Dexibuprofen slow release pellet and preparation method thereof
A technology of dextro-ibuprofen and sustained-release pellets, applied in the field of medicine, can solve the problems of sudden drug release, difference in release degree, increase of related substances, etc., achieve low content of total related substances, improve patient compliance, and improve the frequency of taking medicine reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
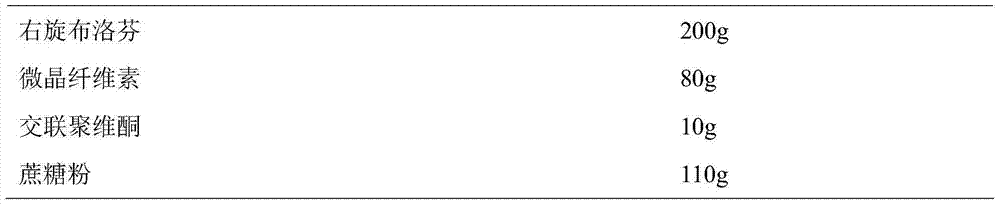
[0037] Prepare Dexibuprofen sustained-release pellets according to the following ratio:
[0038]
[0039] Weigh D-ibuprofen and auxiliary materials according to the prescription amount, mix them evenly, add 80ml of 10% ethanol aqueous solution to make soft materials, sieve the soft materials to make wet granules, and spheronize the wet granules in a centrifugal granulator to obtain D-ibuprofen microparticles. Dried the dextrobuprofen pellets at 40°C to obtain the dextrobuprofen sustained-release pellets.
Embodiment 2
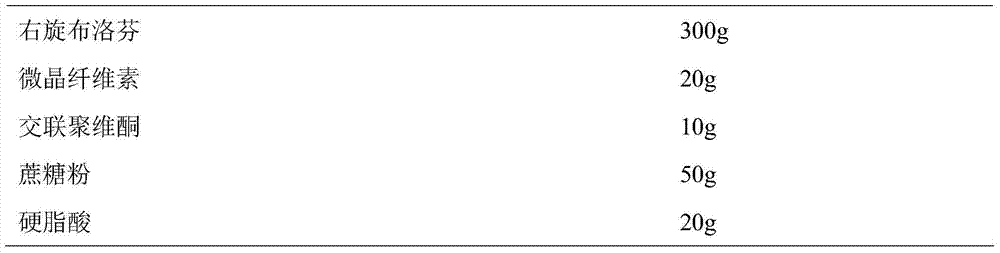
[0041] Prepare Dexibuprofen sustained-release pellets according to the following ratio:
[0042]
[0043]Weigh dextroibuprofen and auxiliary materials according to the prescription amount, mix them evenly, add 100ml of purified water to make soft material, sieve the soft material to make wet granules, and round the wet granules in a sugar-coated pan to obtain dextroibuprofen pellets, dextrobuprofen pellets The ibuprofen sustained-release pellets were obtained by drying the ibuprofen pellets at 35°C.
Embodiment 3
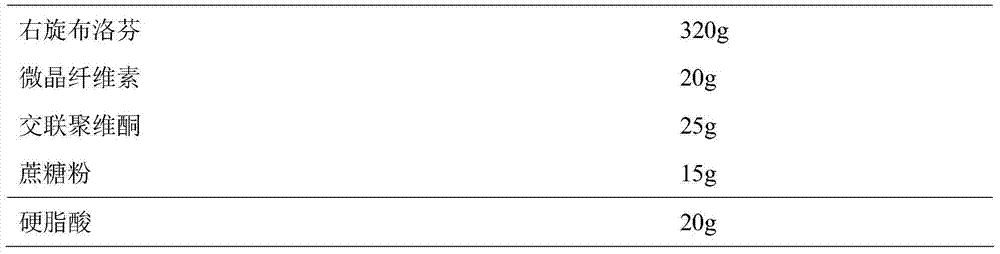
[0045] Prepare Dexibuprofen sustained-release pellets according to the following ratio:
[0046]
[0047] Weigh D-ibuprofen and auxiliary materials according to the prescription amount and mix evenly, add 110ml of purified water to make soft materials, sieve the soft materials to make wet granules, and spheronize the wet granules in a centrifugal granulator to obtain D-ibuprofen pellets. Dexibuprofen pellets were dried at 30°C to obtain Dexibuprofen sustained-release pellets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com