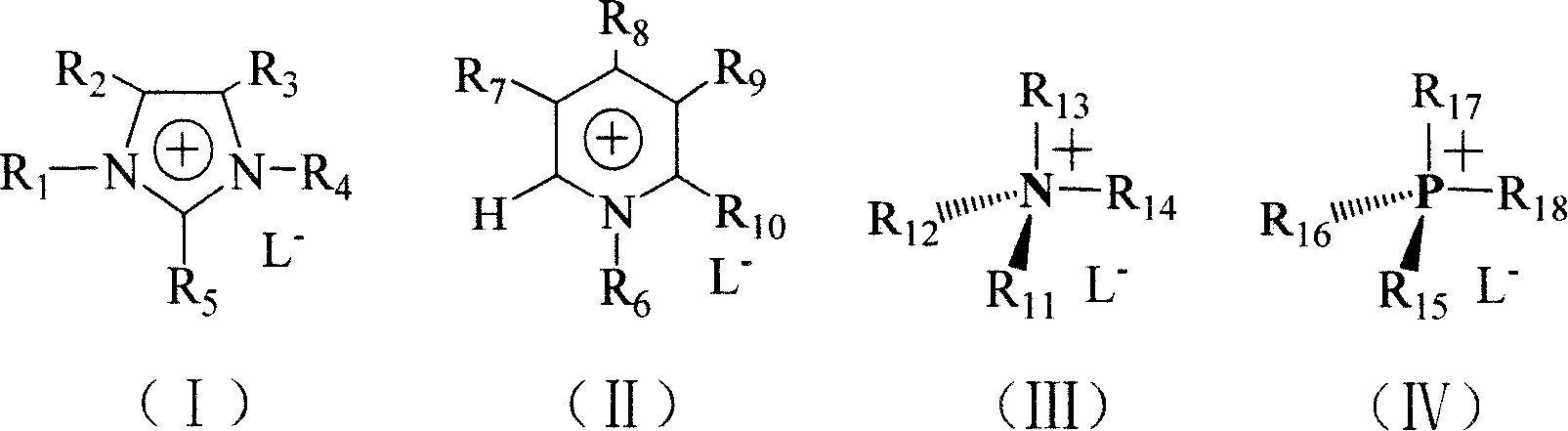
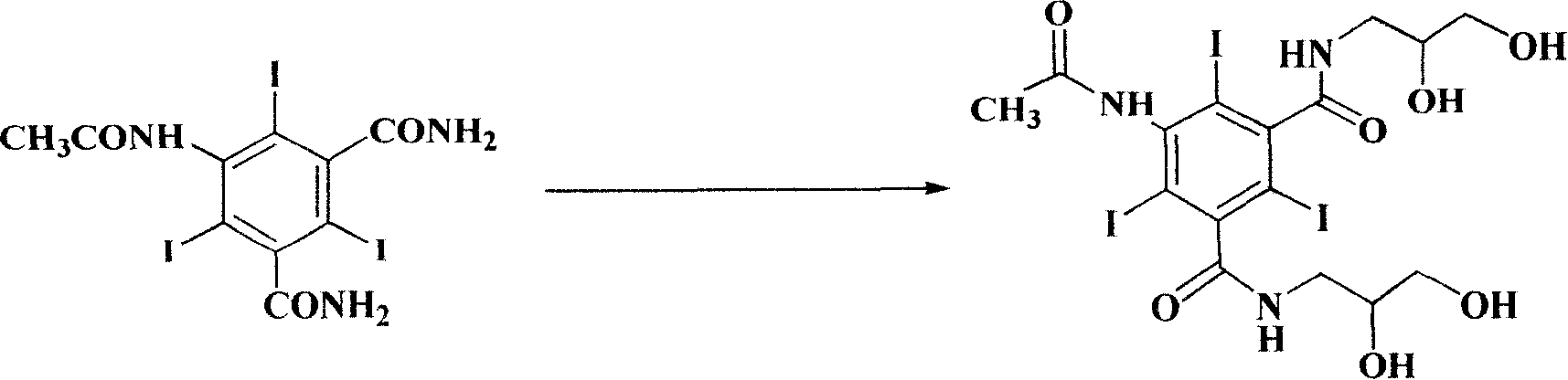
Production method for lodixanol hydrolysate
A technology for iodixanol hydrolyzate and substance, which is applied in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problem of low yield, reduced reaction yield, difficult scale industrial production, etc problems, to achieve the effect of improving yield, reducing environmental pollution, and simplifying production operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 1000 ml reaction flask, add 59.9 g (0.1 mol) of 5-acetylamino-2,4,6-triiodo-1,3-benzenedicarboxamide, 16.6 g (0.15 mol, 13.6 ml) of α-chloroglycerol , a catalytic amount of pyridine, 300 milliliters of 1-butyl-3-methylimidazolium tetrafluoroborate, heated up to 100 ° C, stirred, and reacted for 4 hours. After the reaction was completed, cooled to room temperature and post-treated to obtain 67 grams of the product. Yield 90%.
Embodiment 2
[0035] In a 1000 ml reaction flask, add 59.9 g (0.1 mol) of 5-acetylamino-2,4,6-triiodo-1,3-benzenedicarboxamide, 11 g (0.1 mol, 9 ml) of α-chloroglycerol , a catalytic amount of triethylamine, 300 ml of 1-butyl-3-methylimidazolium tetrafluoroborate, heated up to 100°C, stirred, and reacted for 4 hours. After the reaction was completed, cooled to room temperature and post-processed to obtain the product 62 grams, yield 83%.
Embodiment 3
[0037] In a 1000 ml reaction flask, add 59.9 g (0.1 mol) of 5-acetylamino-2,4,6-triiodo-1,3-benzenedicarboxamide, 16.6 g (0.15 mol, 13.6 ml) of α-chloroglycerol , a catalytic amount of pyridine, using 300 ml of 1-butyl-3-methylimidazolium tetrafluoroborate, heated up to 100 ° C, stirred, and reacted for 4 hours. After the reaction was completed, cooled to room temperature and post-processed to obtain the product 67 grams, yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com