and an upper gabion dam, wherein the steel pipe pile group is formed by arranging a plurality of steel pipe piles in the transverse direction of a debris flow channel, the lower pile bodies of the steel pipe piles are embedded into a debris flow gulley bed, and the upper portions of the steep pipe piles are exposed out of the gulley surface and are connected with the gabion dam in a penetrating mode. The multiple gabion dam prevention and control structural single bodies are arranged adjacently in the longitudinal direction of the debris flow channel. Through the optimization design, the gabion dam is of a multi-layer structure, each layer of the gabion dam is composed of a plurality of gabion nets, and the gabion nets of each layer are arranged in a staggered mode. The invention further provides the design method of the debris flow gabion prevention and control structural body. The debris flow gabion prevention and control structural body can fully utilize the water permeability of the gabion combined structure and deformation feedback of the gabion combined structure on the impact to achieve a good prevention and control effect.
Owner:INST OF MOUNTAIN HAZARDS & ENVIRONMENT CHINESE ACADEMY OF SCI
Telmisartan tablet composition
ActiveCN101897676ALow packaging requirementMeet stability requirementsOrganic active ingredientsPill deliveryMedicineMagnesium stearate
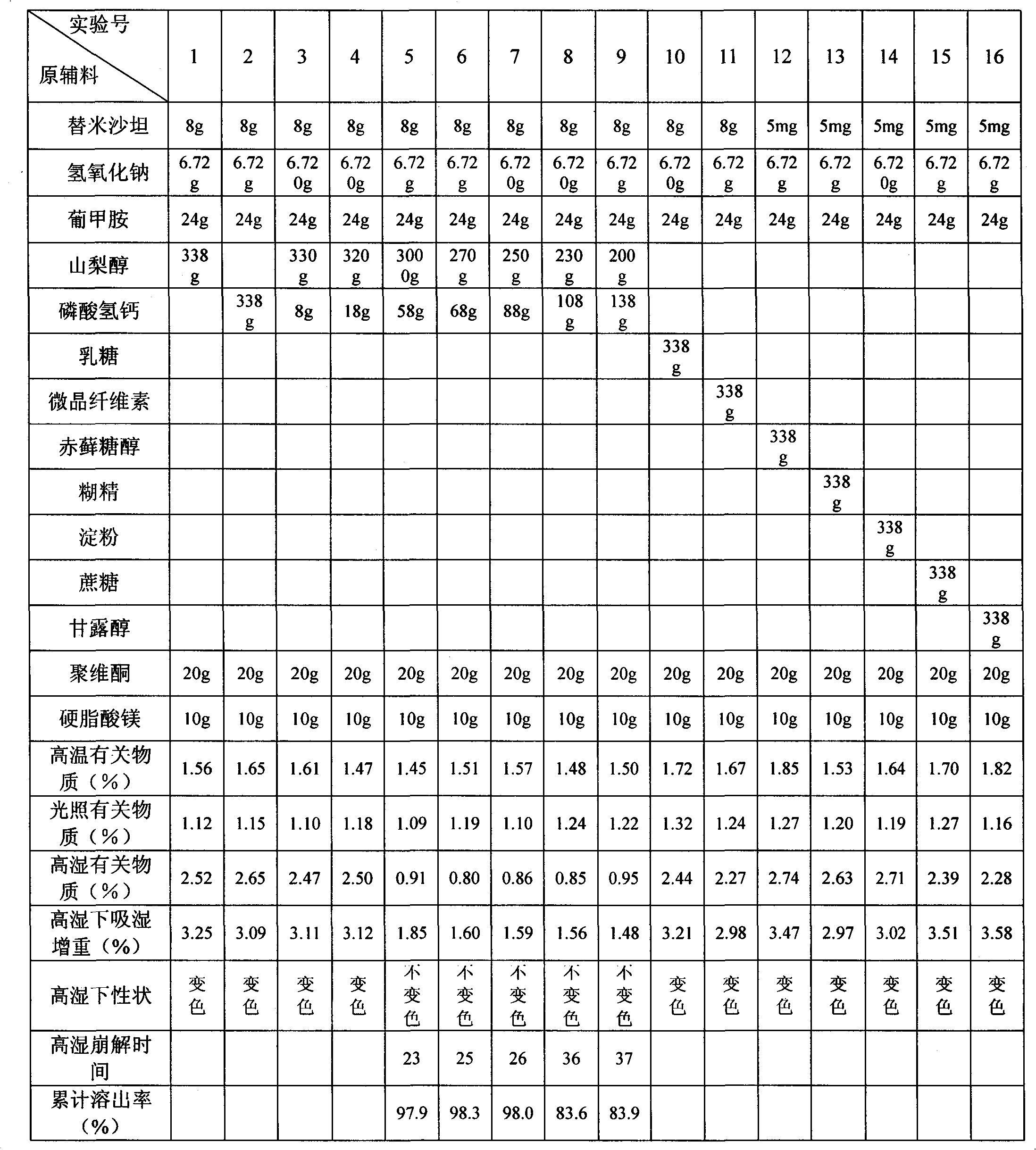
The invention belongs to the technical field of medicinal preparation, and in particular relates to a telmisartan tablet composition. The telmisartan tablet composition is characterized by being prepared from 80 parts of telmisartan, 6.72 parts of sodium hydroxide, 24 parts of meglumine, 250 to 280 parts of sorbierite, 58 to 88 parts of calcium hydrophosphate, 20 parts of polyvidone and 10 parts of magnesium stearate. By selecting and using the combination of sorbierite and calcium hydrophosphate as filler, the composition can obviously improve the stability of the preparation and is favorable for production and application of the preparation.
Owner:BEIJING JINGFENG PHARMA GRP
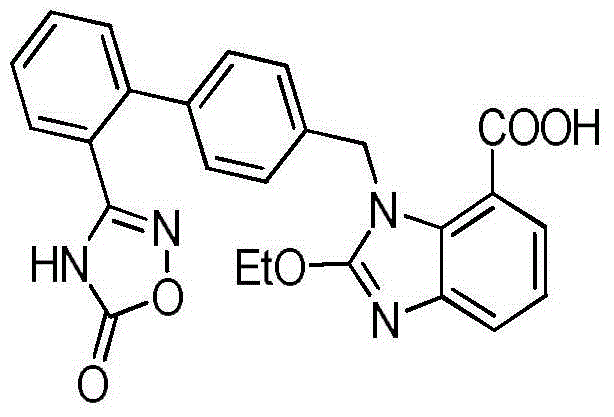
Azilsartan tablet
ActiveCN104523632ADissolution rate is fastSimple processOrganic active ingredientsPill deliveryCelluloseDiethylene glycol monoethyl ether
The invention belongs to the technical field of medicines, and particularly relates to an azilsartan tablet. The azilsartan tablet contains azilsartan, hydroxy propyl cellulose and fumed silica, and is prepared by the following steps: dissolving the azilsartan and the hydroxy propyl cellulose in diethylene glycol monoethyl ether, adding the fumed silica to adsorb, uniformly mixing with pharmaceutically acceptable auxiliary materials and pressing by a direct tableting process. Compared with the prior art, the azilsartan tablet is high in drug dissolution speed and simple in process.
Owner:SHANDONG NEWTIME PHARMA
Dura-mater biological patch and preparation method thereof
InactiveCN107320777ALow content of related substancesReduce immune rejectionTissue regenerationProsthesisLipid formationCell-Extracellular Matrix
The invention discloses a dura-mater biological patch and a preparation method thereof. The dura-mater biological patch adopts a process of using small intestinal submucosa tissues as materials and systemically removing immunogenic substances. The preparation method comprises the following steps of: using alcohol to treat and remove lipids, using trypsin and alkali solution to treat and remove cells, using DNA enzyme to treat and remove DNA, and using alpha-galactosidase to treat and remove alpha-Gal antigens and the like. The dura-mater biological patch and the preparation method disclosed by the invention have the advantages that not only can the immunogenic substances be effectively removed, but also the normal structure of extracellular matrix can not be damaged; and when the dura-mater biological patch is clinically applied in repairing dura-mater defects, the leakage of cerebrospinal fluid can be effectively prevented, the tissue can be guided for ingrowth, the tissue growing speed is matched with the patch decomposing speed, the immunological rejection is low and the biocompatibility is good.
Owner:上海白衣缘生物工程有限公司
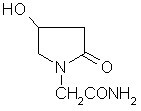
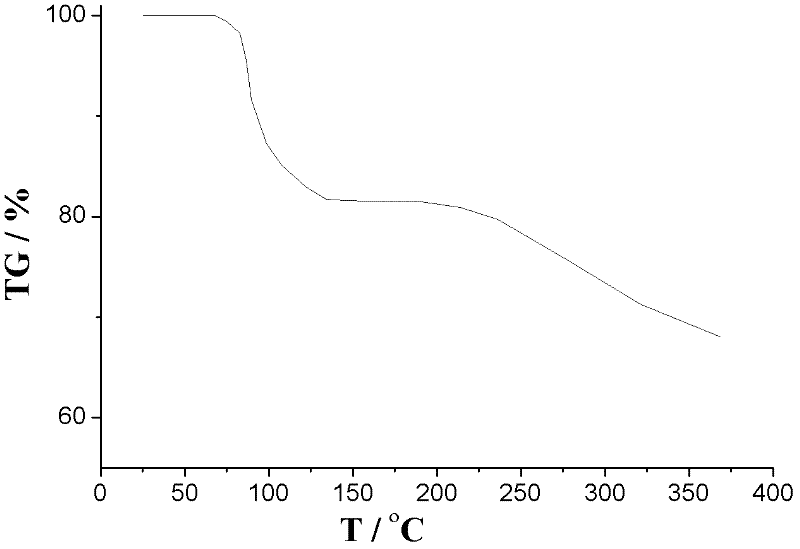
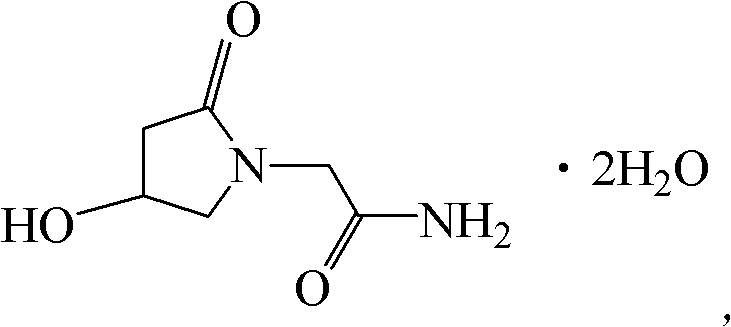
Oxiracetam compound with steady crystal form
The invention belongs to the technical field of medicine, and particularly relates to an oxiracetam compound. The oxiracetam compound provided by the invention contains semi-crystalline water. The oxiracetam compound has the advantages that related substances are small, the purity is high, the stability is good, and a moisture-absorption and weight-gaining effect is not obvious even if under a high humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
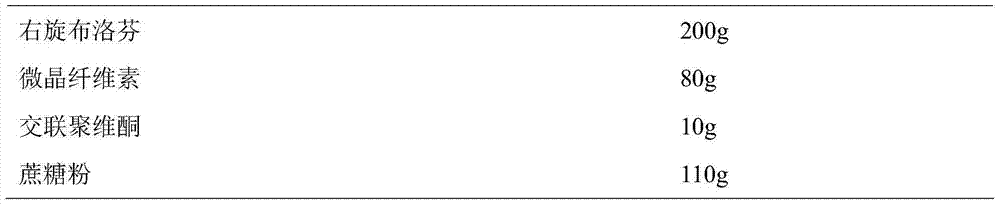
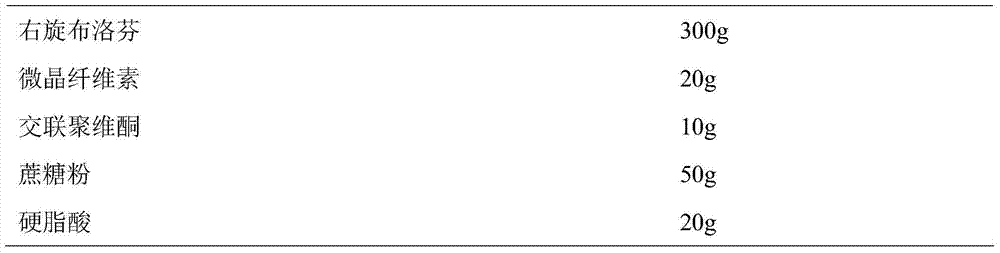
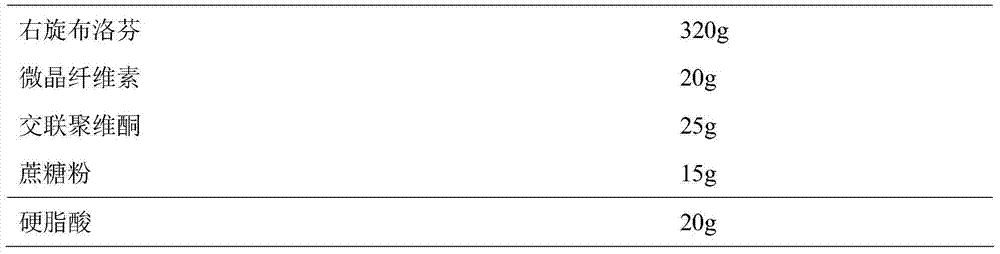
Dexibuprofen slow release pellet and preparation method thereof
ActiveCN104739773AReduce the number of dosesImprove complianceOrganic active ingredientsAntipyreticAdhesiveIn vitro dissolution
The invention discloses a dexibuprofen slow release pellet and a preparation method thereof, and belongs to the field of medicinal preparations. The dexibuprofen slow release pellet comprises 50-80% of dexibuprofen, 0-5% of a slow release material, 0-2.5% of an adhesive, 8.75-47.5% of a filler, and 1.25-6.25% of a disintegrating agent, and all above percentages are based on the total weight of the pellet. The dexibuprofen slow release pellet has the advantages of good stability, stable quality, simple device and process, strong operationality, and suitableness for industrial large scale production. In vitro dissolution test shows that the accumulative release rate within 1h is 10-35%, the accumulative release rate within 2h is 25-55%, the accumulative release rate within 4h is 50-80%, and the accumulative release rate within 7h is 75% or above, so the dexibuprofen slow release pellet has a good release curve.
Owner:LUNAN BETTER PHARMA
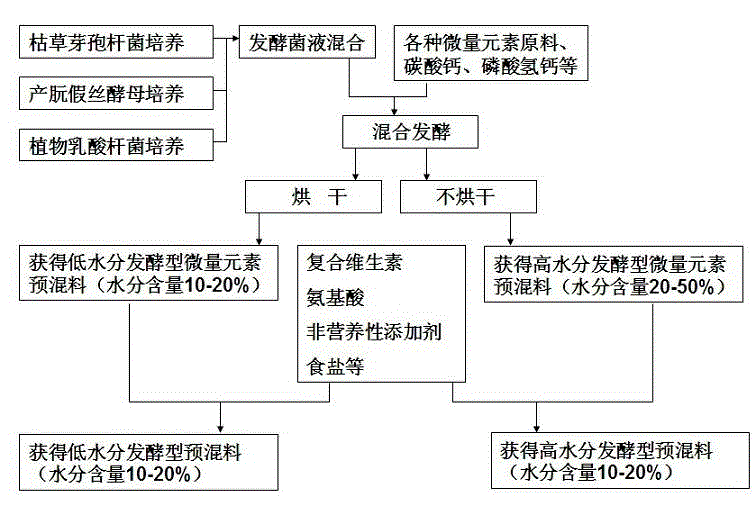
Fermentation trace element premix and preparation method thereof as well as fermentation composite premix and preparation method thereof
ActiveCN104798990AAdd lessImprove utilization efficiencyAnimal feeding stuffManganesePotassium iodine
The invention discloses a fermentation trace element premix and a preparation thereof as well as a fermentation composite premix and a preparation method thereof. The fermentation trace element premix comprises raw materials of bacillus subtilis, lactobacillus plantarum, candida utilis, a fermentation carrier, copper sulfate, ferrous sulfate, zinc sulfate, manganese sulfate, sodium selenite, calcium carbonate, calcium hydrophosphate, cobaltous sulfate and potassium iodide. The preparation method of the fermentation trace element premix comprises the following steps of uniformly mixing the raw materials according to a certain proportion; adjusting the water content; packaging the mixture with an airtight packaging bag; tightening a bag mouth and fermenting; drying or not drying a fermented product to obtain the fermentation trace element premix. The preparation method of the fermentation composite premix comprises the following steps of mixing the prepared fermentation trace element premix with vitamin, edible salts, an enzymic preparation, a non-nutritional additive, amino acid and the like according to the certain proportion, and packaging. According to the fermentation trace element premix and the fermentation composite premix, the formula is reasonable, the use is convenient, the animal feed intake is improved, the utilization rate of feed is improved, the animal diarrhea rate is reduced, the immunity and disease resistance of animals are improved, the environmental pollution is reduced, and the sustainable development of animal husbandry is promoted.
Owner:王升平
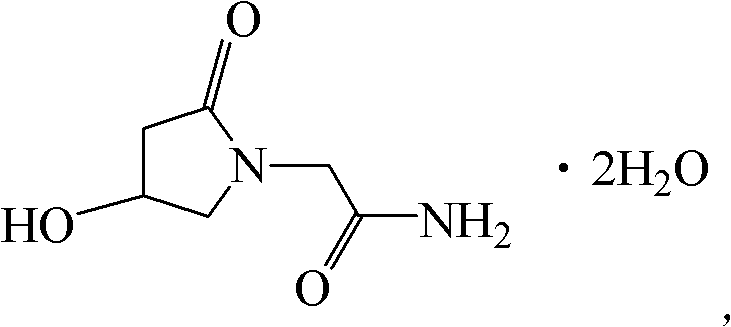
Oxiracetam compound and pharmaceutical composition thereof
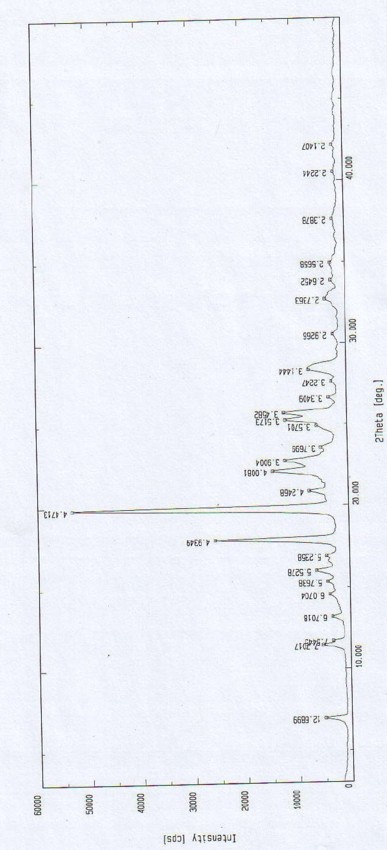
ActiveCN102351770BQuality is easy to controlReduce contentOrganic active ingredientsOrganic chemistryDihydrogen oxideX-ray
The invention relates to an oxiracetam compound, which has two crystal water molecules. In X-ray powder diffraction pattern obtained through Cu-Kalpha ray measurement, the characteristic peaks of the oxiracetam compound are shown in positions wherein 2theta is 17.3 degrees, 19.1 degrees, 21.6 degrees, 23.2 degrees, 27.0 degrees, 28.4 degrees, 30.0 degrees, 31.0 degrees, 31.7 degrees, 33.2 degrees, 36.9 degrees, 39.3 degrees, 40.2 degrees, 45.7 degrees and 51.2 degrees. The invention also relates to a preparation method of the oxiracetam compound, and a pharmaceutical composition containing the oxiracetam compound. The oxiracetam compound and the pharmaceutical composition thereof provided by the invention have the characteristic of high stability.
Owner:江西新先锋医药有限公司
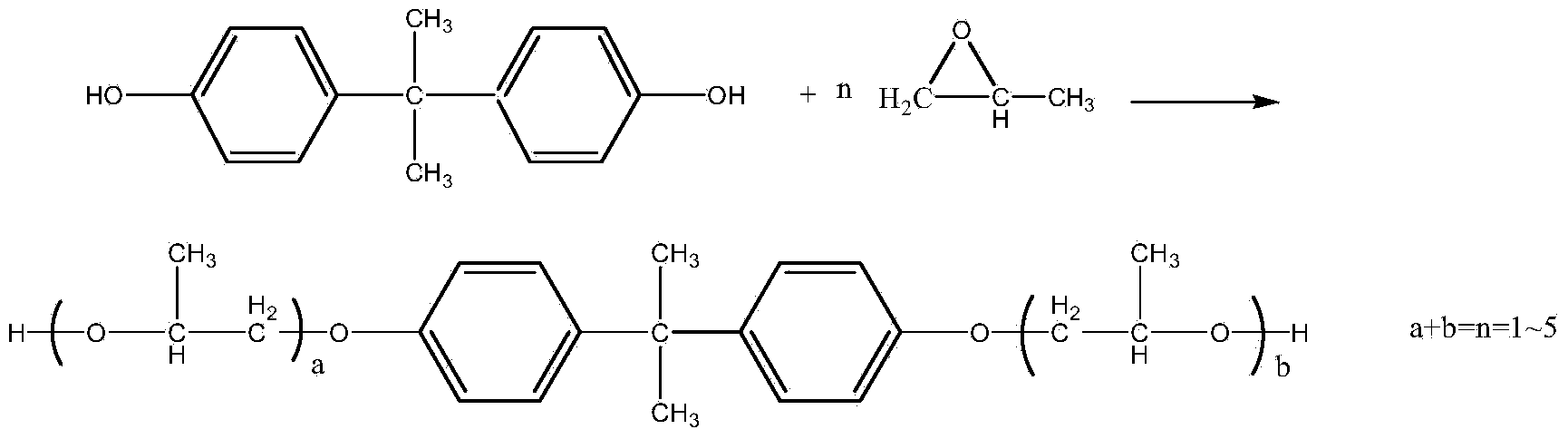
Method for synthesizing dihydroxypropyl bisphenol A ether through one-step process
ActiveCN103641696AHigh activityStrong steric hindranceEther preparation from oxiranesSimple Organic CompoundsHigh energy
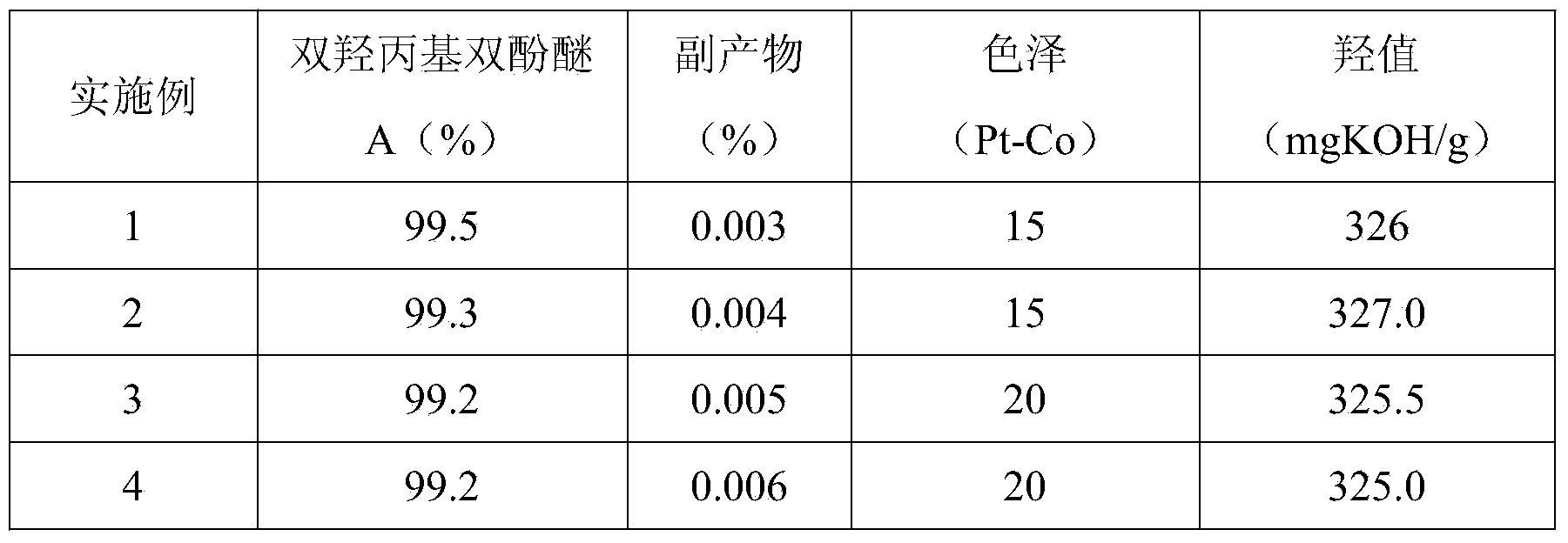
The invention relates to a method for synthesizing dihydroxypropyl bisphenol A ether through a one-step process, and belongs to the technical field of organic compound synthesis. The method comprises the steps of: by taking bisphenol A as the raw material, orderly carrying out material melting and synthesis by adding a chain extension agent, and finally obtaining the dihydroxypropyl bisphenol A ether, wherein the hydroxyl value of the dihydroxypropyl bisphenol A ether is 325-330mgKOH / g, and the color of the dihydroxypropyl bisphenol A ether is smaller than No.30. The technical scheme of the method is used for synthesizing the product through the one-step process, and the problems of complex preparation process, many process steps, high energy consumption, high composite cost, poor product quality and so on in the conventional technology are solved; the obtained product is rational in distribution, low in product color; and the content of the by-product allyl alcohol matters is low.
Owner:ZHEJIANG HUANGMA TECH
Method for catalyzing, esterifying and upgrading bio-oil under microwave condition
InactiveCN101812376APromote conversionImprove stabilityFatty acid esterificationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidSolvent
The invention discloses a method for catalyzing, esterifying and upgrading bio-oil under a microwave condition. In the method, acidic materials in the bio-oil are catalyzed, esterifyed and upgraded by adopting a ZnCl2-midified 732 type cation exchange resin as a catalyst and adding ethanol as a solvent and a reactant and under a microwave heating condition, and mixed solution of ethanol, acetic acid and furfural is adopted as imitation of a bio-oil complex system; esterification is performed under the condition of the catalyst of ZnCl2-midified 732 type cation exchange resin by adding a certain amount of hydrogen peroxide containing 30 percent of H2O2, and oxidizing furfural substances into the acidic materials, and a normal pressure microwave-assisted synthesis and extraction microwave reactor is adopted as a reaction device; the ZnCl2-midified 732 type cation exchange resin catalyst has better activity; and the conversion rate of the acetic acid which is obtained by catalyzing, esterifying and upgrading the bio-oil catalysis under the microwave condition reaches 70.37 percent; and the conversion rate of the furfural reaches 51.71 percent.
Owner:ZHEJIANG UNIV
Preparation method of cefepime hydrochloride
ActiveCN101935325ASimple processAvoid the phenomenon of inhomogeneous crystal form and poor fluidityOrganic chemistryCefepime hydrochlorideBetaine
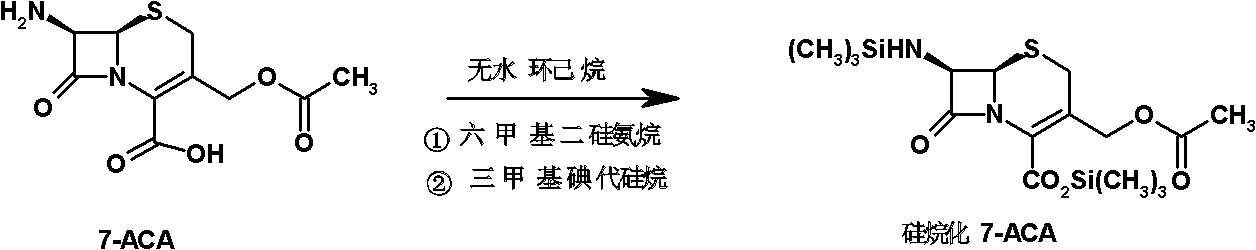
The invention discloses a preparation method of cefepime hydrochloride, comprising the following steps of: reacting oxalyl chloride with 2-methoxyimino-2-(2-aminothiazole-4-yl) acetic acid hydrochloride to obtain a midbody I, i.e. 2-methoxyimino-2-(2-aminothiazole-4-yl) acetyl chloride hydrochloride; mixing silanized 7-aminoce-phalosporanic acid and silanized N-methylpyrrolidine, and reacting to obtain a midbody II, i.e. hydriodic acidification (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydro pyrrolidine) methyl]-3-cephem-4-formic betaine, in the presence of trimethyl idodine silicon hydride, isopropanol and an aqueous solution of hydrogen iodide; dissolving the midbody II into dichloromethane, sequentially adding trimethylchlorosilane and hexamethyldisilazane for reaction, and then adding the midbody I and triethylamine to react to prepare the cefepime hydrochloride. The cefepime hydrochloride prepared by the method has the advantages of uniform crystal form, good flowability and simple process and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Tigecycline freeze-dried injection
ActiveCN101401812AImprove stabilityLow content of related substancesAntibacterial agentsPowder deliverySulfite saltFreeze-drying
The invention relates to a tigecycline freeze-dried powder injection, which consists of tigecycline, dextran, sodium sulfite and sodium citrate according to the following weight proportion: 10 portions of the tigecycline, 10 to 90 portions of the dextran, 0.1 to 3 portions of anhydrous sodium sulfite, and 0.1 to 3 portions of the sodium citrate. The pH value of the tigecycline freeze-dried powder injection is between 7.0 and 9.0. The preparing process comprises the following steps: firstly, dissolving the dextran by 80 percent of water for injection; secondly, adding the sodium citrate and the anhydrous sodium sulfate into the mixture after cooling; thirdly, adding the tigecycline into the mixture after dissolving and evenly stirring the sodium citrate and the anhydrous sodium sulfate, and mixing the mixture evenly; and fourthly, regulating the pH to between 7.0 and 9.0, adding a needle activated carbon into the mixture, stirring and adsorbing the mixture, removing the activated carbon from the mixture, and fixing the capacity. The solution is filtered through two microporous membranes and then are filled in a cillin bottle, and the tigecycline freeze-dried powder injection is obtained through semi-stoppering, spanning, freeze-drying, introducing nitrogen, performing tamponade, taking out the injection from a box, rolling a mouth, passing the quality inspection, and packaging.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Oxiracetam compound and pharmaceutical composition thereof
ActiveCN102351770AQuality is easy to controlReduce contentOrganic active ingredientsOrganic chemistryDihydrogen oxideX-ray
The invention relates to an oxiracetam compound, which has two crystal water molecules. In X-ray powder diffraction pattern obtained through Cu-Kalpha ray measurement, the characteristic peaks of the oxiracetam compound are shown in positions wherein 2theta is 17.3 degrees, 19.1 degrees, 21.6 degrees, 23.2 degrees, 27.0 degrees, 28.4 degrees, 30.0 degrees, 31.0 degrees, 31.7 degrees, 33.2 degrees, 36.9 degrees, 39.3 degrees, 40.2 degrees, 45.7 degrees and 51.2 degrees. The invention also relates to a preparation method of the oxiracetam compound, and a pharmaceutical composition containing the oxiracetam compound. The oxiracetam compound and the pharmaceutical composition thereof provided by the invention have the characteristic of high stability.
Owner:江西新先锋医药有限公司
Polypropylene composite material for automotive interior parts and production process of polypropylene composite material for automotive interior parts
ActiveCN108047560AImprove mechanical propertiesImprove antibacterial propertiesPolypropylene compositesAntioxidant
The invention discloses a polypropylene composite material for automotive interior parts and a production process of the polypropylene composite material for the automotive interior parts. The polypropylene composite material for the automotive interior parts is prepared from, by weight, 55-80 parts of homo-polypropylene, 6-12 parts of ethylene-propylene-diene monomer rubber, 15-25 parts of talcumpowder, 0.2-0.8 part of a light stabilizer, 5-7 parts of a compatibilizer, 0.8-1.2 parts of a coupling agent, 0.3-0.6 part of an antioxidant, 5-8 parts of a flame retardant, 3-6 parts of an antibacterial and 0.2-2.1 parts of an adsorbent. Compared with the prior art, the polypropylene composite material for the automotive interior parts has advantages of great antibacterial and fame retardation performances, low VOC (volatile organic compound) and the like. In addition, the polypropylene composite material for the automotive interior parts has appearance and performances of rubber and can bemade into the automobile interior parts such as driver's seat outer lateral plates, small cover caps, lateral plate inner plates, inner lateral plate plastic parts, passenger seat outer lateral plates, door sheet armrest frameworks, door embedded decorative plate frameworks, door embedded decorative plates and the like through an injection molding process.
Owner:湖北瀚氏汽车零部件有限公司
Ketoralac ammonia butanetriol injection and preparing method thereof
ActiveCN101199514ASolve easy discolorationLow content of related substancesOrganic active ingredientsAntipyreticAcid derivativeKetorolac Tromethamine
Disclosed is ketorolac tromethamine, which is a pyrrole acid derivative developed by the Syntex company in the U.S. The invention can effectively inhibit prostaglandin (PG) from synthesizing with functions of analgesia, anti-inflammation, antipyresis and inhibiting the platelets from aggregating. The invention provides a preparation method of ketorolac tromethamine injection, which well improves the stability and the clarity of the preparation with stable process.
Owner:鲁南新时代生物技术有限公司
Dura mater biopatch and preparation method thereof
ActiveCN108478870ALow content of related substancesReduce immune rejectionTissue regenerationProsthesisAntigenCell-Extracellular Matrix
Owner:上海白衣缘生物工程有限公司
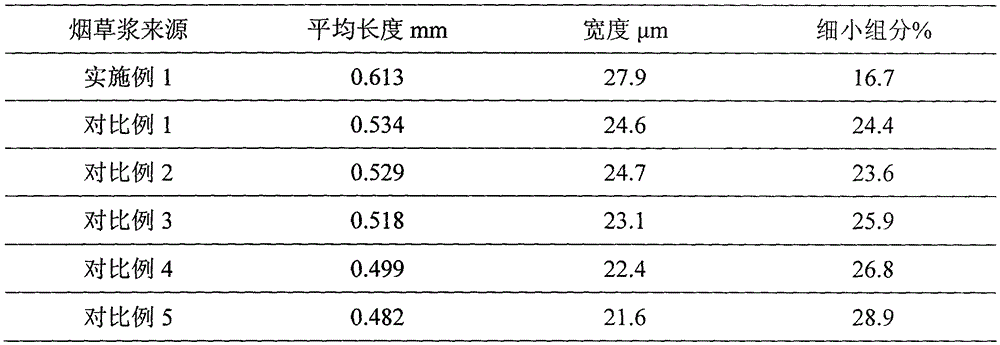
Method for preparing tobacco pulp from regenerated tobacco
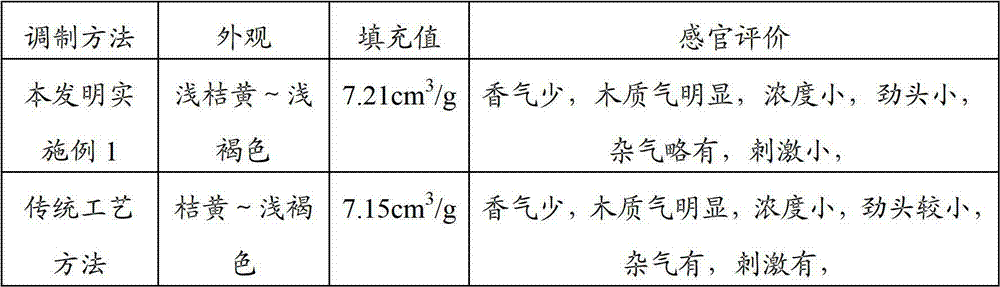
InactiveCN105595400AReduce energy consumption for refiningImprove running performanceTobacco preparationFiberSolvent
The invention discloses a method for preparing tobacco pulp from regenerated tobacco. The method includes the following steps that 1, the tobacco raw material is extracted and then subjected to solid-liquid separation, and an aqueous solvent is added into tobacco filter residues obtained after solid-liquid separation, wherein the aqueous solvent contains salt and alkali and a nonionic surfactant; 2, the tobacco filter residues are fed into a pulping machine for defibering; 3, a complex enzyme preparation is added into the filter residues obtained after defibering for enzymolysis; 4, after enzymolysis is completed, pulping continues till the needed pulping degree is obtained, and the tobacco pulp is obtained. In the method for preparing the tobacco pulp, the pulp forming characteristic of the tobacco is effectively improved by organically combining the physical action, the enzyme preparation and the chemical reagent according to the specificity of the tobacco raw material, so that the effects of improving the papermaking property of the tobacco pulp and increasing the physical indexes of the regenerated tobacco are achieved, and meanwhile the interior quality of the regenerated tobacco is improved.
Owner:宁夏夏盛实业集团有限公司
Preparation method of high-purity breviscapinun material used by breviscapinun injection
ActiveCN102188440AHigh purityImprove securityPowder deliveryOrganic active ingredientsAlcoholSodium Chloride Injection
The invention provides a preparation method of high-purity breviscapinun material used by a breviscapinun injection. The method comprises the preparation steps of: carrying out dissolving, alcohol precipitation, acid precipitation, ultrafitration and the like on the breviscapinun to obtain the high-purity breviscapinun material. The purity of the high-purity breviscapinun material can achieve more than 98 percent by detection through high performance liquid chromatography, and the high-purity breviscapinun material can be used for preparing large-capacity injection such as breviscapinun sodium chloride injection or breviscapinun glucose Injection.
Owner:吉林四长制药有限公司
Preparation method of cefminox sodium
The invention discloses a preparation method of cefminox sodium, which comprises the following steps: 7 beta-bromoacetamide-7 alpha-methoxy-3-(1-methyl-1H-5-tetrazyl)sulfur methyl-3- cephem-4-carboxylic acid and D-cysteine hydrochloride are dissolved in water, the pH value is regulated to 6.0-7.0 by sodium bicarbonate, condensation reaction is carried out, and reaction products are post-treated to obtain the cefminox sodium. In the method, cefminox sodium raw material can be prepared through low-temperature reaction, a nonpolar macroporous resin X5 chromatography column is used for purification, ethanol-aqueous solution or anhydrous alcohol recrystallization and other simple operations are adopted to obtain target products, the yield and the purity of the target products are high, the products have uniform crystal forms and good fluidity, no special equipment is needed for the production, and the method is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
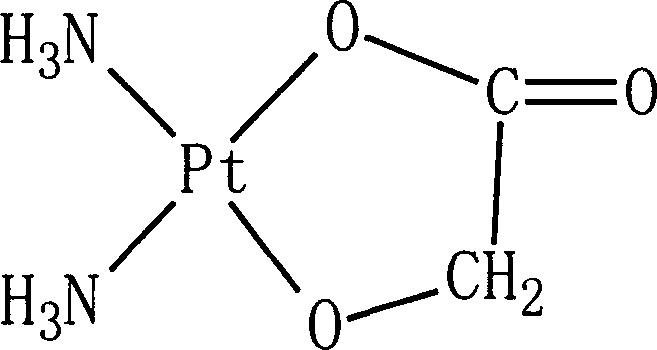
Preparation of nedaplatin freeze-dried powder injection
ActiveCN101015539AImprove stabilityLow content of related substancesPowder deliveryLyophilised deliveryFreeze-dryingBottle
This invention relates to a preparation method of freeze-dried injection of nedaplatin.nedaplatin freeze-dried injection prepared with the inventive method can be used as therapeutic drug of cancer. The invention improves the stability of nedaplatin during lyophilization and shortens the period of lyophilization through adding ethanol during preparation process. the preparation method comprises adding water for injection 80% of nedaplatin into nedaplatin, stirring for dissolving, adding dextran, stirring for dissolving, determing the content of intermediate, adding ethanol 1-10% of the cumulative volume of the solution, adding water for injection to full dose, filtrating with 0.22 mu m millipore filter under aseptic condition, encapsulating in sirin bottle, plugging, freeze drying, rolling the opening, testing, and packaging. The optimum amount of ethanol is 1-5% of cumulative volume of the solution.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
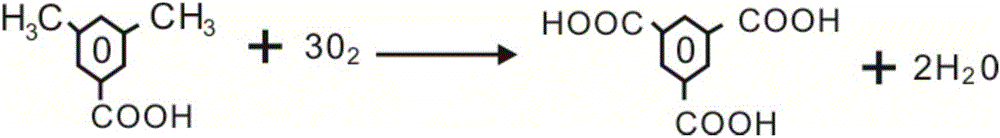
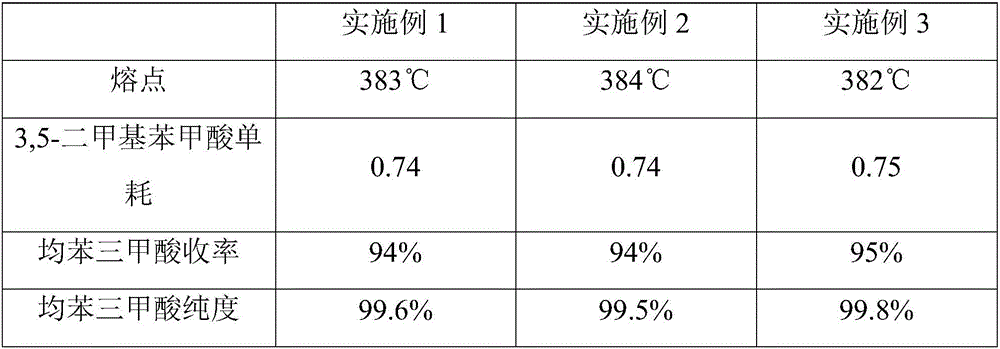
Method for preparing trimesic acid
ActiveCN106431887AReduce generationEasy to purifyOrganic compound preparationCarboxylic compound preparationTarCoal tar
The invention relate to the field of compound production and preparation, in particular to a method for preparing trimesic acid. The method includes oxidizing 3, 5-dimethylbenzoic acid under the effects of catalytic systems by the aid of liquid-phase oxidation processes to generate the trimesic acid. Compared with the traditional method for preparing trimesic acid from mesitylene which is used as an initial raw material, the method has the advantages that tar substances generated by the 3, 5-dimethylbenzoic acid in reaction procedures are low in content, and accordingly the method is high in yield and favorable for environmental protection and product purification; mother liquor is recycled, accordingly, the utilization rates of various raw materials can be increased, and the productivity can be improved.
Owner:黄石市利福达医药化工有限公司
Processing technology for lowering pectin content in cut stem
ActiveCN102783704AReduce pectin contentReduce manufacturing costTobacco treatmentSODIUM METAPHOSPHATEHydrogen
The invention provides a processing technology for lowering pectin content in a cut stem, which comprises the following steps of: A) sieving and peeling: cutting a thick stem into even stem sections of 8-10mm, sieving according to the thickness, enabling the thick stem section of which a diameter is less than 2.38mm to directly enter the next working procedure, peeling and polishing the thick stem of which a diameter is more than or equal to 2.38mm, and removing the bast part of a tobacco stem by mechanical force; B) chemical stem cleaning and dipping: after the tobacco stem is cleaned with sodium metaphosphate contained citric acid solution of which the pH (potential of hydrogen) is 3.0+ / -0.5, keeping the temperature of 50-60DEG C, and dipping for half hour; C) washing the stem with clean water; D) after feeding, pressing and cutting off the stem; and E) drying and storing the stem. Compared with the cut stem prepared by the traditional technology, the cut stem prepared with the method disclosed by the invention has the advantages that the physical behavior does not have obvious difference, but the aesthetic quality is obviously improved, the irritation is reduced, and the miscellaneous qi is reduced. After the cut stem is added into rolled cigarettes, the cigarette sucking comfort can be improved, and the tar content is lowered.
Owner:HUBEI CHINA TOBACCO IND
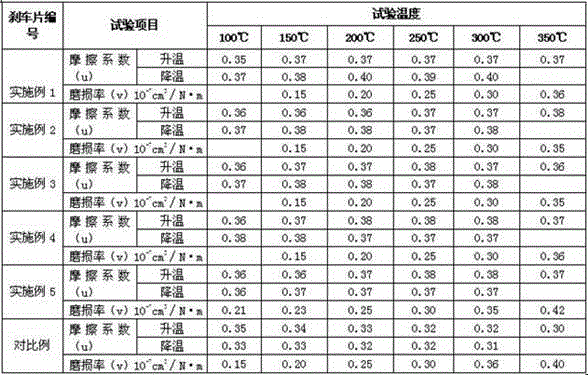
Micro-copper-formulated ceramic brake pad
InactiveCN103821859ALight weightUniform surface distributionOther chemical processesFriction liningSulfideBoron nitride
A micro-copper-formulated ceramic brake pad is made with aramid pulp, carbon fiber, magnesium hydroxide, copper fiber, brown fused alumina, antimony sulfide, ceramic fiber, superfine iron oxide powder, calcium sulfate whisker, potassium titanate, barite, crystalline flake graphite, calcined petroleum coke, nano hollow float beads, flake aluminum powder, nitrile butadiene rubber powder, cashew nut shell oil modified phenolic resin, zirconite, boron nitride, zinc oxide, and benzoxazine resin. The components are reasonably matched, and advantages of the materials can be given to full play in abrasive materials. The novel fiber materials are reasonably composited, performance defects of the materials can be mutually complemented, and high performance is achieved through mutual connective action. The micro-copper-formulated ceramic brake pad has low content of heavy metals and smaller than 0.5% of copper content and therefore, is very environment friendly.
Owner:RUIYANG AUTOMOTIVE MATERIALS XIANTAO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com