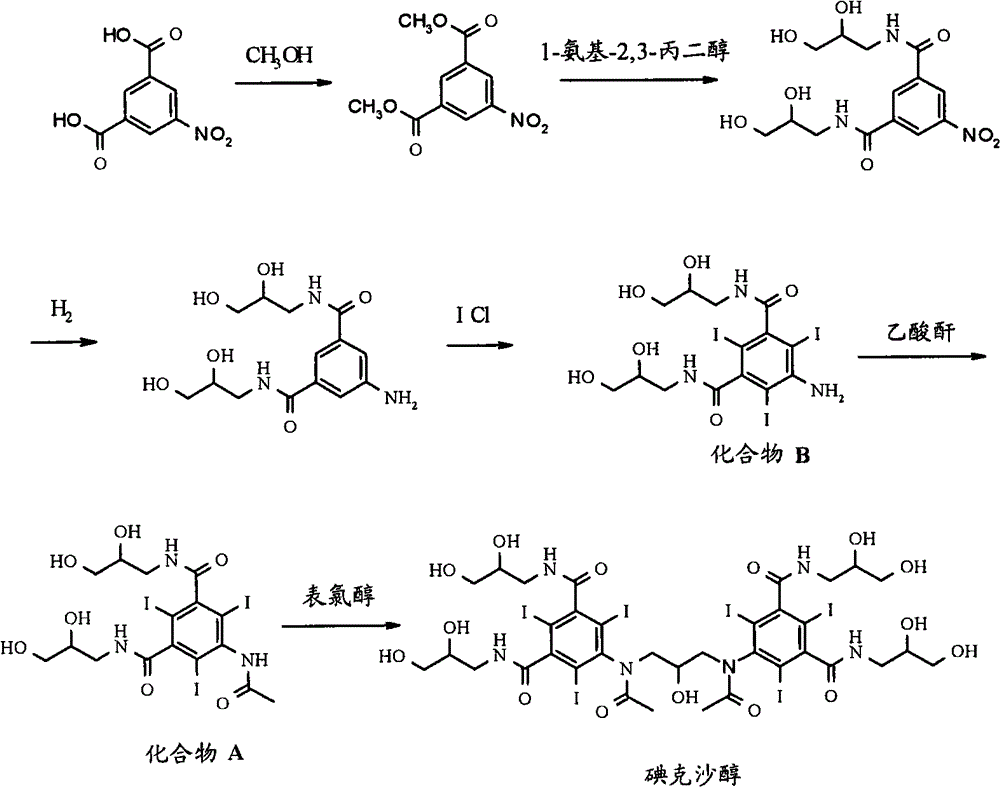
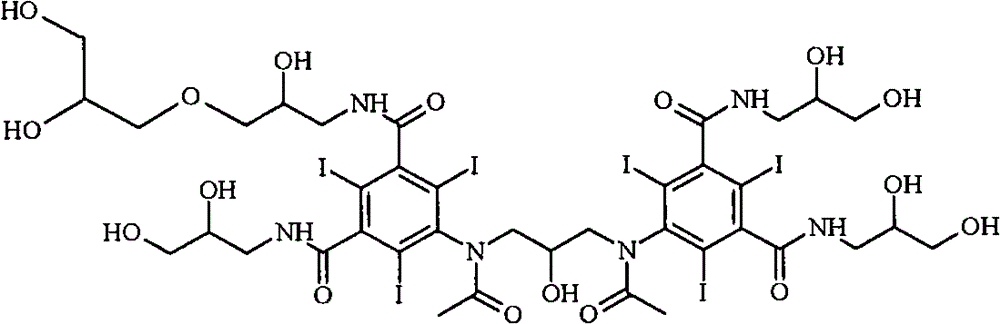
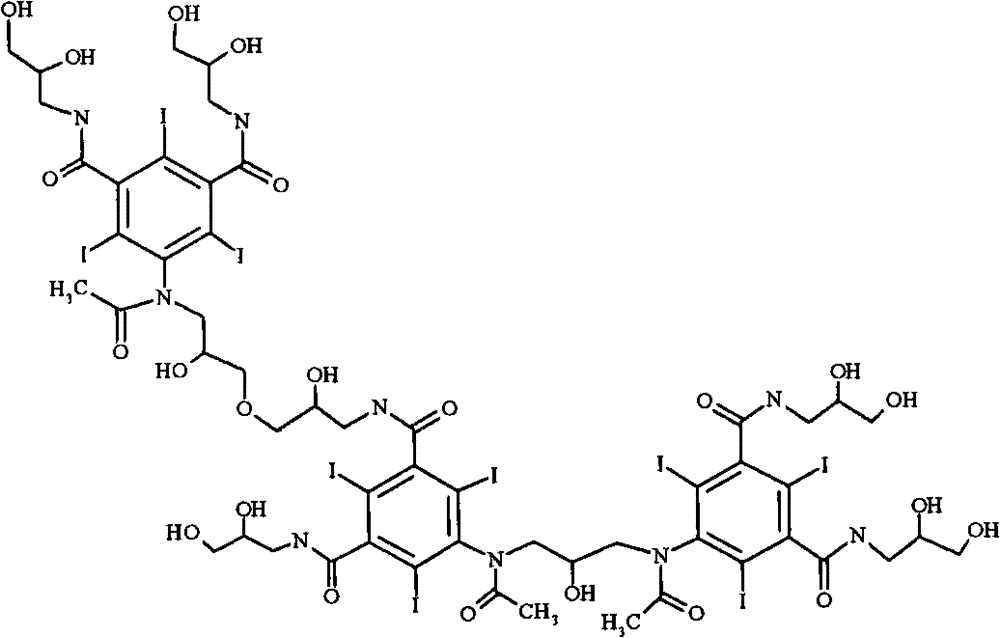
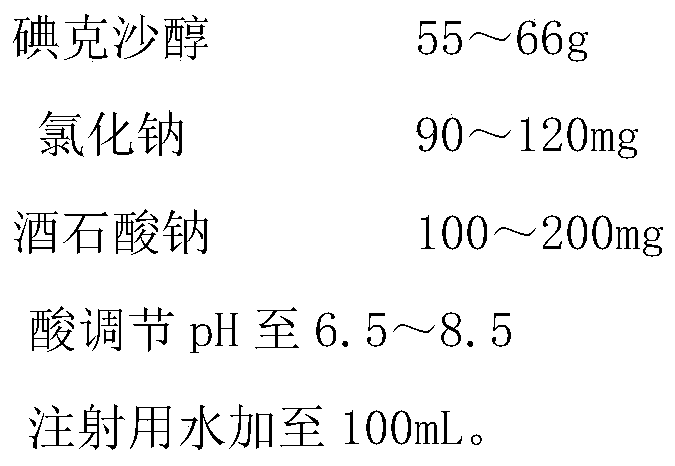
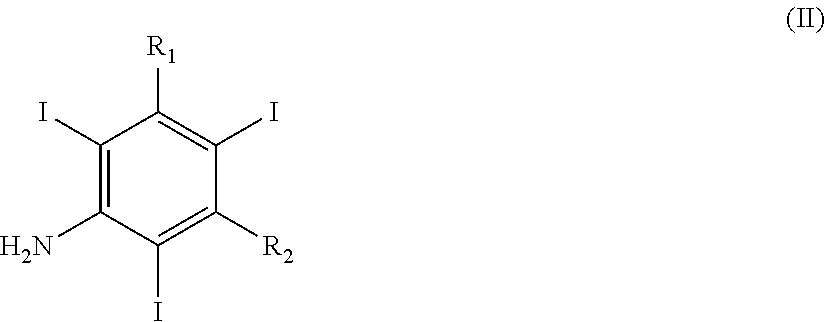Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Iodixanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
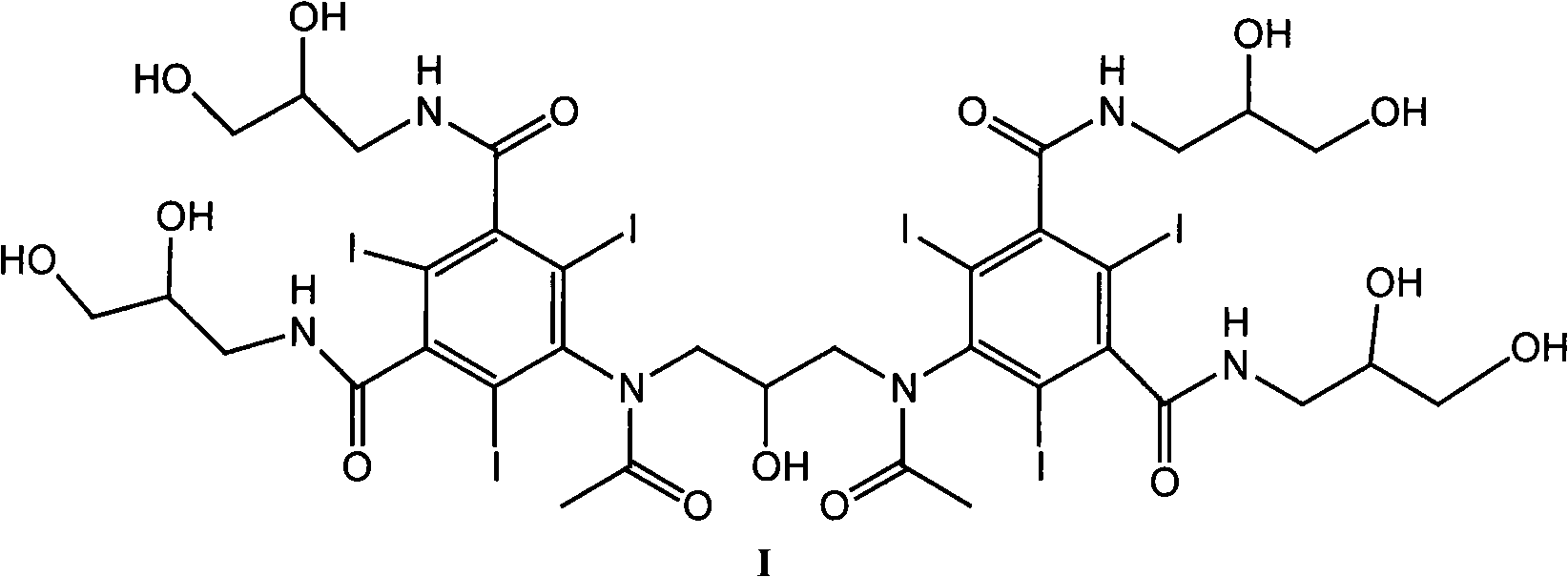
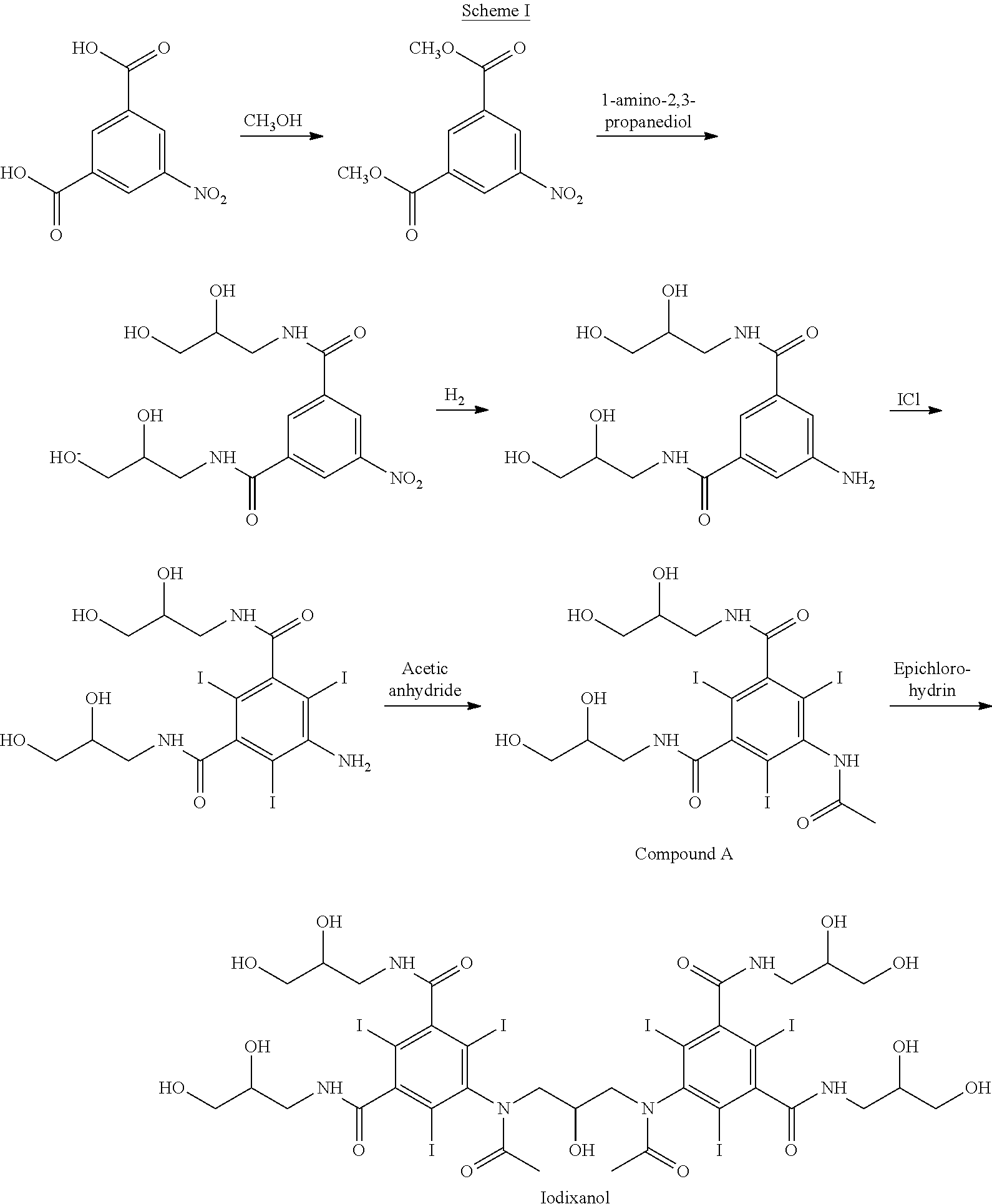
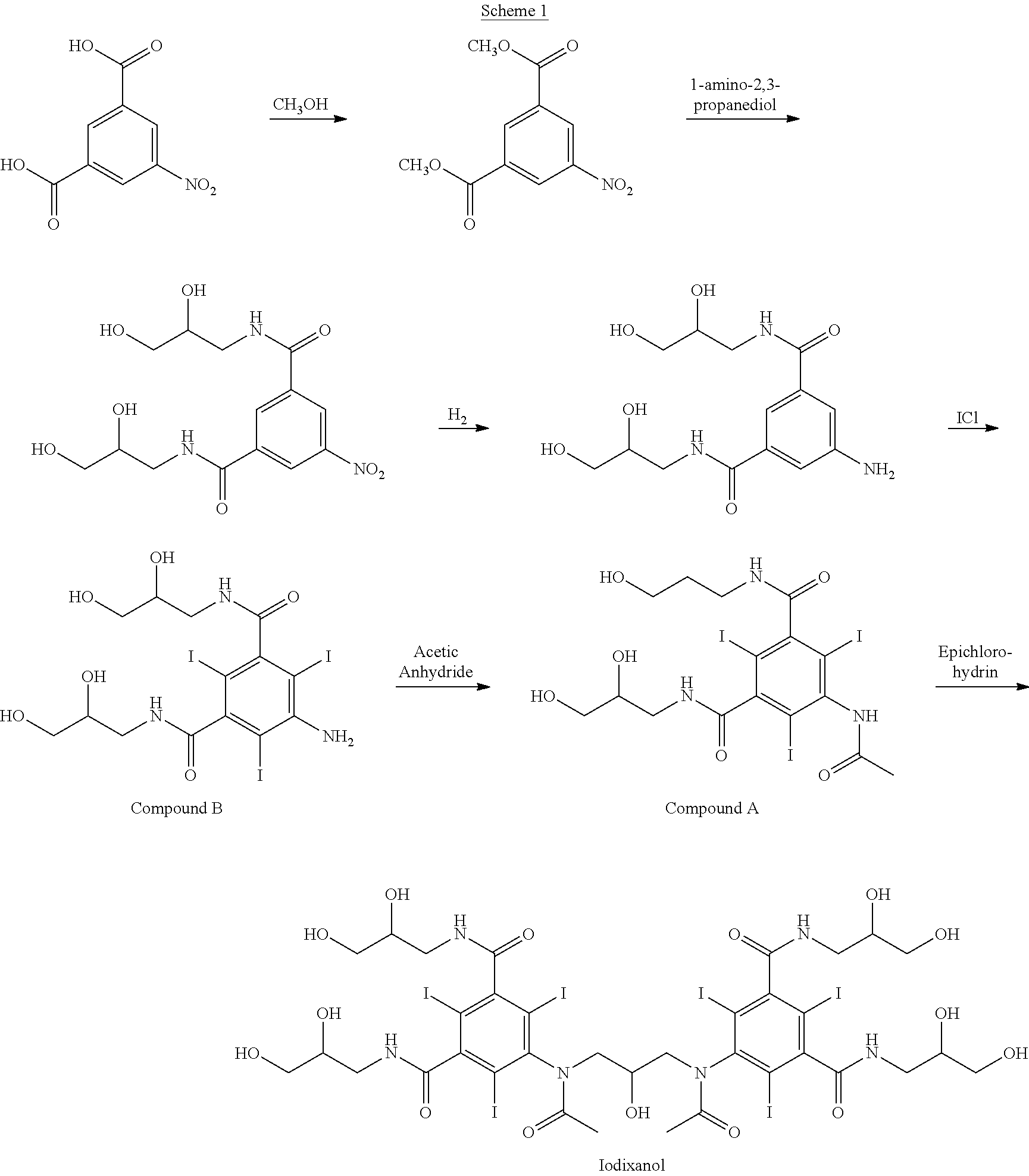
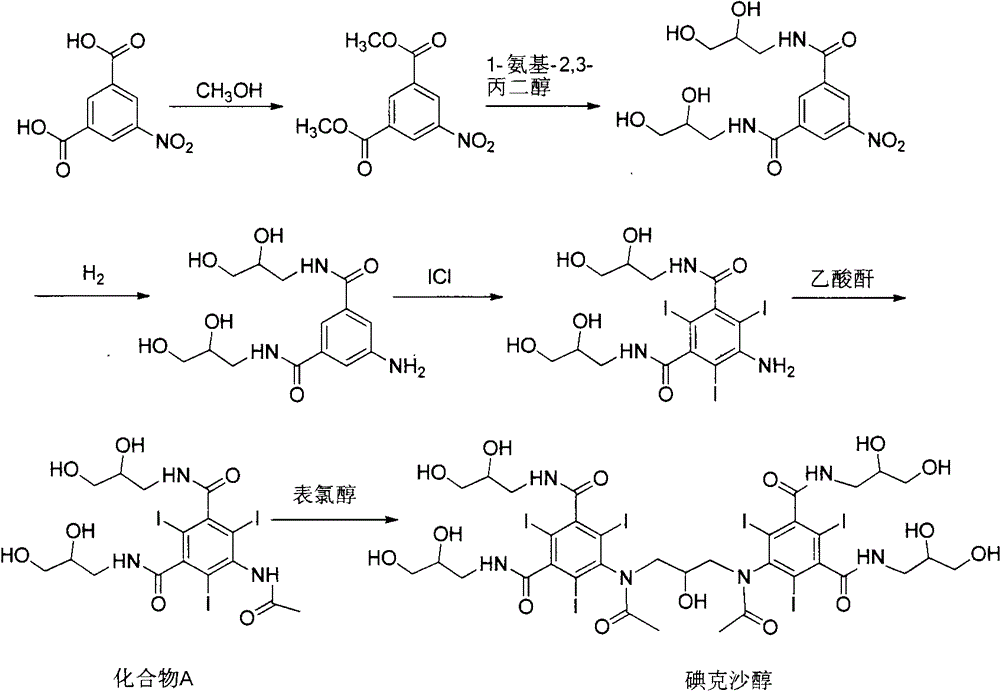
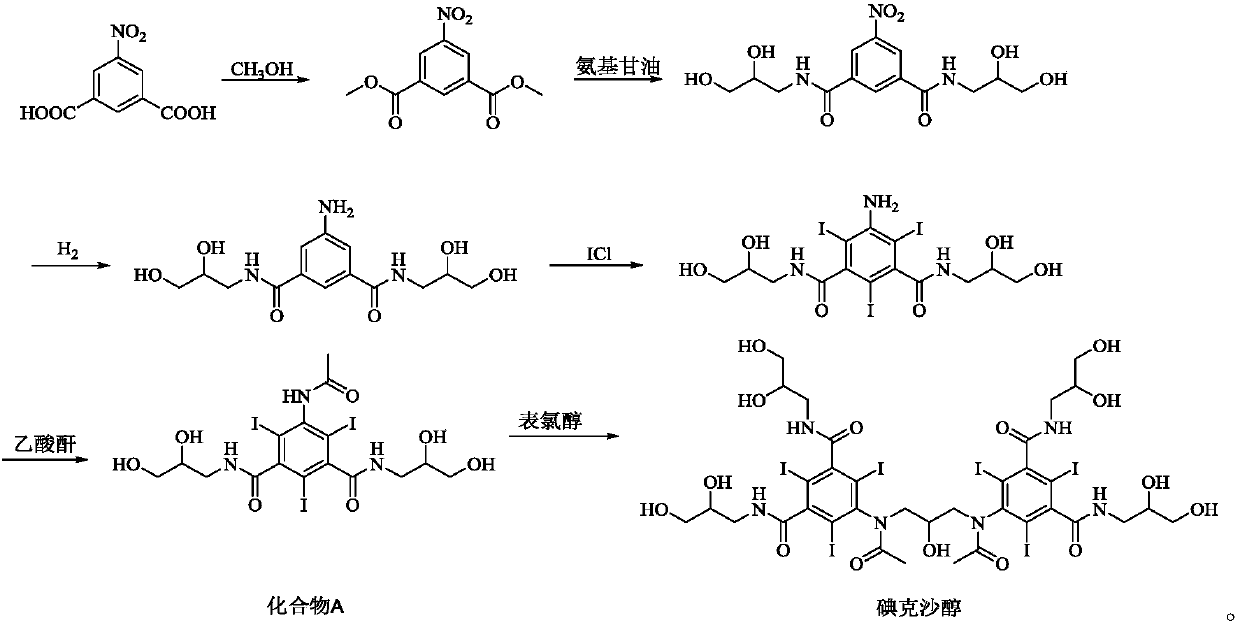
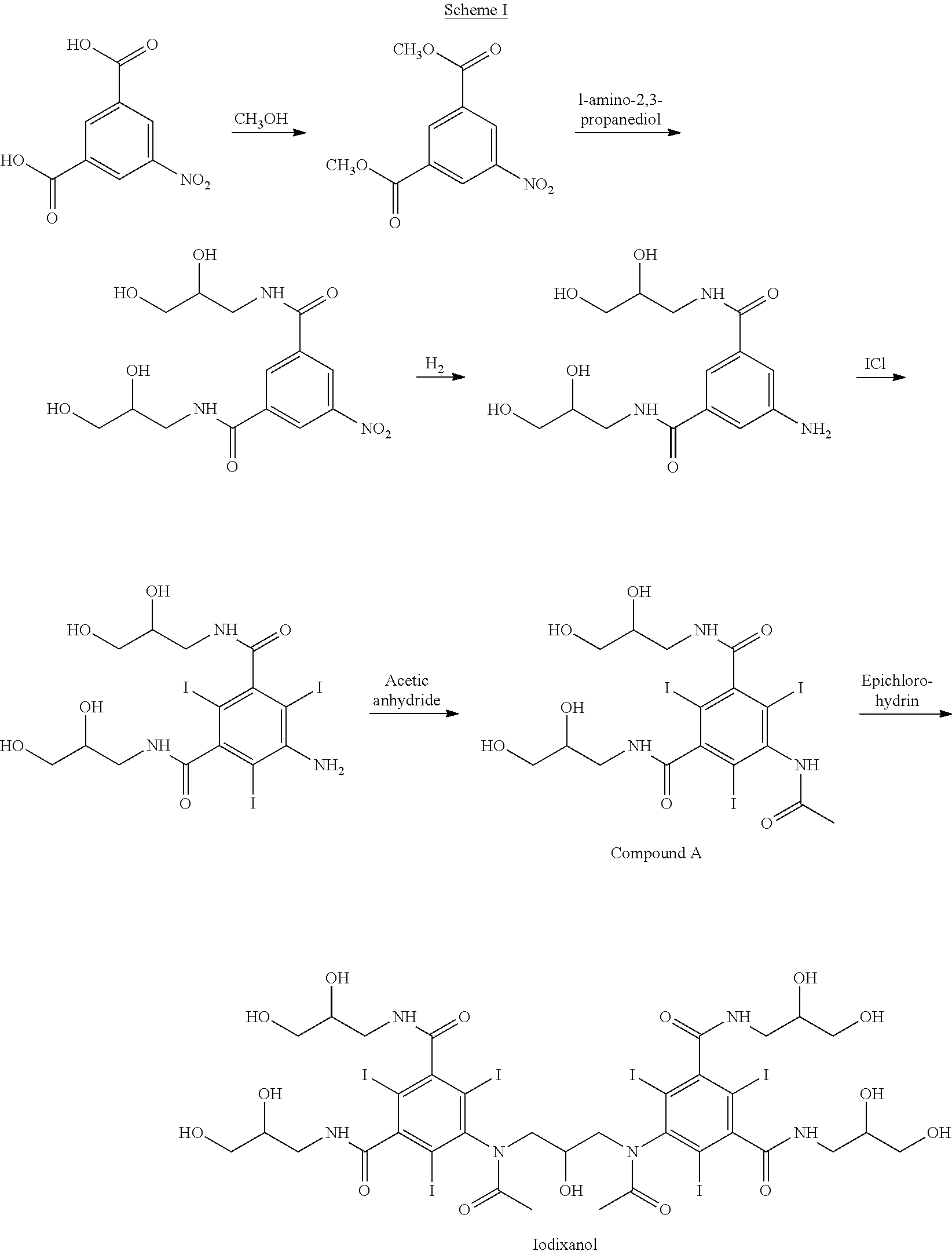
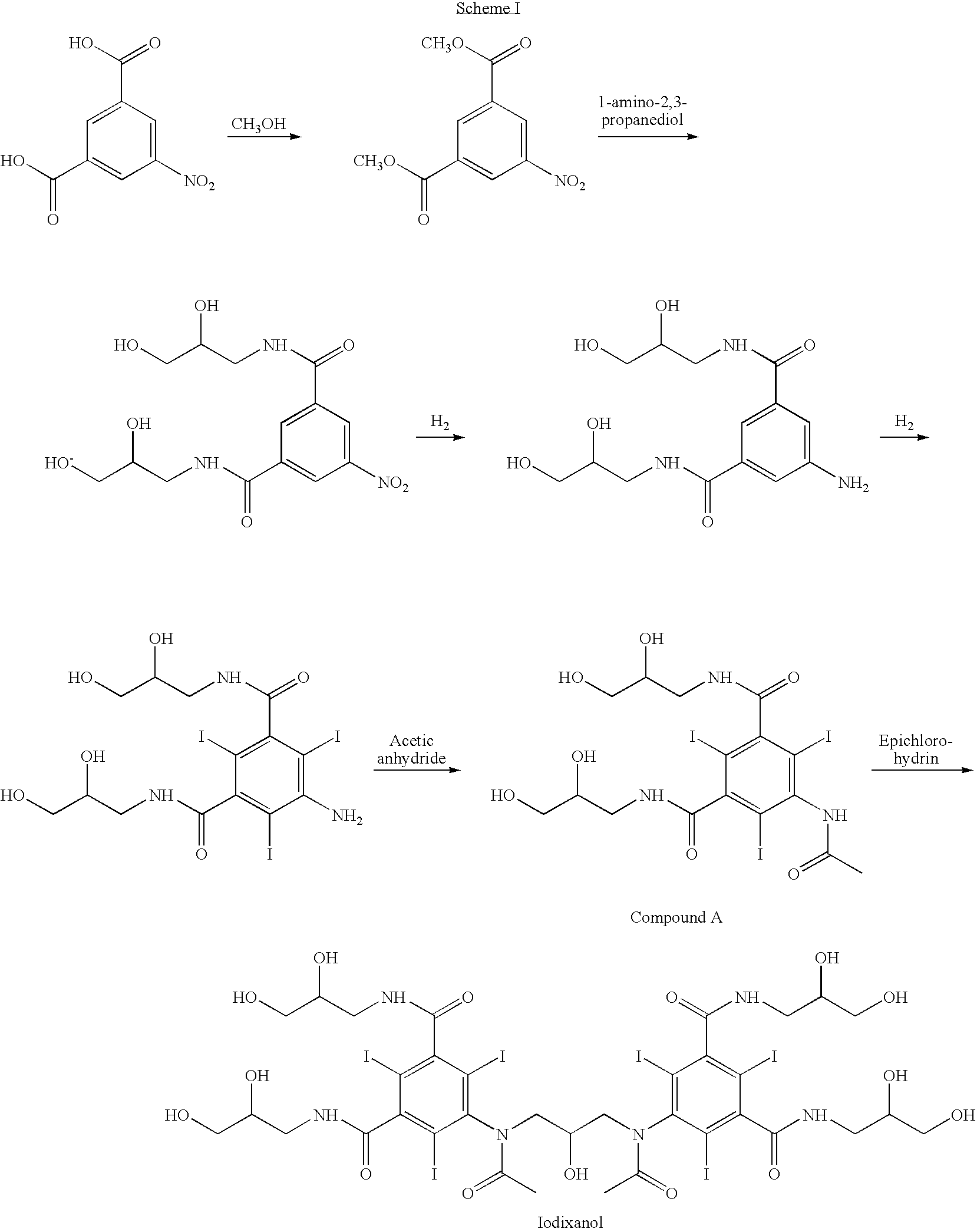
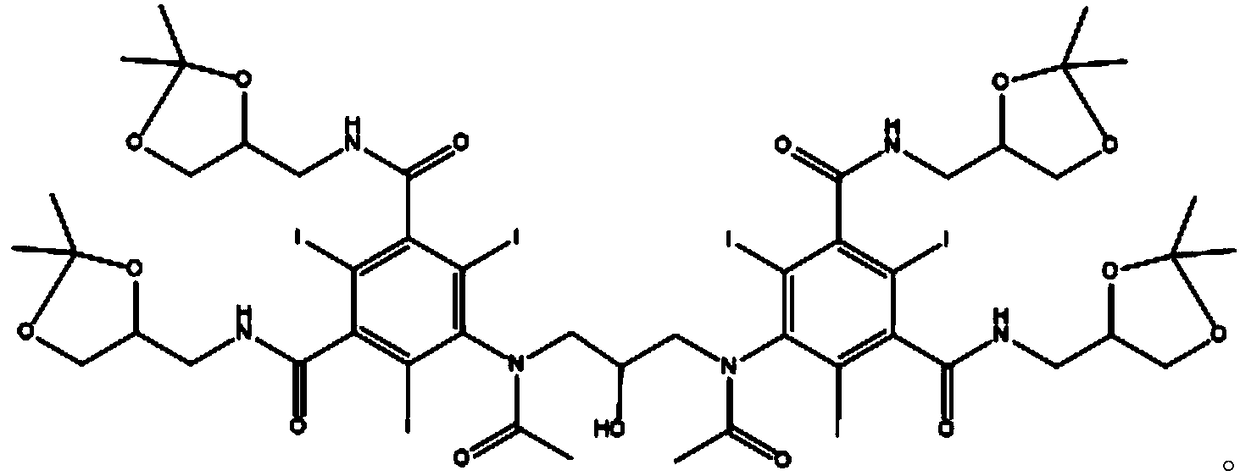
Iodixanol is an iodine-containing non-ionic radiocontrast agent. It is sold under the trade name Visipaque; it is also sold as a density gradient medium under the name OptiPrep. Visipaque is commonly used as a contrast agent during coronary angiography. It is the only iso-osmolar contrast agent, with an osmolality of 290 mOsm/kg H₂O, the same as blood. It is sold in 2 main concentrations 270 mgI/ml and 320 mgI/ml - hence the name Visipaque 270 or 320. It is sold in single dose units and a large 500ml plastic bottle for multi-dose dispensing.
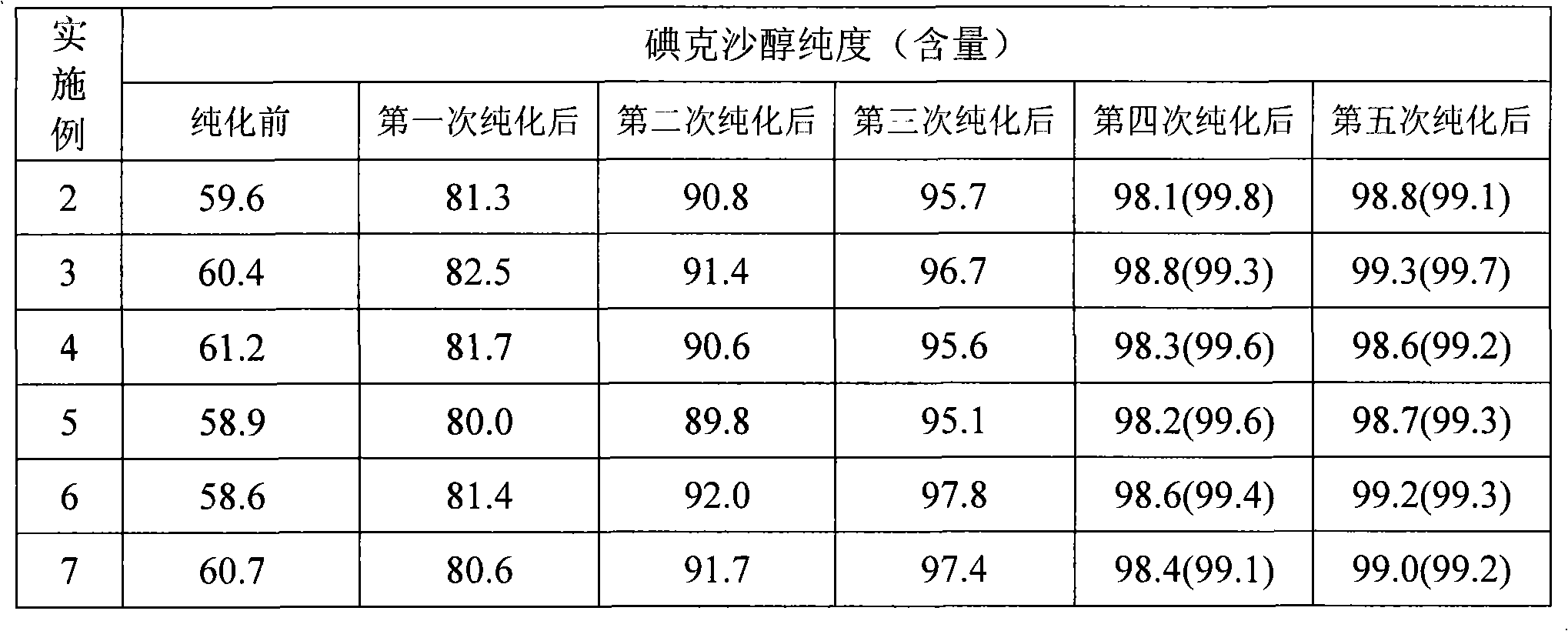
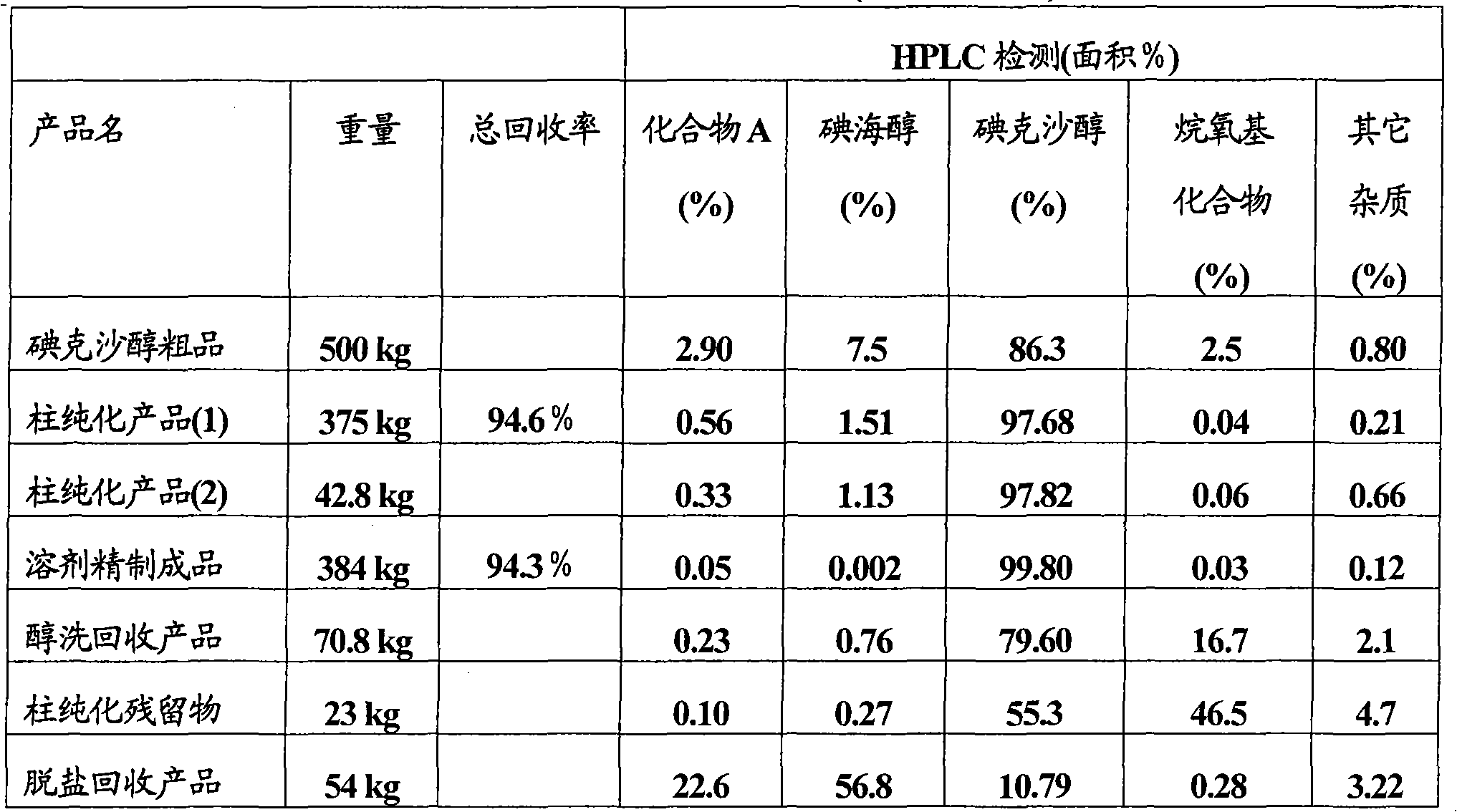
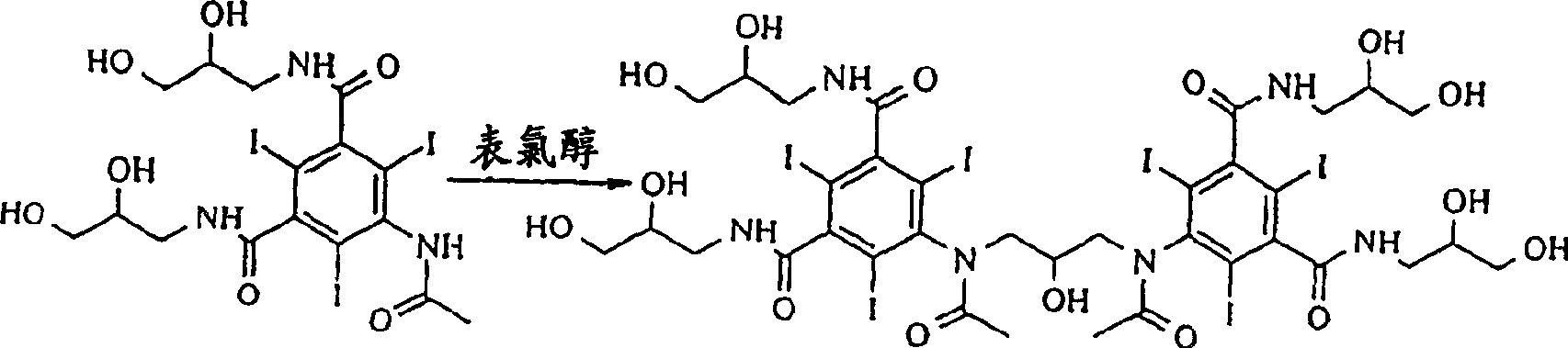
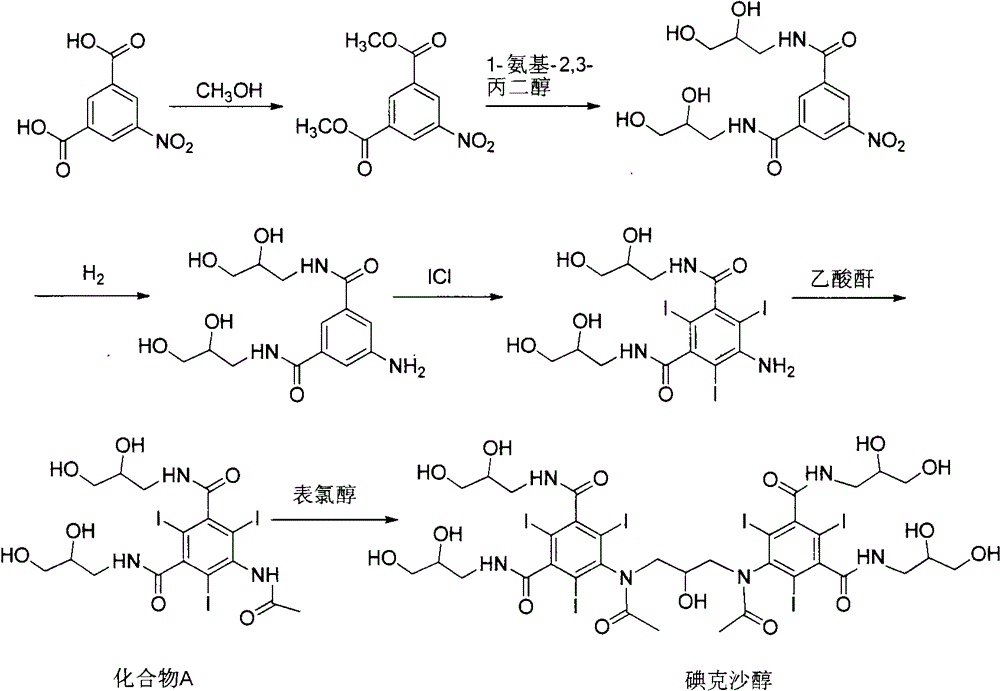
Purification process for Iodixanol
ActiveCN101293855ASuit one's needsFulfill the standardCarboxylic acid amide separation/purificationPurification methodsIodixanol
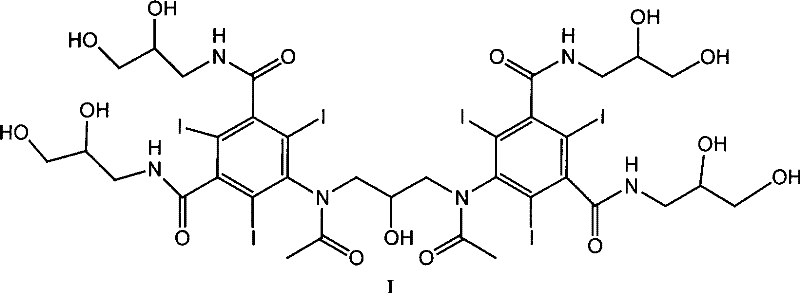
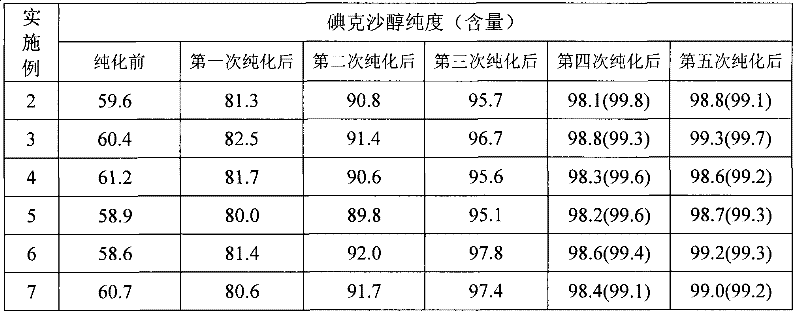
The invention provides a method for purifying iodixanol. The invention adopts a recrystallization method to purify crude iodixanol prepared from dipolymer of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo isophthalamide, after several times of repeated purification, an iodixanol product qualified to Pharmacopoeia standards is obtained. The inventive purification method can be effectively used for purification of crude iodixanol prepared from dipolymer of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo isophthalamide, and the used solvent has low cost, so as to remarkably reduce cost compared with the prior purification method. In addition, the inventive purification method is a non-chromatographic solvent purification method and can obtain iodixanol with high purity and high content after several times of repeated purification, so as to meet the industrial production requirements of iodixanol product, as well as Pharmacopoeia standards.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Composite developing thermosensitive gel embolizing agent as well as preparation method and application thereof
ActiveCN107281502AIncrease success rateReduce chanceOrganic active ingredientsHeavy metal active ingredientsCarboplatinMiriplatin
The invention relates to a composite developing thermosensitive gel embolizing agent as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly, preparing a mixed aqueous solution of an anticancer active substance, a thermosensitive material and a developing agent into composite developing thermosensitive gel; secondly, forming a composite developing thermosensitive gel embolizing agent by the composite developing thermosensitive gel and a coagulant on the scene, wherein the thermosensitive material is hydroxyl C1-4 alkyl cellulose, Pluronic, alginate or a mixture of the substances; the anticancer active substance is arsenic trioxide, docetaxel, cisplatin, carboplatin, nedaplatin, oxaliplatin, lobaplatin, miriplatin, siRNA or a mixture of the substances; the developing agent is a water-soluble developing agent of iodixanol, ioversol or iohexol and the like. The preparation method disclosed by the invention is simple and convenient, is suitable for industrial large-scale production, is particularly suitable for preparing an embolizing agent which is biodegradable and good in biocompatibility and is used for hemorrhagic diseases, and is especially suitable for preparing the composite developing thermosensitive gel embolizing agent for treating liver cancer, kidney cancer, lung cancer, prostate cancer, uterine myoma or splenic tumor and the like.
Owner:苏州申润医疗科技有限公司 +1
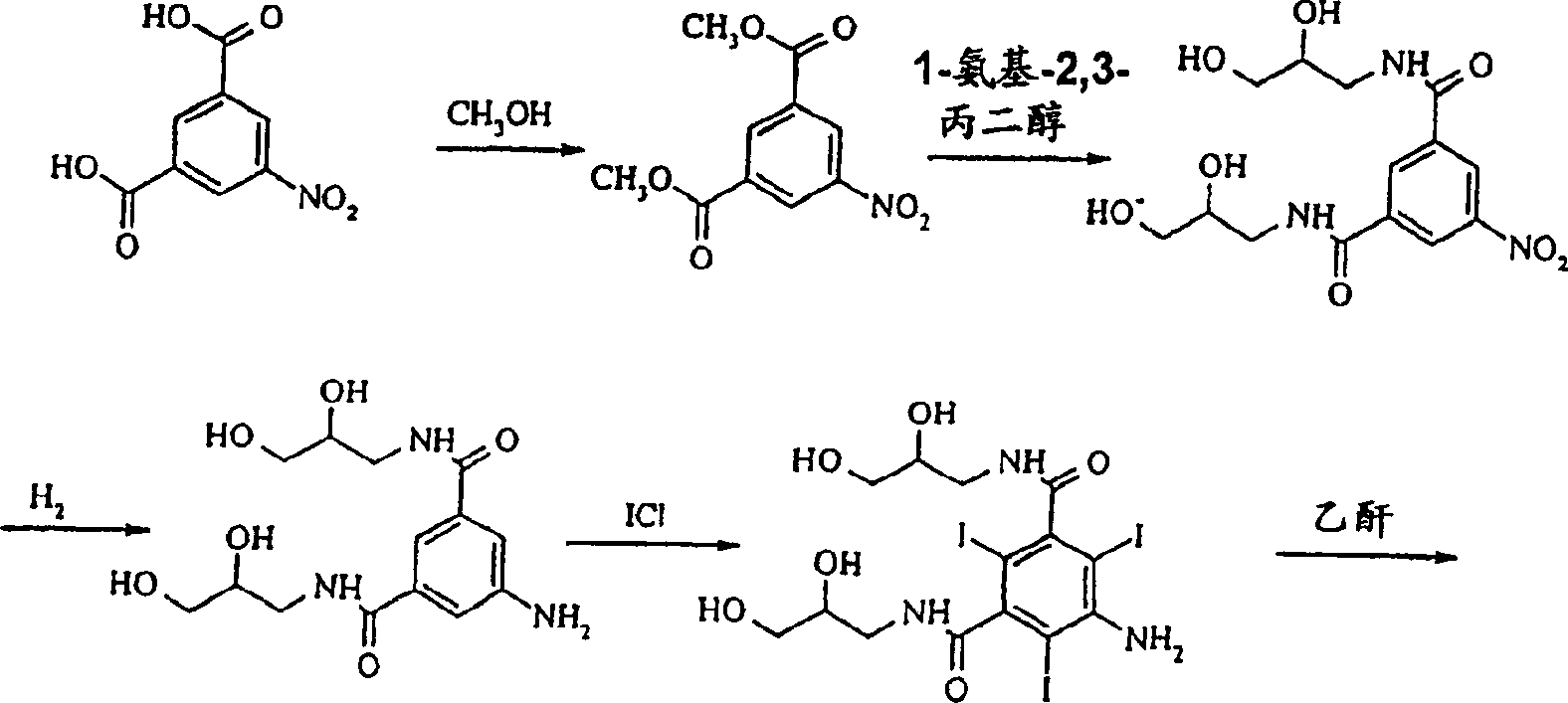
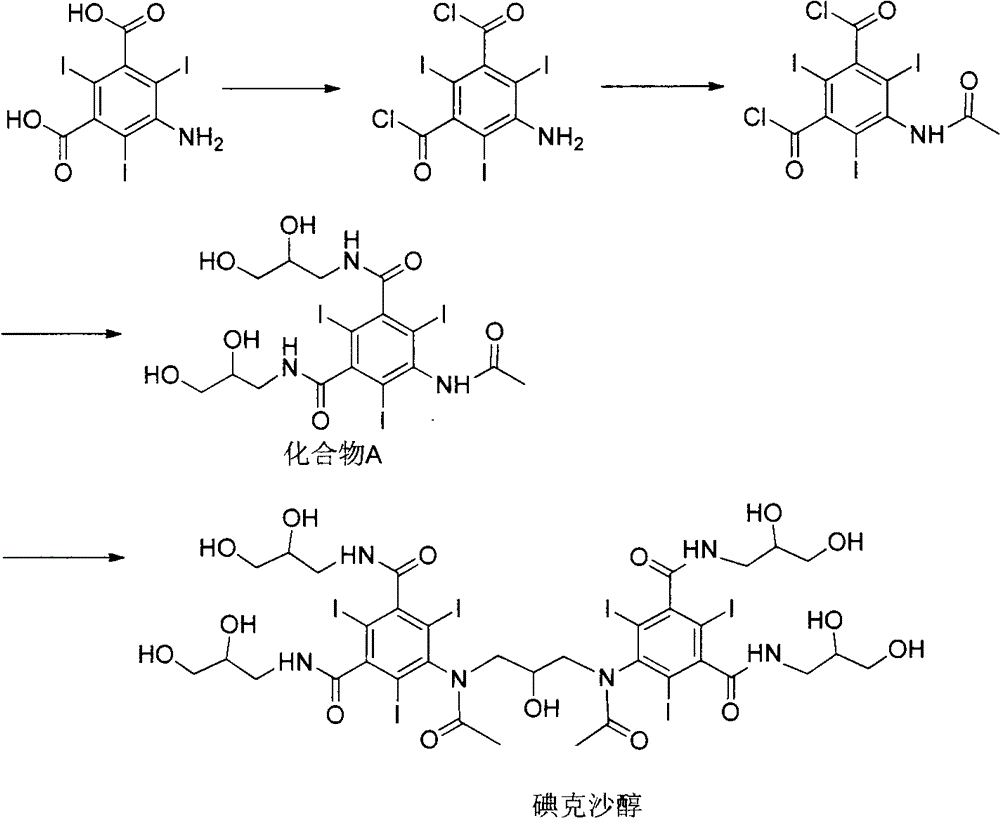
Preparation of iodixanol
InactiveCN1340042AOrganic compound preparationCarboxylic acid amide separation/purificationCompound aIodine
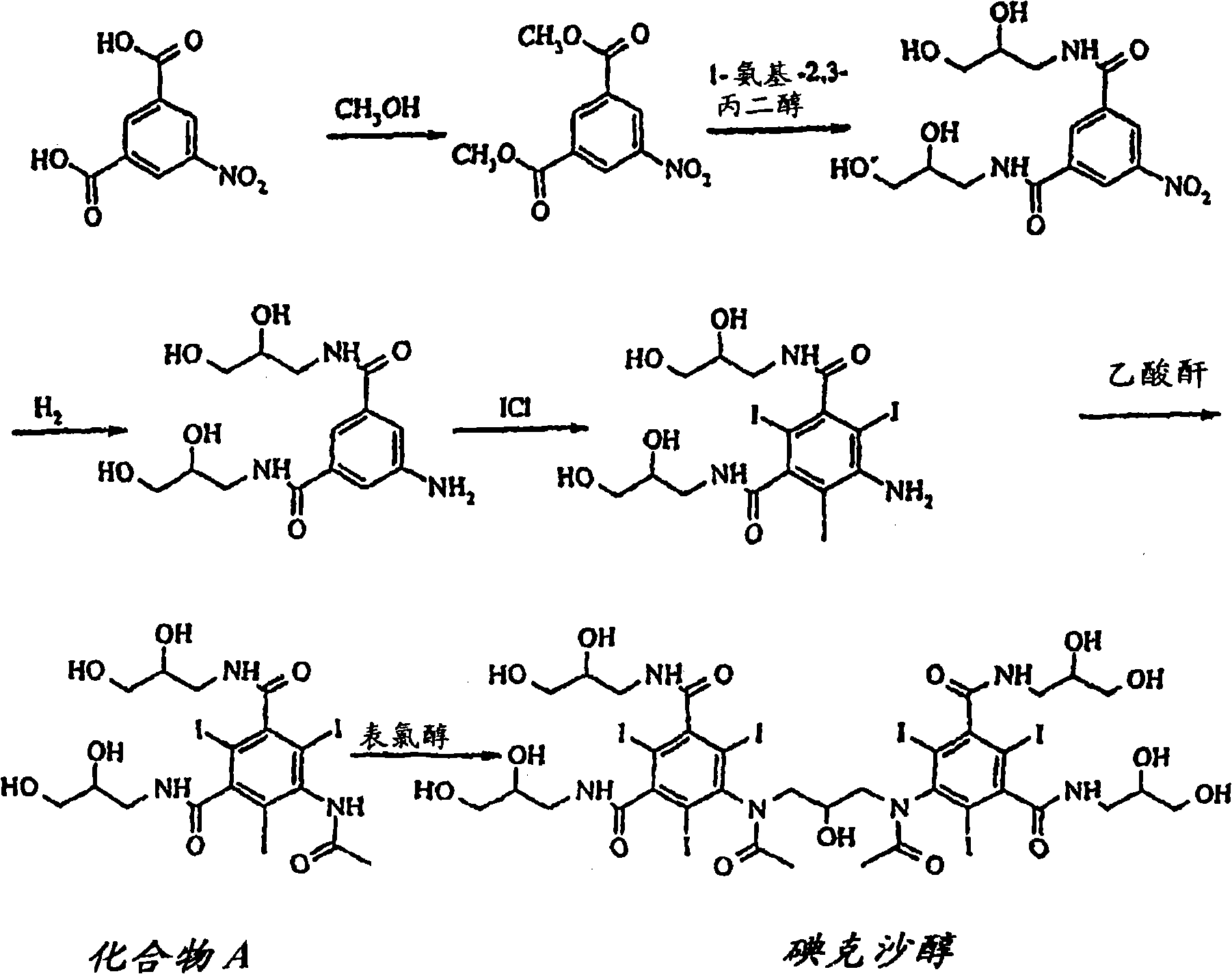
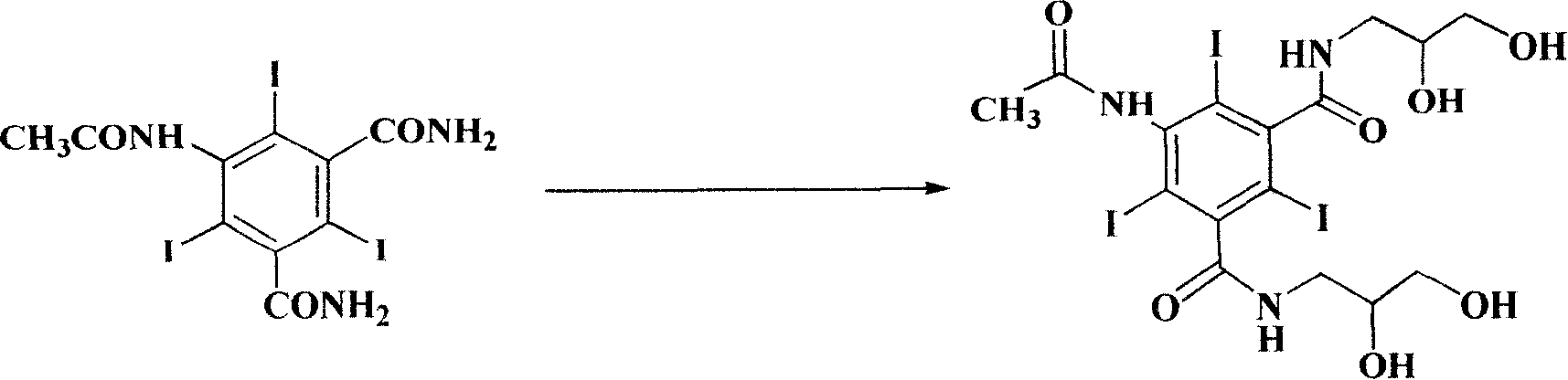
A process for the preparation of iodixanol by dimerisation of 5-acetamido-N, N'- bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide ('Compound A') in whic h, after the dimerisation step, unreacted Compound A is precipitated from the reaction mixture and recovered for re-use. The process substantially increases the net yield of iodixanol and simplifies its purification.
Owner:GE HEALTHCARE AS
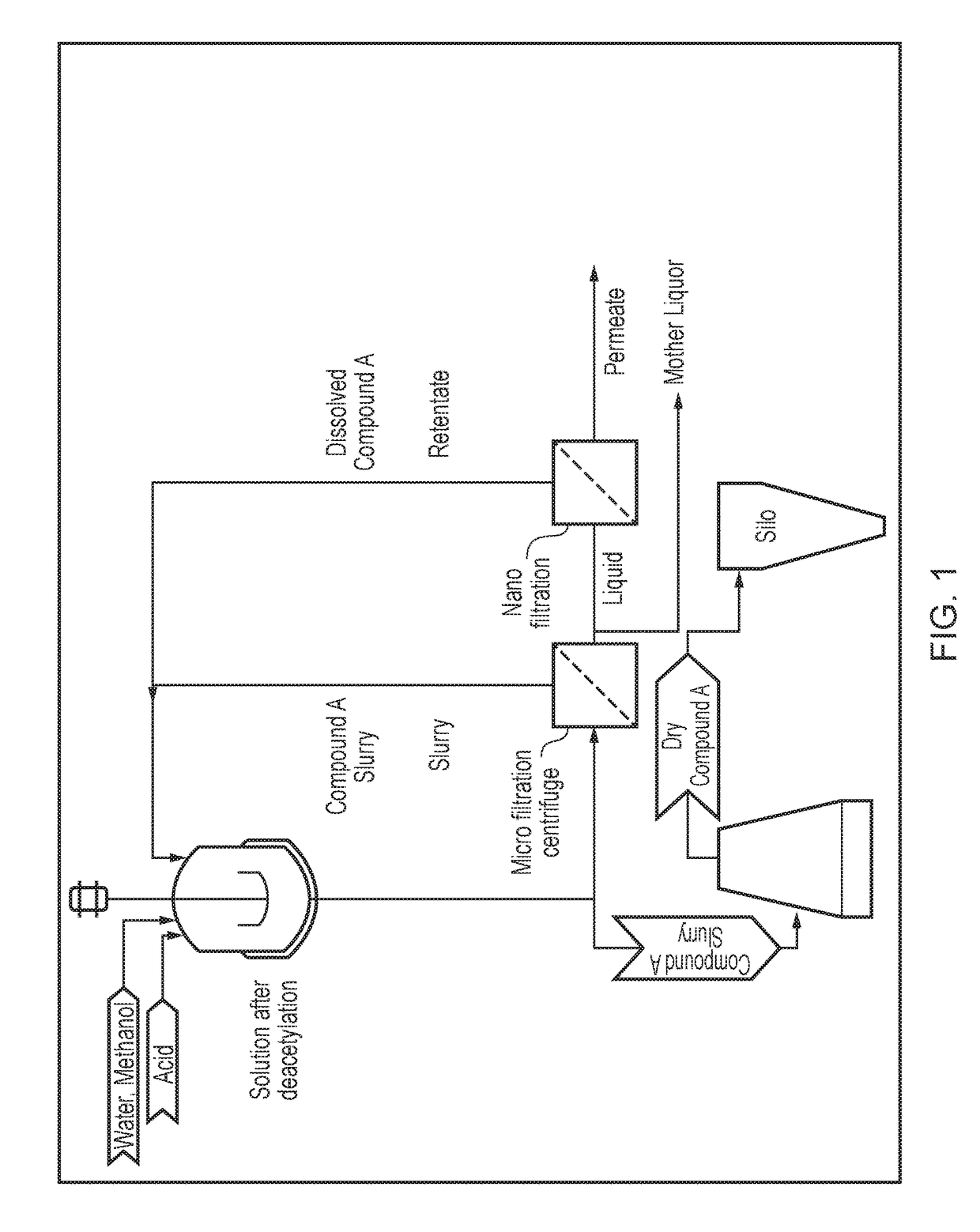
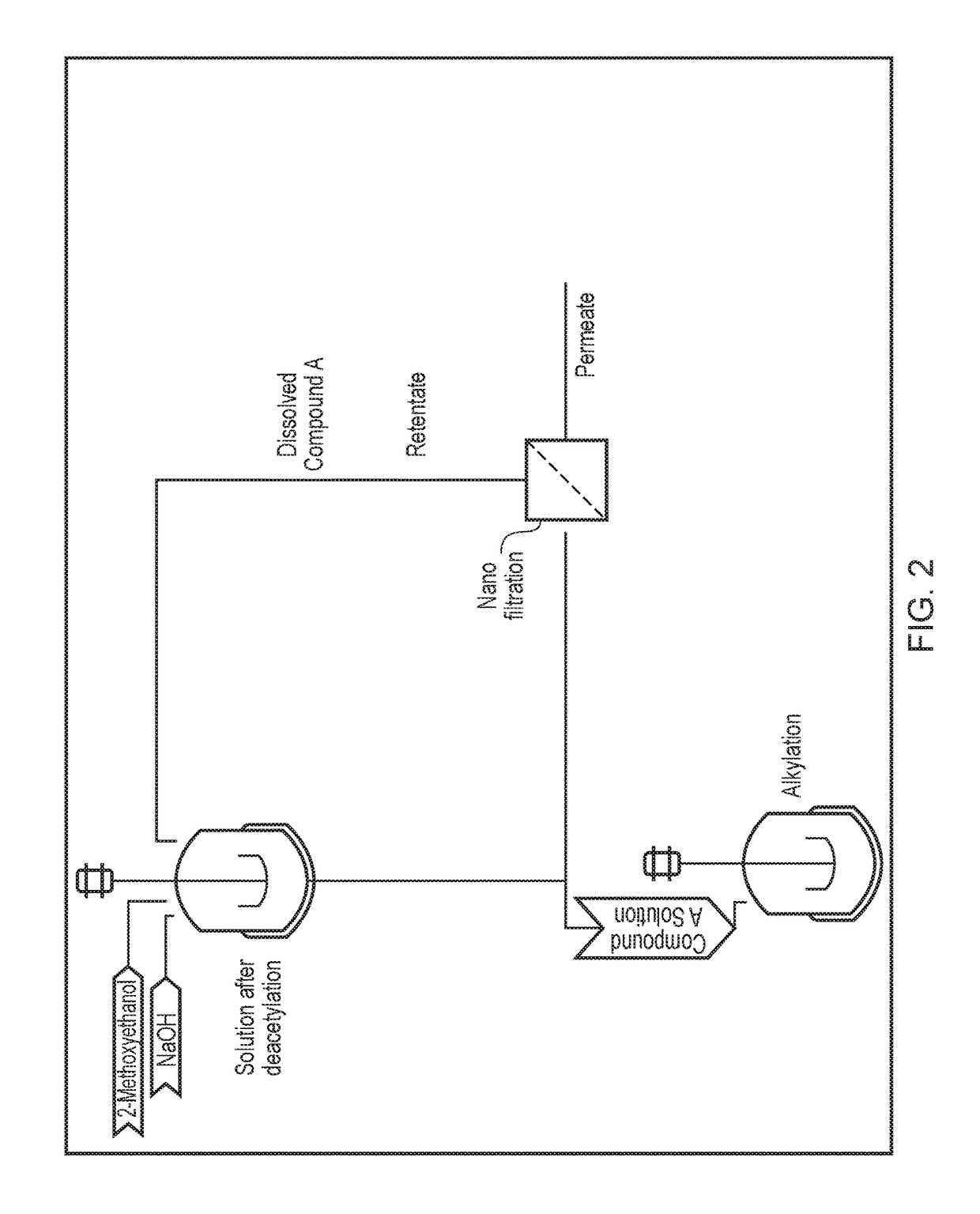
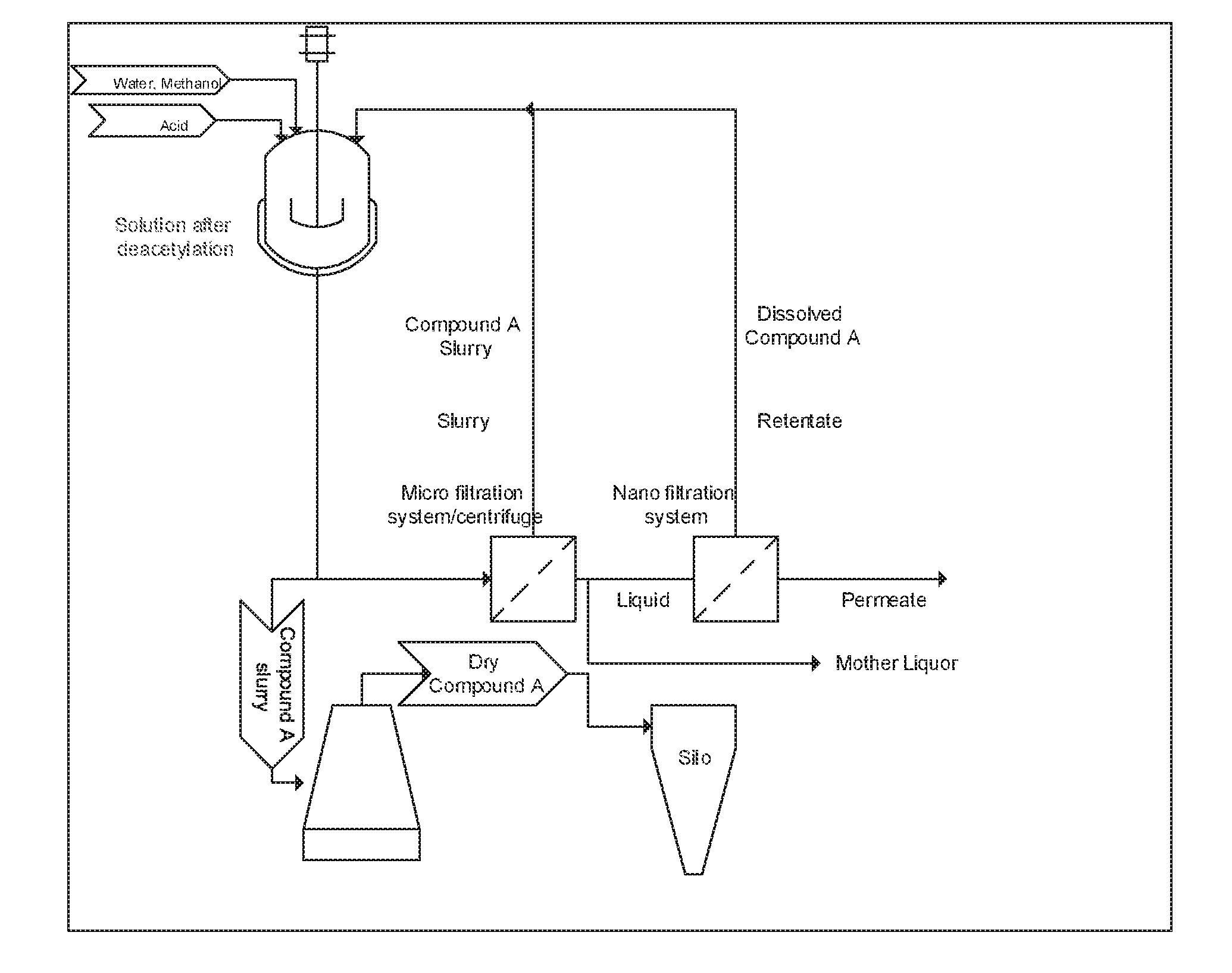
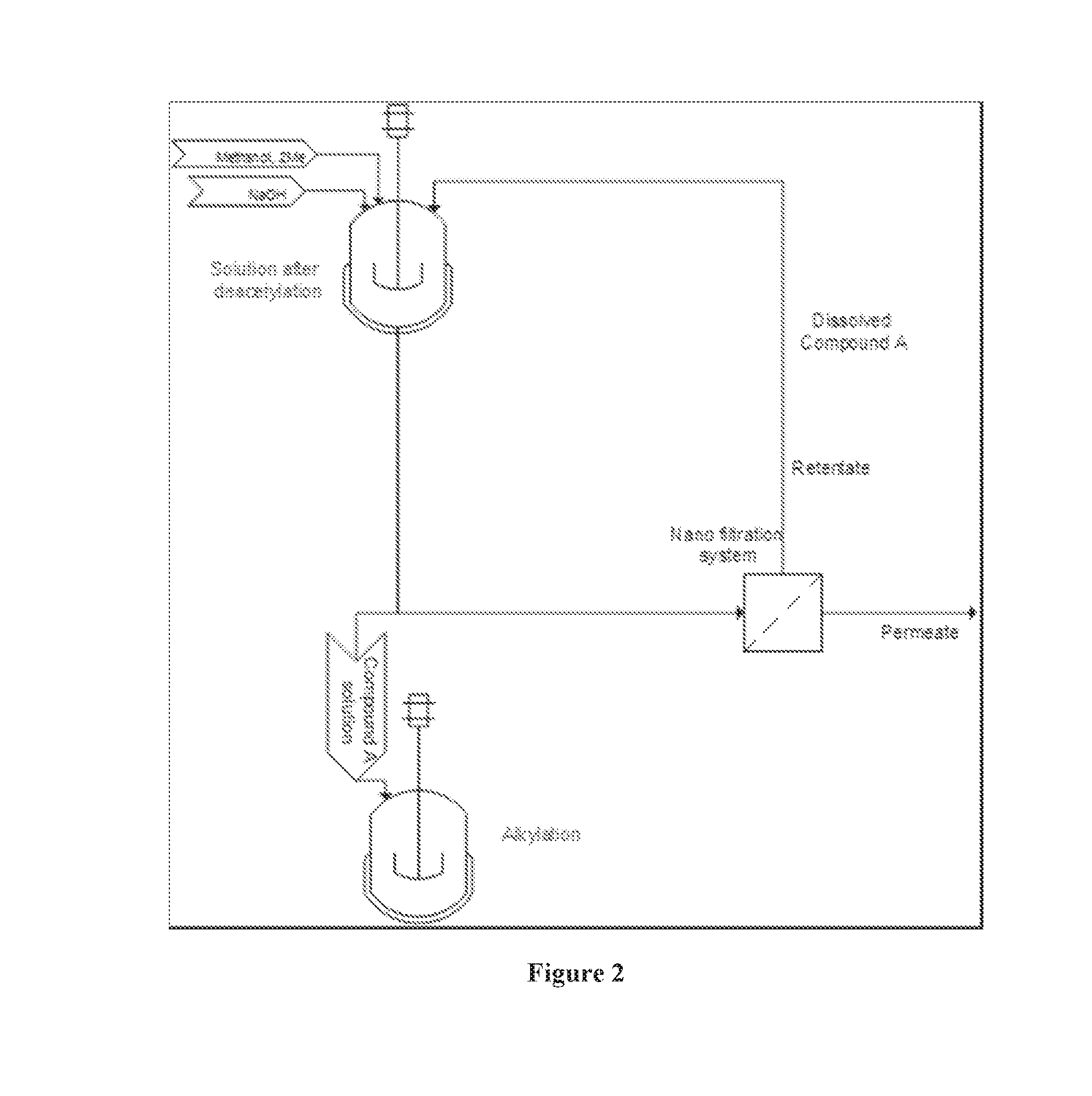
Processing crude iodixanol mixture by nanofiltration
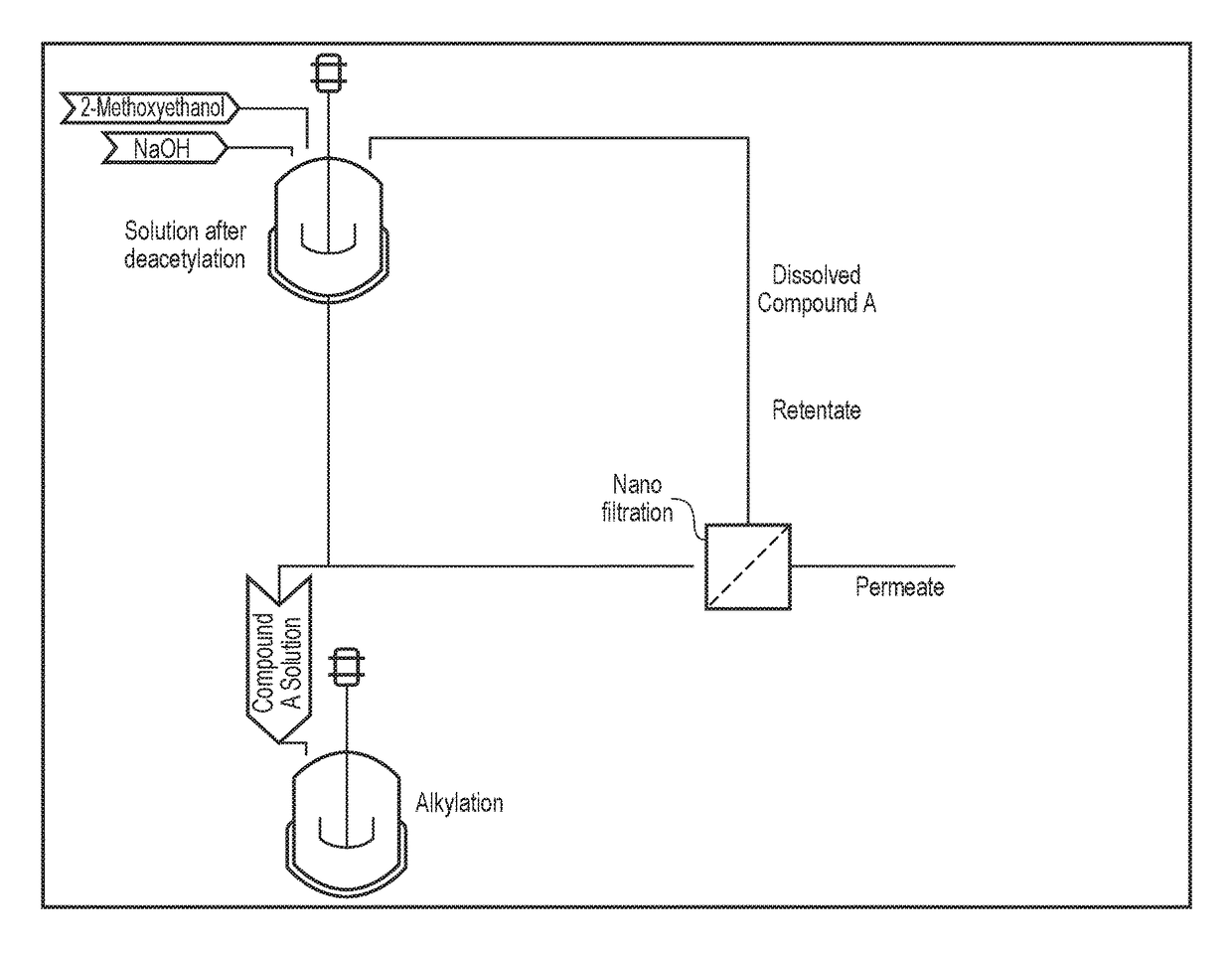
This invention relates generally to industrial preparation of iodixanol (1,3-bis(acetamido)-N,N′-bis[3,5-bis(2,3-dihydroxypropylaminocarbonyl)-2,4,6-triiodophenyl]-2-hydroxypropane), a non-ionic X-ray contrasting agent. It further relates to a method for preparing a crude mixture of the dimerisation reaction from 5-acetamido-N,N-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (“Compound A”) to iodixanol for the crystallization of iodixanol. In particular, it relates to an industrial procedure of simultaneously reducing the salt content and the alcoholic dimerisation solvent using a nanofiltration system prior to the crystallization of iodixanol.
Owner:GE HEALTHCARE AS
Recovering unreacted intermediate from desalinated and desolventized dimerisation reaction mixture by ultrafiltration
InactiveUS20110021828A1Wastage of Compound A is minimizedReduce wasteOrganic compounds purification/separation/stabilisationSemi-permeable membranesCompound aUltrafiltration
This invention relates generally to industrial preparation of iodixanol (1,3-bis(acetamido)-N,N′-bis[3,5-bis(2,3-dihydroxypropylaminocarbonyl)-2,4,6-triiodophenyl]-2-hydroxypropane), a non-ionic X-ray contrasting agent. It further relates to a method of recovering intermediate 5-acetamido-N,N-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (“Compound A”) from the desalinated and desolventized dimerisation reaction mixture. In particular, the present invention employs ultrafiltration to recover non-crystalline Compound A to reduce the overall cost of iodixanol manufacture, increase the yield of iodixanol, and facilitate the subsequent purification procedures to meet the regulatory purity requirement of iodixanol.
Owner:GE HEALTHCARE AS
Iodination method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine compound
InactiveCN103086915AOrganic compound preparationCarboxylic acid amides preparationIodixanolIodination reaction
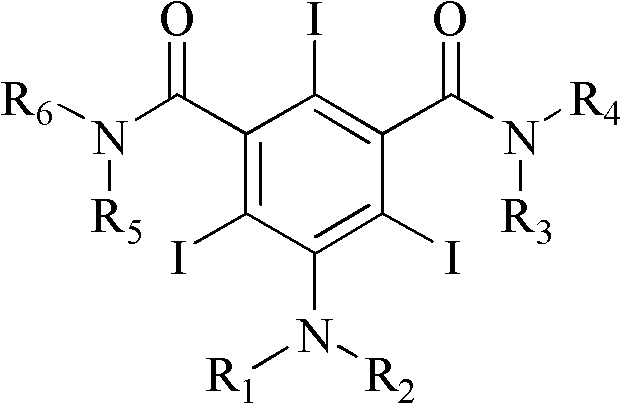
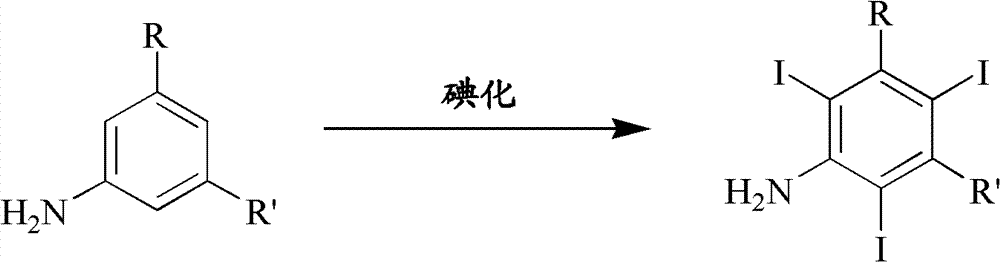
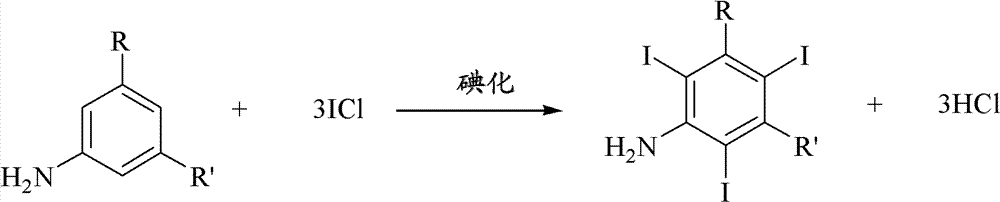
The invention discloses an improved method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by a formula (II), wherein R1 and R2 are defined in the instruction, and the compound represented by the formula (II) is a key intermediate for synthesizing iopamidol, iohexol, iodixanol and a series of non-ionic contrast agents. The method comprises: adopting a chlorine-free iodination reagent and a 3,5-disubstituted aromatic amine compound to carry out an iodination reaction to obtain the 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by the formula (II), wherein a mole yield of the iodination reaction can be 89%.
Owner:上海亿脉利医药科技有限公司
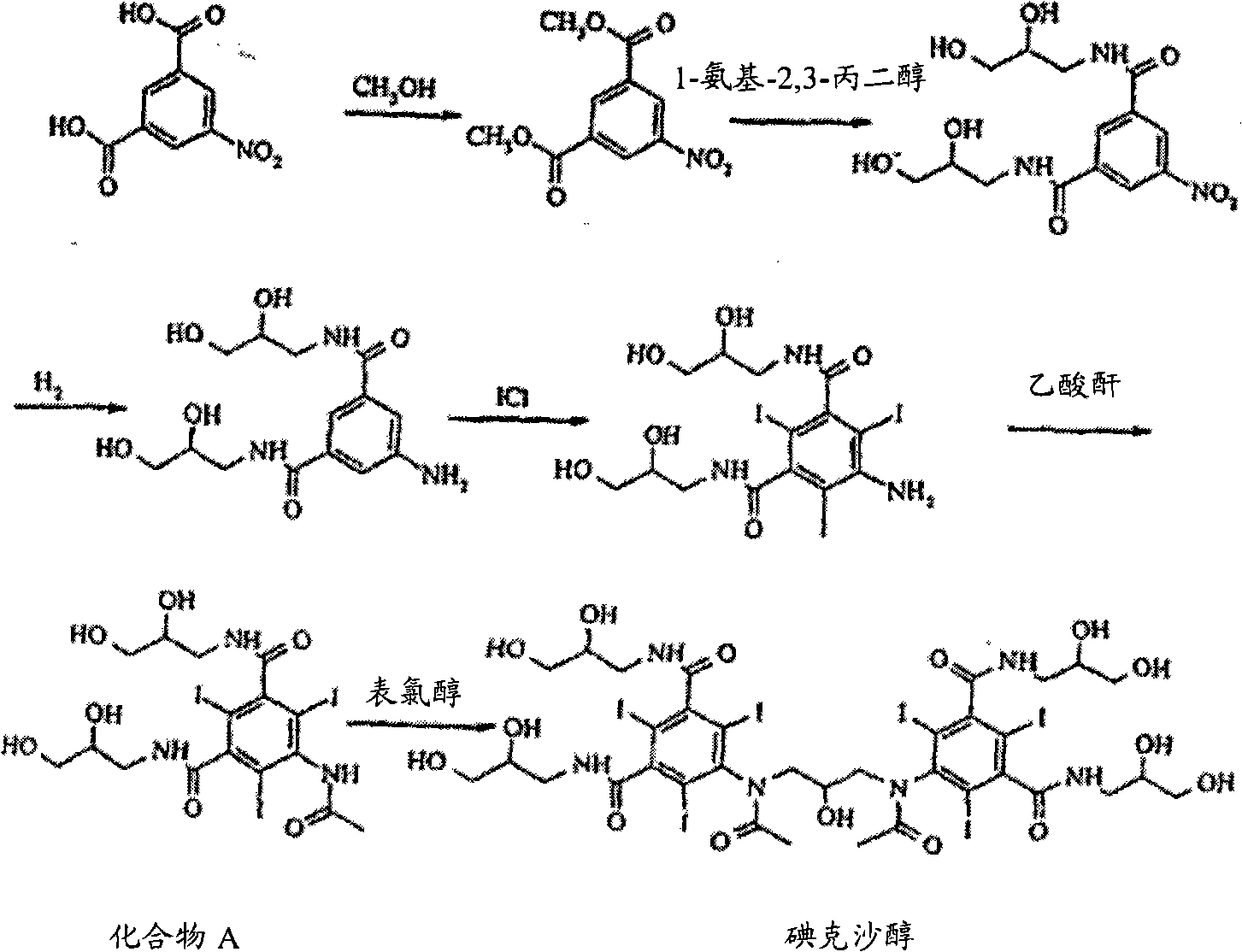
Preparation and purification of iodixanol
ActiveCN102079716AOrganic compound preparationCarboxylic acid amide separation/purificationIodixanolSolvent
The invention provides an improved synthesis method of iodixanol and macroporous adsorbent resin chromatography column purification and solvent recrystallization purification processes which are easy to be used for industrialization. In the synthesis method, iodixanol is synthesized by dimerisation of 5-acetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide ('Compound A'), wherein hydroxyacid containing boron or borate and the like are used to form a buffer solution for controlling the pH value of a reaction process, thus side reactions such as excessive alkylation and the like are effectively inhibited and the transformation ratio for transforming into the iodixanol is improved, the crude product containing 85-90% of iodixanol is obtained and then is purified by a macroporous adsorbent resin chromatography column, the total recovery of the iodixanol is 90-95%, the purity of the iodixanol is more than 97%; and the iodixanol is subjected to recrystallization in a solvent containing 2-methoxy ethanol, the recovery of the iodixanol is 90-95%, and the purity of the iodixanol is more than 99%.
Owner:ZHEJIANG HISYN PHARMA
Fluorescent-CT bimodal imaging probe and preparation method thereof
InactiveCN104721841AReduce the risk of adverse reactionsLow equipment requirementsX-ray constrast preparationsFreeze-dryingIodixanol
The invention relates to a fluorescent-CT bimodal imaging probe and a preparation method thereof, specifically relates to a fluorescent-CT bimodal iodine doped carbon quantum dot prepared by a one-step hydrothermal carbonization method and application of the fluorescent-CT bimodal iodine doped carbon quantum dot in biomedical images, and belongs to the field of biomedical materials. The specific scheme is as follows: dissolving iodixanol and glycine in an aqueous solution according to a certain mass ratio to serve as precursors, putting the solution in a hydrothermal reaction kettle with polytetrafluoroethylene lining, heating up to 120-200 DEG C to react for 4 hours, dialyzing and freeze drying nigger-brown reaction liquid to remove residues and water to obtain black powdery nanoparticles, namely iodine doped carbon quantum dots. The iodine doped carbon quantum dot prepared by the method has such excellent properties as stable fluorescence, efficient X-ray attenuation capacity and good biocompatibility, and is successfully applied to live cell fluorescent imaging in vitro and CT imaging in vivo. The method has the advantages of simple operation, environmental protection and easy mass production.
Owner:JIANGSU UNIV
Method for separating and purifying iodixanol injection raw materials by macroporous resin
InactiveCN102875411AIon-exchange process apparatusIon-exchanger regenerationIodixanolBiological activation
The invention relates to a method for separating and purifying iodixanol injection raw materials by macroporous resin. The method comprises the following steps: dissolving and synthetizing the iodixanol raw materials with water, carrying out adsorption separation and purification with PIPO-02 macroporous resin, adopting a non-linear chromatography method to perform pressing continuous chromatography, carrying out segmentation elution with ethanol, activating and recycling parting materials. The process flow is simple, the cost is low, the efficiency is high, the environment is protected, posts can be subjected to on-line activation and recycle, and the method is suitable for producing iodixanol products for injection industrially. PIPO-02 macroporous resin has remarkable advantages as compared with C-18 separating material, the cost of PIPO-02 macroporous resin is only about 20% of C-18 reverse phase silica gel, the adsorption capacity of PIPO-02 macroporous resin is about 2.5 times of C-18 separating material, the non-linear chromatography method is adopted to produce 98% iodixanol products for injection to the maximum limit, and the 98% iodixanol products have obvious advantages.
Owner:PI & PI BIOTECH
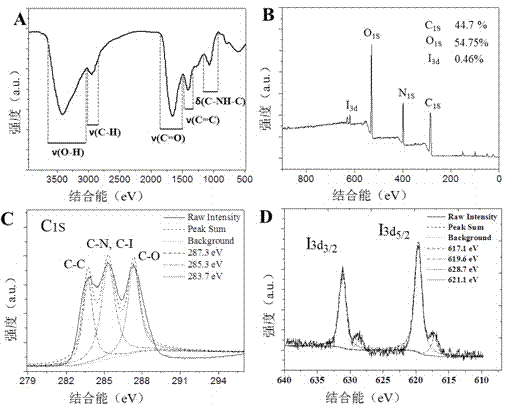
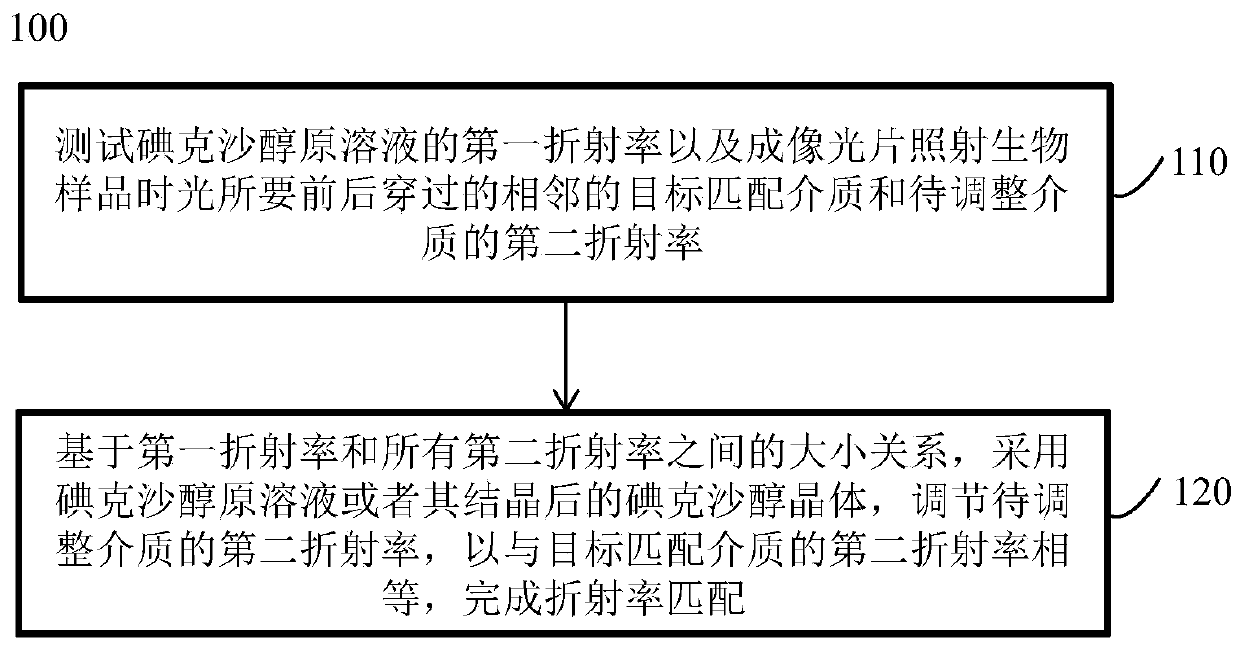
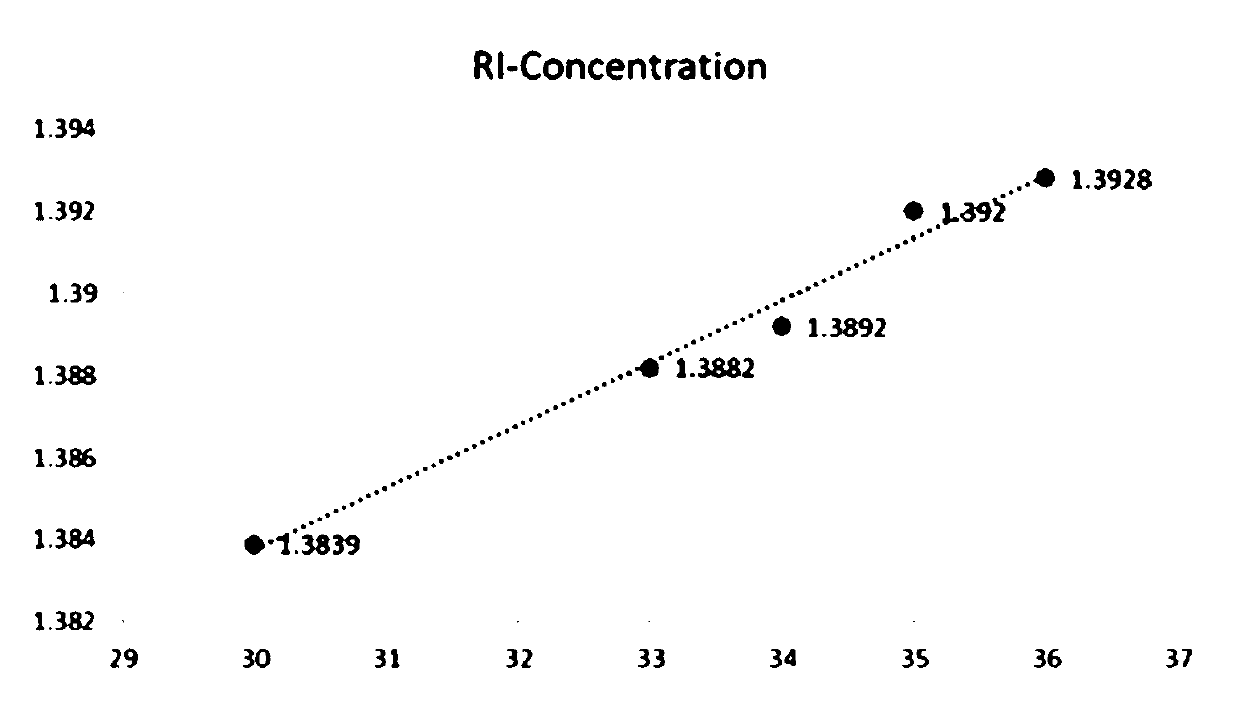
Refractive index matching method adaptive to microfluidic-X-ray plate imaging and application thereof
ActiveCN110579442AImprove efficiencyHigh precisionMaterial analysis by optical meansLaboratory glasswaresImaging qualityBiocompatibility Testing
The invention discloses a refractive index matching method adaptive to microfluidic-X-ray plate imaging and an application thereof. The method comprises the following step: an imaging environment of biological sample X-ray plate imaging is adjusted by using iodixanol, so that the refractive indexes of adjacent media in the imaging environment are matched, wherein the adjacent media are respectively a microfluidic chip used for fixing a biological sample and imaging liquid containing the biological sample, or the adjacent media are respectively oil and water on water-in-oil droplets containingthe biological sample and formed by the micro-fluidic chip. An iodixanol solution is introduced, and the refractive index of the imaging environment is adjusted by changing the concentration of iodixanol. The adjustment range is wide, and the refractive indexes of adjacent media in the imaging environment can be matched. The scattering phenomenon of illumination light on an oil-phase and water-phase interface and a solid-liquid interface of a PDMS-solution is eliminated. The problems of scattering and aberration of imaging are solved. Meanwhile, iodixanol has biocompatibility, real-time imaging can be achieved, and the imaging quality is improved.
Owner:HUAZHONG UNIV OF SCI & TECH
Crystallization of iodixanol in isopropanol and methanol
The present invention relates to crystallization of iodixanol in isopropanol and methanol, concretely relates to a process for the manufacture of iodixanol by performing a crystallization process of the crude product in a solvent mixture comprising water, methanol and isopropanol. The crude product may be obtained in aqueous solution from dimerisation of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide (''Compound A'').
Owner:GE HEALTHCARE AS
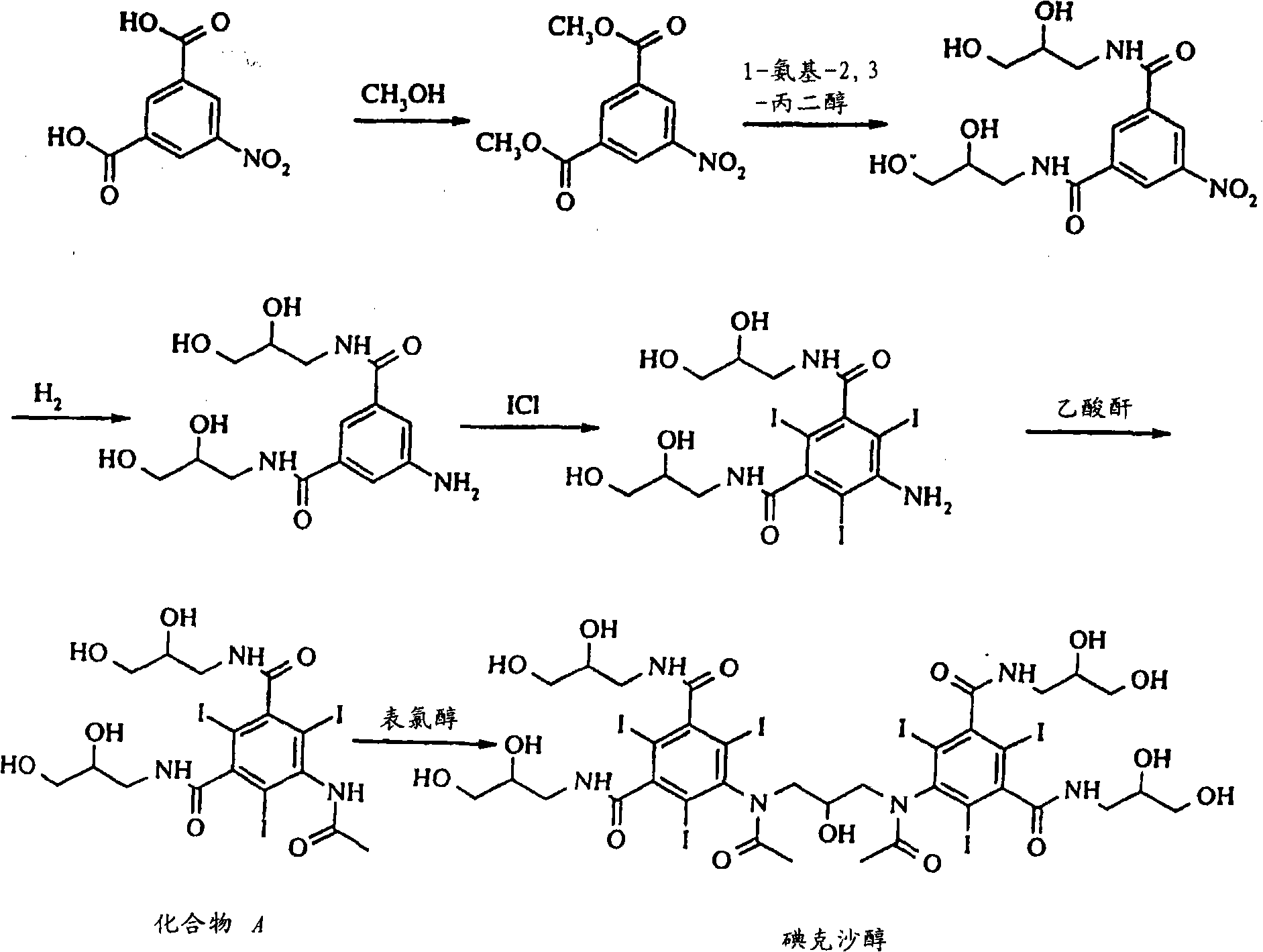
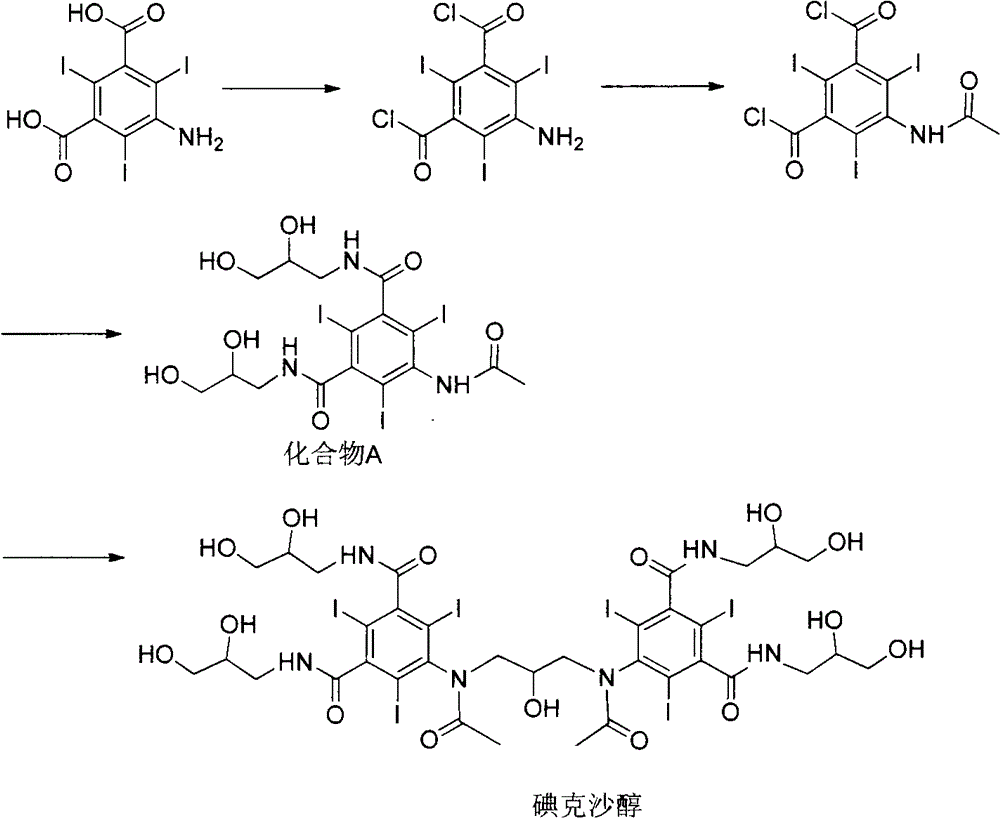
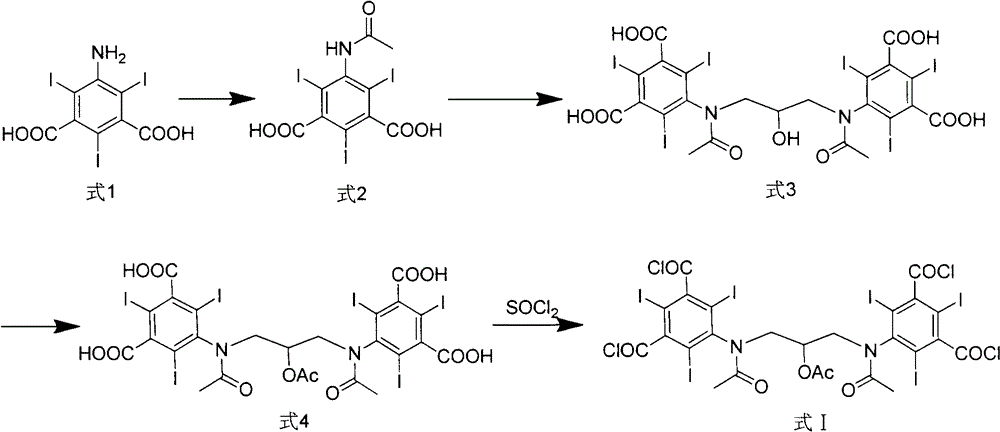
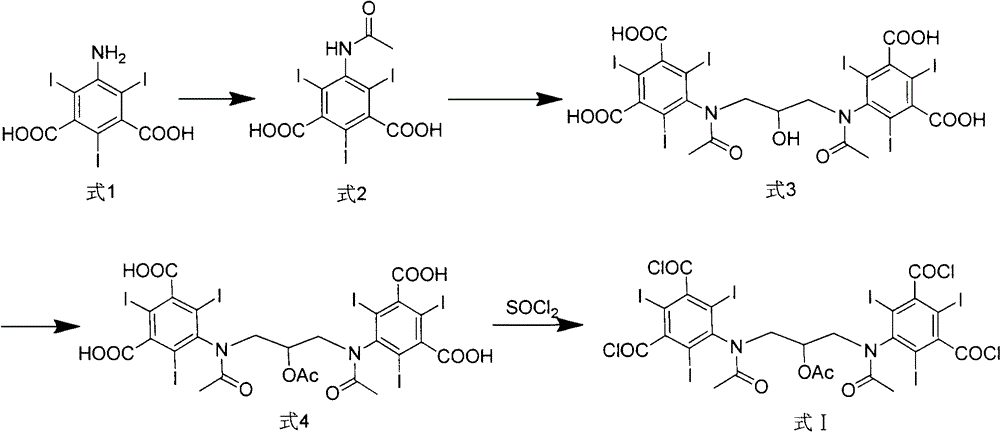
Iodixanol and preparation method of synthetic intermediate thereof
ActiveCN103058880AIncrease contentReduce difficultyOrganic compound preparationCarboxylic acid amides preparationIodineIodixanol
The invention discloses iodixanol and a preparation method of a synthetic intermediate 1, 3-bi (acetamino)-N, N'-bi [3, 5-bi (chloroformyl)-2, 4, 6-triiodo-phenyl]-2-propanol acetate of iodixanol. A dimerization condensation reaction of 5-acetamino-N, N'-bi (2, 3-dihydroxy propyl)-2, 4, 6-triiodo-isophthalamide as a final step is avoided by the preparation method, so that the content of iodixanol in a product is effectively improved, the purity of the obtained iodixanol crude product is greater than 90%, and the highest content of single impurity is less than 2%, so that the difficulty of purifying the product is reduced.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Production method for lodixanol hydrolysate
ActiveCN101195587AReduce pollutionEasy to operateOrganic compound preparationCarboxylic acid amides preparationTriiodideHydrolysate
The invention provides a method for preparing 5-(acetylamino)-N, N'-di (2, 3-dihydroxy propyl)-2, 4, 6-triiodide-1, 3-phenyl diformamide as Iodixanol hydrolysate and the method is that 5-acetylamino-2, 4, 6-triiodide-1, 3-phenyl diformamide is adopted as raw material to react with alpha-halogenated glycerin or 1-halogenated-2, 3-epoxypropane under the temperature of 30-150 DEG C for 1-20 h in the molecular organic solvent or ion liquid such as alkyl benzene, DMSO, DMF, acetonitrile, C1-C10 alcohol and the like acted by inorganic or organic alkaline such as catalyst quantity of alkaline metal carbonate, alkaline hydroxide, organic amine and the like. The obtained material is separated and purified to produce Iodixanol hydrolysate. The invention has the advantages of simplifying production operation, fitting for industrial batch production, reducing environmental pollution, improving the yield, thereby reaching the goal of reducing energy consumption.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Novel method for separating and preparing technical raw materials by multi-grade resin
InactiveCN107200696AIon-exchange process apparatusIon-exchanger regenerationEnvironmental resistanceIodixanol
The present invention relates to a process method for separating and purifying iodixanol by using a macroporous adsorption resin material, comprising dissolving and synthesizing raw materials of iodixanol in water, using a PIPO-02 macroporous resin column to adsorb, separate and purify, and adopting a non-linear chromatography method, Pressurized continuous chromatography, ethanol segmental elution, and the separation material is activated and recycled. The technological process is simple, the cost is low, the benefit is high, environmental protection, the column can be activated and regenerated on-line, and it is suitable for industrial production of iodixanol products for injection. Compared with C-18 separation material, PIPO-02 macroporous adsorption resin has obvious advantages, the cost is only about 20% of C-18 reversed-phase silica gel, the adsorption capacity is about 2.5 times that of C-18 separation material, and a nonlinear layer is used Analytical method, maximum production of 98% iodixanol for injection products, has obvious advantages.
Owner:JIANGSU WANLI BIOTECH CO LTD
Purification process for Iodixanol
ActiveCN101293855BFulfill the standardMeet the needs of industrialized mass productionCarboxylic acid amide separation/purificationPurification methodsIodixanol
The invention provides a method for purifying iodixanol. The invention adopts a recrystallization method to purify crude iodixanol prepared from dipolymer of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo isophthalamide, after several times of repeated purification, an iodixanol product qualified to Pharmacopoeia standards is obtained. The inventive purification method can be effectivelyused for purification of crude iodixanol prepared from dipolymer of 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo isophthalamide, and the used solvent has low cost, so as to remarkably reduce cost compared with the prior purification method. In addition, the inventive purification method is a non-chromatographic solvent purification method and can obtain iodixanol with high purity and high content after several times of repeated purification, so as to meet the industrial production requirements of iodixanol product, as well as Pharmacopoeia standards.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Alternative process for the purification of an intermediate in the synthesis of non-ionic X-ray contrast agents
ActiveUS9695113B2Organic compound preparationCarboxylic acid amide separation/purificationCompound aX-ray
Alternative continuous downstream processes for the production of 5-acetamido-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (“Compound A”) are described. Compound A is a key intermediate in the production of iodixanol and iohexol, which are two of the biggest commercially available non-ionic x-ray contrast media agents.
Owner:GE HEALTHCARE AS
Crystallization purification method for iodixanol
ActiveCN107698457AHigh purityResidue reductionCarboxylic acid amide separation/purificationIodixanolAqueous solution
The invention provides a crystallization purification method for iodixanol. The crystallization purification method uses a simple methanol / ethanol system for recrystallization of a crude iodixanol solid product, does not use an aqueous solution of a crude iodixanol product, and unexpectedly prepares the iodixanol with less solvent residue and high purity. Meanwhile, the crystallization purification method provided by the invention has the advantages of simple operation, less time consumption, high yield, and applicability to industrial mass production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Alternative process for the purification of an intermediate in the synthesis of non-ionic x-ray contrast agents
ActiveUS20160297749A1Organic compound preparationCarboxylic acid amide separation/purificationCompound aX-ray
Alternative continuous downstream processes for the production of 5-acetamido-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide (“Compound A”) are described. Compound A is a key intermediate in the production of iodixanol and iohexol, which are two of the biggest commercially available non-ionic x-ray contrast media agents.
Owner:GE HEALTHCARE AS
Preparation and Purification of Iodixanol
ActiveUS20120283474A1Speed up the processCurbing formationOrganic compounds purification/separation/stabilisationOrganic compound preparationCompound aSynthesis methods
An improved synthesis method for preparation of iodixanol, and a purification process through macroporous adsorption resin chromatographic column and recrystallization are provided. The synthesis method relates to dimerization of 5-acetamido-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide (compound A) to prepare iodixanol, wherein excessive side reactions such as alkylation are effectively inhibited by controlling the pH of the reaction mixture with a boron-containing acidic substance or salts thereof such as boric acid. In this way, the conversion rate of compound A to iodixanol is 85-90%. The iodixanol crude product is purified by a macroporous adsorption resin chromatographic column, obtaining iodixanol product with recovery of 90-95% and purity of 96-98%. The iodixanol crude product is recrystallized in mixed solvent containing 2-methoxyethanol, obtaining iodixanol product with recovery of 90-95% and purity of greater than 99%.
Owner:IMAX DIAGNOSTIC IMAGING HLDG
Crystallization of iodixanol in isopropanol and methanol
A process for the manufacture of iodixanol by performing a crystallization process of the crude product in a solvent mixture comprising water, methanol and isopropanol. The crude product may be obtained in aqueous solution from dimerisation of 5-acetamido-N,N′-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-isophthalamide (“Compound A”).
Owner:GE HEALTHCARE AS
Iodixanol and synthesis method thereof
InactiveCN108530312AHigh yieldHigh purityOrganic compound preparationCarboxylic acid amides preparationAcetic acidSynthesis methods
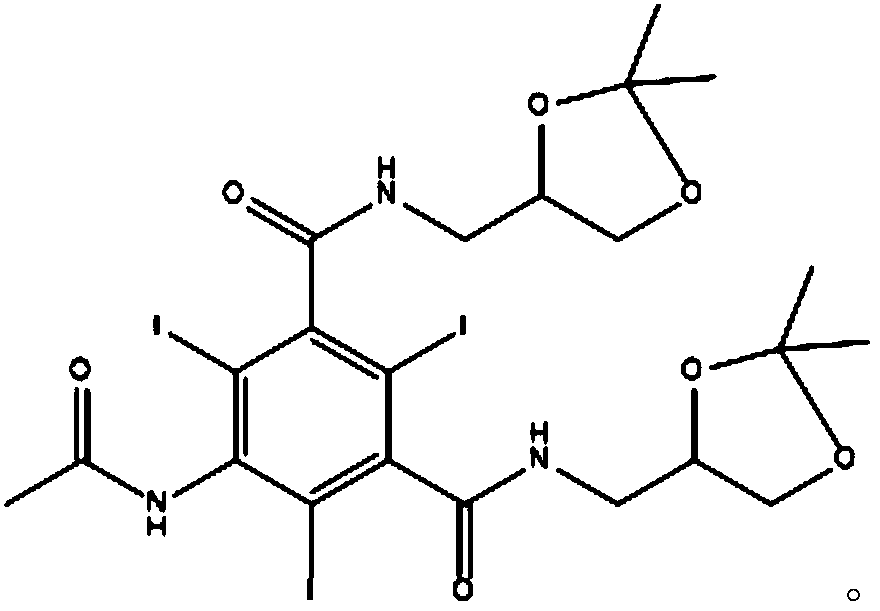
The invention belongs to the technical field of macromolecules, and discloses iodixanol and a synthesis method thereof. With a compound 5-acetamido-N, N'-bis(2, 3-dihydroxypropyl)-2, 4, 6-triiodo-isophthalamide as a raw material, an intermediate compound 5-acetamido-N, N'-bis(2, 3-dimethyl-1, 3-dioxolan-4-yl)methyl)-2, 4, 6-triiodo-isophthalamide is produced through addition of acetic acid and concentrated sulfuric acid. In allusion to the defects of the existing methods for preparing the iodixanol, through production of the novel intermediate compound, production of difficultly removed O-alkylated impurities during a reaction is avoided. The invention provides a method for synthesizing the iodixanol, which is more reasonable, higher in yield and higher in purity. By the method, the reaction cost is lower.
Owner:川金药业有限公司 +1
Iodination Process for the Preparation of 3, 5-Disubstituted-2, 4, 6-Triiodo Aromatic Amines Compounds
ActiveUS20150025275A1Organic compound preparationCarboxylic acid amides preparationIodixanolIodination reaction
The invention discloses a improved process for the preparation of 3,5-disubstituted-2,4,6-triiodo aromatic amines of formula (II), wherein R1 and R2 are defined as herein. The compounds of formula (II) are the key intermediates for the synthesis of a series of non-ionic contrast agents such as Iopamidol, Iohexol and Iodixanol. The process comprises reacting chlorine-free iodinating reagents with 3,5-disubstituted-2,4,6-triiodo aromatic amines to obtain 3,5-disubstituted-2,4,6-triiodo aromatic amines of formula (II), wherein the molar yield of the iodination reaction can reach to 89%.
Owner:IMAX DIAGNOSTIC IMAGING HLDG
Preparation of iodixanol
InactiveCN1178899COrganic compound preparationCarboxylic acid amide separation/purificationCompound aIodixanol
Owner:GE HEALTHCARE AS
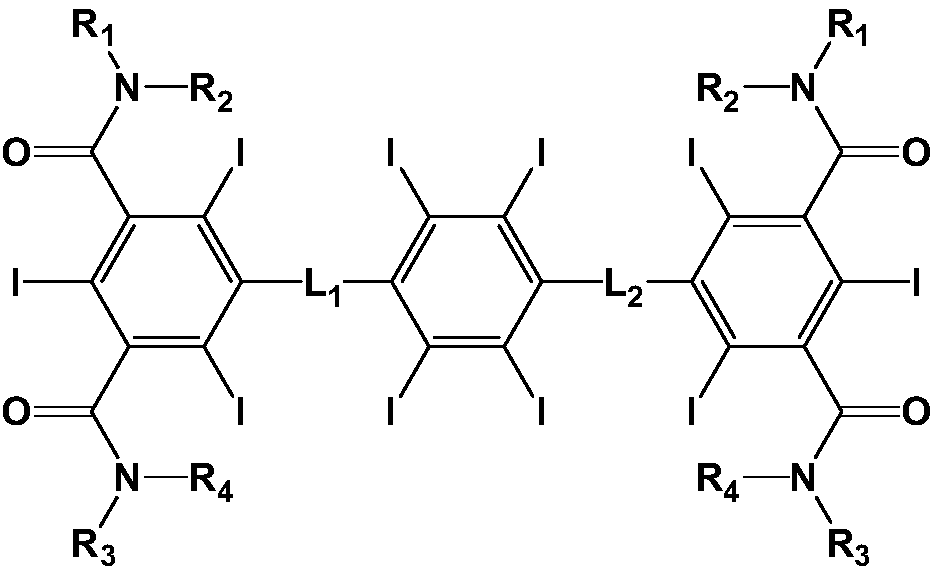
A New Contrast Agent for CT
ActiveCN108191695BHigh in iodineLow viscosityOrganic compound preparationX-ray constrast preparationsIotrolanIodine
The invention relates to an iodine contrast agent. The iodine contrast agent has high iodine content; a contrast agent obtained by using a small amount of a substance under a same iodine / mL concentration can obtain proper osmotic pressure close to human blood osmotic pressure (300 mOsm / kg) and relatively-low viscosity; compared with iotrolan and iodixanol, the iodine contrast agent has more excellent comprehensive physical and chemical properties; in addition, an image obtained by using the iodine contrast agent provided by the invention is clearly-visible, has high distinguishability which iseven superior to the distinguishability of iodixanol, and has broad application prospect.
Owner:MUDANJIANG MEDICAL UNIV
Iodixanol injection and method for preparing same
ActiveCN105106976AImprove shrinkageReduce the impactPharmaceutical delivery mechanismX-ray constrast preparationsHydrogenIrritation
The invention belongs to the technical field of medicine preparations, and discloses iodixanol injection and a method for preparing the same. The iodixanol injection comprises iodixanol, sodium chloride and sodium sulphate. The method includes adding the balance water for injection into the iodixanol, the sodium chloride and the sodium sulphate to obtain mixed liquid; regulating the mixed liquid by the aid of acid until the pH (potential of hydrogen) of the mixed liquid is within an appropriate pH range. Compared with the prior art, the iodixanol injection and the method have the advantages that the types and the quantities of excipients added into the iodixanol injection are greatly reduced, the viscosity of the injection is further lowered, and accordingly the application compliance of clinical patients can be improved; as studied via high-temperature sterilization testes, low-temperature freezing and thawing experiments, irritation tests and the like, the iodixanol injection is stable in quality and low in vascular irritation, and requirements on safe medication can be completely met.
Owner:NANJING CHIA TAI TIANQING PHARMA
Method for culturing contrast agent damage model based on renal tubular epithelial cells
InactiveCN108300687APromote growthReduce toxinsCulture processEpidermal cells/skin cellsSerum free mediaIodixanol
The invention discloses a method for culturing a contrast agent damage model based on renal tubular epithelial cells. The method includes the steps of: (1) conducting HK-2 cell resuscitation; (2) performing cell subculture; (3) observing the culture period cell morphology under a microscope, when the cell edge crumples become round and the refractivity is enhanced, conducting centrifugation, and adding a complete medium to make a cell resuspension solution; (4) transferring the cell resuspension solution into a complete medium according to an inoculation density of 40%-50%; (5) when the cell growth density reaches 80%-90%, performing centrifugation, adding a complete medium to prepare a cell resuspension solution (with a cell concentration of 1.0-1.5*10<6> / ml); and (6) inoculating every 40-60microl of the prepared cell resuspension solution into a 2ml complete medium, conducting culture for 40-50h, then conducting replacing with a serum-free medium and performing culture for 4-8h, thenadding a 45-55mgI / ml iodixanol solution, and further conducting culture for 5-7h, thus obtaining damage model cells. The method provided by the invention can culture the cell damage model that has similar content to contrast-induced nephropathy patient postoperative serum HMGB-1 content, closer damage and fewer system toxins.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
Preparation method of iodixanol and its synthetic intermediate
ActiveCN103058880BIncrease contentReduce difficultyOrganic compound preparationCarboxylic acid amides preparationPropanolIodine
The invention discloses iodixanol and a preparation method of a synthetic intermediate 1, 3-bi (acetamino)-N, N'-bi [3, 5-bi (chloroformyl)-2, 4, 6-triiodo-phenyl]-2-propanol acetate of iodixanol. A dimerization condensation reaction of 5-acetamino-N, N'-bi (2, 3-dihydroxy propyl)-2, 4, 6-triiodo-isophthalamide as a final step is avoided by the preparation method, so that the content of iodixanol in a product is effectively improved, the purity of the obtained iodixanol crude product is greater than 90%, and the highest content of single impurity is less than 2%, so that the difficulty of purifying the product is reduced.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Stabilizing aqueous solution of iodine chloride by adding sodium chloride
This invention relates generally to non-ionic X-ray contrast agents. It further relates to the preparation of iodine chloride, a key reagent in the synthesis of non-ionic X-ray contrast agents such as iodixanol and iohexol. In particular, the iodine chloride is produced in a reaction involving iodine, sodium chlorate, and hydrochloric acid as the starting materials. The instant invention relates to a method of stabilizing aqueous iodine chloride solutions by adding about one to about four molar equivalents of sodium chloride relative to sodium chlorate to an aqueous reaction mixture of sodium chlorate, hydrochloric acid, and iodine.
Owner:GE HEALTHCARE AS
Synthesis of iodixanol in water
ActiveCN102001965BHigh yieldHigh purityOrganic compound preparationCarboxylic acid amides preparationIodixanolSolvent
Owner:GE HEALTHCARE AS
A kind of iodixanol injection and preparation method thereof
ActiveCN105106976BAvoid adverse reactionsSide effects should not be ignoredPharmaceutical delivery mechanismX-ray constrast preparationsFreeze thawingPatient compliance
The invention belongs to the technical field of pharmaceutical preparations, and discloses an iodixanol injection and a preparation method thereof. The iodixanol injection of the present invention comprises the following components: iodixanol, sodium chloride, sodium tartrate, adding water for injection to the full amount, and finally adjusting the pH to a suitable pH range with acid. Compared with the prior art, the types and quantities of auxiliary materials added to the iodixanol injection of the present invention are greatly reduced, the viscosity of the injection is further reduced, and the compliance of clinical application by patients is improved. At the same time, through high-temperature sterilization test, low-temperature freeze-thaw test and irritation test, etc., the iodixanol injection of the present invention has stable quality, low blood vessel irritation, and fully meets the requirements of safe drug use.
Owner:NANJING CHIA TAI TIANQING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com