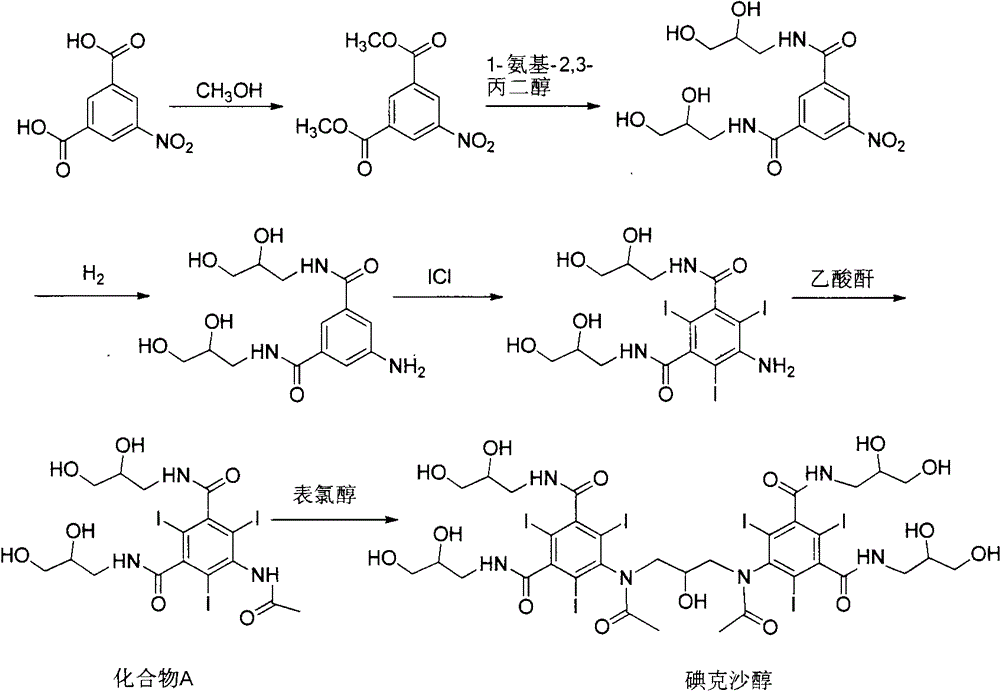
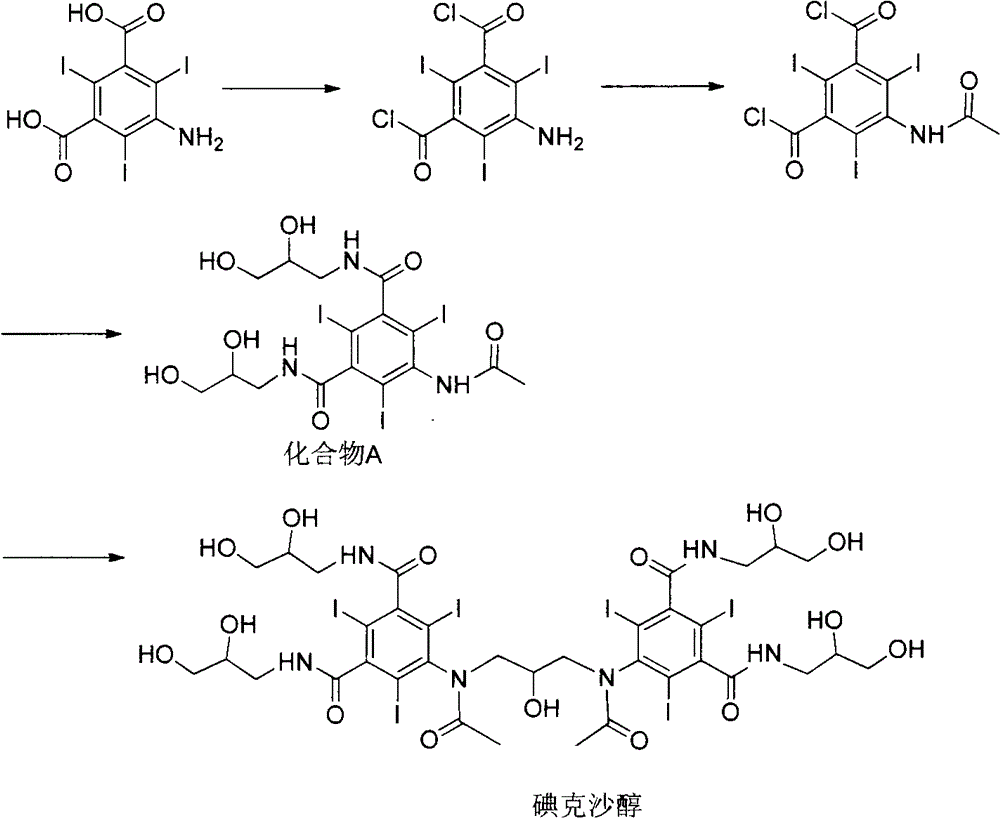
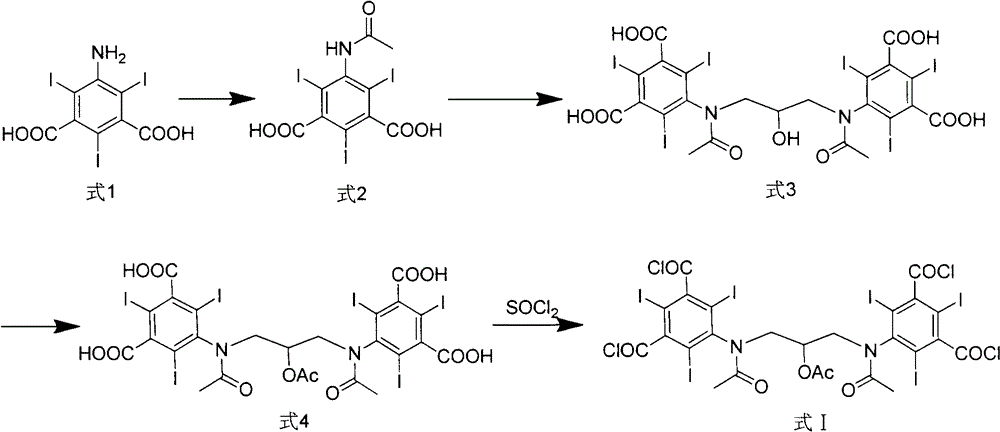
Iodixanol and preparation method of synthetic intermediate thereof
A technology of iodixanol and intermediates, applied in the field of medicinal chemistry, can solve the problems of unreported synthesis reaction conditions and yields, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The synthesis of embodiment 1,5-acetylamino-2,4,6-triiodoisophthalic acid (formula 2)
[0050] Add 500g of 5-amino-2,4,6-triiodoisophthalic acid (1) and 500mL of acetic anhydride in sequence in the reaction flask, raise the temperature to 80°C, keep stirring for 18 hours, and evaporate under reduced pressure after the reaction is completed. solvent to obtain 505 g of off-white solid with a yield of 90%.
Embodiment 2、1
[0051] Example 2, 1,3-bis(acetylamino)-N,N'-bis(3,5-dicarboxy-2,4,6-triiodophenyl)-2-propanol Synthesis of (Formula 3)
[0052] method one
[0053] 500 g of the intermediate (formula 2) directly obtained in Example 1, and 3000 mL of 1-methoxy-2-propanediol were successively added to the reaction flask, sodium hydroxide was added to adjust the pH of the solution to 12, and 1,3-dichloro- 64g of 2-propanol was reacted for 24h-48h. After the reaction, concentrated hydrochloric acid was added to acidify to pH 0-1, and an off-white solid was precipitated. After filtering, the filter cake was dried to obtain 310g of off-white solid with a yield of 59%.
[0054] Method Two
[0055] 500 g of the intermediate (formula 2) directly obtained in Example 1, and 3000 mL of 1-methoxy-2-propanediol were successively added to the reaction flask, potassium hydroxide was added to adjust the pH of the solution to 12, and 1,3-dichloro- 64g of 2-propanol was reacted for 24h-48h. After the r...
Embodiment 3、1
[0060] Example 3, 1,3-bis(acetylamino)-N,N'-bis(3,5-biscarboxy-2,4,6-triiodophenyl)-2-propanol ethyl Synthesis of esters (Formula 4)
[0061] 500 g of the intermediate (formula 3) directly obtained in Example 2, 1000 mL of acetic anhydride and 2 g of 4-dimethylaminopyridine were successively added to the reaction flask, the temperature was raised to 70 ° C, and the reaction was carried out for 12 hours. The solvent was evaporated under reduced pressure to obtain an off-white solid 510g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com