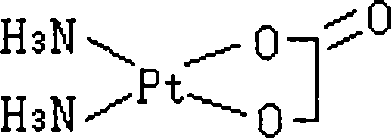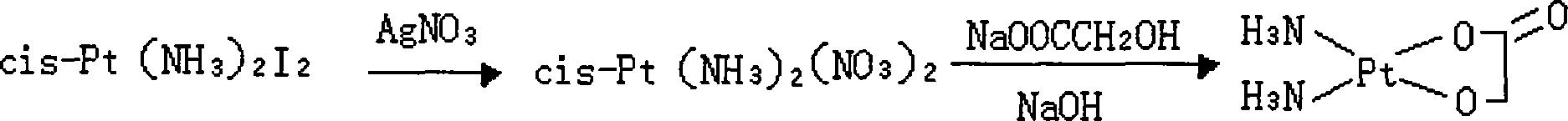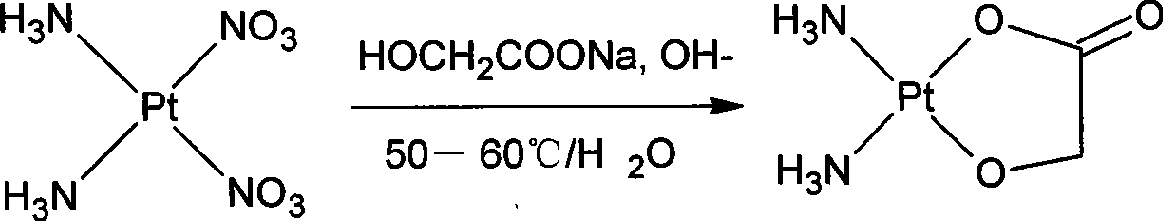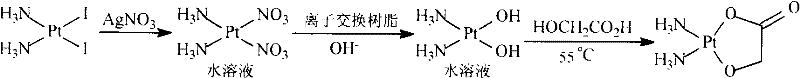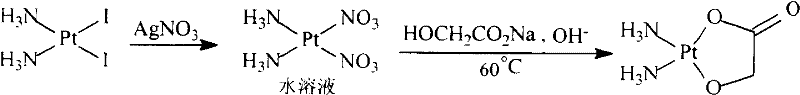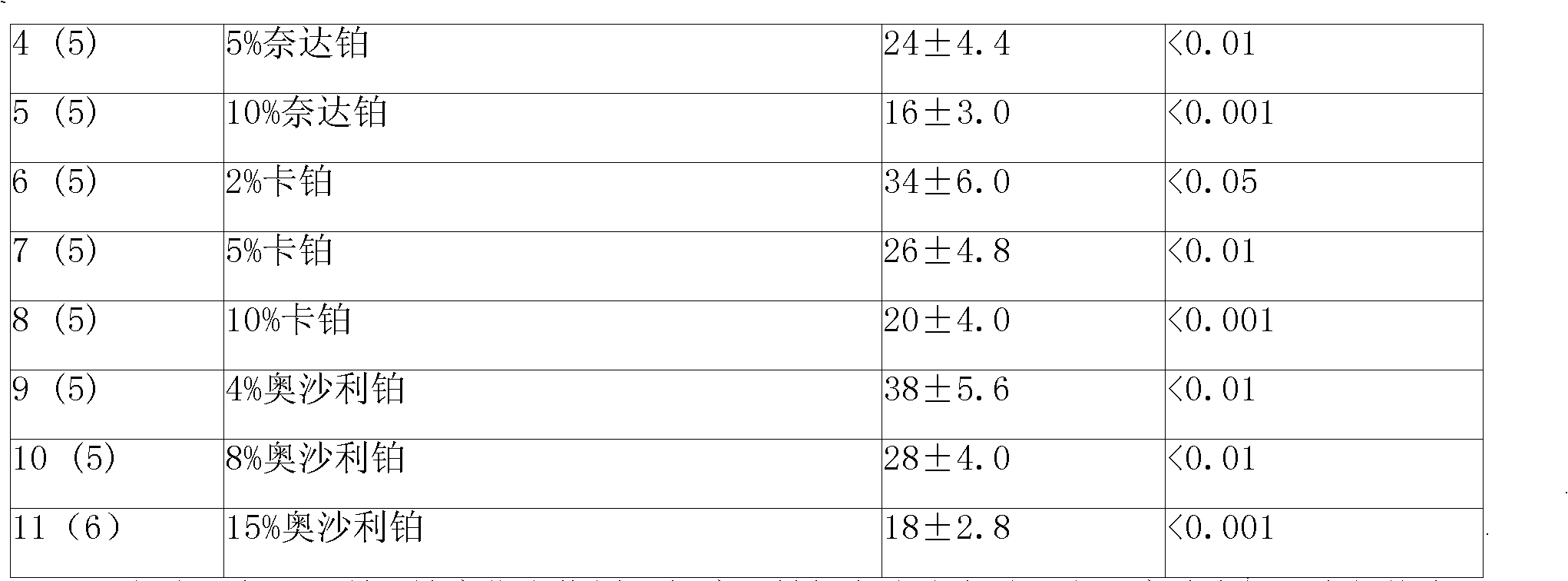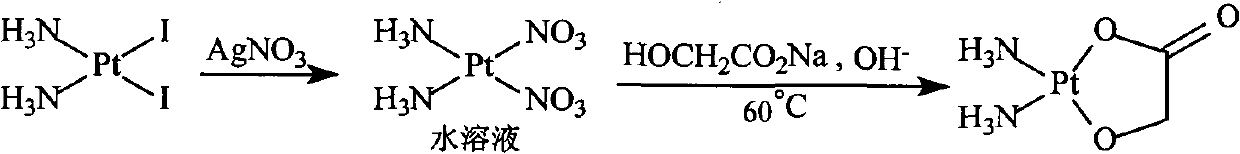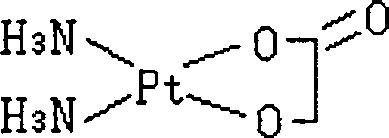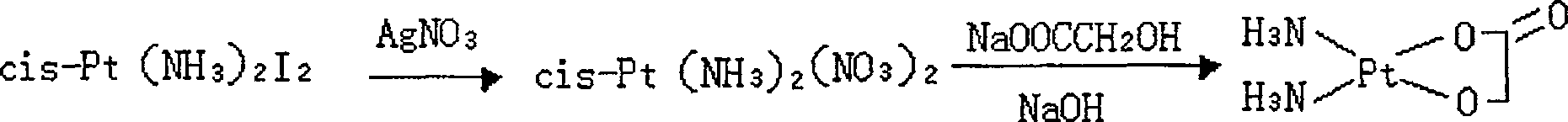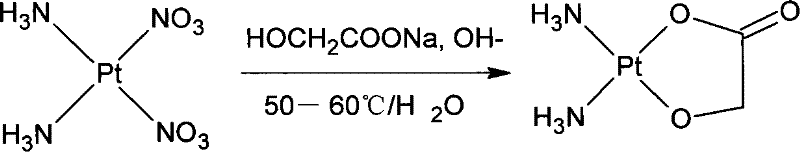Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
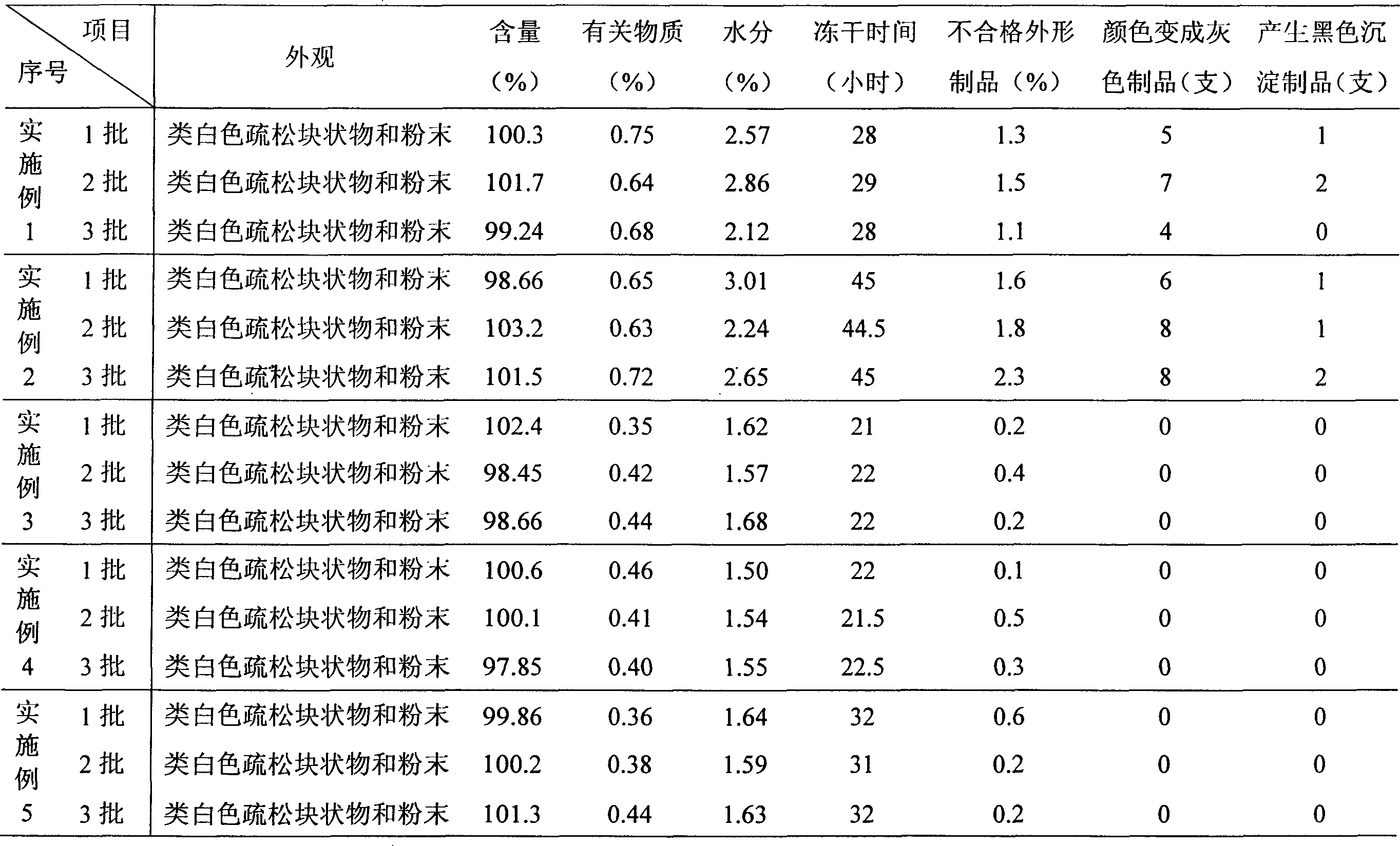
46 results about "Nedaplatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
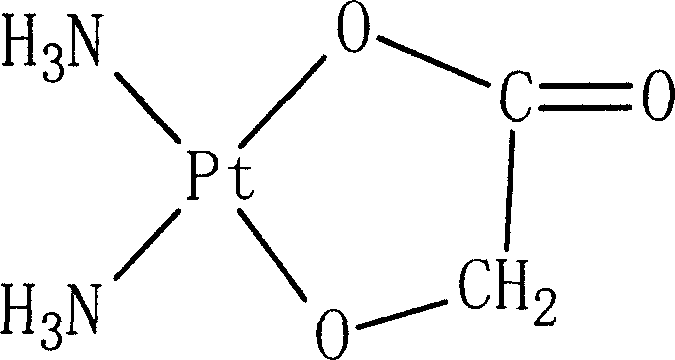
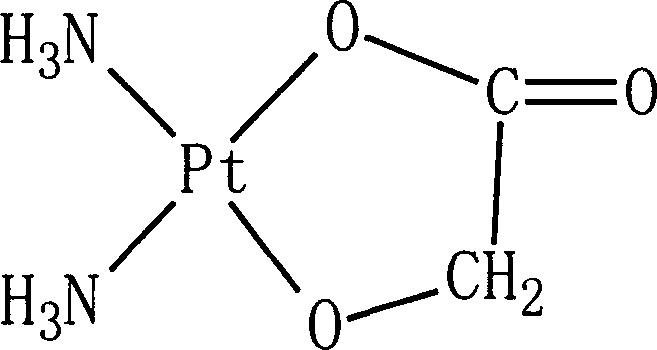
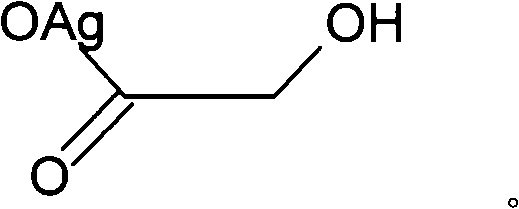
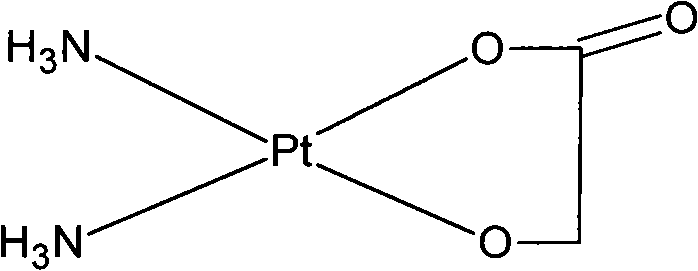
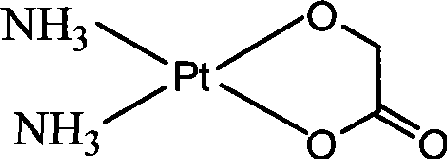
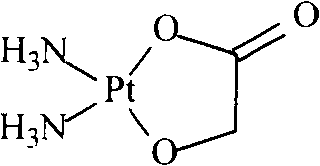
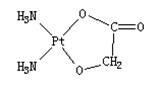
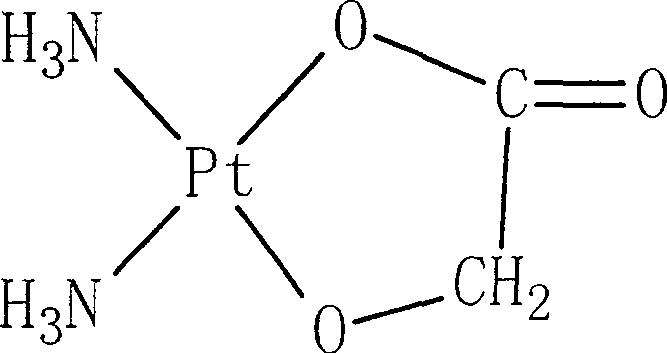
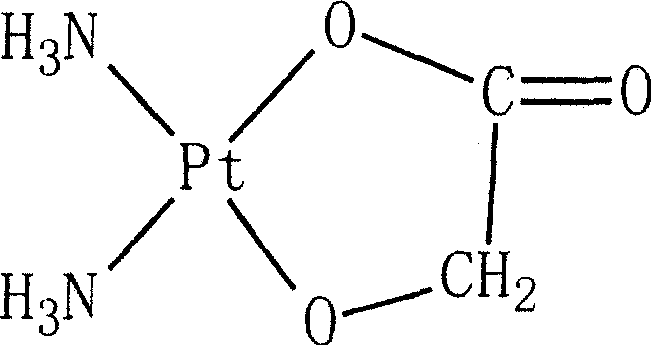
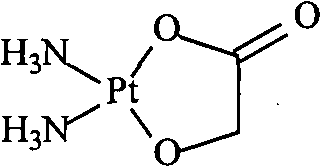
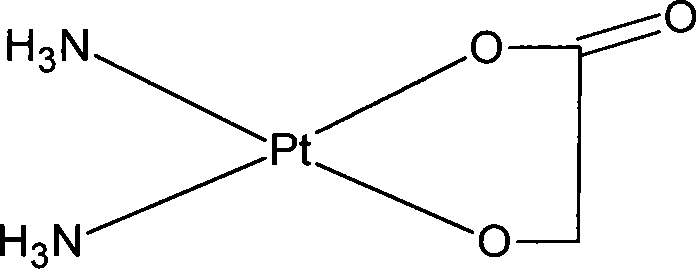
Nedaplatin (INN, marketed under the tradename Aqupla) is a platinum-based antineoplastic drug which is used for cancer chemotherapy. The complex consists of two ammine ligands and the dianion derived from glycolic acid.
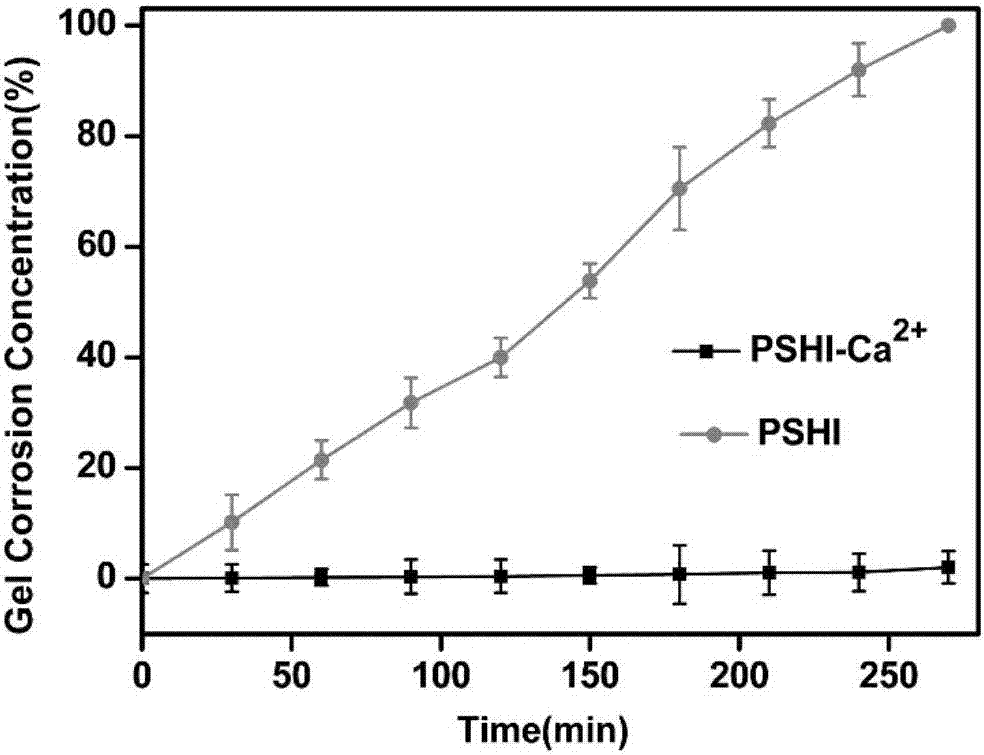
Composite developing thermosensitive gel embolizing agent as well as preparation method and application thereof
ActiveCN107281502AIncrease success rateReduce chanceOrganic active ingredientsHeavy metal active ingredientsCarboplatinMiriplatin
The invention relates to a composite developing thermosensitive gel embolizing agent as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly, preparing a mixed aqueous solution of an anticancer active substance, a thermosensitive material and a developing agent into composite developing thermosensitive gel; secondly, forming a composite developing thermosensitive gel embolizing agent by the composite developing thermosensitive gel and a coagulant on the scene, wherein the thermosensitive material is hydroxyl C1-4 alkyl cellulose, Pluronic, alginate or a mixture of the substances; the anticancer active substance is arsenic trioxide, docetaxel, cisplatin, carboplatin, nedaplatin, oxaliplatin, lobaplatin, miriplatin, siRNA or a mixture of the substances; the developing agent is a water-soluble developing agent of iodixanol, ioversol or iohexol and the like. The preparation method disclosed by the invention is simple and convenient, is suitable for industrial large-scale production, is particularly suitable for preparing an embolizing agent which is biodegradable and good in biocompatibility and is used for hemorrhagic diseases, and is especially suitable for preparing the composite developing thermosensitive gel embolizing agent for treating liver cancer, kidney cancer, lung cancer, prostate cancer, uterine myoma or splenic tumor and the like.
Owner:苏州申润医疗科技有限公司 +1
Combination therapy for cancer
ActiveUS20180271889A1High plasma concentrationPositive clinical outcomeOrganic active ingredientsPharmaceutical delivery mechanismSatraplatinAnticarcinogen
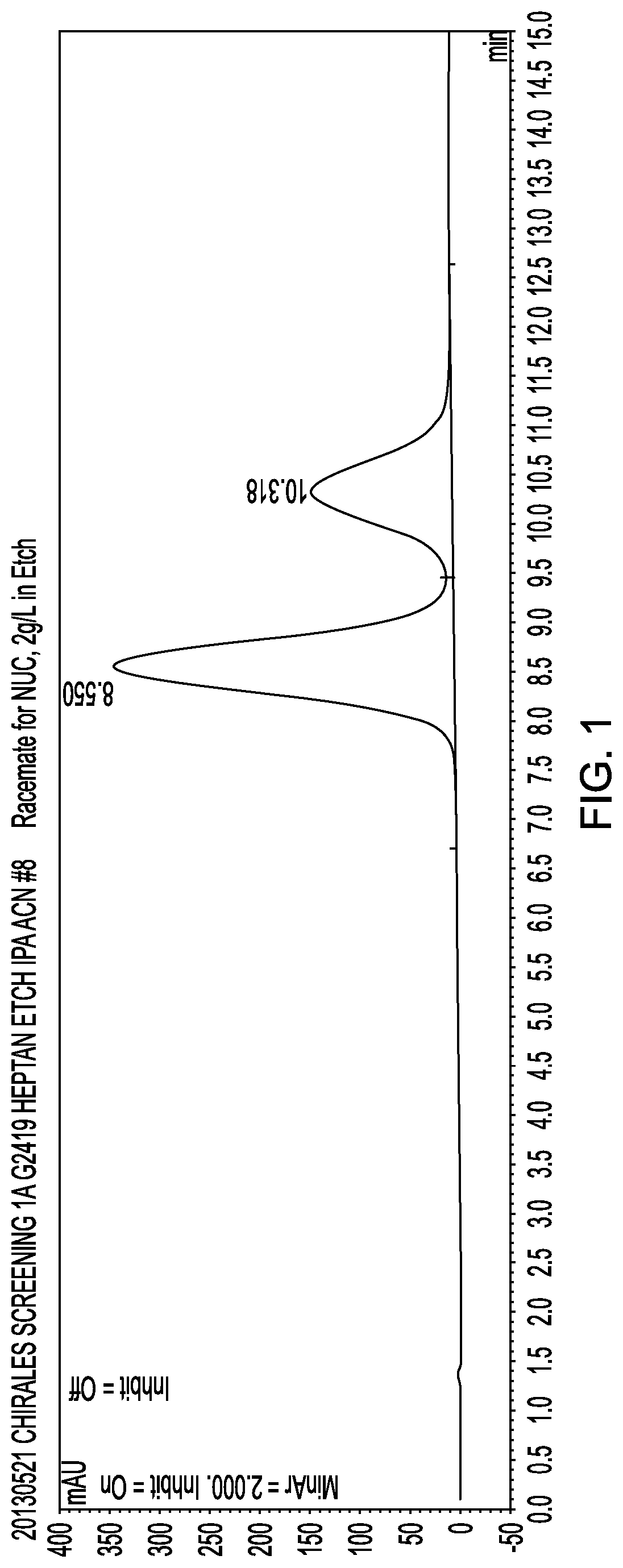
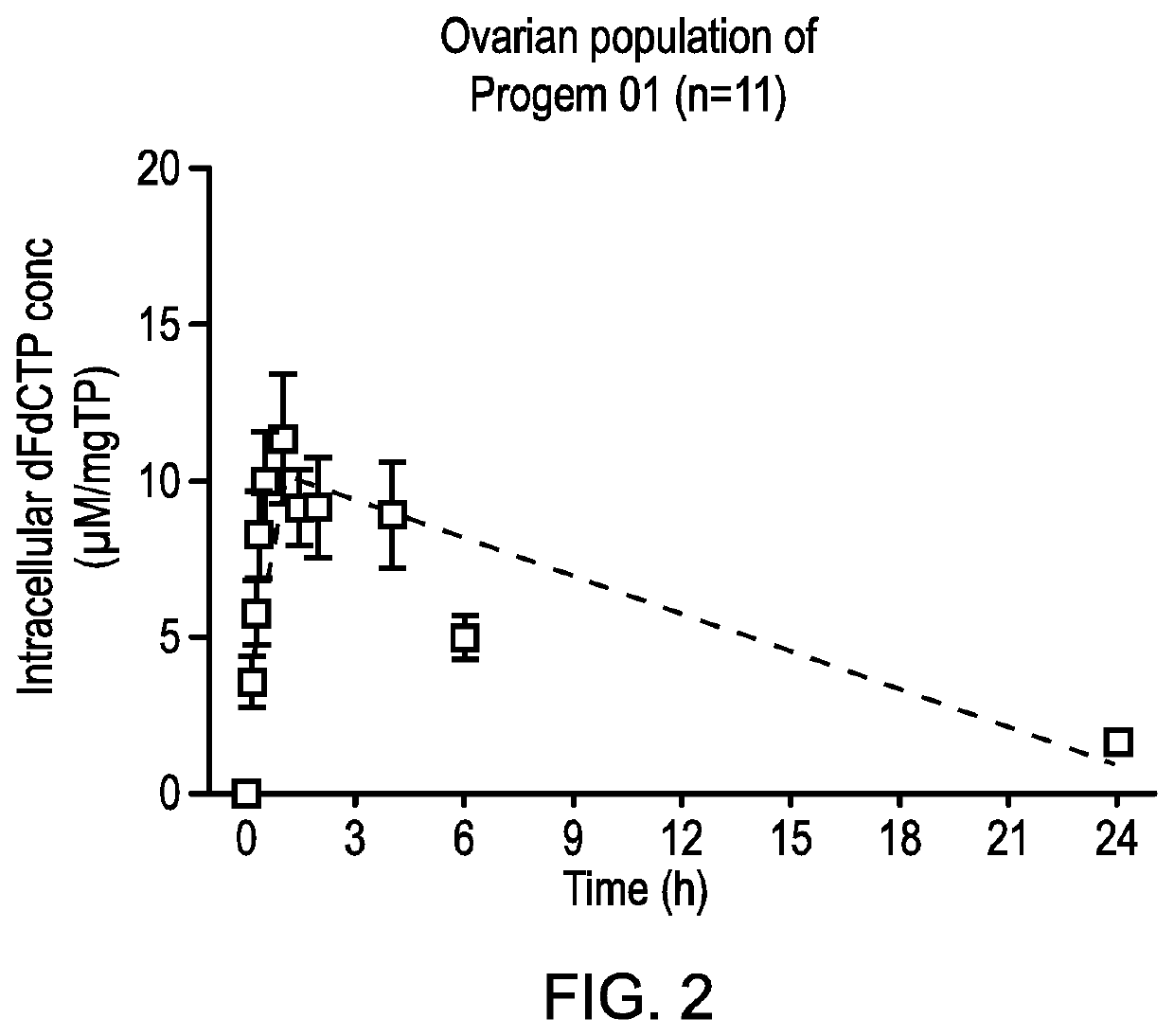
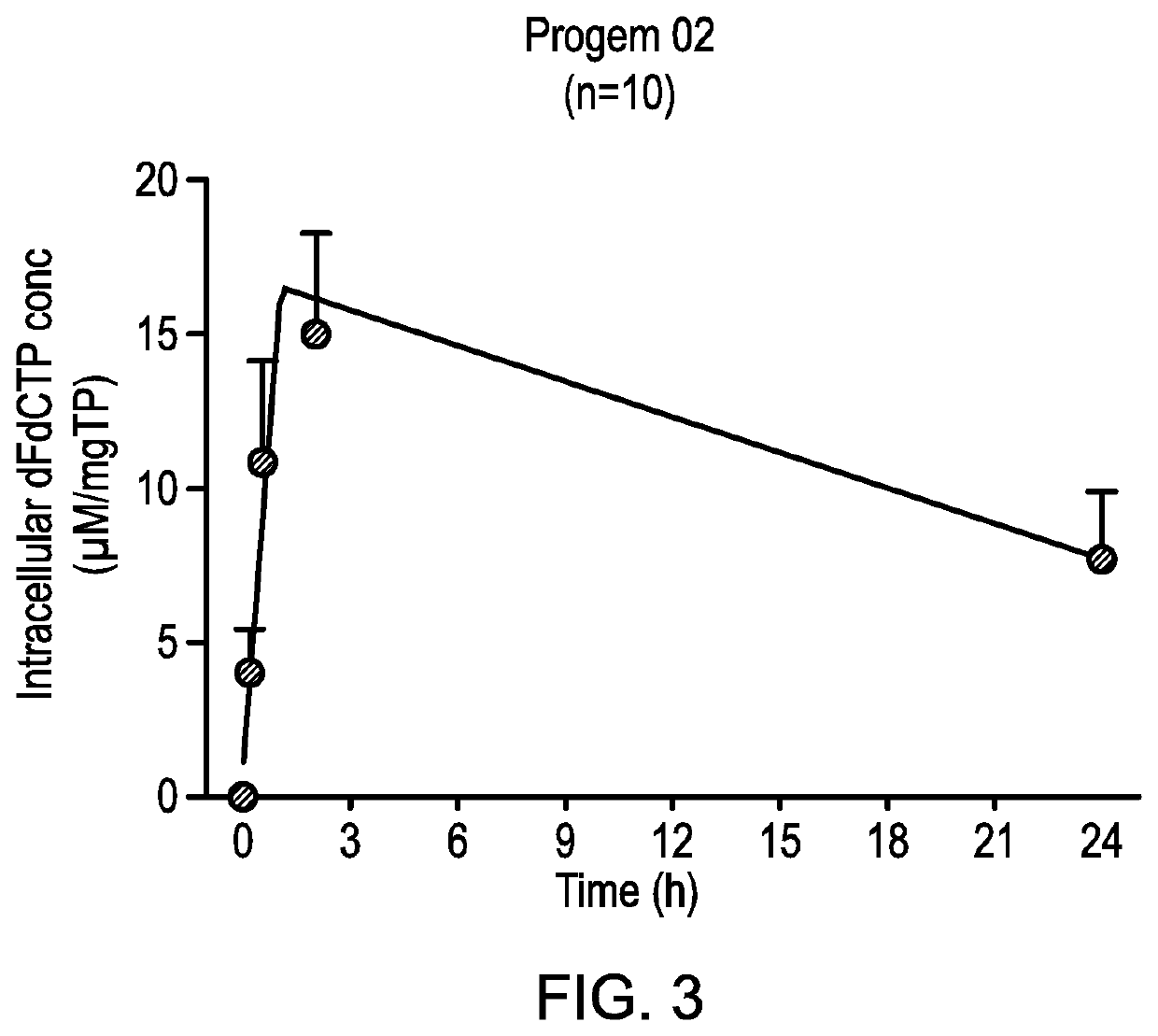
Disclosed is a combination of gemcitabine-[phenyl-benzoxy-L-alaninyl)]-phosphate (chemical name: 2′-Deoxy-2′,2′-difluoro-D-cytidine-5′-O-[phenyl (benzoxy-L-alaninyl)]phosphate) (NUC-1031) and a platinum-based anticancer agent selected from carboplatin, dicycloplatin, oxaliplatin, satraplatin and nedaplatin. The combinations are useful in the treatment of cancer and particularly ovarian cancer.
Owner:NUCANA PLC
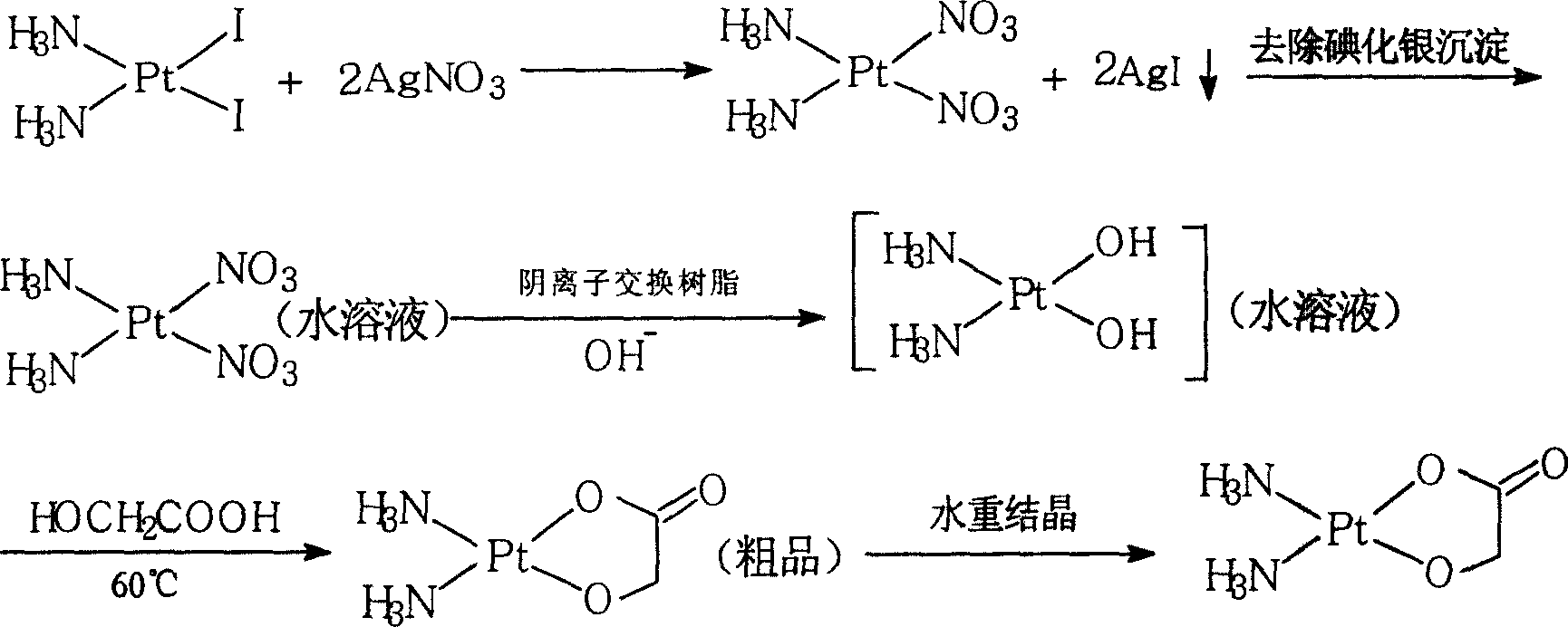
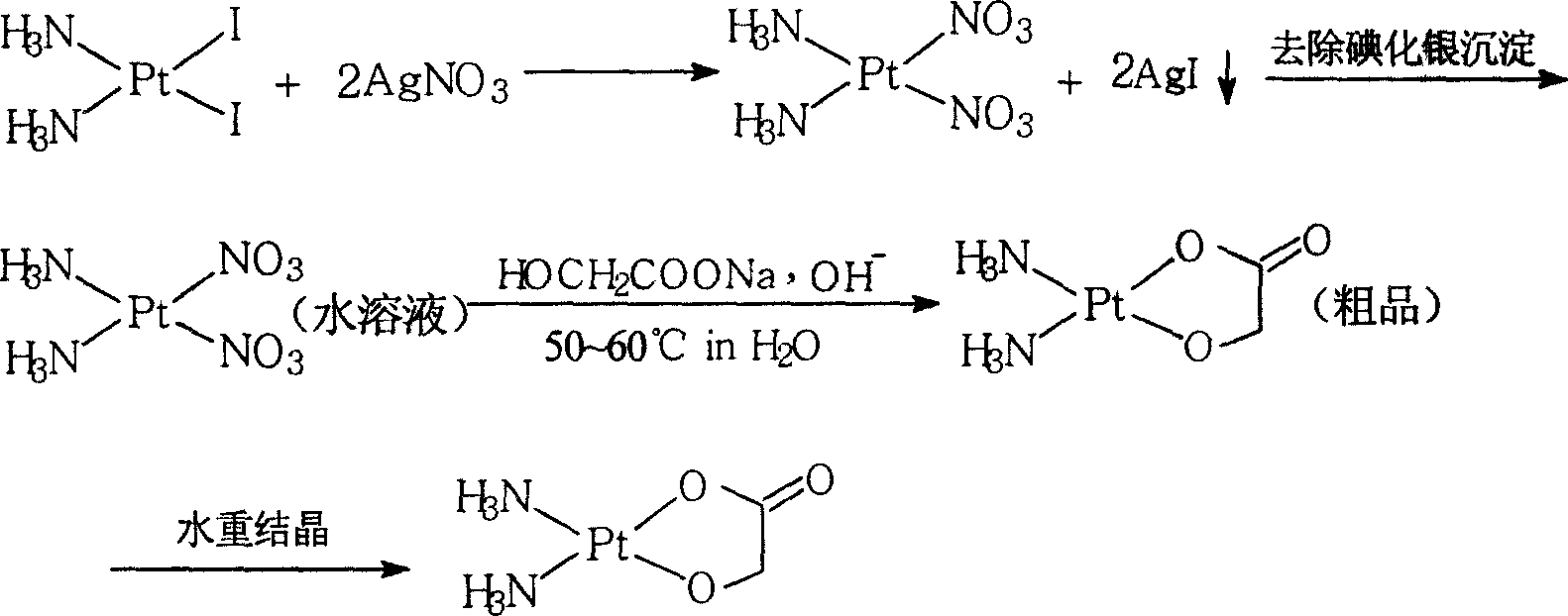
Method of preparing nedaplatin with ultra-low content of silver
ActiveCN101012244ALow silver contentQuality improvementGroup 8/9/10/18 element organic compoundsPlatinumCis-platinum
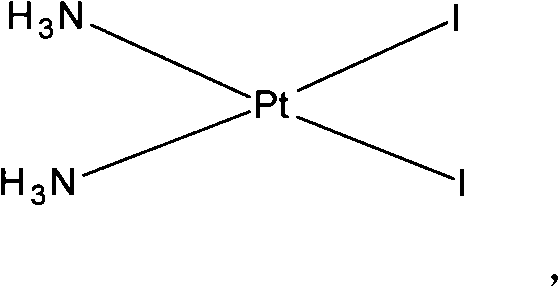
The invention discloses a making method of naiad platinum with low-silver content, which comprises the following steps: reacting cis-platinum diiododiamide and silver nitrate with molar rate at 1: 2 for 3-8 h under 10-30 deg.c; filtering; adding halogenate MX [M is Na, K, Ca, Ba, NH4 or M is (CnH2n+1) 4N (n=1-4), X=Cl, Br, I] in the filtrate to remove trace silver; reacting for 0.5-1h under 10-30 deg.c; filtering; adding sodium glycolate in the solution; adjusting pH value to neutral to react for 3-10 h under 50-60 deg.c; decompressing; condensing; filtering; drying; obtaining the product; setting the molar rate of cis-platinum diiododiamide and sodium glycolate at 1: 1 and the molar rate of halogenate MX and silver nitrate at 0.1-0.01:1.
Owner:NANJING HAIRUN PHARM CO LTD
Preparation of nedaplatin freeze-dried powder injection
ActiveCN101015539AImprove stabilityLow content of related substancesPowder deliveryLyophilised deliveryFreeze-dryingBottle
This invention relates to a preparation method of freeze-dried injection of nedaplatin.nedaplatin freeze-dried injection prepared with the inventive method can be used as therapeutic drug of cancer. The invention improves the stability of nedaplatin during lyophilization and shortens the period of lyophilization through adding ethanol during preparation process. the preparation method comprises adding water for injection 80% of nedaplatin into nedaplatin, stirring for dissolving, adding dextran, stirring for dissolving, determing the content of intermediate, adding ethanol 1-10% of the cumulative volume of the solution, adding water for injection to full dose, filtrating with 0.22 mu m millipore filter under aseptic condition, encapsulating in sirin bottle, plugging, freeze drying, rolling the opening, testing, and packaging. The optimum amount of ethanol is 1-5% of cumulative volume of the solution.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
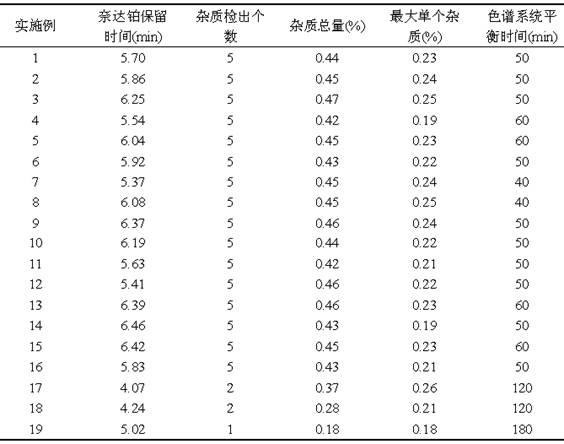
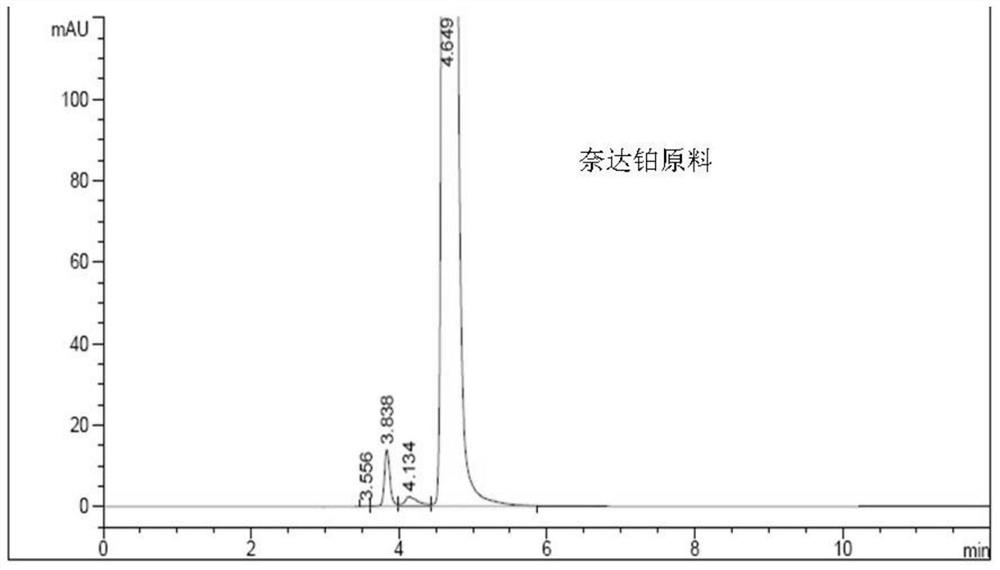
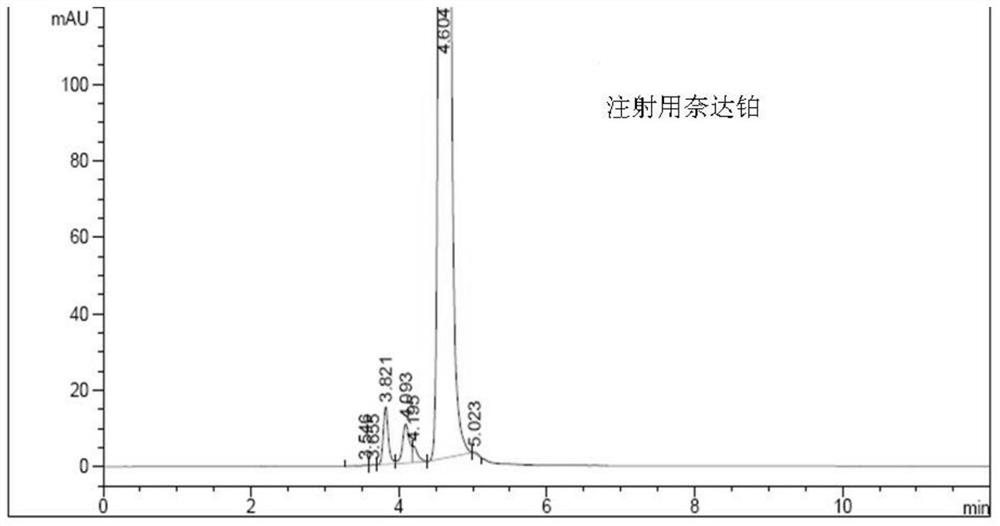
Method for detecting impurities in freeze-dried powder injection of nedaplatin
The invention relates to a method for detecting impurities in a freeze-dried powder injection of nedaplatin. In particular, the method comprises the following steps of: 1. adoption of a high performance liquid chromatography, wherein the chromatographic conditions are as follows: octyl chemically bonded silica is used as a bulking agent, a mobile phase is a mixture of water and acetonitrile or methanol, into which diethylamine or triethylamine is added, the detection wavelength is 205 to 222nm, the column temperature is between 20 and 45 DEG C, and the flow rate of the mobile phase is 0.5 to 2.0ml / min; 2. sample solution preparation, wherein the solution of the freeze-dried powder injection sample of nedaplatin, of which the nedaplatin content is 0.5 to 2mg / ml, is prepared by using a mixed solvent of water and methanol; and 3. measurement, wherein 5 to 20 micro liters of the sample solution is poured into a high performance liquid chromatograph, and chromatograms are recorded for analysis. By the method, the interference of skeleton excipient dextran with impurity detection is eliminated, and the impurities in nedaplatin raw materials can be easily, quickly and stably detected while the cost is lowered.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Process for reifining Nedaplatin
ActiveCN101070331AStable traitsHigh purityGroup 8/9/10/18 element organic compoundsFiltrationCrystallization temperature
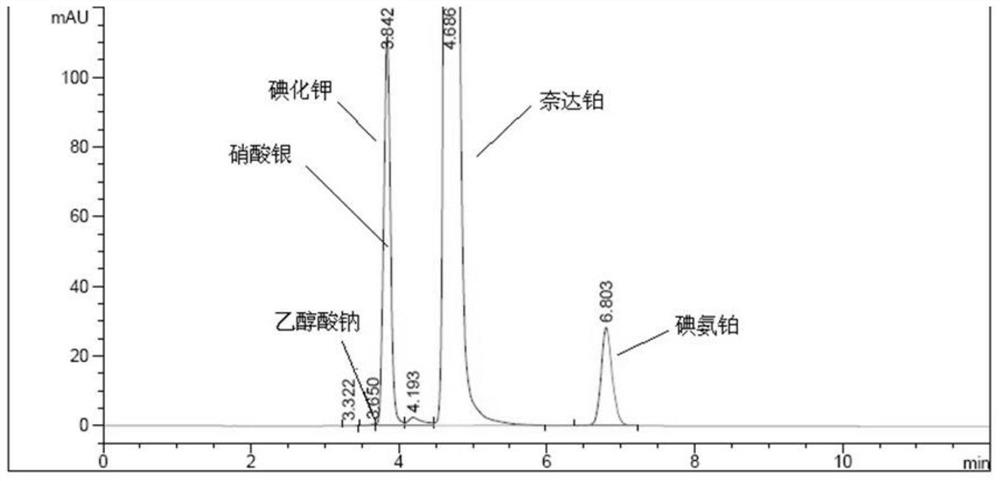
This invention relates purifing method a refined Nedaplatin. By weight proportion of 1:15 to 80, dissolve Nedaplatin crude product in water of 25 to 70 deg, after completeness dissolving add KI, by filtration, remove excessive Ag+, add 1 to 5 times volume C1 to C2 alkyl alcoho filtrate, through crystallization, cooling, filtration, dryness, gain Nedaplatin refiend product; the cooling crystallization temperature is -25 to 25deg, time for 5 to 30h,drying temperature is 40 to 70deg, and drying time is 4 to 15h. The Ag content of this refined Nedaplatin is very low.
Owner:SIMCERE ZAIMING PHARM CO LTD
Novel method for synthesizing antineoplastic medicine nedaplatin
ActiveCN101302236AHigh yieldShort reaction processGroup 8/9/10/18 element organic compoundsAntineoplastic agentsNedaplatinReagent
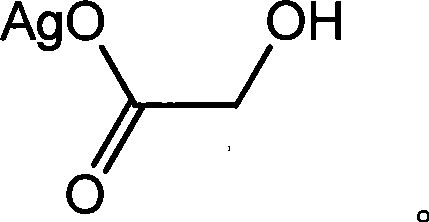
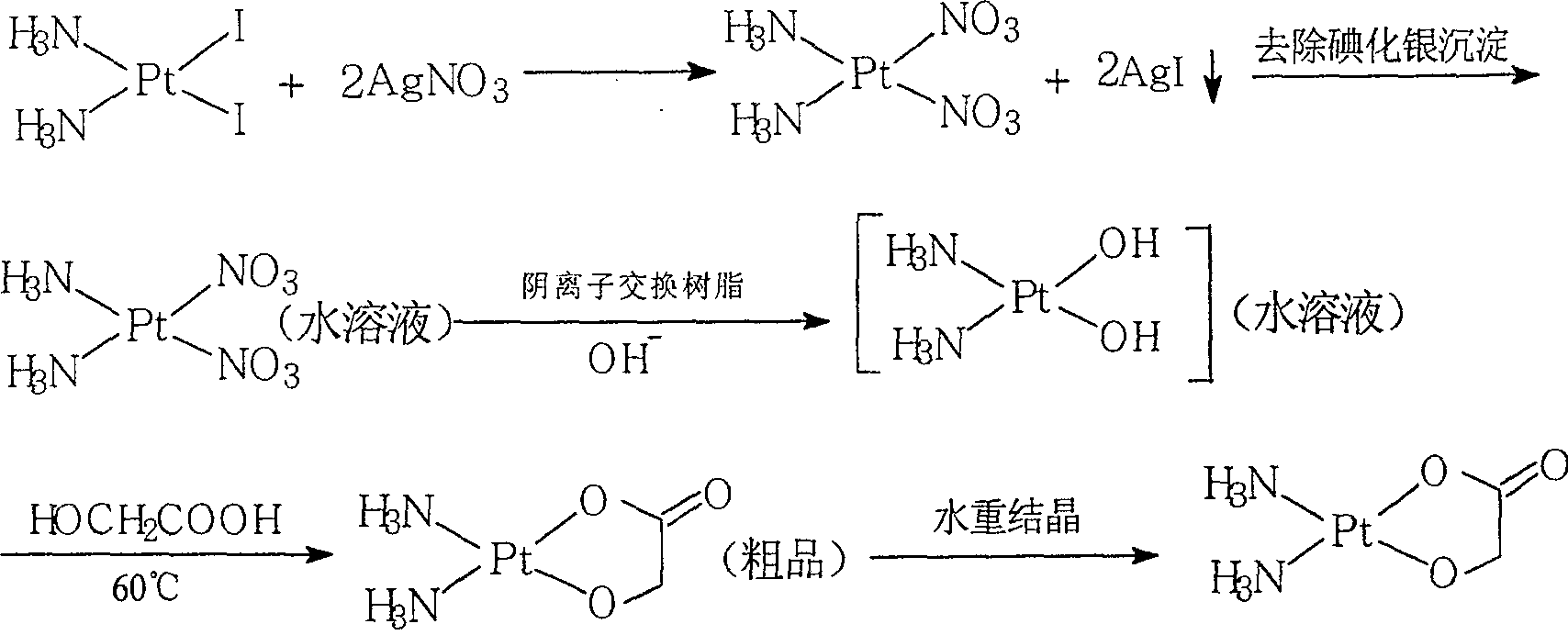
The invention discloses a method for making antineoplastic medicine nedaplatin C2H8N2O3Pt. The method comprises the following technological processes that: cis-diiododiaminoplatinum (II) is taken as reactant; an amount of water is added in the reactant and is evenly stirred; then, argentum glycolate C2H3O3Ag and AgNO3 are slowly added in the solution with the molar ratio Pt (NH3) 2I2: C2H3O3Ag:AgNO3 equal to 1:1:1, and are reacted at a temperature of between 50 and 60 DEG C for 2 to 3 hours; after solid is filtered out, the filtrate is heated to 40 DEG C, and the pH value of the filtrate is adjusted to 7 by NaOH; the obtained solution is heated to 50 to 60 DEG C so as to carry out reaction for 4 hours; finally, the reaction liquid undergoes decompression condensation to obtain nedaplatin solid. The method has short process flow, high yield (above 56 percent) and high product purity.
Owner:KUNMING GUIYAN PHARMA
Novel method for synthesizing antineoplastic medicament nedaplatin
InactiveCN101475599AHigh yieldShort reaction processGroup 8/9/10/18 element organic compoundsAntineoplastic agentsNedaplatinReagent
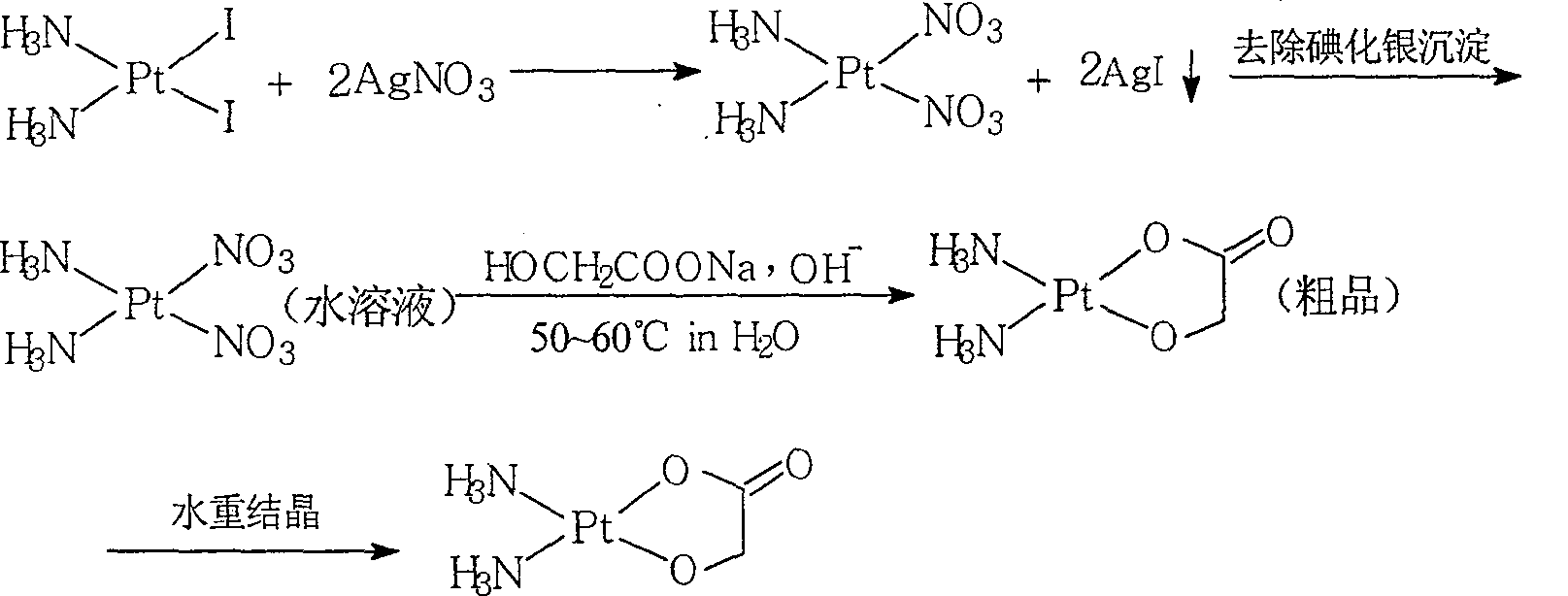
The invention relates to a method for preparing an antitumor drug of nedaplatin C2H8N2O3Pt. The method comprises that: a reactant of cis-diiododiammineplatinum(II) is added with a proper amount of water, uniformly stirred, added with silver glycollate C2H3O3Ag slowly according to a molar ratio of Pt(NH3)2I2 to C2H3O3Ag of 1:2 for reaction at 50-60 DEG C for 2 to 3 hours; and after solid is removed, the pH value of the solution is adjusted by using NaOH to maintain about 7, the solution is heated to 50 to 60 DEG C for reaction for 6 hours totally, reaction liquid is concentrated under reduced pressure and thus solid nedaplatin is obtained. The method for preparing the nedaplatin has the advantages of short technical process, high yield up to more than 60 percent and good product purity.
Owner:KUNMING GUIYAN PHARMA
Preparation method of nedaplatin with extremely low silver content
InactiveCN102417522AGood process reproducibilityEase of industrial productionPhotography auxillary processesGroup 8/9/10/18 element organic compoundsSilver ionPt element
The invention relates to a preparation method of nedaplatin with an extremely low silver content. The preparation method comprises the following steps: allowing cis-diiododiammine platinum to react with silver nitrate at 10-90 DEG C for 0.5-8 hours, filtering, adding the filtrate in an electrolytic cell composed of an anode (a graphite rod) and a cathode (stainless steel), adjusting the electrolytic voltage and current to allow residual silver ions in the solution to be reduced and precipitated, filtering, adding sodium glycollate into the filtrate, adjusting the pH to become neutral, reacting at 40-70 DEG C for 3-10 hours, performing reduced-pressure concentration and crystallization, filtering, washing the filter cake, drying to obtain the nedaplatin with an extremely low silver content. The reaction molar ratio of cis-diiododiammine platinum and silver nitrate is 1:2, and the reaction molar ratio of cis-diiododiammine platinum and sodium glycollate is 1:1. The electrolytic voltage is 0.6-3.0 V, and the current is 5-200 mA. The nedaplatin prepared by the method of the invention has a silver content of less than 1.0 ppm; the content of nedaplatin in the product is more than 99.00%, and the content of related substances is less than 1.00%; the product quality is stable.
Owner:NANJING UNIV OF TECH
Method for detecting impurities in nedaplatin
The invention relates to a method for detecting impurities in nedaplatin. In particular, the method comprises the following steps of: 1. adoption of a high performance liquid chromatography, wherein the chromatographic conditions are as follows: octyl chemically bonded silica is used as a bulking agent, a mobile phase is a mixture of water and acetonitrile or methanol, into which diethylamine or triethylamine is added, the detection wavelength is 205 to 222nm, the column temperature is between 20 and 45 DEG C, and the flow rate of the mobile phase is 0.5 to 2.0ml / min; 2. sample solution preparation, wherein a solution of the sample, of which the nedaplatin content is 0.5 to 2mg / ml, is prepared by using the mobile phase, and the obtained solution is stored in a dark place at the temperature of between 2 and 8 DEG C; and 3. measurement, wherein 5 to 20 micro liters of the sample solution is poured into a high performance liquid chromatograph, and chromatograms are recorded for analysis. By the method, the impurities in nedaplatin raw materials can be easily, quickly and stably detected while the cost is lowered.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
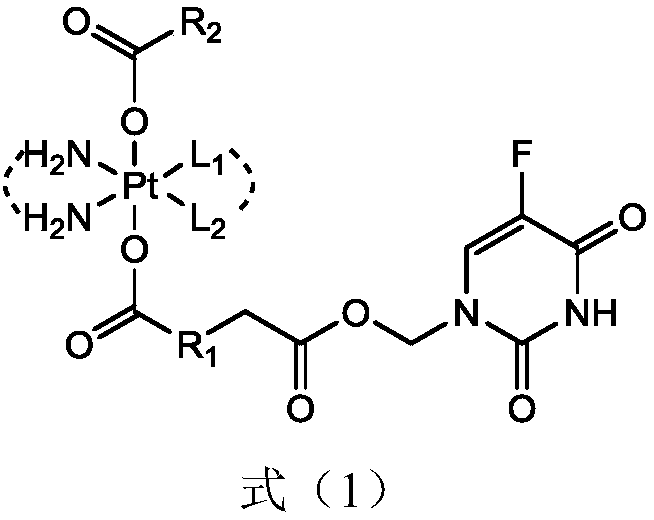
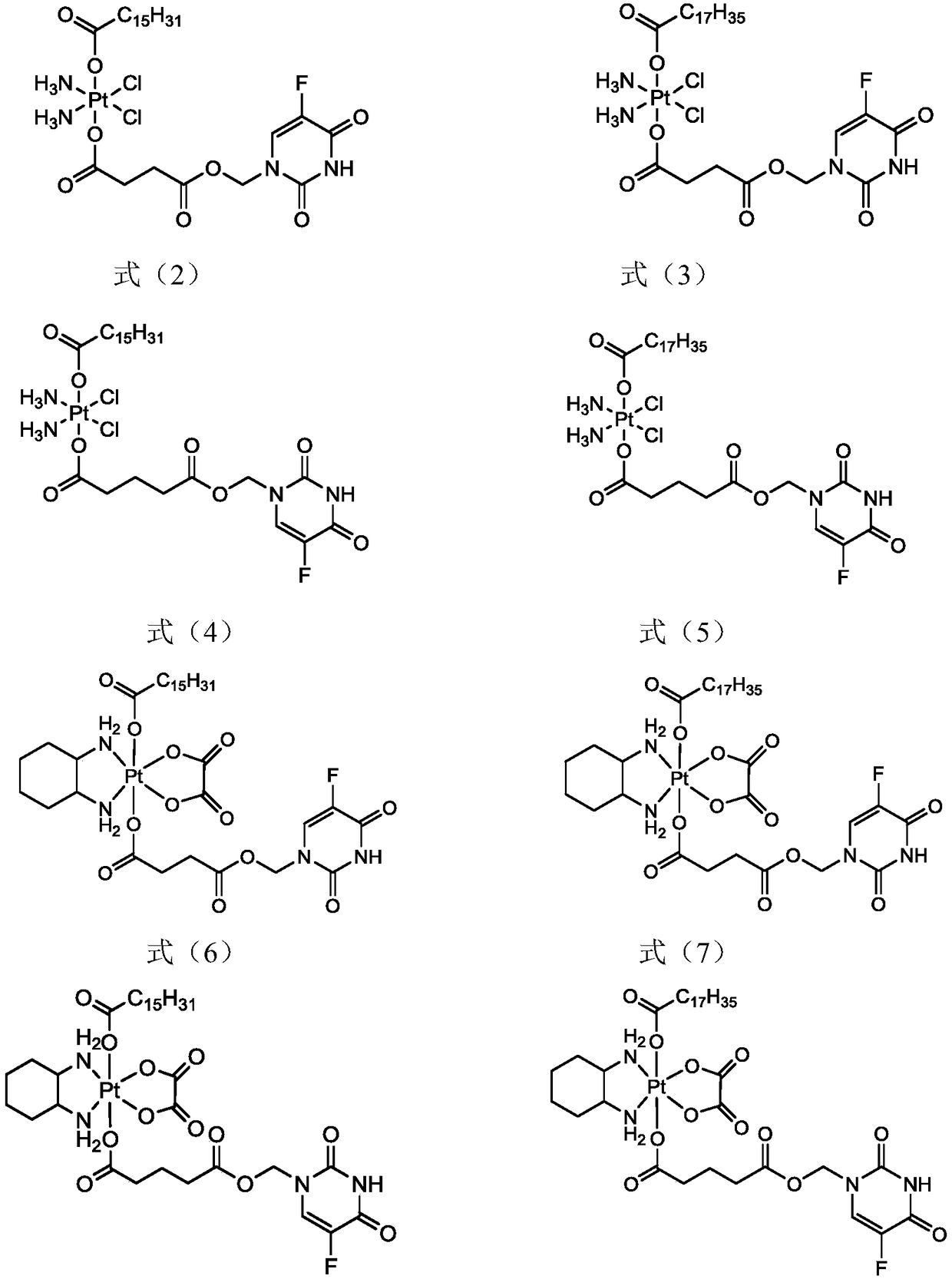
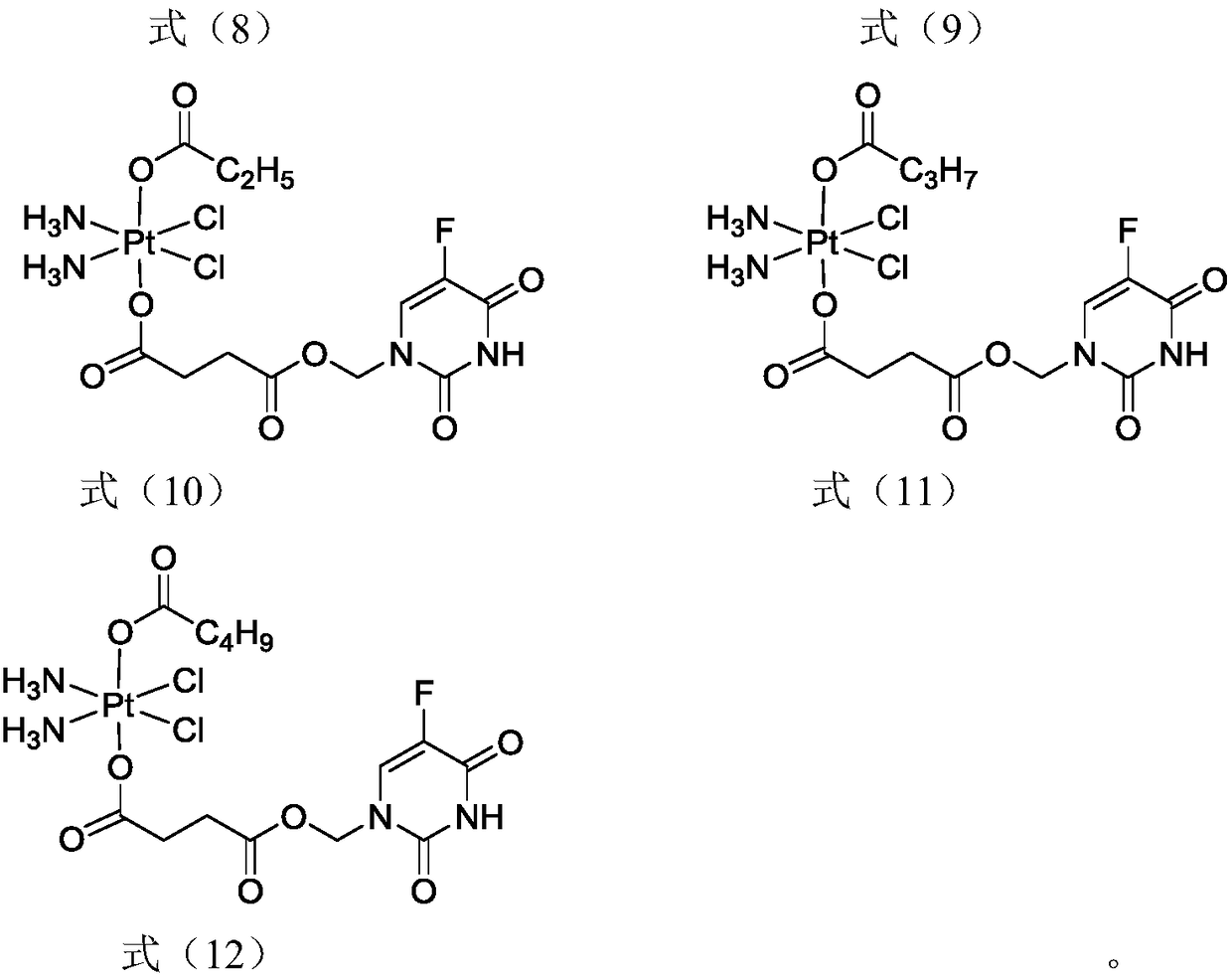
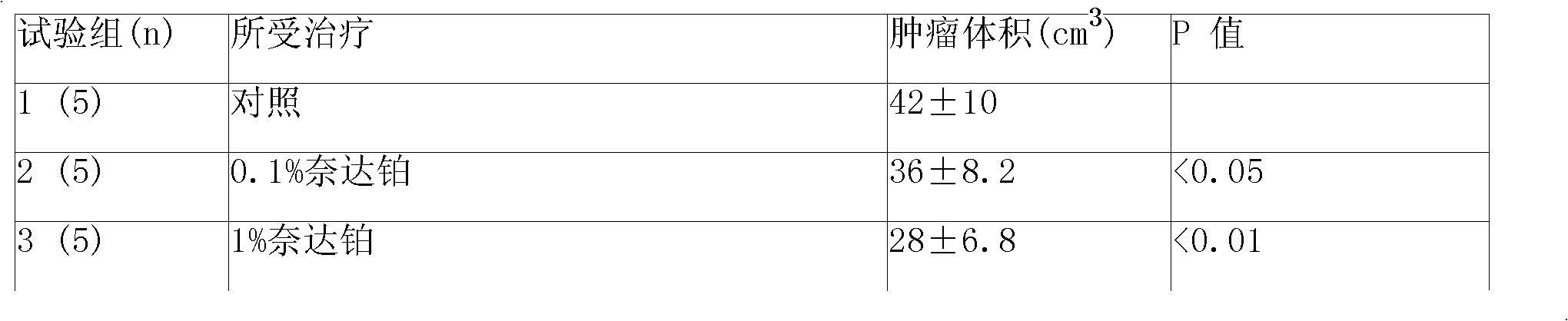
5-fluorouracil-platinum (IV) complex, intermediate, as well as preparation method and application thereof
ActiveCN109438522AGood choiceGood anti-proliferation abilityPlatinum organic compoundsAntineoplastic agentsPlatinumSide effect
The invention provides a 5-fluorouracil-platinum (IV) complex, an intermediate, and a preparation method. The 5-fluorouracil-platinum (IV) complex is represented by a formula (1) shown in the description, wherein the part shown in the description is selected from cis-platinum, oxaliplatin, carboplatin, eptaplatin, nedaplatin or miriplatin, and is preferably the cis-platinum or the oxaliplatin; R1is -CnH2n-, n is an integer and is larger than or equal to 1, and preferably, -CnH2n- is a straight chain group; and R2 is -CmH2m+1, m is an integer and is larger than or equal to 1, and preferably, -CmH2m+1 is a straight chain group. According to the 5-fluorouracil-platinum (IV) complex provided by the invention, the toxic and side effects of the cis-platinum, the oxaliplatin and 5-fluorouracil can be reduced, and meanwhile, the 5-fluorouracil-platinum (IV) complex has a synergistic sensitization effect when being used in medicines for treating cancers and antitumor drugs.
Owner:TIANJIN MEDICAL UNIV
Temperature controlled sustained-release injection containing platinum compound
InactiveCN101273964AHeavy metal active ingredientsPharmaceutical delivery mechanismPolyesterPicoplatin
The invention relates to a temperature-controlled sustained-release injection containing a platinum compound and a preparation method thereof, the temperature-controlled sustained-release injection comprises effective anti-cancer amount of the platinum compound, an amphiphilic block copolymer and a certain amount of drug release regulator, wherein, the amphiphilic block copolymer is composed of polyethylene glycol and polyester, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained platinum compound to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the anti-cancer sustained-release gel injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus being used for the treatment of the tumors in different stages. The platinum compound is selected from cisplatin, carboplatin, cycloplatin, eptaplatin, denaplatin, zeniplatin, enloplatin, sulfatodiaminocyclohexane platin, cis-spiroplatin, dexormaplatin, iproplatin, lobaplatin, miboplatin, picoplatin, nedaplatin, ormaplatin, oxaliplatin and so on.
Owner:SHANDONG LANJIN PHARMA +1
Anticancer sustained-release gel injection containing platinum compound
InactiveCN101283978APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPicoplatinSolvent
An anticancer sustained-release gel injection sustained-release microspheres containing platinum-based compound, amphiphilic block copolymer, solvent and release regulator, the mixture of amphiphilic block copolymer and solvent has a temperature sensitive gelatinization characteristic, and can automatically become non-flowing degradable water insoluble gel, which can locally and slowly release drug at tumor foci for several weeks to several months, after injection into body. The adjuvant in the sustained-release microspheres is poly(lactic acid)-glycollic acid copolymer; and the amphiphilic block copolymer is PLGA-PEG-PLGA copolymer. The preparation can be injected into artery, inside a tumor or around a tumor with remarkably reduced systemic toxicity of drug, and can be used for solid tumors at different stages. The platinum-based compound is selected from nedaplatin, carboplatin, cisplatin, enloplatin, ormaplatin, sulfato-1,2-diaminocyclohezane-platinum (SHP), picoplatin, cyclopentylamine platium, zeniplatin, spiroplatin, lobaplatin, iproplatin, oxaliplatin, cycloplatin, and dexormaplatin. The preparation can be used with radioactive particles and can enhance chemotherapy effect.
Owner:济南基福医药科技有限公司
Method for preparing hydroxy carboxylic acid platinum complexes
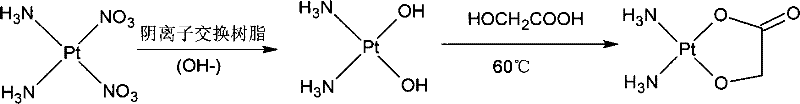
InactiveCN101787052AThe synthesis method is simpleShort processGroup 8/9/10/18 element organic compoundsFiltrationCarboxylic acid
The invention relates to a method for preparing hydroxy carboxylic acid platinum complexes, in particular to a method for preparing platinum anticancer drugs of nedaplatin and lobaplatin. The preparation method includes that: compounds (I) and (II) and silver oxide in molar ratio of 1:1: 1, and certain quantity of water are added to a reactor and stirred to conduct light-shielding reaction for 1-20 hours at the temperature of 0-80 DEG C. The filtrate after filtration is concentrated to be dry, is cooled and rinsed by water, filtrated and rinsed by ethanol and is finally pumped out to obtain the product (III) after vacuum drying for 2-3 hours at the temperature of 40-45 DEG C. The invention obtains the product water solution through one-step reaction, concentrates the water solution to obtain the final product, has no need to adjust the pH value in the synthesis process, avoids the Na<+>, NO<3->, SO4<2->, Ba<2+> interference reaction, and accordingly realizes high productivity and good purity.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Anti-cancer composition loading both platinum compound and synergist
InactiveCN101011351APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinMitozolomide
Disclosed is a slow release injection agent of anticancer composition containing platinum-group compounds and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the platinum-group compounds are selected from cisplatin, Carboplatin, Nedaplatin or Oxaliplatin, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or Temozolomide, and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Combination therapy for cancer
ActiveUS10660912B2High concentrationReduce riskPharmaceutical delivery mechanismUnknown materialsSatraplatinAnticarcinogen
Disclosed is a combination of gemcitabine-[phenyl-benzoxy-L-alaninyl)]-phosphate (chemical name: 2′-Deoxy-2′,2′-difluoro-D-cytidine-5′-O-[phenyl (benzoxy-L-alaninyl)]phosphate) (NUC-1031) and a platinum-based anticancer agent selected from carboplatin, dicycloplatin, oxaliplatin, satraplatin and nedaplatin. The combinations are useful in the treatment of cancer and particularly ovarian cancer.
Owner:NUCANA PLC
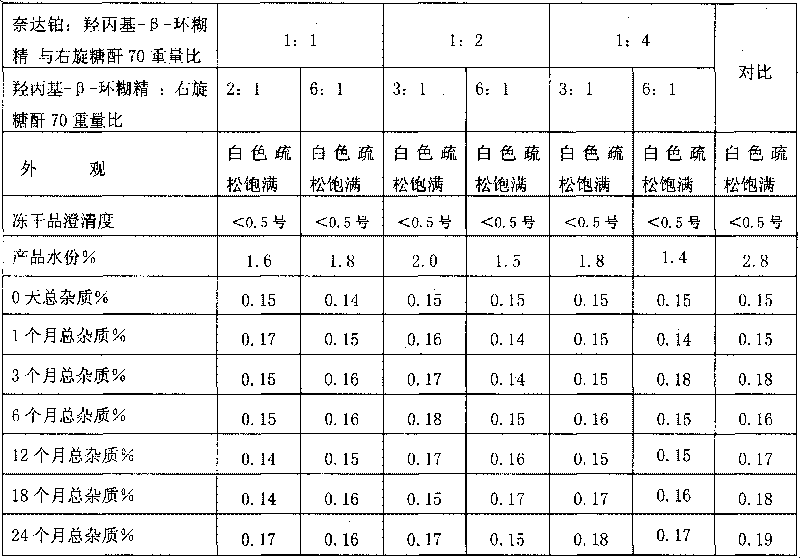
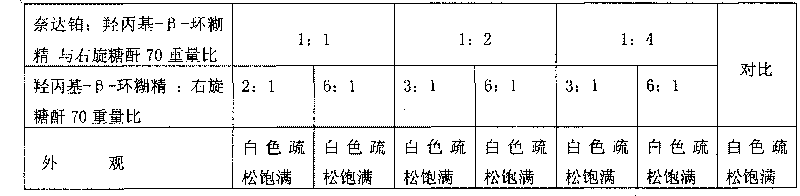
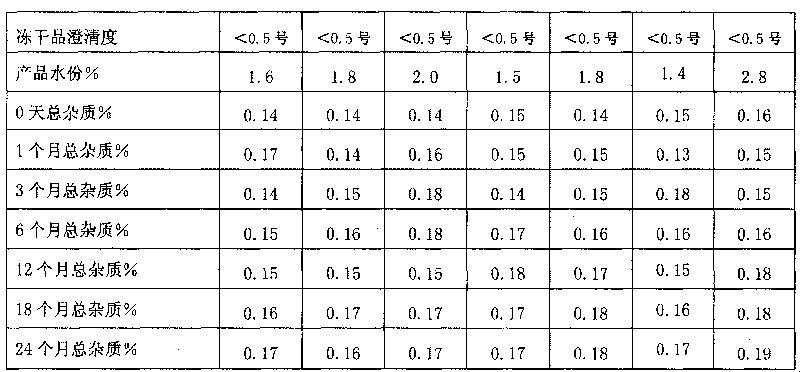
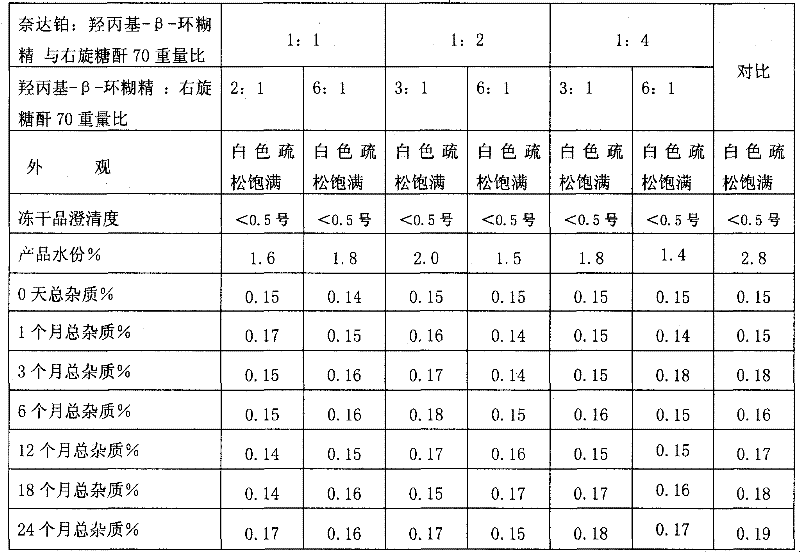
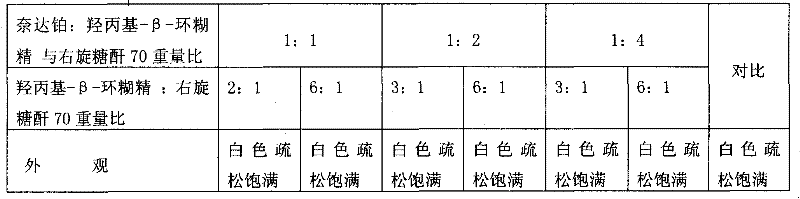
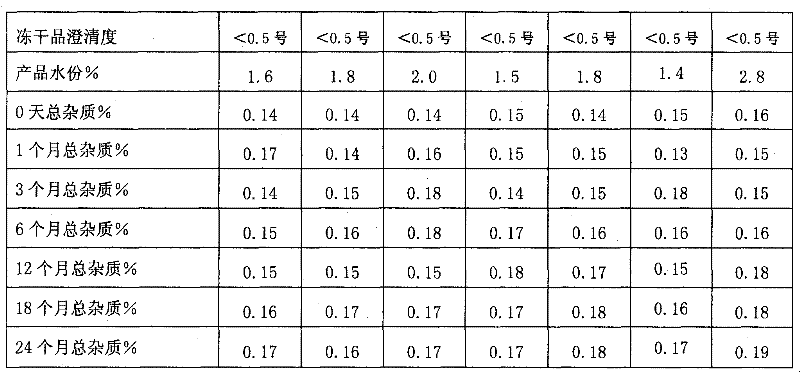
Nedaplatin medical composition and application
ActiveCN101744800AMacromolecular non-active ingredientsAntineoplastic agentsFreeze-dryingMedical product
The present invention relates to a nedaplatin medical composition, particularly to a nedaplatin freeze-dried powder preparation made by mixing nedaplatin with cyclodextrin or cyclodextrin with dextranum. The present invention relates to a method for preparing the composition and an application of the medical composition to the cancer treating prospect as a non-gastrointestinal administration medical product.
Owner:QILU PHARMA CO LTD
Sustained release anticancer agent carrying angiogenesis inhibitor and cytotoxic drug
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents being the combination of anti-angiogenesis agent selected from Marimastat, SU5416, SU6688, Fumagillin or TNP-470, with cytotoxic drugs selected from Eptaplatin, Nedaplatin, Melphalan, 4-hydroperoxycyclophosphamide, hydroxyl radical Paclitaxel, 10-desacetyltaxuyunnanin, 7-epi-taxol, Vinorelbine, Tamoxifen, Amethopterin, Adriamycin, pidorubicin, actinomycin D, Tallimustine, Atrimustine, Semustine or Ranimustine, the slow release auxiliary materials are selected from di-aliphatic acid and sebacylic acid copolymer, ethylene-vinylacetate copolymer or lactic acid polymer, the viscosity of the suspension adjuvant is 100-3000cp and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
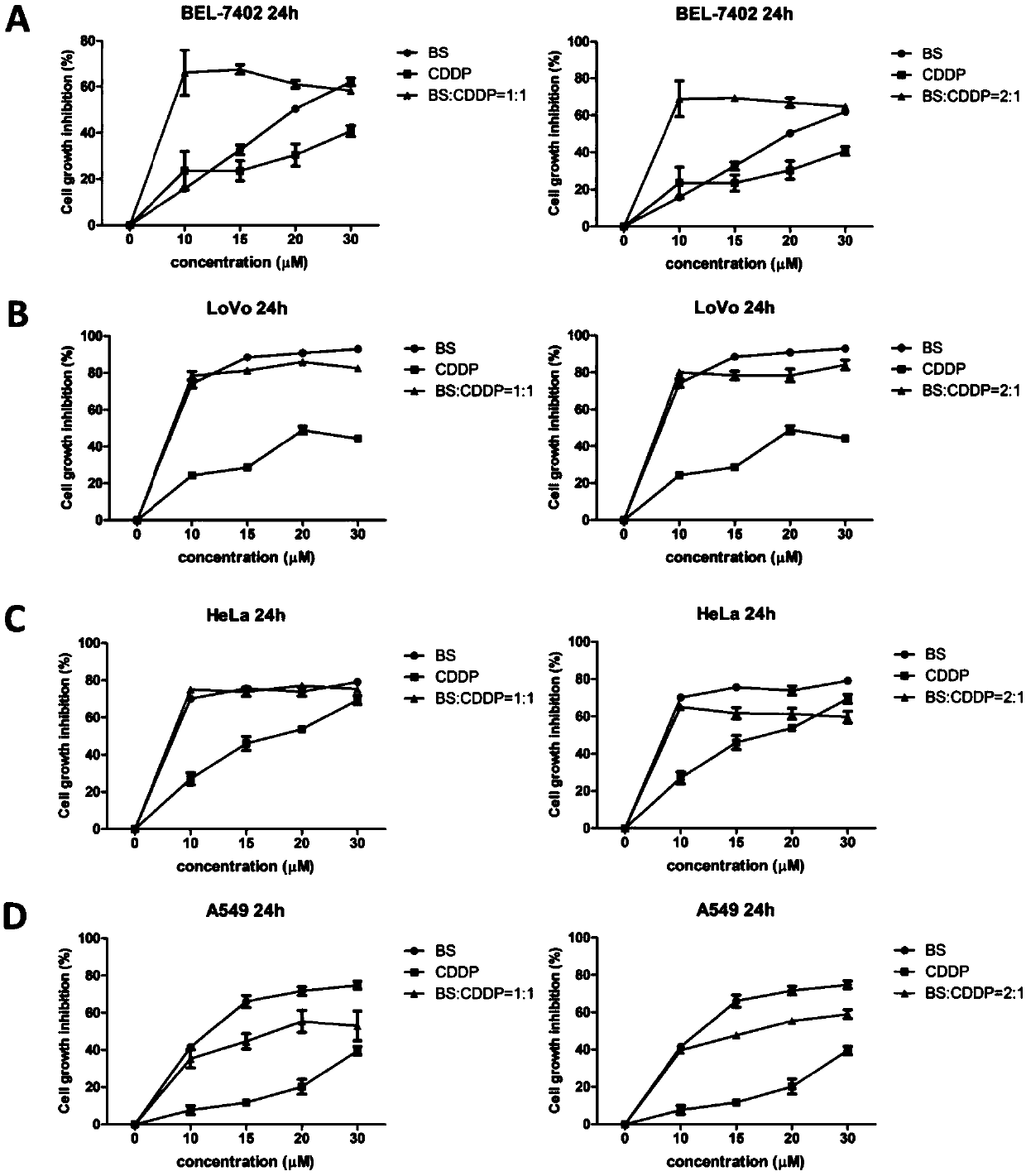
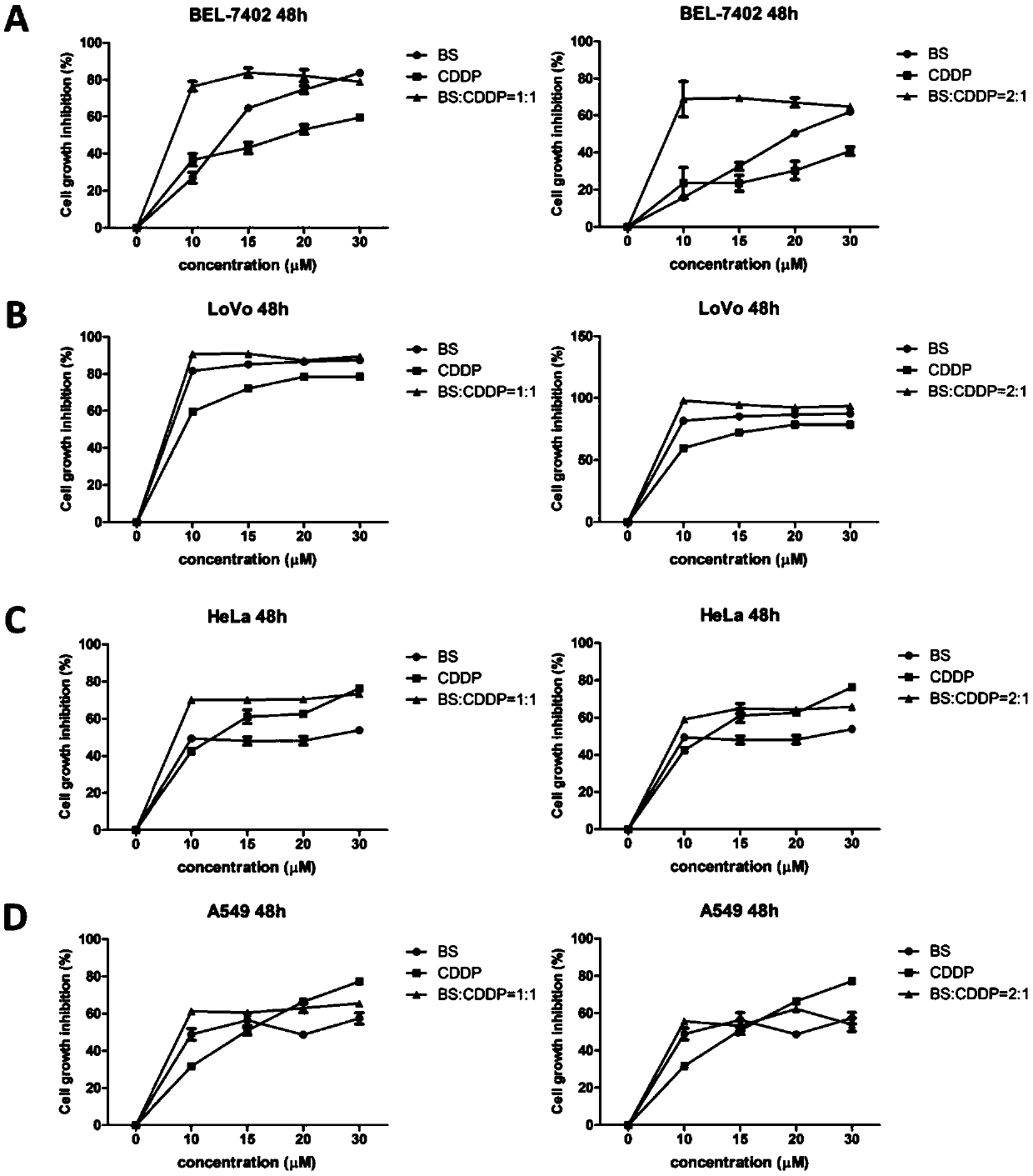
Application of benzisoselenazole derivative combined with platinum drug to preparation of tumor treatment drugs and postoperative tumor recurrence drugs
PendingCN110856718APrevent proliferationInhibition of proliferation rateHeavy metal active ingredientsAntineoplastic agentsCarboplatinTumor therapy
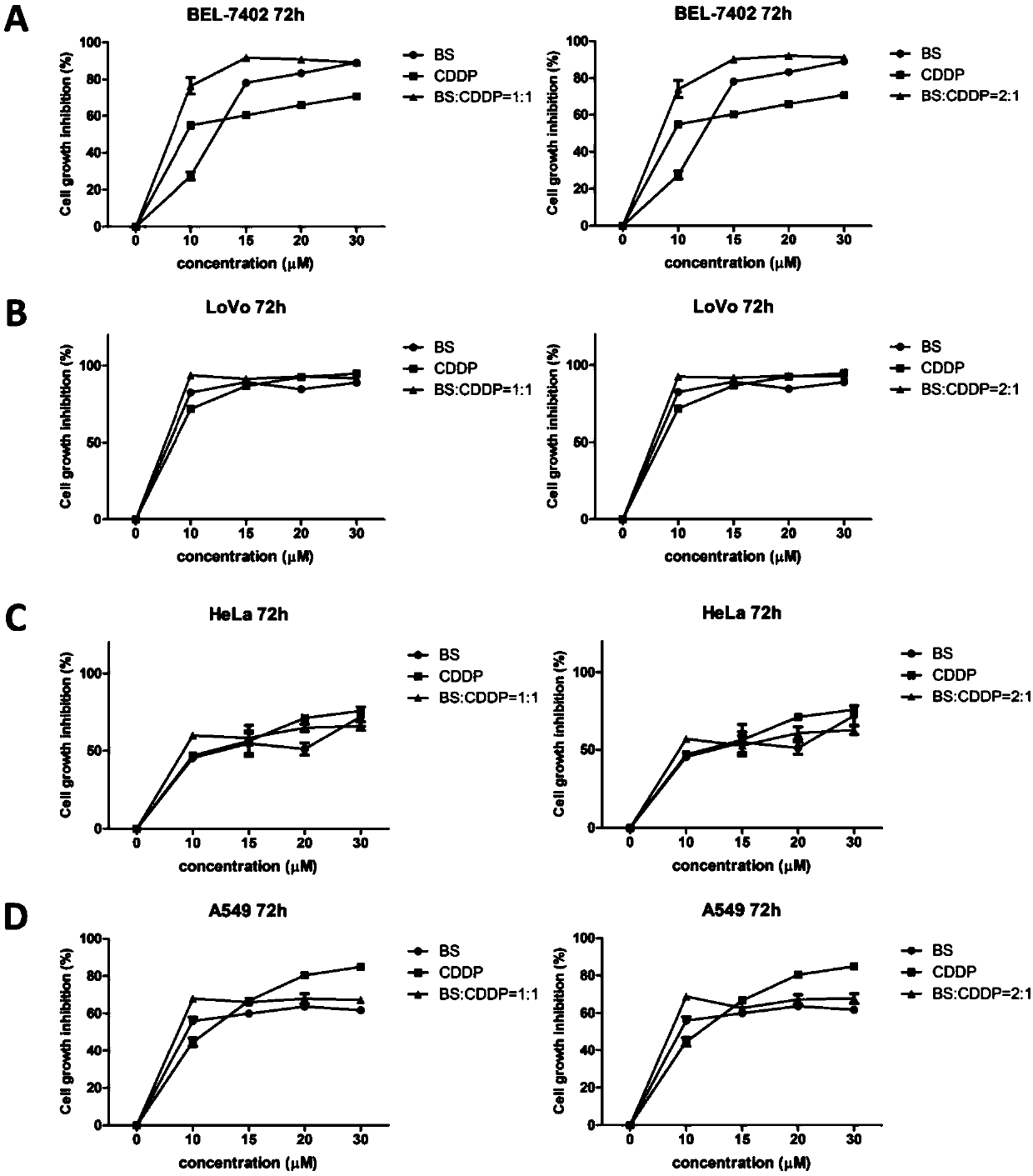
The invention belongs to the technical field of tumor treatment, and discloses application of a benzisoselenazole derivative combined with a platinum drug to the preparation of tumor treatment drugs and postoperative tumor recurrence drugs. The benziselenazole derivative has a structure as shown in the formula A, the platinum-based anticancer drug is selected from at least one of cisplatin, carboplatin, oxaliplatin, nedaplatin, and the like. The molar ratio of the benziselenazole derivative to the platinum-based anticancer drug is (1-99): (1-99). With the combination of the benzisoselenazole derivative and the platinum drug, the dosage of platinum-based anticancer drug with high toxicity can be effectively reduced and the safety of anticancer drugs can be improved; and Bel-7402 cell apoptosis can be induced by reducing Bcl-2 / Bax protein expression ratio, and the expression of TrxR in tumor tissues can be synergistically inhibited, the proliferation rate of tumor cells after operation can be significantly reduced, and the growth inhibition rate of tumor cells after operation can be improved.
Owner:SHANGHAI YUANXI MEDICINE CORP
An anticancer sustained release injection carrying tumor drug resistance reversal agent and cytotoxic drug simultaneously
Disclosed is an anticancer slow release injection carrying both tumor drug resistance reversal agents and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active ingredient is tumor drug resistance reversal agent selected from Pemetrexed, Raltitrexed, Nolatrexed, Nelarabine, Tipifarnib, Ronafarnib or Valspodar, and / or cytotoxic drugs selected from Eptaplatin, Nedaplatin, Melphalan, 4-hydroperoxycyclophosphamide, Vinorelbine, Tamoxifen, Tallimustine, Atrimustine, Semustine, Ranimustine, the slow release auxiliary materials are selected from poly(lactic acid), Polifeprosan, di-aliphatic acid and sebacylic acid copolymer, polyglycolic acid and glycolic acid copolymer, ethylene-vinylacetate copolymer and lactic acid polymer.
Owner:JINAN KANGQUAN PHARMA TECH
Method of preparing nedaplatin with ultra-low content of silver
ActiveCN100500681CLow silver contentQuality improvementGroup 8/9/10/18 element organic compoundsPlatinumCis-platinum
The invention discloses a making method of naiad platinum with low-silver content, which comprises the following steps: reacting cis-platinum diiododiamide and silver nitrate with molar rate at 1: 2 for 3-8 h under 10-30 deg.c; filtering; adding halogenate MX [M is Na, K, Ca, Ba, NH4 or M is (CnH2n+1) 4N (n=1-4), X=Cl, Br, I] in the filtrate to remove trace silver; reacting for 0.5-1h under 10-30 deg.c; filtering; adding sodium glycolate in the solution; adjusting pH value to neutral to react for 3-10 h under 50-60 deg.c; decompressing; condensing; filtering; drying; obtaining the product; setting the molar rate of cis-platinum diiododiamide and sodium glycolate at 1: 1 and the molar rate of halogenate MX and silver nitrate at 0.1-0.01:1.
Owner:NANJING HAIRUN PHARM CO LTD
Preparation of nedaplatin freeze-dried powder injection
ActiveCN100463677CImprove stabilityLow content of related substancesPowder deliveryLyophilised deliveryFreeze-dryingBottle
This invention relates to a preparation method of freeze-dried injection of nedaplatin.nedaplatin freeze-dried injection prepared with the inventive method can be used as therapeutic drug of cancer. The invention improves the stability of nedaplatin during lyophilization and shortens the period of lyophilization through adding ethanol during preparation process. the preparation method comprises adding water for injection 80% of nedaplatin into nedaplatin, stirring for dissolving, adding dextran, stirring for dissolving, determing the content of intermediate, adding ethanol 1-10% of the cumulative volume of the solution, adding water for injection to full dose, filtrating with 0.22 mu m millipore filter under aseptic condition, encapsulating in sirin bottle, plugging, freeze drying, rolling the opening, testing, and packaging. The optimum amount of ethanol is 1-5% of cumulative volume of the solution.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Combined medicine capable of simultaneously amplifying immunogenic cell death and enhancing anti-tumor effect
ActiveCN112535735AEffectively induce apoptosisInduce apoptosisOrganic active ingredientsInorganic active ingredientsCarboplatinBULK ACTIVE INGREDIENT
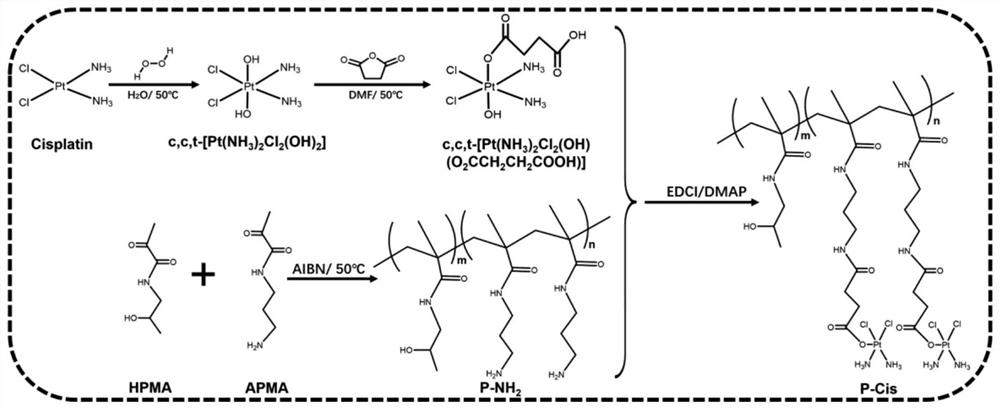
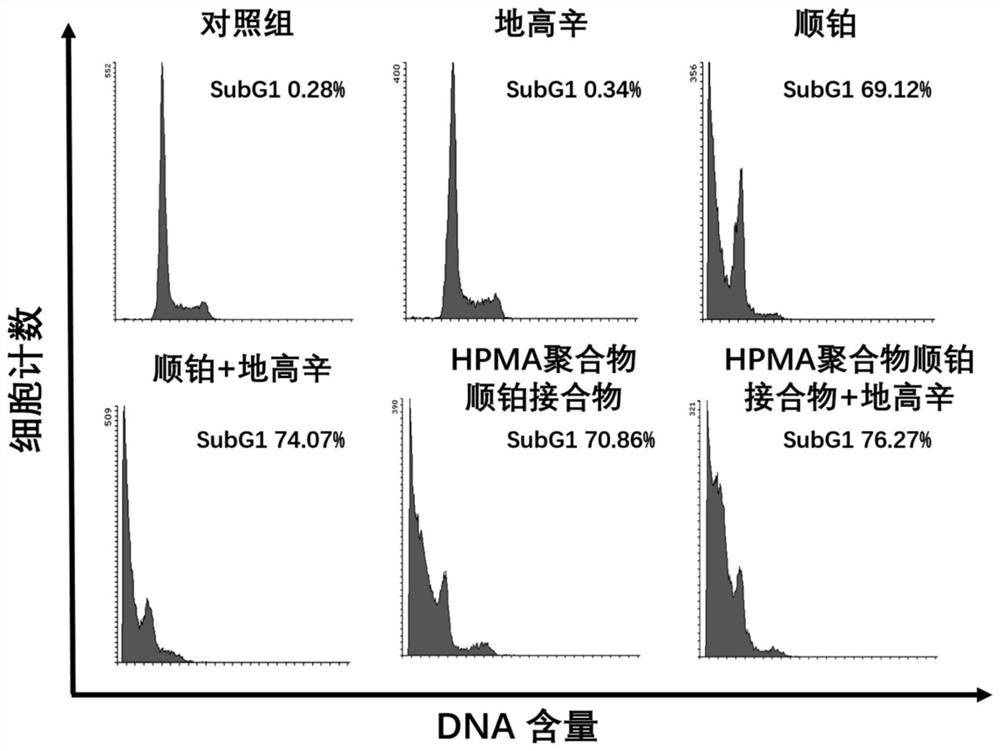
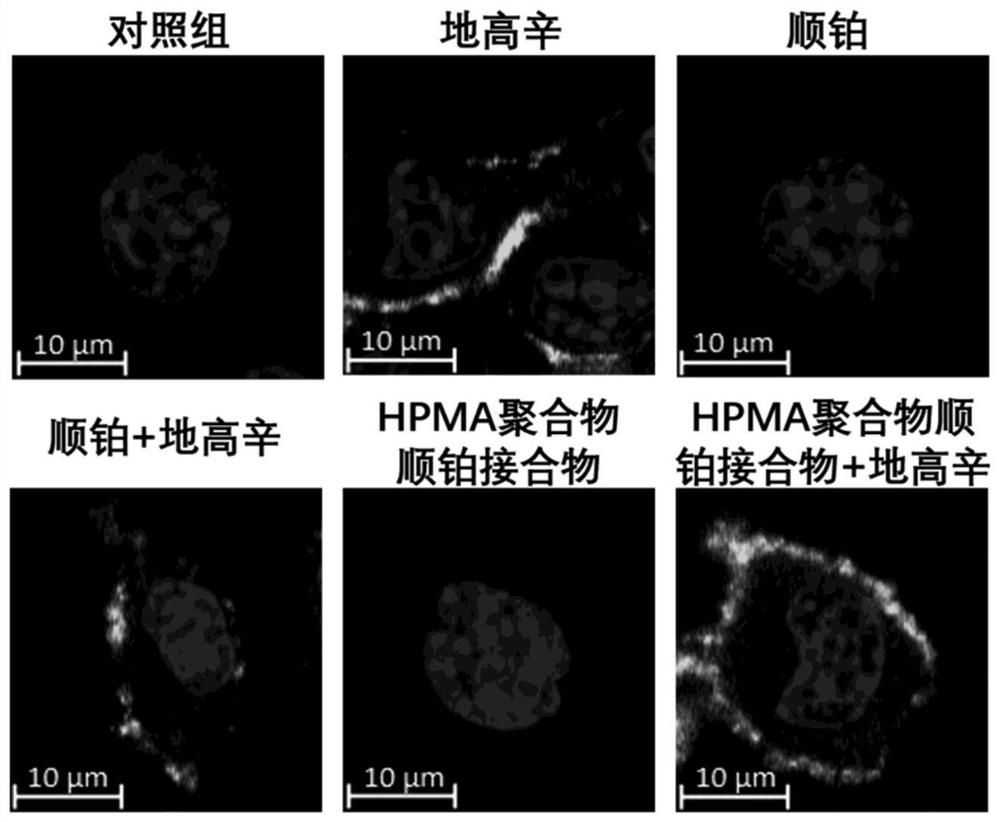
The invention discloses combined medicine capable of simultaneously amplifying immunogenic cell death and enhancing an anti-tumor effect. The anti-tumor combined medicine comprises a cardiac glycosidecompound used as an active ingredient and a cytotoxic N-(2-hydroxypropyl) methacrylamide HPMA polymer medicine conjugate, wherein the cardiac glycoside compound is at least one of clinical medicine including digoxin, digitonin, deacetylated erioflorin, erioflorin C, G erioflorin, oleandrine and erioflorin K. The cytotoxic HPMA polymer medicine conjugate is an HPMA conjugate carrying at least oneof cis-platinum, carboplatin, nedaplatin, oxaliplatin, lerplatinum and heptplatinum. According to the invention, the cardiac glycoside compound and the N-(2-hydroxypropyl) methacrylamide HPMA polymermedicine conjugate are combined for use, so that the capability of causing anti-tumor immune response is improved, and the anti-tumor effect is enhanced.
Owner:SICHUAN UNIV
Refining method of nedaplatin
InactiveCN102352516BGood process reproducibilityEase of industrial productionElectrolysis componentsElectrolytic organic productionPower flowElectrolysis
The invention relates to a refining method of nedaplatin. The refining method comprises the following steps of: dissolving nedaplatin in water at 30-90 DEG C, wherein the weight ratio of nedaplatin to water is 1:(10-100); then, putting the nedaplatin in an electrolytic tank composed of an anode (graphite rod) and a cathode (stainless steel); regulating electrolysis voltage and current to enable the residual silver ions in the solution to be reduced and separated out on the cathode; filtering, and concentrating the filtered solution under the reduced pressure; crystallizing; filtering, and washing the filter cake; and drying to obtain the nedaplatin with very low silver content, wherein the electrolysis voltage is 0.6-3.0V, and the current is 5-200mA. The nedaplatin prepared by the method provided by the invention has the characteristics that the silver content is less than 1.00ppm, the nedaplatin content is greater than 99.00%, the content of related substances is less than 1.00%, and the quality is stable.
Owner:NANJING TECH UNIV
Anticancer traditional Chinese medicine composition
InactiveCN106075182AEffective treatmentImprove protectionHeavy metal active ingredientsAntineoplastic agentsTherapeutic effectBud
The invention discloses an anticancer traditional Chinese medicine composition. The anticancer traditional Chinese medicine composition is prepared from 15-25 parts of platinum metal, 10-15 parts of taxol, 10-18 parts of gallium salt, 20-30 parts of coix seeds, 15-20 parts of salvia miltiorrhiza, 13-18 parts of evening primrose, 8-10 parts of camptothecin and 15-18 parts of lilac daphne flower buds. Nedaplatin is used as a material, the composition further effectively treat multiple cancers except reduction of the toxic and side effects, the Nedaplatin is matched with the taxol and the gallium salt in use, an anticancer effect is more obvious, natural lilac daphne flower bud extracting solution contains palmitic acid, oleic acid, linoleic acid, stearic acid, gamma-linolenic acid and trace elements including magnesium, zinc, copper, vitamins C, E, B6, B5 and the like, has a very good protection effect on a human body, enhances immunity, and the salvia miltiorrhiza and the camptothecin can improve the cancer cell inhibiting effect of drugs. The anticancer traditional Chinese medicine composition can effectively play a very good cancer cell growth inhibiting effect, can also improve the body's resistance and immunity, meanwhile has the very good therapeutic effect on multiple cancers and is wide in application range and convenient to popularize.
Owner:聂超
Process for refining Nedaplatin
ActiveCN100494210CStable traitsHigh purityGroup 8/9/10/18 element organic compoundsFiltrationCrystallization temperature
This invention relates purifing method a refined Nedaplatin. By weight proportion of 1:15 to 80, dissolve Nedaplatin crude product in water of 25 to 70 deg, after completeness dissolving add KI, by filtration, remove excessive Ag+, add 1 to 5 times volume C1 to C2 alkyl alcoho filtrate, through crystallization, cooling, filtration, dryness, gain Nedaplatin refiend product; the cooling crystallization temperature is -25 to 25deg, time for 5 to 30h,drying temperature is 40 to 70deg, and drying time is 4 to 15h. The Ag content of this refined Nedaplatin is very low.
Owner:SIMCERE ZAIMING PHARM CO LTD
Novel method for synthesizing antineoplastic medicine nedaplatin
ActiveCN101302236BHigh yieldShort reaction processGroup 8/9/10/18 element organic compoundsAntineoplastic agentsGlycolic acidNedaplatin
The invention discloses a method for making antineoplastic medicine nedaplatin C2H8N2O3Pt. The method comprises the following technological processes that: cis-diiododiaminoplatinum (II) is taken as reactant; an amount of water is added in the reactant and is evenly stirred; then, argentum glycolate C2H3O3Ag and AgNO3 are slowly added in the solution with the molar ratio Pt (NH3) 2I2: C2H3O3Ag:AgNO3 equal to 1:1:1, and are reacted at a temperature of between 50 and 60 DEG C for 2 to 3 hours; after solid is filtered out, the filtrate is heated to 40 DEG C, and the pH value of the filtrate is adjusted to 7 by NaOH; the obtained solution is heated to 50 to 60 DEG C so as to carry out reaction for 4 hours; finally, the reaction liquid undergoes decompression condensation to obtain nedaplatin solid. The method has short process flow, high yield (above 56 percent) and high product purity.
Owner:KUNMING GUIYAN PHARMA
Nedaplatin medical composition and application
ActiveCN101744800BMacromolecular non-active ingredientsAntineoplastic agentsFreeze-dryingMedical product
Owner:QILU PHARMA CO LTD
A kind of impurity detection method of nedaplatin
The invention discloses an impurity HPLC analysis method for nedaplatin. In the method, octadecylsilane bonded silica gel is used as a filler (recommendation: AQ-C18), the detection wavelength is 205-222nm, and the column temperature is 20~45℃, the mobile phase is a mixture of pH6.0~8.5 buffer and polar organic solvent, characterized in that the mobile phase is to elute the impurities in the nedaplatin sample by gradient elution, pH6. The initial ratio of 0-8.5 buffer solution to polar organic solvent is 100:0-90:10, and the final ratio is 90:10-60:40; the flow rate of mobile phase is 0.5-1.0ml / min; prepared with mobile phase into a solution containing 0.5 mg to 2 mg / ml of nedaplatin; inject 5 μl to 20 μl of the sample solution into a high performance liquid chromatograph, record the chromatogram and perform sample analysis. After using the HPLC analysis method of the present invention, the theoretical plate number of the main peak of the product is obviously increased, and more impurities can be separated, so that the accuracy of analyzing the nedaplatin sample is improved.
Owner:SIMCERE ZAIMING PHARM CO LTD
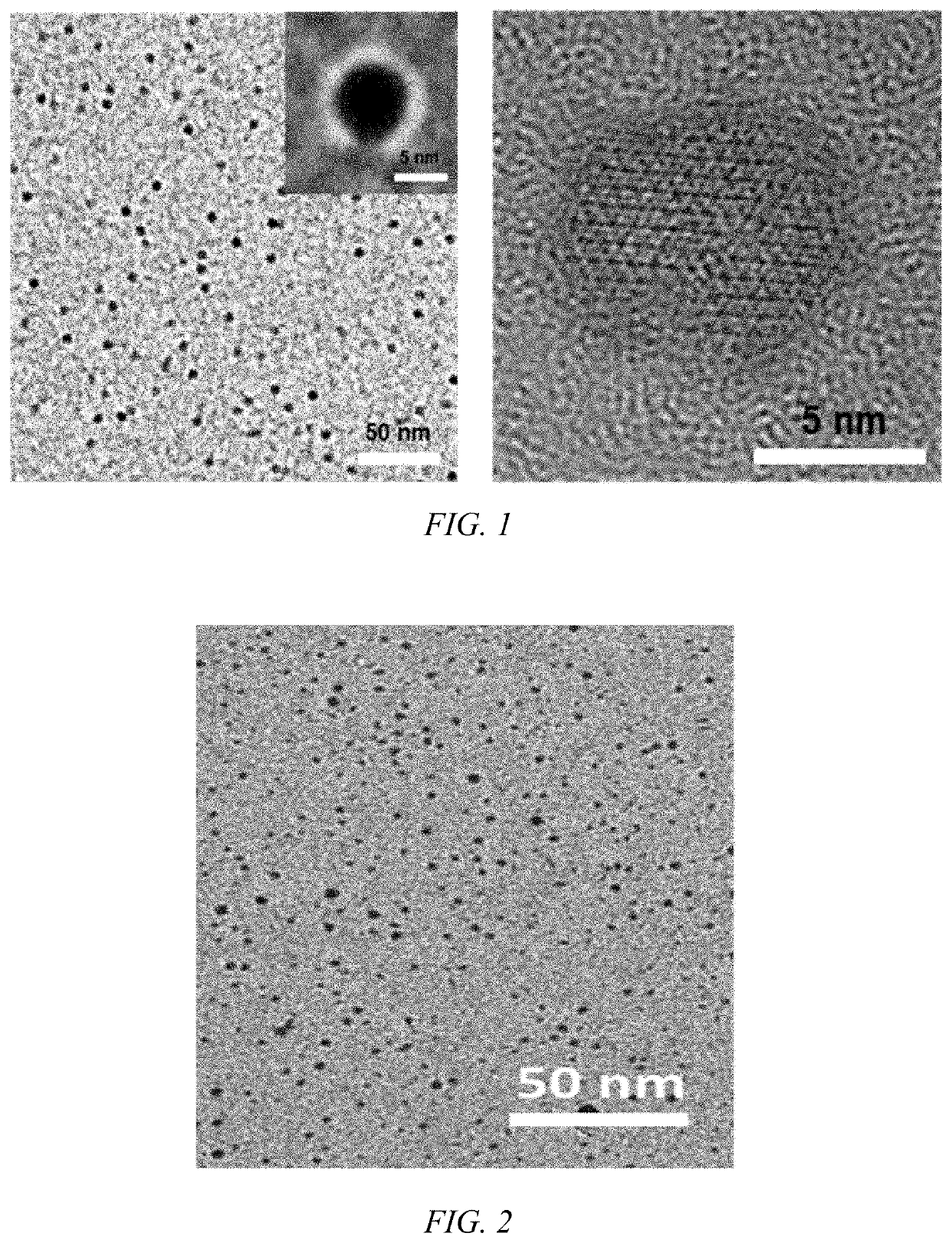
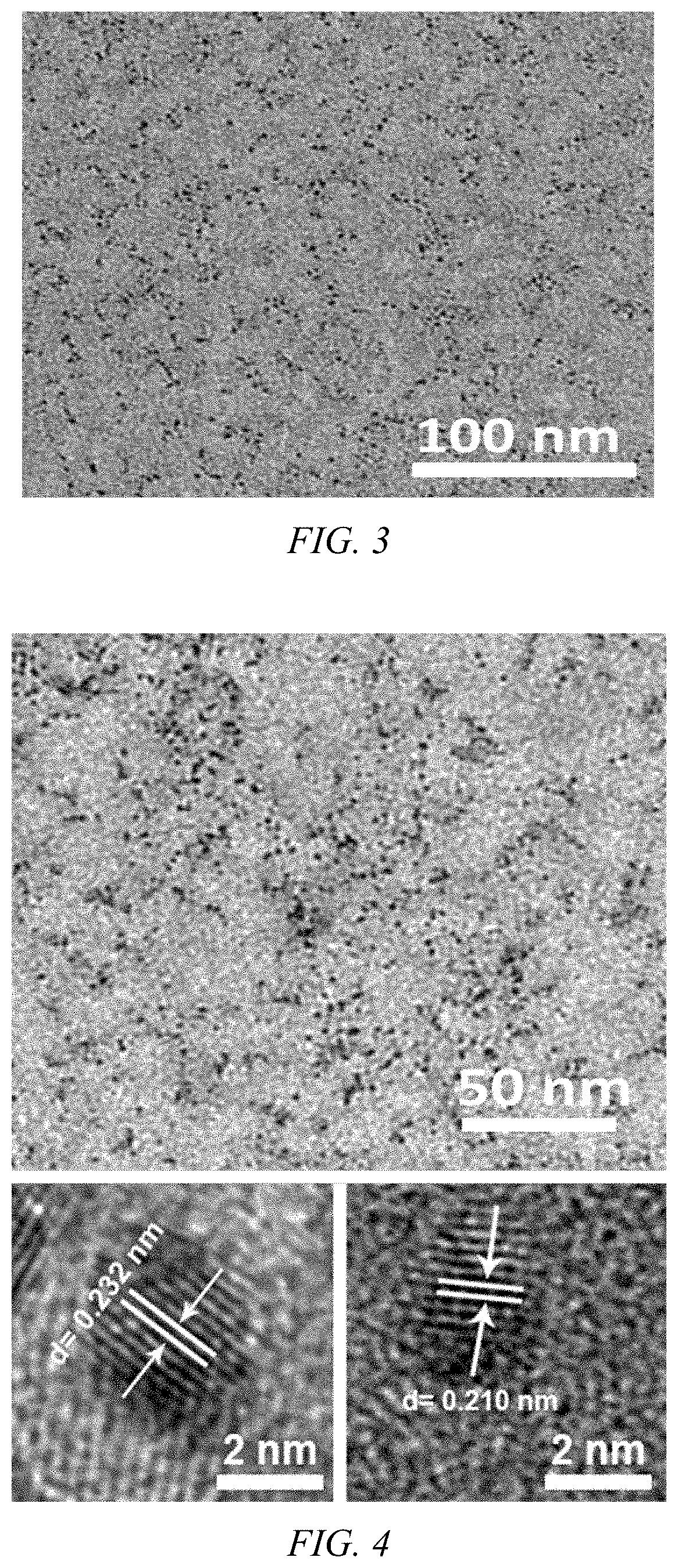
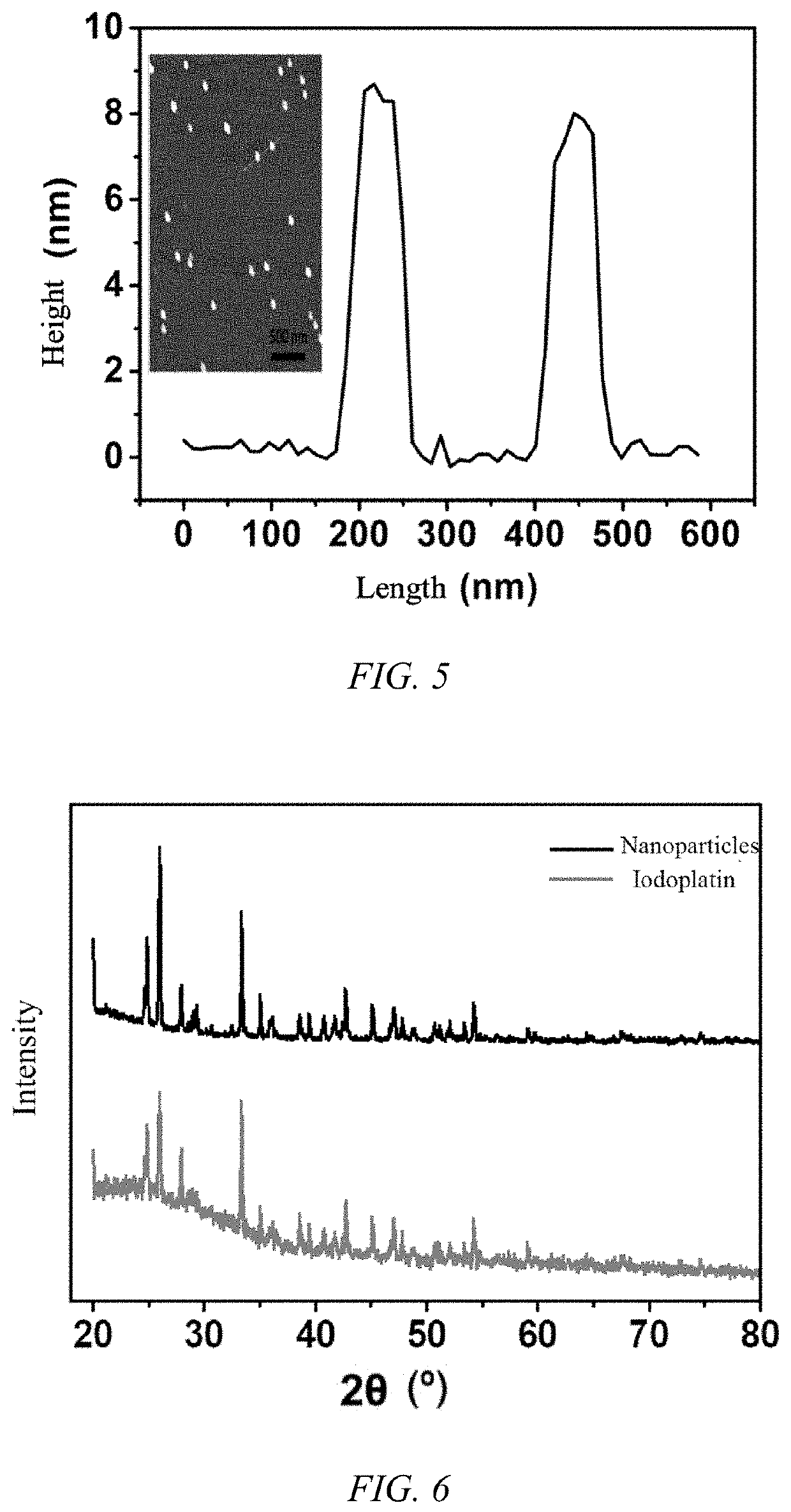
Anti-tumor platinum-based drug mineralized protein nanoparticle, preparation method therefor and use thereof
PendingUS20220110883A1Good tumor cytotoxicityEasy to makeInorganic active ingredientsAntineoplastic agentsCarboplatinA lipoprotein
Anti-tumor platinum drug mineralized protein nanoparticles and a preparation method therefor are disclosed. The anti-tumor platinum-based drug mineralized protein nanoparticles include a platinum drug and a protein. The protein is one or more selected from the group consisting of albumin, transferrin, hemoglobin, and low-density lipoprotein. The platinum drug is cisplatin, iodoplatin, bromoplatin, oxaliplatin, carboplatin, or nedaplatin. A drug loading of the anti-tumor platinum-based drug mineralized protein nanoparticles is 1% to 50%.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com