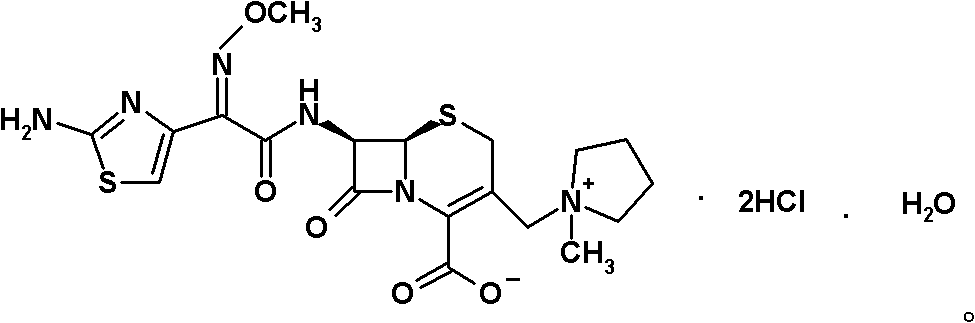
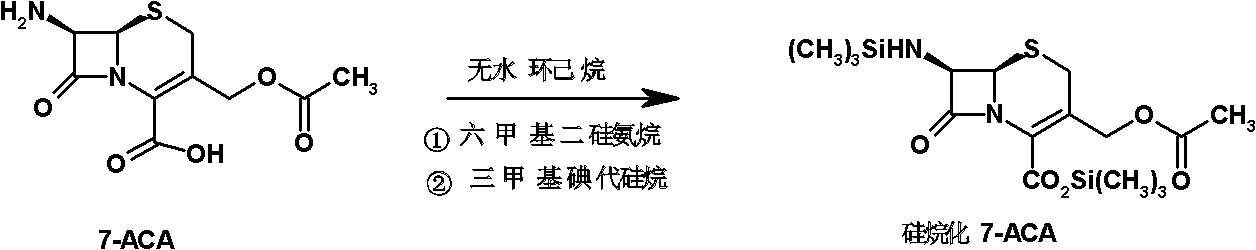
Preparation method of cefepime hydrochloride
A technology of cefepime hydrochloride and acetyl chloride hydrochloride, which is applied in the field of preparation of cefepime hydrochloride, can solve the problems of unavailable raw materials, complicated process, harsh reaction conditions, etc., and avoid harsh reaction conditions and temperature, and simple operation process , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Intermediate I: Preparation of 2-methoxyimino-2-(2-aminothiazol-4-yl)acetyl chloride hydrochloride: Add DMF (8.76L, 113mol) and dichloromethane (375L) to a reaction flask , control the temperature at 5°C, add dropwise oxalyl chloride (9.64L, 111mol), stir for 10 minutes, cool down to -25°C, and add 2-methoxyimino-2-( 2-aminothiazol-4-yl)acetic acid hydrochloride (25kg, 104mol), stirred at this temperature for 2.5 hours, filtered under nitrogen atmosphere, and the resulting solid was washed with 80L of dichloromethane and dried in vacuum at -25°C (via P 2 o 5 ), to obtain 23.48kg (90mol) of light yellow solid, based on the mass of 2-methoxyimino-2-(2-aminothiazol-4-yl) acetic acid hydrochloride, the yield of light yellow solid is 93.92% .
Embodiment 2
[0055] Intermediate II: Preparation of hydriodated (6R,7R)-7-amino-3-[(1-methyl-1-tetrahydropyrrolidine)methyl]-3-cephem-4-carboxylic acid betaine :
[0056] (1) Preparation of silylated 7-ACA slurry: add 7-ACA (20.0kg, 73.5mol) and 140L of anhydrous cyclohexane into the reaction flask, add hexamethyldisilazane (18.6 L, 88.0mol) and trimethyliodosilane (0.4L, 2.8mol) were refluxed at 55°C for 12 hours to obtain (19.92kg, 73.21mol) silylated silylated 7-ACA, which was cooled for later use.
[0057] (2) Preparation of silylated N-methyltetrahydropyrrolidine slurry: add N-methyltetrahydropyrrole (10.68L, 102.7mol) and 40L cyclohexane into the reaction flask, and add trimethylpyrrole at a controlled temperature of 20°C Iodosilane (14.6 L, 102.7 mol) was stirred for 10 minutes to obtain (10.12 L, 97.31 mol) of silylated N-methyltetrahydropyrrolidine.
[0058] (3) Preparation of Intermediate II: Under nitrogen protection, mix the above-mentioned silylated 7-ACA slurry and silylate...
Embodiment 3
[0060] Preparation of cefepime hydrochloride: Under nitrogen atmosphere at 20°C, under stirring, hydroiodate (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydropyrrole) of intermediate II Alkyl)methyl]-3-cephem-4-formic acid betaine (18.82kg, 42.38mol) was dissolved in 300L of dichloromethane, and 5.8L of trimethylsilyl chloride and 5.8L of hexamethyldisilazane were added successively. L to obtain the reaction solution. Gradually raise the temperature of the reaction solution to 25°C and keep it warm for 1.5 hours, then cool down to -40°C, within -40 to -20°C, within 40 minutes, sequentially add intermediate I 2-methoxyimino-2-( 2-aminothiazol-4-yl) acetyl chloride hydrochloride 23.48kg and triethylamine 22.9L, the resulting slurry was stirred at a temperature of 10°C and a stirring speed of 246r / min for 45 minutes, and then added within 10 minutes 70L of water was stirred at room temperature at a stirring speed of 246.5r / min for 1 hour to dissolve the solids, and the insolubles were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com