Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "Iohexol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used before X-ray imaging tests (such as CT scans).
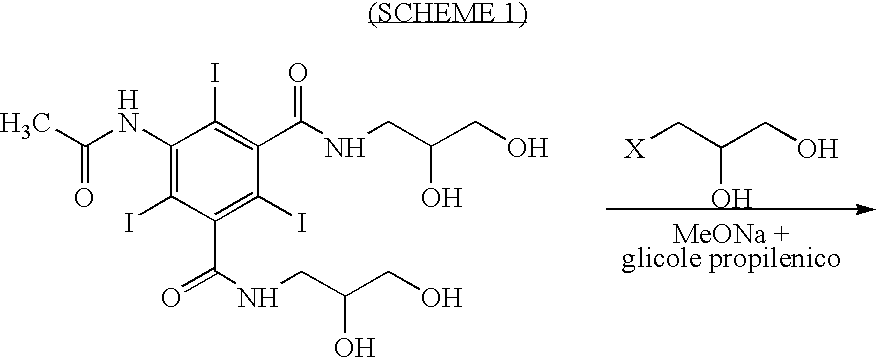
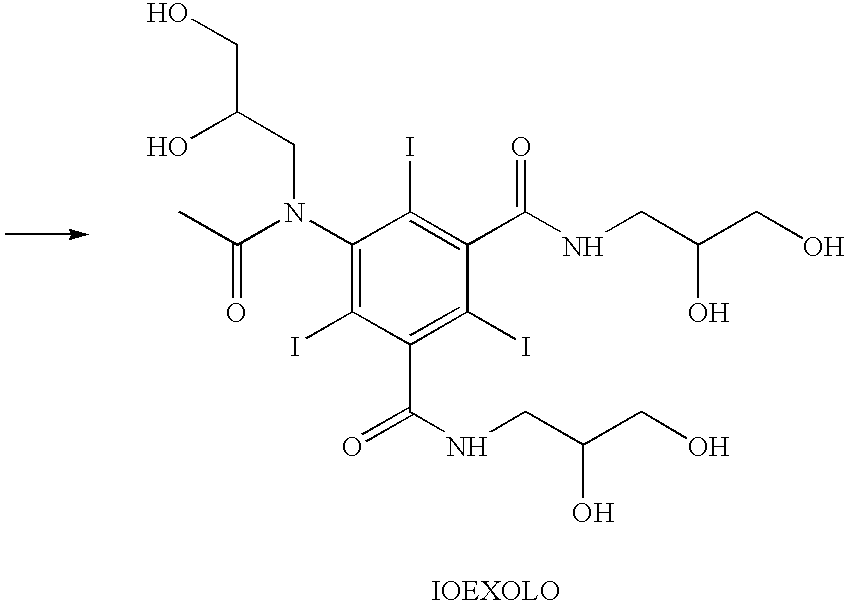
Process for the production of high purity iohexol
InactiveUS6897339B2Reduce impurityEliminate effectiveOrganic compound preparationCarboxylic acid amides optical isomer preparationPropanolPurification methods
Owner:CHEMI SPA
CT imaging contrast agent and preparation method thereof
InactiveCN101732733ALow costNo pollution in the processX-ray constrast preparationsImage contrastBiocompatibility Testing
The invention discloses a CT imaging contrast agent and a preparation method thereof, relating to a contrast agent and providing a CT imaging contrast agent with higher chemical stability, better CT contrast effect and biocompatibility and controllable grains and a preparation method thereof. The CT imaging contrast agent contains nano gold and iohexol and is structurally characterized in that nano gold grains are cores, and the iohexol are packed on the peripheries of the nano gold grains. The preparation method comprises the following steps of: dissolving an iohexol solution in hyperpure water to prepare an iohexol solution the iohexol concentration of which is 1.2-6mg I / ml; adding a chloroauric acid solution in the iohexol solution to prepare a reaction precursor solution the chloroauric acid concentration of which is 0.2-2mM; pouring the reaction precursor solution into an agitated reactor for reaction, and cooling reaction system to the room temperature; and centrifuging the cooled system to enable the synthetized nano gold grains to be separated from the surplus iohexol in the solution and then cleaning to obtain the CT imaging contrast agent.
Owner:XIAMEN UNIV
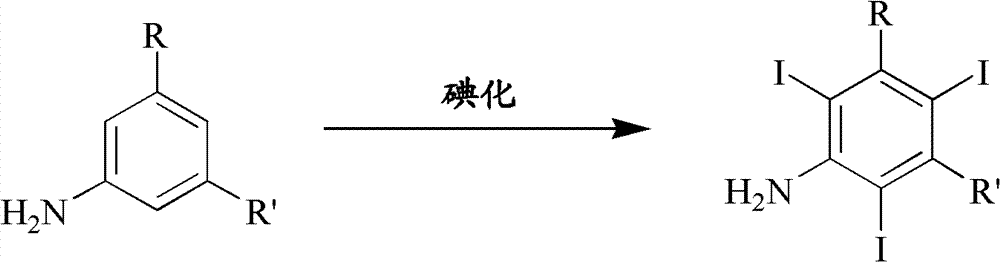
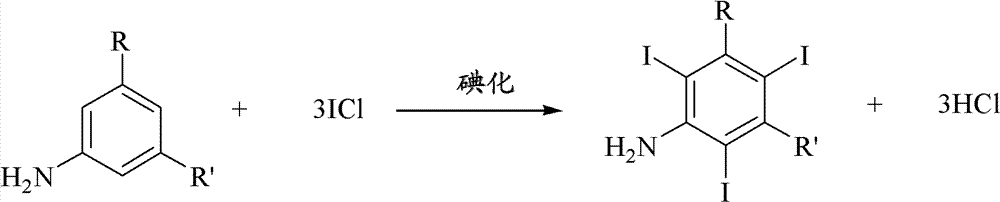
Iodination method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine compound
InactiveCN103086915AOrganic compound preparationCarboxylic acid amides preparationIodixanolIodination reaction
The invention discloses an improved method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by a formula (II), wherein R1 and R2 are defined in the instruction, and the compound represented by the formula (II) is a key intermediate for synthesizing iopamidol, iohexol, iodixanol and a series of non-ionic contrast agents. The method comprises: adopting a chlorine-free iodination reagent and a 3,5-disubstituted aromatic amine compound to carry out an iodination reaction to obtain the 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by the formula (II), wherein a mole yield of the iodination reaction can be 89%.
Owner:上海亿脉利医药科技有限公司
Preparation method of iohexol impurity
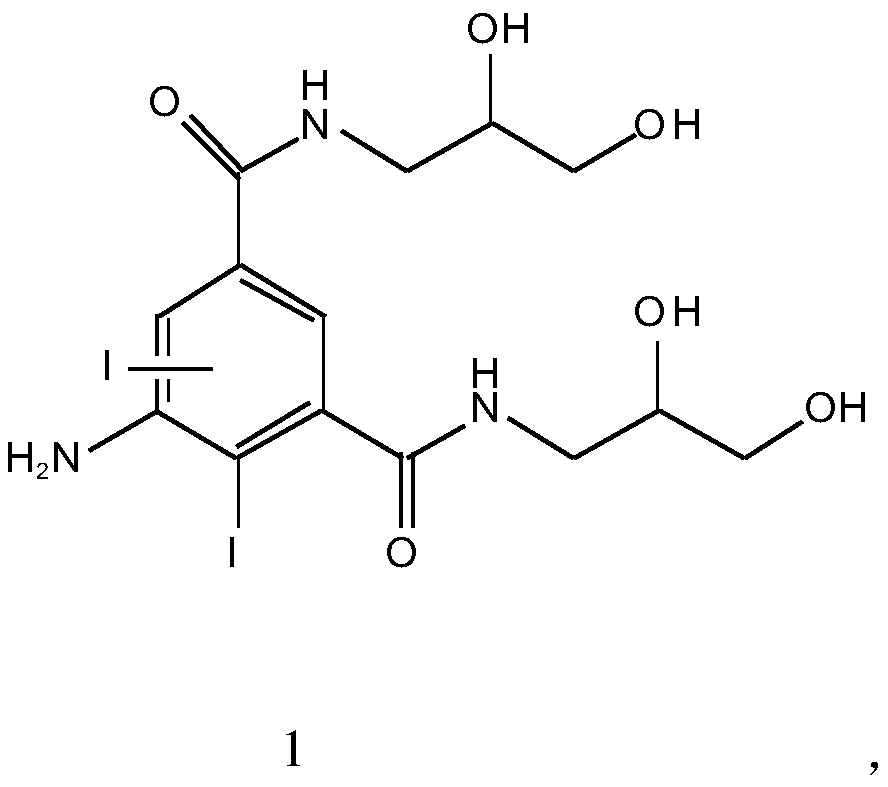
The invention relates to a preparation method of an iohexol impurity shown in Formula (1) 5-[N-(2, 3-dihydroxy-propyl) acetamido]-3-N-(2, 3-dihydroxy-propyl)-2, 4, 6-triiodo-1, 3-benzenedicarboxamide. Due to synthesis of the iohexol impurity, a reference substance can be provided for qualitative and quantitative analysis of iohexol impurities, the quality standards of iohexol can be improved, andimportant guiding significance can be achieved for safe use of the iohexol.
Owner:ZHEJIANG STARRY PHARMA +1
Preparation of dendrimer/ gold-silver alloy nanoparticle with computed tomography (CT) radiography function
InactiveCN101987202AEasy to makeEasy to operateNMR/MRI constrast preparationsEmulsion deliveryDendrimerFifth generation
The invention relates to an in-situ preparation method of dendrimer / gold-silver alloy nanoparticle with a computed tomography (CT) radiography function, comprising the following steps: (1) weighing the fifth generation of polyamide-amine dendrimer of which the tail end is an amino, and preparing 9.7-14.9mg / mL of solution; and (2) in the above solution, adding silver nitrate solution, then addingchloroauric acid solution, adding sodium borohydride solution after stirring, and continuing stirring and reacting for 2-3 hours, dialyzing the reacted solution, freezing and drying to obtain an acetylated pre-product. The dendrimer / gold-silver alloy nanoparticle prepared by the method of the invention has narrow size distribution, good dispersibility and no agglomeration phenomenon. The X-ray attenuation intensity test shows that the X-ray attenuation intensity of alloy nanoparticle is in rising tendency and gradually shows the CT imaging property superior to that of the traditional contrast agent Iohexol.
Owner:DONGHUA UNIV
Process for iohexol manufacture
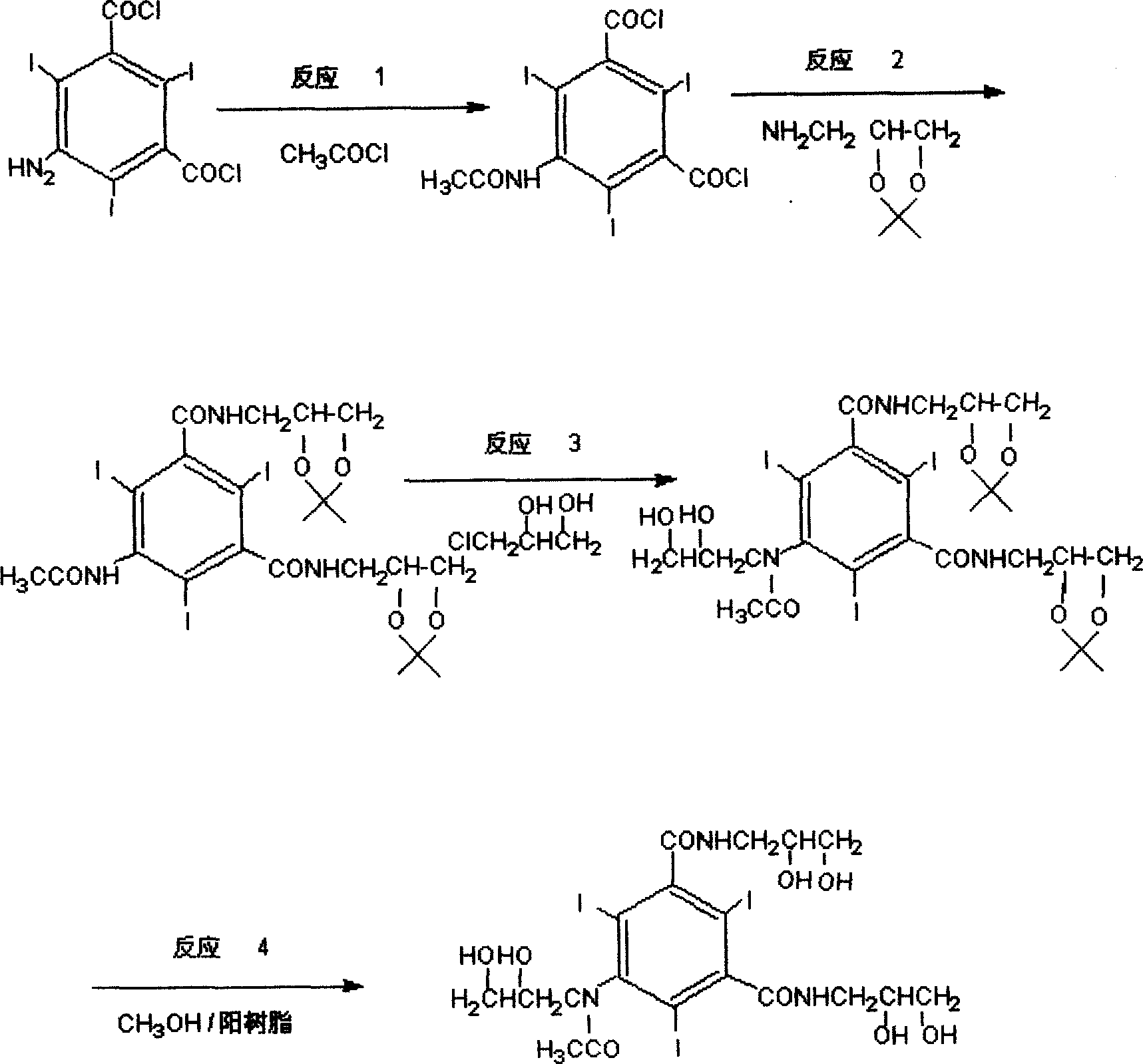
ActiveCN1907961AAvoid it happening againShort reaction timeOrganic compound preparationCarboxylic acid amides preparationMethanol waterAlkyl transfer
The invention provides a preparation method for an iohexol of 5-[N-(2, 3-dihydroxypropyl)acetyl]-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-3-triiodo-1,3-benzamide, belonging to a non-ionic X-CT contrast agent (organic compound) technology field. The invention improves the existing technology by protecting side-chain hydroxyl with amino-acetonide before N-alkylation so as to avoid O-alkylation at the time of N-alkylation. Positive resin / methanol-water system is used as protection system. The invention greatly reduce main impurity oxygen alkyl compound in iohexol and improve the yield.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Preparation and use of injectable isopropyl acrylamide tripolymer as thrombus material and its use
InactiveCN1679620AHigh strengthAvoid false boltsSuture equipmentsOrganic active ingredientsThrombusPropylamine
An injection of three-element isopropyl acrylamide material used as the vascular blocking material for treating the deformity of human cerebral artery and vein is prepared through reacting between propencyl chloride and n-propylamine to obtain propyl propenoyl chloride, synthesizing PNINAVP from ammonium persulfate and tetramethyl ethyldiamine in water, and dissolving iohexol in the solution of PNINAVP.
Owner:TIANJIN UNIV
Synthetic method of iohexol impurity F and application thereof in synthesis of iohexol impurity G, impurity H and impurity M
InactiveCN109912445ARaise quality standardsOrganic compound preparationCarboxylic acid amides preparationHydrogenChloride
The invention relates to a synthetic method of iohexol impurities, in particular to a synthetic method of an iohexol impurity F and an application thereof in synthesis of an iohexol impurity G, an impurity H and an impurity M and belongs to the technical field of medicines. The method comprises the steps as follows: a compound in formula 3 is prepared from raw materials including 5-nitro-1,3-dimethyl phthalate (a compound in formula 2) and 2,3-dihydroxyl propylamine, the compound in formula 3 and hydrogen are subjected to a reduction reaction to prepare a compound in formula 4, and the compound in formula 4 reacts with iodine chloride to obtain the iohexol impurity F. 5-amino-N,N'-bis(2,3-dihydroxypropyl)-diiodo-1,3-benzenedicarboxamide (a compound in formula 1) is synthesized from 5-nitro-1,3-dimethyl phthalate (the compound in formula 2) as the initial material, the impurity G, the impurity H and the impurity M are synthesized from the compound in formula 1 as the initial material, and qualified impurity reference substances are provided for quality control of iohexol.
Owner:BROTHER ENTERPRISES HLDG CO LTD
Process for the manufacture of iohexol
InactiveCN101336228AReduce moisture contentOrganic compound preparationCarboxylic acid amides preparationPotassium hydroxideSolvent
A process for the production of iohexol comprises alkylating 5-Acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6- triiodoisophthalamide using 2(2-methoxy-ethoxy)-ethanol as solvent in the presence of a base, and optionally isolating crude iohexol from the reaction mixture. Preferably, the alkylating agent is 1-chloro-2,3 propanediol and the base is an alkali metal hydroxide such as sodium hydroxide or potassium hydroxide.
Owner:霍维奥恩联合有限公司
Novel bimodal micromolecular contrast agent and preparation method and application thereof
InactiveCN109985252AStrong penetrating powerGood water solubilityOrganic chemistryGeneral/multifunctional contrast agentsSolubilityInfrared
The invention discloses a novel bimodal micromolecular contrast agent and a preparation method and application thereof. According to the contrast agent, by utilizing the characteristic that ICG can beselectively enriched in a liver cancer area and form strong contrast with surrounding tissue, a key structure of ICG is selected as a carrier to be connected with diatrizoic acid, iohexol and other functional groups of CT contrast agents rich in iodine group in order to obtain a liver cancer targeting property and enhance the penetration capacity at the same time, and correspondingly the micromolecular near-infrared / CT bimodal contrast agent is designed and synthesized. The micromolecular contrast agent has high water solubility and low toxicity, and two contrast modes are mutually verified;diagnosis information is enriched, the diagnosis precision is improved, and the feasible novel contrast agent is provided for clinical early diagnosis of liver cancer.
Owner:NANJING DRUM TOWER HOSPITAL
Purification method of high-load iohexol
ActiveCN111100029AIncrease capacityLarge apertureCarboxylic acid amide separation/purificationFluid phaseMicrosphere
The invention discloses a purification method of high-load iohexol. The purification method comprises the following specific operation steps: dissolving a crude iohexol product with deionized water, and filtering the iohexol solution; loading the iohexol solution into a chromatographic column filled with polymer microspheres for adsorption; performing elution by using deionized water as a mobile phase; collecting effluent in sections, carrying out liquid chromatography detection, and gathering the effluent meeting the requirements to obtain a purified iohexol solution; and after purification is finished, regenerating the chromatographic packing by using a low-toxicity reagent. The method is simple to operate and mild in condition, the resin for purification can be recycled, the iohexol loading capacity is high, the production cost is reduced, and the method is particularly suitable for large-scale production.
Owner:SUNRESIN NEW MATERIALS CO LTD GAOLING
PDI-loaded biochar photocatalyst and preparation method and use method thereof
ActiveCN111905812ASimple preparation processIncrease productionWater/sewage treatment by irradiationWater treatment compoundsPtru catalystOrganic synthesis
The invention discloses a PDI-loaded biochar photocatalyst and a preparation method and a use method thereof. The photocatalyst is prepared from the following raw material components: PDI, charcoal and acid in a mass ratio of 1 to 45: 1: 3000 to 7000. According to the invention, perylene-3, 4, 9, 10-tetracarboxylic dianhydride, beta alanine, imidazole and biochar are used as raw materials, an organic synthesis method is adopted to obtain PDI, the photocatalyst is obtained through synchronous acidification, and the application method of the photocatalyst is provided, so that the PDI-loaded biochar photocatalyst prepared by the invention is simple in preparation process and high in yield, and has good application prospects under the condition of not introducing metal elements. A system can perform efficient catalytic degradation on iohexol in water.
Owner:NANJING NORMAL UNIVERSITY
Energy-saving environment-friendly continuous preparation method of iohexol
InactiveCN108191690ASave processing powerOrganic compound preparationCarboxylic acid amides preparationSodium methoxideAcetic anhydride
The invention relates to an energy-saving environment-friendly continuous preparation method of iohexol. The preparation method comprises the following steps: (1) acylation reaction: carrying out a reaction on a compound of a formula [2] with acetic anhydride in an environment of hydrochloric acid to obtain a compound of a formula [3]; (2) transesterification: carrying out a reaction on the compound of the formula [3] with a small-molecule liquid alcohol under an appropriate conditions to obtain a compound of a formula [4]; (3) alkylation reaction: carrying out a reaction on the compound of the formula [4] with and 3-chloro-1,2-propanediol under a condition of a mixed solution of methanol and sodium methoxide to obtain an iohexol crude product; and (4) purification: purifying the iohexol crude product to obtain an iohexol pure product. According to method, the transesterification reaction is carried out on the liquid alcohol with 5-acetylamino-2,4,6-triiodo-N,N'-bis(2,3-diacetoxypropyl)-1,3-phthalamide, then the alkylation reaction is carrred out with the alkylation reagent 3-chloro-1,2-propanediol in a methanol solution of the sodium methoxide, and the obtained iohexol crude product is purified to obtain the pure iohexol product.
Owner:天津河清化学工业有限公司
Temperature-responsive supra-molecular copolymer hydrogel embolization material and preparation method thereof
InactiveCN107754025ASmooth entryFast transitionSurgeryTissue regenerationMolten statePolymer science
The invention discloses a temperature-responsive supra-molecular copolymer hydrogel embolization material and a preparation method thereof, wherein two monomers such as acrylamide and N-acryloyl chloride glycinamide are copolymerize under the action of an initiator to obtain a polymer gel PNAGA-PAAm, the polymer gel and iohexol are mixed at a high temperature to obtain a uniform mixture, the iohexol-containing hydrogel can be injected into renal artery through a microcatheter in a molten state at a temperature of slightly above the body temperature to embolize the kidney so as to be used for embolizing large arteries, the hydrogel mixture can undergo sol-gel transition at the temperature near body temperature, and the transition is fast. According to the present invention, the iohexol-containing polymer can be developed under X-rays, such that the embolization material can smoothly enter the target site, and the false embolization can be avoided.
Owner:TIANJIN UNIV
Contrast media injection being suitable for MR arthrography
InactiveCN106362170AHigh strengthSimplify work proceduresGeneral/multifunctional contrast agentsX-ray constrast preparationsNervous systemSide effect
The invention belongs to the medical field and particularly relates to a contrast media injection being suitable for MR arthrography. The injection is prepared from the following raw materials: 0.05-0.25 ml of gadopentetate dimeglumine, 1.25-5 ml of iohexol, 1.25-5 ml of lidocaine and 0-10 ml of physiological saline. The injection has following advantages: 1) under guidance of X ray or CT, the injection, when being injected into articular cavity for performing the MR inspection to the arthrosis that needs to inspect, can be used for development under both X-ray and MR inspection, wherein according to different inspection positions, only does corresponding proportion adjustment need to carry out, thus greatly simplifying operation procedure of medical staffs; 2) the injection has low permeability, low toxic and side effects, high bio-safety, low toxicity on nervous system, low generation rate of side reactions, and good body tolerance; and 3) the injection can generate high-intensive magnetic resonance signal and increase signal intensity of magnetic resonance imaging, so that an image can be displayed clearly.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Fine purification method for iohexol
InactiveCN107721875ASolution to short lifeEasy to reuseComponent separationCarboxylic acid amide separation/purificationStationary phasePurification methods
The invention provides a fine purification method for iohexol. Crude iohexol is dissolved and filtered; the filtered iohexol solution is loaded into a chromatographic column of high-molecular polymermicrospheres containing hydrophilic and lipophic functional groups for chromatography; water is adopted as a mobile phase to elute the iohexol solution; iohexol solutions of target peaks are collectedstage by stage, and component solutions which meet requirement are gathered, so that a finely purified iohexol solution is obtained. Iohexol which is finely purified by the method disclosed by the invention is high in purity and yield and stable; moreover, operation is simple and convenient, conditions are mild, and high temperature is not needed; since the used stationary phase can be recycled and the used mobile phase is water, environmental protection pressure and cost are greatly reduced; and the fine purification method for iohexol is suitable for large-scale application.
Owner:SUZHOU NANOMICRO TECH CO LTD
Control and regulation device for safeguarding a conveyor device, conveyor device and crane unit
ActiveCN101336205AAccurate and reliable detectionEasy to adjustWinding mechanismsCranesSignal onContainer crane
The invention relates to a control and regulation device for safeguarding a conveyor device (1), in particular, a crane unit in the case of overload. A brake device (11, 18) acting on the conveyor device (1) is provided, along with a controller (30) for the brake device (11, 18), an overload sensor (29), recording the overload and emitting an overload signal and a speed sensor (31, 24), recording a cA process for the production of iohexol comprises alkylating 5-Acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6- triiodoisophthalamide using 2(2-methoxy-ethoxy)-ethanol as solvent in the presence of a base, and optionally isolating crude iohexol from the reaction mixture. Preferably, the alkylating agent is 1-chloro-2,3 propanediol and the base is an alkali metal hydroxide such as sodium hydroxide or potassium hydroxide.onveyed item speed and emitting a speed signal. The controller (30) reacts to the overload signal on the braking device (11, 18), such that this device stops the conveyor device and thus safeguards a conveyed item (16).; On a subsequent unload signal the controller (30) releases the conveyor device (1) such that the conveyor device (1) is moved into an unload position by the effect of the load of the conveyed item (16) and the effect of the braking device (11, 18) on the conveyor device (1) is regulated according to the speed signal from the speed sensor (24, 31) such that the conveyed item (16) is moved into the unload state at an essentially constant conveyor speed. The invention further relates to a conveyor device with such a control and regulation arrangement and a container crane unit with two conveyor devices provided with such a control and regulation device.
Owner:PINTSCH BUBENZER
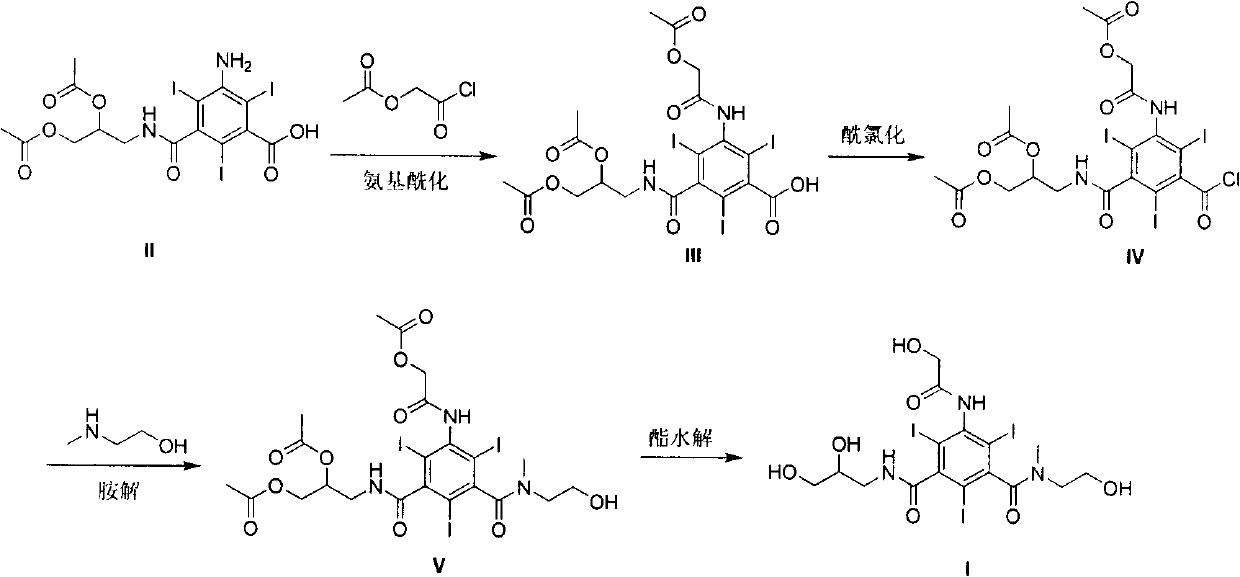
Preparation method of iohexol impurity
ActiveCN104402758ALow priceNo side effectsOrganic compound preparationCarboxylic acid amides preparationMedicineIodine
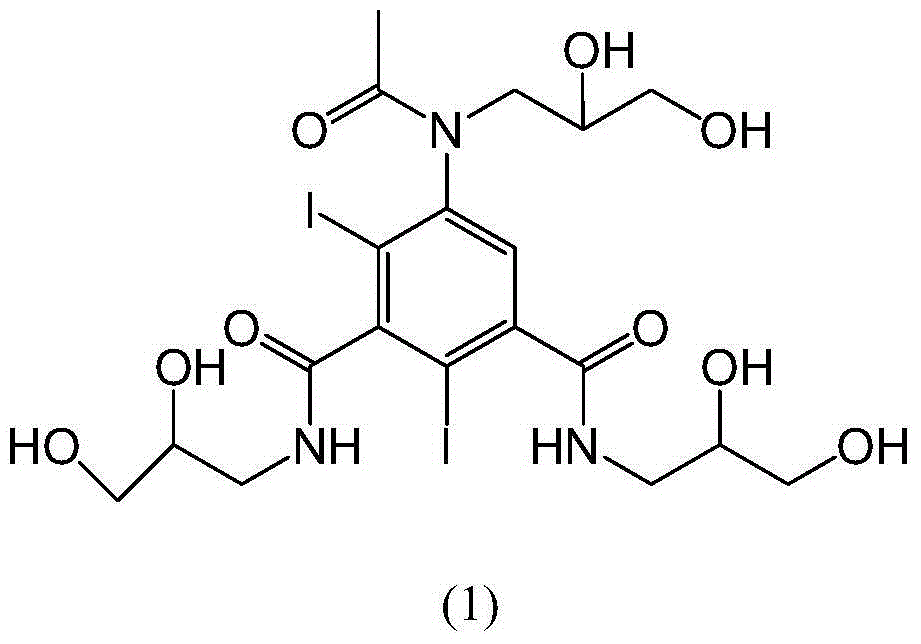
The invention relates to a preparation method of an iohexol impurity component, namely, 5-(N-(2,3-dihydroxypropyl)acetamido)-N,N'-bis(2,3-dihydroxypropyl)-2,4-diiodo-1,3-benzenedicarboxamide, represented by formula (1). A reference substance is provided for qualitative and quantitative analysis of an iohexol impurity through preparation of the iohexol impurity, so that the quality standard of iohexol is improved, and the important guiding significance is provided for safe medication of the iohexol. The formula (1) is shown in the specification.
Owner:SHANGHAI STARRY PHARMA CO LTD
Formula of density gradient solution used for tissue and cell purification
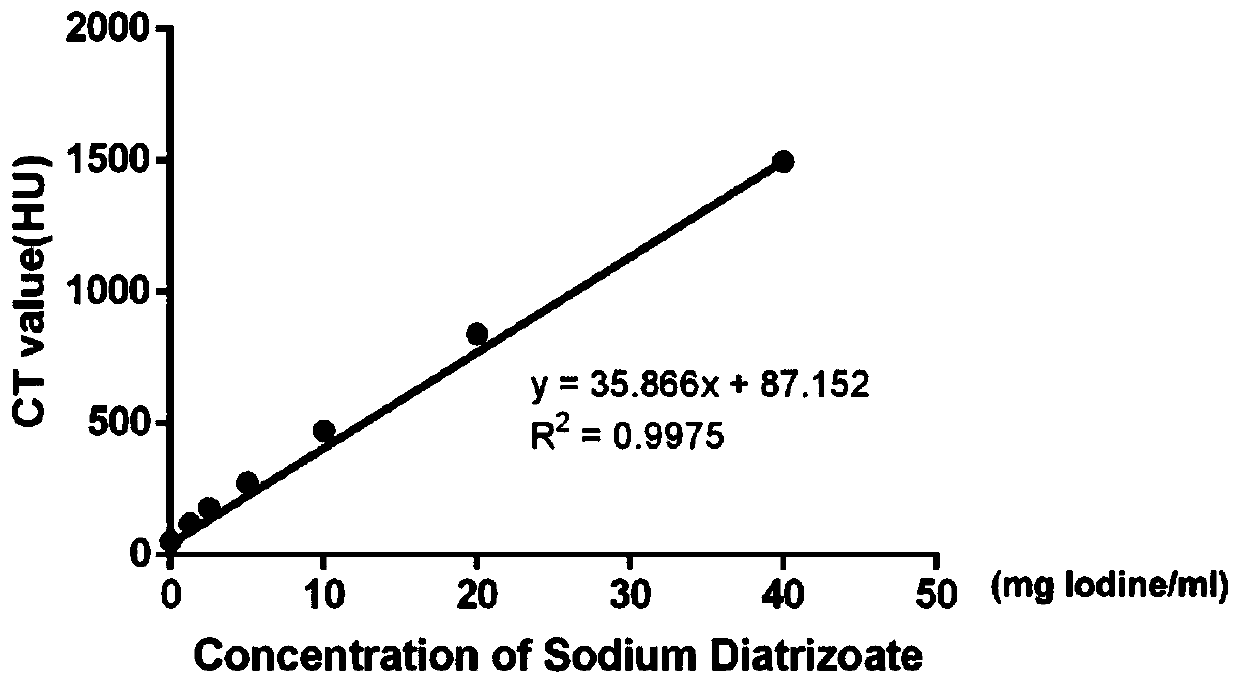
InactiveCN105886454AGood separation resultEasy to separateCell dissociation methodsPancreatic cellsSodium DiatrizoateAdditive ingredient
The invention provides a formula of a density gradient solution used for tissue and cell purification. According to the formula, the density gradient solution contains density gradient ingredients, such as iohexol, sodium diatrizoate and iodixanol, wherein the substances capable of improving the osmotic pressure and preventing tissue and cell edema, such as raffinose and mycose are added, then magnevist solution is also added, and culture solutions such as DMEM culture solution and buffer solutions such as HBSS buffer solution are adopted as the solvents. With the adoption of the formula, the problems that at present, the density gradient separation for tissues and cells of large animals and human beings is difficult, and the separated tissues and cells are low in activity are solved, and the cells and tissues with different densities after dissociation or mechanical dispersion can be separated by the density gradient solution under the centrifugal condition. Due to the design of the density gradient solution, a certain application value is achieved for promoting the domestic and overseas study on the separation of tissues and cells. The principle is simple, the preparation is easy, the use is convenient, and the large-scale preparation can be realized; due to the declaration of the patent, a foundation is laid for the development and application of the density gradient separating solution.
Owner:WENZHOU YIKANG CELL TRANSPLANTATION CO LTD
Preparation method of iohexol impurity
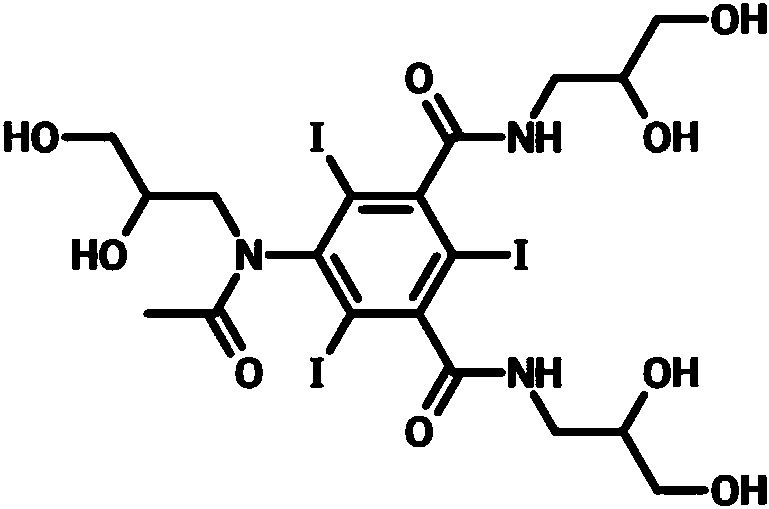
The invention relates to a preparation method of an iohexol impurity as shown in formula (1), namely 5-(N-(2, 3-dihydroxy-n-propyl) acetamido)-N-(2, 3-dihydroxy-n-propyl)-N'-(1-hydroxymethyl-2-hydroxyethyl)-2, 4, 6-triiodo-isophthalamide. Through the synthesis of the iohexol impurity, a control article is provided for qualitative and quantitative analysis of the iohexol impurity, and so that the quality standard of iohexol is improved, which provides important guide significance for safe using of the iohexol.
Owner:ZHEJIANG HISYN PHARMA
Compositions and methods for clearing tissue
ActiveUS20200224129A1Simple methodPreparing sample for investigationOrganic non-surface-active detergent compositionsTissue clearingPolyethylene glycol
A composition and a method for clearing tissue for subsequent three-dimensional analysis. The clearing composition comprises: (1) a homogenizing agent such as N-methylglucamine, urea, or ethylenediamine; (2) a cytoplasmic, water-soluble refractive index adjusting agent such as iohexol, sodium thiosulfate, or polyethylene glycol; and (3) a membrane, lipid-soluble RI adjusting agent such as 2,2′-thiodiethanol (TDE) or propylene glycol. The tissue clearing composition is particularly suitable for use with long-term fixed human tissues.
Owner:THE UNIVERSITY OF HONG KONG
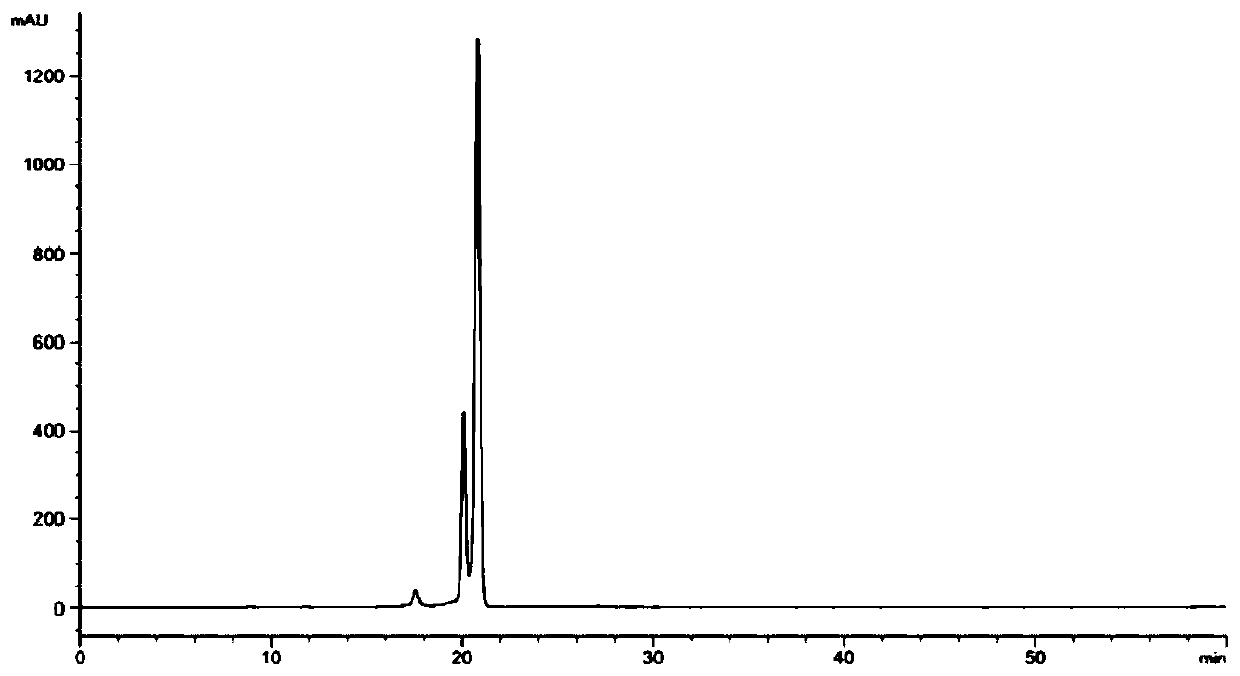
Method for detecting content of iohexol in plasma of rhesus monkeys and application thereof to evaluation of influence of drug on GFR (Glomerular Filtration Rate)
The invention discloses an exclusive method for detecting the content of iohexol in plasma of rhesus monkeys and application thereof to evaluation of the influence of a drug on GFR (Glomerular Filtration Rate), and belongs to the field of drug evaluation. The exclusive method comprises the following steps: (1) standard product working solution and standard curve preparation: preparing standard production working solutions in various concentrations; (2) preparation of an internal standard production solution preparing an iohexol impurity I working solution; (3) sample pretreatment; (4) determination of iodexol: determining under the conditions that a C18 is used as a chromatography column, a mobile phase comprises a mobile phase A which is methanol and a mobile phase B which is phosphoric acid water, isocratic elution with elution time of 10 minutes , 20 to 40 DEG C in temperature and 0.2 to 1.5 mL / min in flowing speed, the detection wavelength of a chromatogram is 190 to 320 nm and thesampling volume is 1 to 20 mu L. The exclusive method disclosed by the invention is capable of detecting the content of the iohexol, is accurate and simple and is time-saving and labor-saving; the dosage of the iohexol required for determining and evaluating the GFR by utilizing the iodexol clearance rate is smaller, and the safety of kidney is greatly improved; the exclusive method is simple andreliable, the exclusiveness is strong, and effective experimental data can be provided for clinical application.
Owner:SICHUAN PRIMED BIO TECH CO LTD
Applications of a temperature-responsive supra-molecular copolymer hydrogel
InactiveCN107754006ASmooth entryFast transitionSurgical adhesivesX-ray constrast preparationsMolten stateX-ray
The invention discloses applications of a temperature-responsive supra-molecular copolymer hydrogel. According to the present invention, two monomers such as acrylamide and N-acryloyl chloride glycinamide are copolymerize under the action of an initiator to obtain a polymer gel, the polymer gel and iohexol are mixed at a high temperature to obtain a uniform mixture, the iohexol-containing hydrogelcan be injected into renal artery through a microcatheter in a molten state at a temperature of slightly above the body temperature to embolize the kidney so as to be used for embolizing large arteries, the hydrogel mixture can undergo sol-gel transition at the temperature near body temperature, and the transition is fast. According to the present invention, the iohexol-containing polymer can bedeveloped under X-rays, such that the embolization material can smoothly enter the target site, and the false embolization can be avoided.
Owner:TIANJIN UNIV
Method and kit for separating stem cells
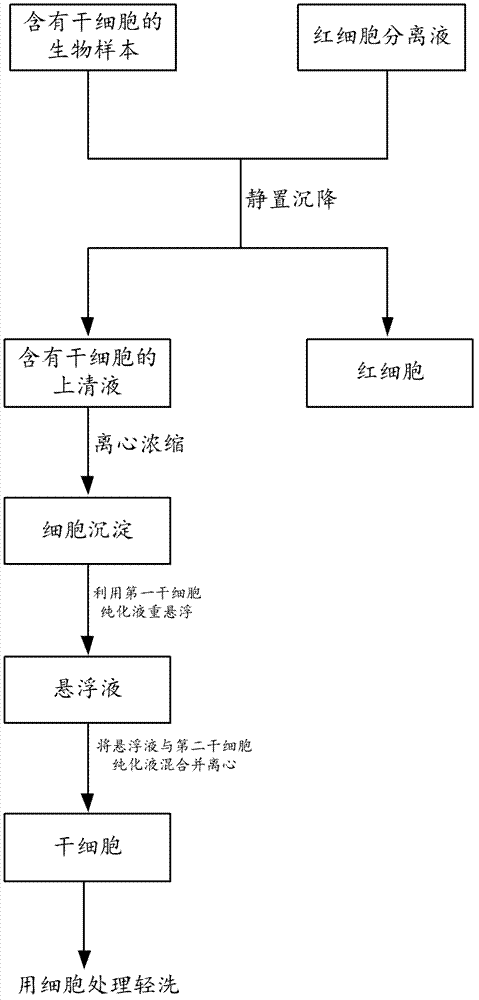
ActiveCN103361299AEfficient separationArtificial cell constructsBlood/immune system cellsBiologyBalanced salt solution
The invention relates to a method and a kit for separating stem cells and provides a composition for separating stem cells. The composition comprises iohexol and balanced salt solution, wherein the iohexol is dissolved in the balanced salt solution. With the adoption of the composition, the stem cells can be effectively separated.
Owner:江苏元生细胞工程科技发展有限公司
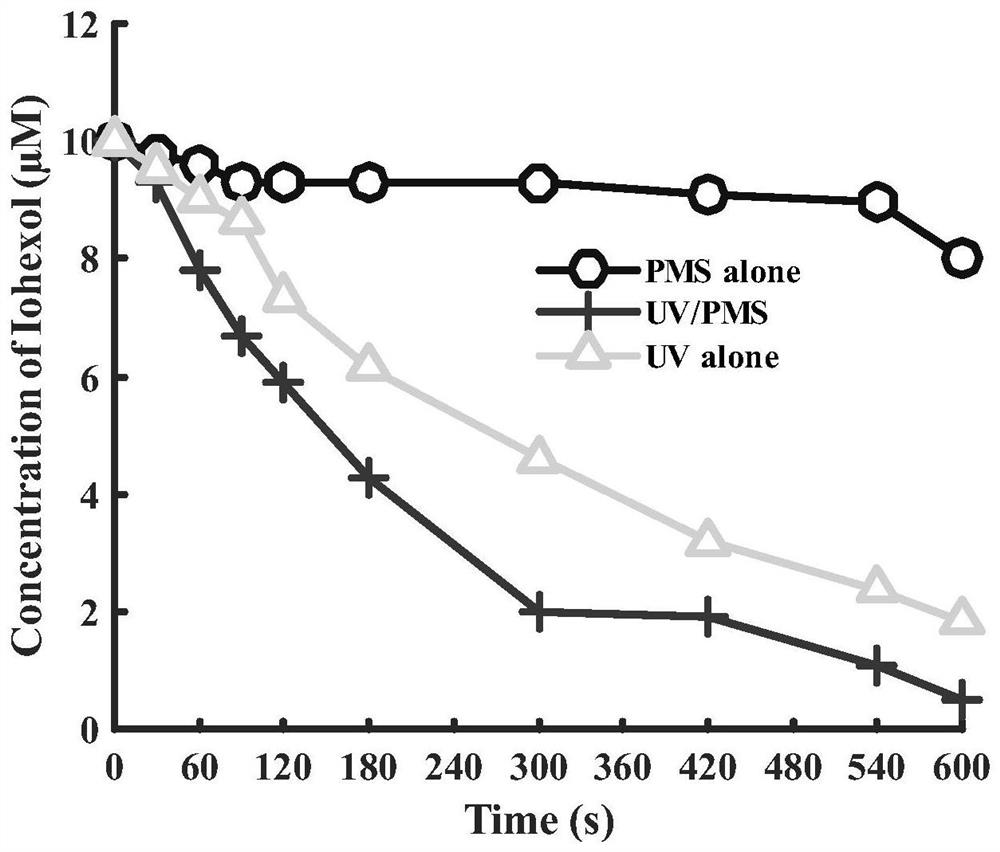
Method for removing high-stability iodinated developer in water
InactiveCN113087116AReduce concentrationEasy to operateWater/sewage treatment by irradiationWater treatment compoundsPersulfateHazardous substance
The invention relates to a method for removing a high-stability iodination developer in water. The method comprises the following steps: (1) pretreating a water sample to be treated; and (2) adding persulfate into the pretreated water sample, adjusting the pH value, and carrying out photocatalytic oxidation reaction by adopting ultraviolet irradiation to remove the iodinated developer in the water. Compared with the prior art, the method has the advantages that the removal effect of iohexol can reach more than 95%, the concentration of harmful substances which are difficult to degrade in water is effectively reduced, the operation is simple, the reaction conditions are easy to control, the used chemical reagents and materials are conventional products for water treatment, other toxic and harmful substances are not introduced, the safety and the practicability are relatively outstanding, and the method is suitable for industrial production. The reaction environment is easy to realize, treatment can be performed at room temperature, and the feasibility and operability of the method are effectively improved.
Owner:SHANGHAI INST OF TECH
Triiodobenzene compound and contrast medium with same
The invention relates to a triiodobenzene compound and a contrast medium with same and also provides a preparation method of the compound. The compound in the invention is 5-(2-hydroxylacetamido)-N-methyl-N-(2-hydroxyethyl)-N'-(2,3-dihydroxyl-n-propyl)-2,4,6-triiodoisophthalamide (I). Compared with the existing clinical nonionic contrast medium monomers such as Iohexol and Iomeprol, the contrast medium in the invention has lower osmotic pressure and viscosity.
Owner:ZHEJIANG STARRY PHARMA
PDI/MOF heterojunction photocatalyst and preparation method and use method thereof
ActiveCN112718009ANo secondary pollutionEasy to operateWater/sewage treatment by irradiationWater treatment compoundsHeterojunctionPtru catalyst
The invention discloses a PDI / MOF heterojunction photocatalyst and a preparation method and a use method thereof. The photocatalyst is prepared from the following raw material components: PDI, MIL-101 (Cr) and acid in a mass ratio of 1: 0.11-1: 37. According to the invention, perylene-3, 4, 9, 10-tetracarboxylic dianhydride, beta-alanine, imidazole and MOF are used as raw materials, PDI is obtained by an organic synthesis method, the PDI / MOF heterojunction photocatalyst is obtained by a water bath heating method, and the use method of the photocatalyst is provided, so that the prepared PDI / MOF photocatalyst has the characteristics of simple preparation process, high yield, large specific surface area and multiple reaction sites; and the use of hydrofluoric acid is avoided, and the material can activate persulfate under visible light to perform efficient catalytic degradation on iohexol in water.
Owner:NANJING NORMAL UNIVERSITY +1
Nano contrast agent and preparation method thereof
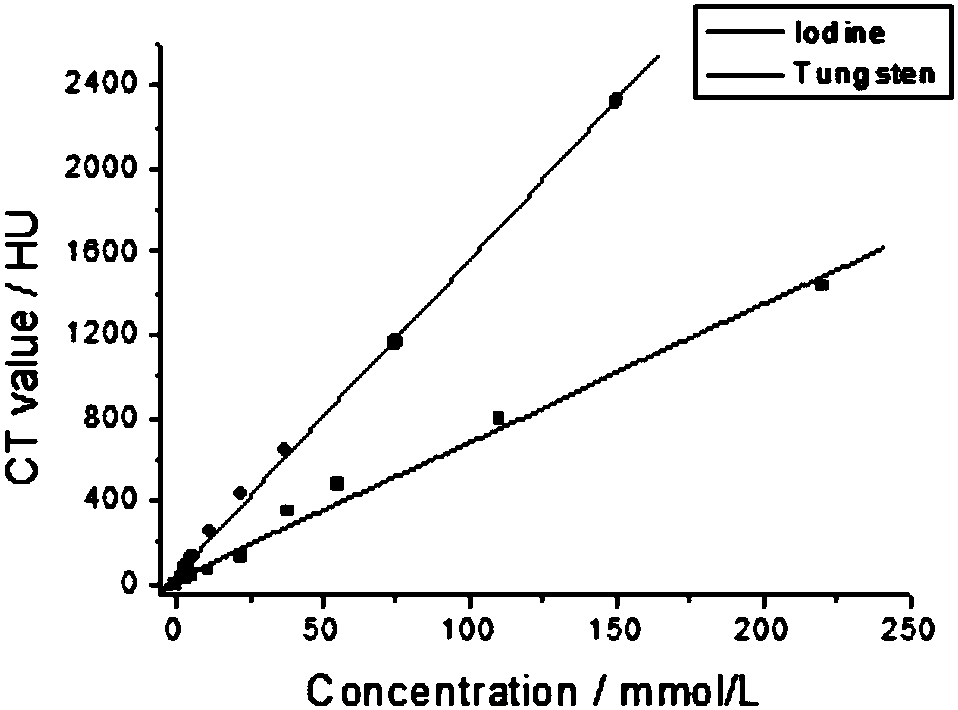
InactiveCN107899023AAccurate diagnosisX-ray constrast preparationsMacromolecular non-active ingredientsCancer cellBiocompatibility Testing
The invention belongs to the technical field of imaging methods, and discloses a nano contrast agent and a preparation method thereof. The preparation method of the nano contrast agent comprises the following steps of synthesizing blue tungsten oxide nano particles with particle size of 60nm by using a hydrothermal method, and dispersing the nano particles in water uniformly, so as to synthesize suspension liquid; preparing a series of iodine and tungsten solutions of different concentrations by using iohexol and tungsten oxide nano particles, and detecting and imaging by using CT. The methoddiscloses the targetability of WO3-X at PEG-Tf, compares the difference of normal cells and cancer cells in endocytosis of targeted tungsten oxide nano particles and in vivo distribution of tumor-bearing rats and rats, gives an explanation that the WO3-X at PEG-Tf can be identified specifically and highly by cancer cells (the cancer cells have rich Tf receptors on the surface), argues the advantage of covalent modification of transferrin, and discloses SPECT / CT imaging and biocompatibility of the WO3-X at PEG-Tf.
Owner:山西省肿瘤医院
Preparation method of iohexol hydrolysate
PendingCN114736132ALow priceFewer reaction by-productsOrganic compound preparationCarboxylic acid amides preparationAcetyl chlorideIodide
The invention provides a preparation method of iohexol hydrate, which comprises the following steps: by taking iodide and acetyl chloride as raw materials, after acetylation reaction is completed, hydrolyzing under an alkaline condition, and then adjusting acid to obtain iohexol hydrolysate; the method is convenient to operate, mild in reaction condition, free of separation in the middle process, high in yield, high in purity and suitable for large-scale industrial production, and preparation is completed through a one-pot method.
Owner:安庆朗坤药业有限公司
Synthesis method of iohexol
ActiveCN113816868AReduce generationReduce lossOrganic compound preparationCarboxylic acid amide separation/purificationOrganic baseCombinatorial chemistry
The invention discloses a synthesis method of iohexol, which comprises the following steps: taking a compound (I) as a raw material, carrying out amidation reaction to obtain a compound (II), then carrying out alkylation reaction on the compound (II) and glycidol under the catalytic action of organic alkali to obtain a compound (VIII), and finally carrying out hydrolysis or alcoholysis deacetylation protection to obtain a target compound (IV). The reaction formula is shown in the specification. The reaction route can effectively avoid generation of byproducts.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com