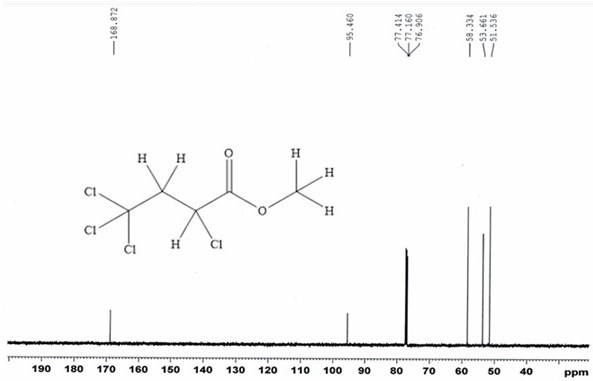
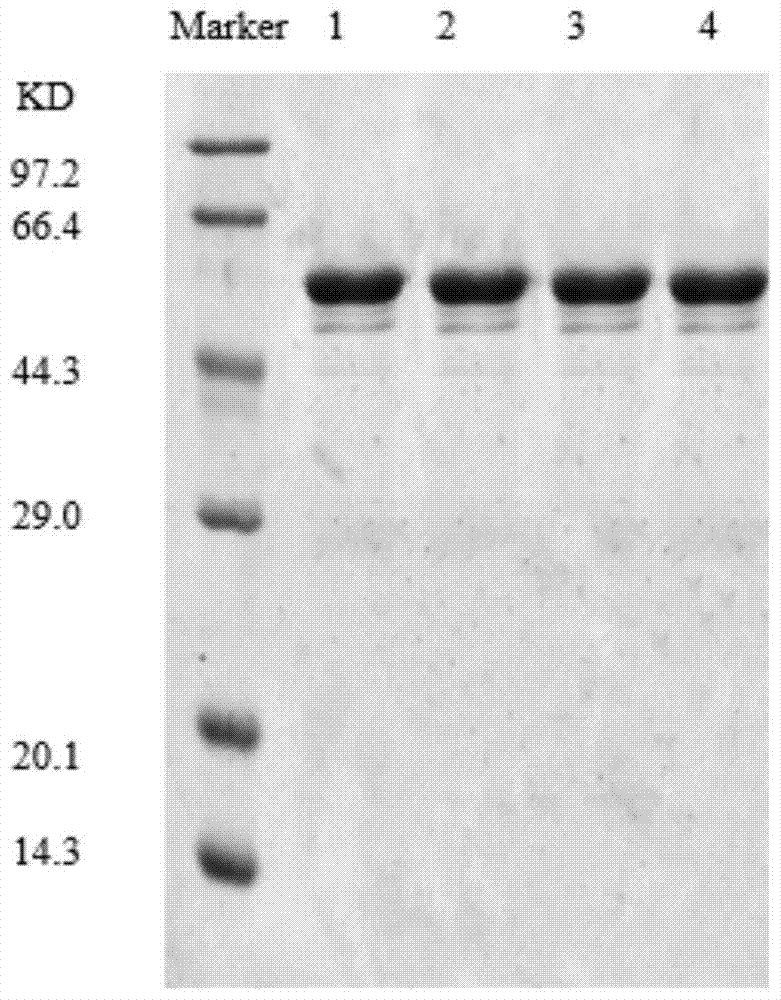
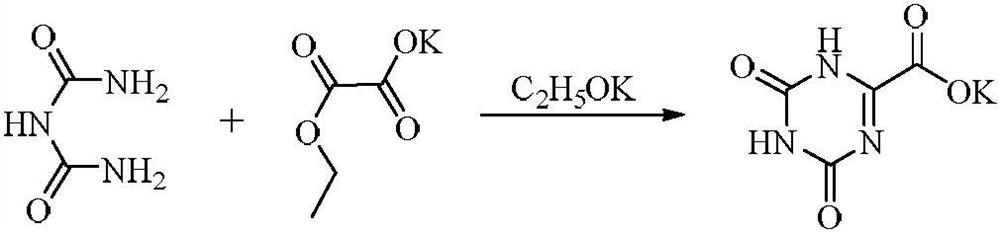
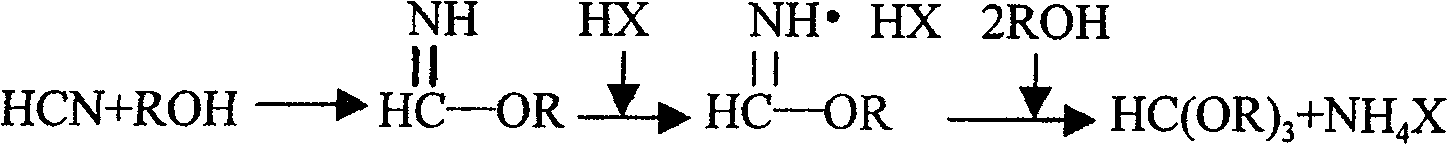Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40results about How to "Fewer reaction by-products" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Application of heat-resistant beta-glucosidase and mutants thereof
InactiveCN103589702AHigh activityImprove conversion efficiencyMicroorganism based processesFermentationAlgluceraseHeat resistance
The invention discloses application of heat-resistant beta-glucosidase and mutants thereof. The mutants of the heat-resistant beta-glucosidase G224T, N223S and N223S / G224T have the amino acid sequences shown in SEQ: ID NO. 1, SEQ: ID NO. 2 and SEQ: ID NO. 3; the heat-resistant beta-glucosidase and the mutants thereof can be used for preparing quercetin through enzymolysis of quercetin glycoside. The preparation of the quercetin through the heat-resistant beta-glucosidase and the mutants thereof has the advantages of high conversion efficiency, few reaction byproducts, easy separation and purification of the product, less chemical pollution, and high yield and high purity of the obtained quercetin product, and the like.
Owner:NANJING FIRST HOSPITAL +1
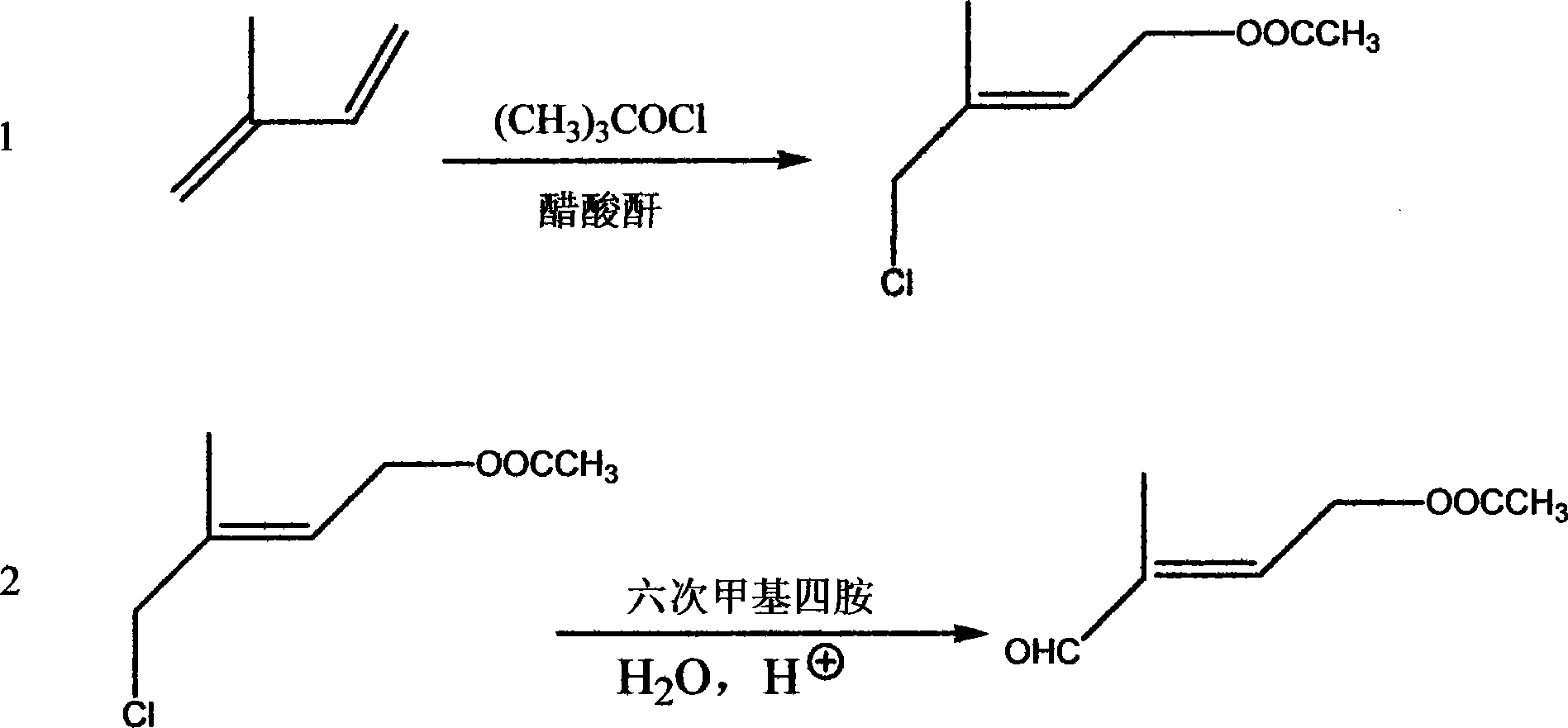
Method for preparing antiform - 4 - acetoxy - 2 - methyl - butenoic aldehyde
InactiveCN101092355AFew reaction stepsFewer reaction by-productsPreparation from carboxylic acid halidesOrganic compound preparationSolventAldehyde
This invention provides a method for preparing trans-4-acetoxy-2-methyl-2-crotonaldehyde. The method comprises: reacting isoprene and tert-butyl hypochlorite in acetic anhydride solution to obtain chlorine-containing ester, reacting with urotropine, and hydrolyzing to obtain trans-4-acetoxy-2-methyl-2-crotonaldehyde. The method has such advantages as few reaction procedures, easy operation, high isoprene conversion rate, high yield (40-56%), no need for DMSO during the oxidation process, no byproduct, low raw material cost, easy recovery of solvent, little environmental pollution and low cost, and is suitable for industrial production.
Owner:GUANGZHOU WISDOM BIO TECH
Preparation method of medical hydroxypropyl starch-based capsule
ActiveCN104800188AAffect qualityFewer reaction by-productsPharmaceutical non-active ingredientsCapsule deliveryDigestionEngineering
The invention belongs to the technical field of medicine preparation, and relates to a preparation method for production of a starch-based capsule. The preparation method specifically comprises the following steps of starch etherifying, glue digestion, defoaming, impurity removal, glue dipping and forming, and the like. The prepared hydroxypropyl starch-based capsule has the advantages that the surface is smooth, the transparency is high, the disintegration time is short, the production technology is simplified, the production cost is reduced, and the hydroxypropyl starch-based capsule is suitable for industrial production.
Owner:HUNAN ER KANG PHARMA
Synthetic method of 3,3'-binitro-5,5'-di-1,2,4-triazole
InactiveCN103965125ASimple and efficient operationFewer reaction by-productsOrganic chemistryAqueous solutionSide reaction
The invention discloses a synthetic method of 3,3'-binitro-5,5'-di-1,2,4-triazole. The structural formula of 3,3'-binitro-5,5'-di-1,2,4-triazole is as follows: as shown in the description. The synthetic method disclosed by the invention comprises the following steps: taking 5,5'-diamido-3,3'-di-1,2,4-triazole as a raw material, dissolving 5,5'-diamido-3,3'-di-1,2,4-triazole in organic sulfonic acid to prepare a salt solution, at normal temperature, dripping the salt solution in a sodium nitride aqueous solution, heating in water bath, when the gas is released completely, cooling to the room temperature, filtering, washing with water and drying the solution to obtain the target product 3,3'-binitro-5,5'-di-1,2,4-triazole. The synthetic method disclosed by the invention is easy in operation, few in side reactions and high in reaction yield, and can be mainly applied to synthesis of 3,3'-binitro-5,5'-di-1,2,4-triazole.
Owner:XIAN MODERN CHEM RES INST
Preparation method of polyfluoro aliphatic carboxylic acid
InactiveCN101774902AProcess environmental protectionFewer reaction by-productsCarboxylic preparation by oxidationCarboxylateAliphatic alcohol
The invention discloses a preparation method of polyfluoro aliphatic carboxylic acid, which comprises the following concrete steps: oxidizing polyfluoro aliphatic alcohol by potassium permanganate to obtain polyfluoro carboxylic acid potassium salt, purifying intermediate products of fluorine-containing potassium carboxylate, namely washing for removing unreacted raw materials, and then, acidizing for releasing polyfluoro carboxylic acid. By utilizing the characteristic that the polyfluoro aliphatic alcohol is oxidized by the potassium permanganate to obtain the polyfluoro carboxylic acid, but the acidity of the polyfluoro carboxylic acid is further stronger than the acidity of acetic acid, so that the generated polyfluoro carboxylic acid is neutralized with potassium hydroxide released by the potassium permanganate in the reaction process to obtain the fluorine-containing carboxylic acid potassium salt, the invention adopts the method of firstly washing the fluorine-containing potassium carboxylate and then acidizing for releasing the polyfluoro carboxylic acid, thereby solving the problem that the polyfluoro aliphatic alcohol and the polyfluoro aliphatic carboxylic acid can not be rectified and separated easily. The polyfluoro aliphatic alcohol which is used as a raw material in the invention is prepared by telomerization of tetrafluoroethylene and belongs to an industrialized raw material. The process for preparing the polyfluoro aliphatic carboxylic acid by oxidizing the polyfluoro aliphatic alcohol has mild conditions and is suitable for industrialized production.
Owner:SUZHOU UNIV
Heat-resisting beta-glucosidase and application of heat-resisting beta-glucosidase mutants to arctigenin preparation
InactiveCN104818261AHigh activityImprove conversion efficiencyFermentationGlycosylasesAlgluceraseTransformation efficiency
The invention discloses an application of heat-resisting beta-glucosidase mutants N223T, G224F and N223T / G224F to transforming arctiin into arctigenin. Amino acid sequences of the heat-resisting beta-glucosidase mutants N223T, G224F and N223T / G224F are indicated in SEQ: ID NO.1, ID NO.2 and ID NO.3. The arctiin is hydrolyzed to prepare the arctigenin by the aid of heat-resisting beta-glucosidase and the mutants thereof, and as compared with wild-type enzyme, the heat-resisting beta-glucosidase has the advantages of high transformation efficiency, fewer reaction by-products, high yield and purity, easiness in separation and purification, less chemical pollution and the like.
Owner:NANJING NORMAL UNIVERSITY
Application of transition metal carbonate nanomaterial in electrocatalytic reduction reaction of nitrate
ActiveCN113788516AEasy to prepareHigh yieldWater contaminantsWater/sewage treatmentPtru catalystPyrrolidinones
The invention relates to application of a transition metal carbonate nanomaterial in electrocatalytic reduction of nitrate, belonging to the technical field of electrocatalytic reduction of nitrate. The preparation method comprises the following steps: preparing the transition metal carbonate nanomaterial Co@Cu2(OH)2CO3 by a simple one-step hydrothermal method, preparing catalyst slurry from the transition metal carbonate nanomaterial Co@Cu2(OH)2CO3, acetylene black and an N-methyl pyrrolidone solution of polyvinylidene fluoride, loading the catalyst slurry on hydrophilic carbon cloth to obtain an electrocatalytic reduction nitrate electrode, and with the electrocatalytic reduction nitrate electrode as a working electrode, forming a three-electrode system, which is used for electrocatalytic reduction of nitrate in sewage, from the working electrode, a platinum electrode and a saturated calomel electrode. The transition metal carbonate nanomaterial disclosed by the invention is simple in preparation method, high in yield, friendly to environment, low in production cost and high in thermal stability and chemical stability, shows high catalytic activity and selectivity and high cycling stability in electrocatalytic reduction of nitrate, and is suitable for large-scale industrial application.
Owner:JIANGNAN UNIV
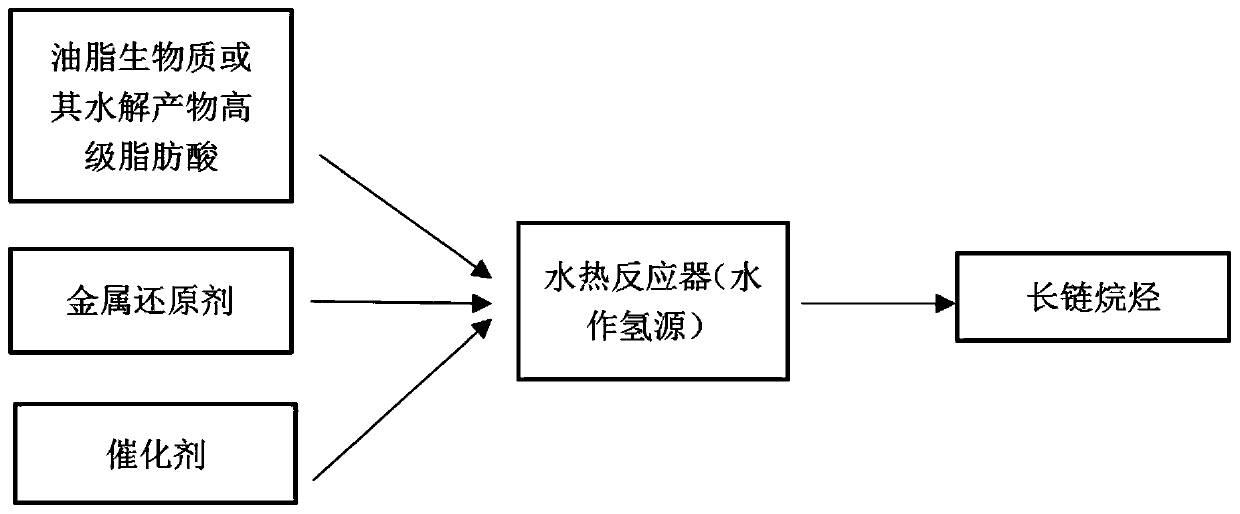
Hydrothermal method for converting oil or higher fatty acid into long-chain alkane
ActiveCN107325835AAlleviate energy problemsLow dielectric constantLiquid carbonaceous fuelsLiquid hydrocarbon mixture productionAlkaneOil and grease
The invention discloses a hydrothermal method for converting oil or oil hydrolysate, namely, higher fatty acid into long-chain alkane. According to the method, oil or oil hydrolysate, namely, higher fatty acid is taken as a raw material to react in a hydrothermal reactor at 150-450 DEG C for 2-24 h with metal and a metal oxide used as reaction catalysts and metal elements or metal waste used as a reducing agent, and thus, long-chain alkane with the highest yield of 90% can be obtained. The long-chain alkane can be synthesized effectively and highly selectively with the method. Biomass oil or oil hydrolysate, namely, higher fatty acid is used as the raw material, the condition that a large number of fossil fuel is consumed in traditional industrial preparation methods is avoided, preparation of complex noble metal catalysts is not required, and the method is simple to operate and high in conversion rate, requires no pure hydrogen, causes small environmental pollution due to adoption of water as a hydrogen source and a reaction solvent and is beneficial to industrial production. The long-chain alkane product can be used for producing aviation fuel to replace traditional fossil fuel.
Owner:SHANGHAI JIAO TONG UNIV
Method for producing 4,4-difluoro cyclohexyl formic ether by using counter cyclohexanone formic ether through fluorination
InactiveCN102531898AResidue reductionHigh purityOrganic compound preparationCarboxylic acid esters preparationCyclohexanoneChemical industry
The invention discloses a method for producing 4,4-difluoro cyclohexyl formic ether by using counter cyclohexanone formic ether through fluorination, which belongs to the technical field of chemical industry. The method comprises the steps of (1) respectively putting the counter cyclohexanone formic ether, sulfur tetrafluoride and hydrogen fluoride in a pressure kettle, starting blending, enabling the reaction temperature in the pressure kettle to be controlled at 0-80 DEG C, and enabling response time to be 0.5-24.0h; (2) opening an outlet valve of the pressure kettle, enabling surplus gas to be led in alkali liquid, and enabling the gas to be absorbed by the alkali liquid; (3) opening the pressure kettle, enabling reaction liquid to be poured in ice water, starting blending simultaneously, stewing and layering after completing pouring to separate an oil layer; and (4) adjusting the separated oil layer to be neutral by using the alkali liquid, stewing to remove a water layer, adding a desiccating agent to the oil layer, and filtering the desiccating agent to obtain the 4,4-difluoro cyclohexyl formic ether. The method synthesizes target products through a one-step reaction, and is simple in reaction, few in steps, few in secondary products, apt to obtain products with high purity through distillation, high in total recovery, good in safety and favorable for scale production.
Owner:江苏华达化工集团有限公司
Biosynthesis method of borneol
InactiveCN101857883AHigh selectivityFewer reaction by-productsMicroorganism based processesFermentationBorneolBiosynthesis
The invention discloses a biosynthesis method of borneol, which comprises the following steps: selecting and separating microbial strains for synthesizing the borneol from the nature, collecting cells after fermentation, taking the cells as a bio-catalyst to be subjected to the hydrolysis reaction of borneol ester in an organic solvent-buffer salt system, and obtaining a borneol product with the borneol content of over 75 percent after separation and refining. The bornrol product is close to the composition of natural borneol, and the method is a novel method for preparing the borneol.
Owner:GUANGXI UNIV
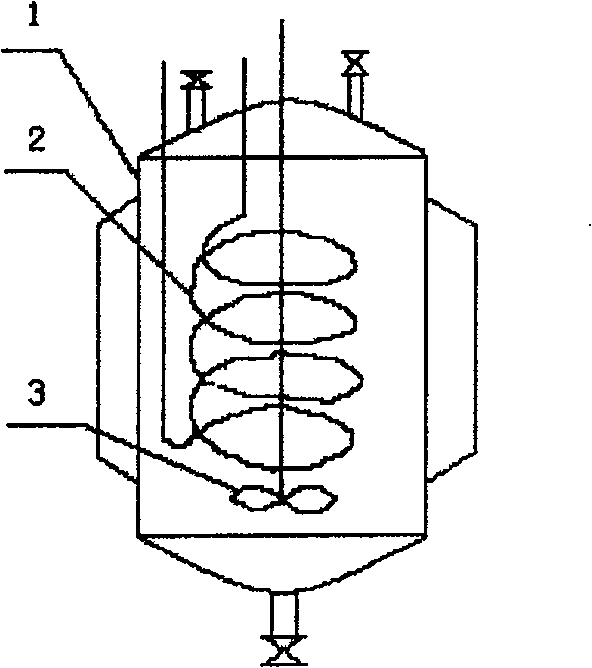
Process and device for preparing orthoformate by using byproduct hydrocyanic acid from acrylonitrile production
ActiveCN100457704CStable reaction temperatureFewer reaction by-productsEther preparationSolventChemistry
The present invention relates to a process for preparing orthoformate by taking advantage of hydrocyanic acid, as by-product in production of acrylic nitrile, and apparatus therefore. The hydrocyanic acid, as by-product in production of acrylic nitrile, is adopted as a starting raw material and, together with alkyl alcohol and halogen hydride, is dissolved in an indifferent solvent; then after halogenation, salt forming and alcoholysis reactions, crystal separation and distillation rectification, the orthoformate is obtained. By adopting a reactor equipped with stirring, built-in plastics bundled tubes or metal dish tube heat interchanger to resolve the heat exchange problem existing in the aggregation exothermic halogenation and salt forming reactions, the present invention prevents blasting boiling and exploding accident states from appearing during the course of reaction, facilitates placidity and controllability of the reaction temperature, and achieves the requirement for safe reactions.
Owner:YINGKOU YINGXIN CHEM TECH CO LTD
Application of heat-resistant alpha-glucuronidase polypeptide fusion body in preparation of glucuronic acid
ActiveCN112592911AImprove conversion efficiencyLarge amount of productHydrolasesFermentationUronic acidGlucuronidase
The invention discloses application of a heat-resistant alpha-glucuronidase polypeptide fusion body in preparation of glucuronic acid. An amino acid sequence of the fusion body is as shown in SEQ ID NO.1. According to the invention, seven amino acid residues are fused at the C end of heat-resistant alpha-glucuronidase protein to form a brand-new polypeptide fusion body, the specific activity of the obtained polypeptide fusion body is improved by 297% compared with that of wild type enzyme TmAguA, the Kcat and Kcat / Km of a corresponding hydrolyzed 4-O-methyl glucuronic acid xylo-oligosaccharidesubstrate are improved by 356% and 363% respectively, and the stability is improved by 21.1%-65.7% compared with that of the wild type enzyme; the fusion body is used for preparing the glucuronic acid, and compared with the wild type enzyme, the fusion body has the advantage that the yield of the glucuronic acid prepared by converting beechwood xylan through the polypeptide fusion body is increased by 21.6-60.3%; and the fusion body has the advantages of high conversion efficiency, few reaction byproducts, high yield and purity, easiness in separation and purification, less chemical pollutionand the like.
Owner:NANJING NORMAL UNIVERSITY
Method for preparing durene from carbon monoxide and methanol
ActiveCN112521241AImprove stabilityHigh space-time yieldMolecular sieve catalystsMolecular sieve catalystMolecular sieveAcid treatment
The invention discloses a method for preparing durene from carbon monoxide and methanol. The method comprises the following steps: contacting a raw material containing carbon monoxide and methanol with a catalyst in a reactor, and reacting to obtain the durene, wherein the catalyst is prepared by modifying a material containing a molecular sieve, and the modification treatment is selected from atleast one of oxide modification, water vapor treatment and acid treatment. According to the method provided by the invention, the defects of a traditional durene process route are overcome, and the method for preparing durene by coupling carbon monoxide and methanol is a new process technology for producing durene. The preparation of durene by using carbon monoxide and methanol as raw materials isnot restricted by the raw materials, the device is easy to realize large-scale production, and the methodhas good economy, and has good industrial application prospect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for separating and detecting related substances of 1-butylsulfonyl chloride
The invention provides a method for separating and detecting related substances of 1-butylsulfonyl chloride, which comprises the following steps: dissolving 1-butylsulfonyl chloride in a solvent to prepare a solution, adding an amine reagent into the solution for reaction, and detecting and analyzing by adopting GC. According to the method, all impurities and main components are effectively separated, the specificity, sensitivity, accuracy, precision and the like of the method meet the requirements, and the method can be used for separating and detecting related substances of 1-butylsulfonyl chloride.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
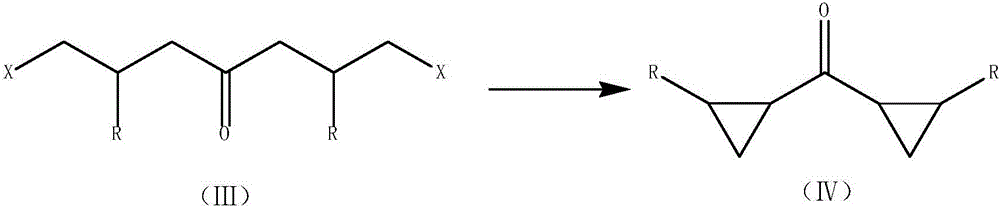
Modified synthetic method of dicyclopropyl ketone
ActiveCN105732352AImprove controllabilityFewer reaction by-productsOrganic compound preparationPreparation from heterocyclic compoundsKetoneClosing loops
The invention discloses a modified synthetic method of dicyclopropyl ketone, comprising: first, in the presence of reaction inert organic solvent and solid sodium alcoholate, subjecting gamma-butyrrolactone to inter-molecular dehydration condensation to generate an intermediate; second, adding concentrated hydrochloric acid into a reaction system and generating 1,7-dichloro-4-heptanone crude product by decarboxylation; third, closing loop under the action of a strong base to generate the dicyclopropyl ketone. The invention has the advantages that the inert organic solvent and solid sodium alcoholate are used, there is no need for recycling alcohols, reaction controllability is greatly improved, reaction byproducts are decreased, product yield is 15-20% as high as that of a conventional technique, production cost is lowered, environment protection burden is lowered, and product quality is improved.
Owner:浜田智子
A kind of method for biotransformation of Polygonin using thermostable β-glucosidase and its mutants
InactiveCN104818262BImprove conversion efficiencyHigh activityFermentationGlycosylasesDrug biotransformationBiology
The invention discloses application of heat-resisting beta-glucosidase mutants N223T, G224F and N223T / G224F in a conversion of polydatin to resveratrol. According to the heat-resisting beta- glucosidase mutants N223T, G224F and N223T / G224F, the amino acid sequences thereof are represented as SEQ: ID NO. 1, ID NO. 2 and ID NO. 3. By the use of the heat-resisting beta- glucosidase and the mutants thereof to hydrolyze the polydatin for preparing the resveratrol, comparing to the use of wild-type enzymes, the conversion efficiency is high, the reaction byproducts are fewer, the yield and purity are high, the separation and purification are easy, and chemical pollution is less.
Owner:NANJING NORMAL UNIVERSITY
Application of heat-resistant beta-glucosidase and mutants thereof
InactiveCN103589702BHigh activityImprove conversion efficiencyMicroorganism based processesFermentationAlgluceraseQuercetin Glycoside
The invention discloses application of heat-resistant beta-glucosidase and mutants thereof. The mutants of the heat-resistant beta-glucosidase G224T, N223S and N223S / G224T have the amino acid sequences shown in SEQ: ID NO. 1, SEQ: ID NO. 2 and SEQ: ID NO. 3; the heat-resistant beta-glucosidase and the mutants thereof can be used for preparing quercetin through enzymolysis of quercetin glycoside. The preparation of the quercetin through the heat-resistant beta-glucosidase and the mutants thereof has the advantages of high conversion efficiency, few reaction byproducts, easy separation and purification of the product, less chemical pollution, and high yield and high purity of the obtained quercetin product, and the like.
Owner:NANJING FIRST HOSPITAL +1
Method for converting oil or higher fatty acid into long-chain alkanes by hydrothermal method
ActiveCN107325835BAlleviate energy problemsLow dielectric constantLiquid carbonaceous fuelsLiquid hydrocarbon mixture productionAlkaneOil and grease
The invention discloses a hydrothermal method for converting oil or oil hydrolysate, namely, higher fatty acid into long-chain alkane. According to the method, oil or oil hydrolysate, namely, higher fatty acid is taken as a raw material to react in a hydrothermal reactor at 150-450 DEG C for 2-24 h with metal and a metal oxide used as reaction catalysts and metal elements or metal waste used as a reducing agent, and thus, long-chain alkane with the highest yield of 90% can be obtained. The long-chain alkane can be synthesized effectively and highly selectively with the method. Biomass oil or oil hydrolysate, namely, higher fatty acid is used as the raw material, the condition that a large number of fossil fuel is consumed in traditional industrial preparation methods is avoided, preparation of complex noble metal catalysts is not required, and the method is simple to operate and high in conversion rate, requires no pure hydrogen, causes small environmental pollution due to adoption of water as a hydrogen source and a reaction solvent and is beneficial to industrial production. The long-chain alkane product can be used for producing aviation fuel to replace traditional fossil fuel.
Owner:SHANGHAI JIAOTONG UNIV
Vitamin D3 C-25 site P450 hydroxylase as well as gene, expression vector, strain and application thereof
ActiveCN114350629AStrong specificityEfficient conversionBacteriaMicroorganism based processesHeterologousBacilli
The invention relates to a vitamin D3 C-25 site P450 hydroxylase as well as a gene, an expression vector, a strain and application thereof, and belongs to the technical fields of enzyme engineering and gene engineering. The amino acid sequence of the vitamin D3 C-25 site P450 hydroxylase CYP109E1-H is shown as SEQ ID NO.1, the gene cyp109E1-H is inserted into a pMA5 plasmid, bacillus subtilis WB600 is used as an expression host to construct a strain, heterologous expression of the vitamin D3 C-25 site P450 hydroxylase CYP109E1-H is achieved, efficient conversion of the vitamin D3 is successfully achieved, meanwhile, specificity is high, reaction by-products are reduced, production cost is reduced, and the application prospect is wide. And a foundation is laid for further industrial application.
Owner:JIANGNAN UNIV
Simple preparation method of 9-hydroxyfluorene-9-carboxylate compound
ActiveCN111718262AFewer reaction by-productsLess wasteOrganic compound preparationCarboxylic acid esters preparationSide productCarboxylate
The invention relates to a simple preparation method of a 9-hydroxyfluorene-9-carboxylate compound. The method comprises the following step: with a benzoyl formate compound I and an iodobenzene compound II as raw materials, under the catalysis of Pd(OAc)2 and trifluoroacetic acid, carrying out a cyclization reaction by using silver trifluoroacetate as an oxidant and 2-fluoro-5-trifluoromethylaniline as a ligand to obtain the 9-hydroxyfluorene-9-carboxylate compound. Compared with an existing method, the method of the invention has the advantages that reaction raw materials are easy to synthesize through a classical reaction; 2, the reaction is simple and convenient, operation is easy, a target product can be obtained in one step, few reaction byproducts are produced, little waste is discharged, the product is directly purified by using column chromatography, strong acid or strong base is not needed for aftertreatment, pressure on the environment is greatly reduced, purification effectis good, and yield can reach 60-90%; and 3, reaction expansibility is strong, a novel method for synthesizing the 9-hydroxyfluorene-9-carboxylate compound is provided, and the compound which is not synthesized before can be synthesized.
Owner:WUHAN UNIV OF TECH
Synthesis method of alpha,gamma,gamma,gamma-tetrachlorobutyrate
InactiveCN112574037AEasy to separateEasy to recycleOrganic compound preparationCarboxylic acid esters preparationPtru catalystOrganosolv
The invention relates to a synthesis method of alpha,gamma,gamma,gamma-tetrachlorobutyrate. The synthesis method comprises the following steps: stirring and mixing carbon tetrachloride and acrylate with a copper salt and / or cuprous salt main catalyst, an organic amine or imidazole cocatalyst and a polar inert organic solvent at room temperature, sealing a reaction kettle, conducting heating for anaddition reaction, preforming cooling to room temperature, carrying out filtering, distilling a filtrate under normal pressure to recover carbon tetrachloride, and performing rectifying under reducedpressure to obtain alpha,gamma,gamma,gamma-tetrachlorobutyrate. The purity of the alpha,gamma,gamma,gamma-tetrachlorobutyrate obtained by using the method is up to 99.5%, one-way reaction yield is upto 96.1%, a product is easy to separate, reaction byproducts are few, the catalytic effect of the catalysts and the ligand is excellent, the raw materials and the catalysts are cheap and easy to obtain, and the solvent and the catalysts are easy to recycle; and the method is mild in reaction condition, simple in technological operation, low in production cost, good in economic benefit and suitable for industrial production.
Owner:HUNAN NORMAL UNIVERSITY
A kind of preparation method of medicinal hydroxypropyl starch capsule
ActiveCN104800188BSignificant progressAffect qualityPharmaceutical non-active ingredientsCapsule deliveryDigestionImpurity
Owner:HUNAN ER KANG PHARMA
Application of a heat-resistant β-glucosidase and its mutants in the preparation of arctigenin
InactiveCN104818261BHigh activityImprove conversion efficiencyFermentationGlycosylasesAlgluceraseTransformation efficiency
The invention discloses an application of heat-resisting beta-glucosidase mutants N223T, G224F and N223T / G224F to transforming arctiin into arctigenin. Amino acid sequences of the heat-resisting beta-glucosidase mutants N223T, G224F and N223T / G224F are indicated in SEQ: ID NO.1, ID NO.2 and ID NO.3. The arctiin is hydrolyzed to prepare the arctigenin by the aid of heat-resisting beta-glucosidase and the mutants thereof, and as compared with wild-type enzyme, the heat-resisting beta-glucosidase has the advantages of high transformation efficiency, fewer reaction by-products, high yield and purity, easiness in separation and purification, less chemical pollution and the like.
Owner:NANJING NORMAL UNIVERSITY
A kind of preparation method of oteracil potassium
ActiveCN110655492BMild reaction conditionsShort reaction timeOrganic chemistryAlcoholOTERACIL POTASSIUM
The invention provides a preparation method of oteracil potassium. The method comprises the steps of: dissolving allantoin, alkali and potassium iodide in purified water at room temperature, adding N-halogenated amides in batches after the reaction solution is cooled, and heating up the reaction after the addition is completed. After the reaction is detected, the reaction solution is Lower the temperature, adjust the pH with acid, stir and grow the crystal, and filter with suction. The filter cake is washed with cold purified water until it is colorless, then rinsed with cold anhydrous alcohol solvent, and dried in vacuum to obtain oteracil potassium. Compared with the prior art, the whole process has simple and easy raw materials, easy operation, mild reaction conditions, stable quality, high yield, no pollution to the environment, and is suitable for industrialized production.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of 1-bromonaphthalene
InactiveCN109651070AThe reaction device is simpleFewer reaction by-productsHalogenated hydrocarbon preparationBromineChemical synthesis
Belonging to the technical field of chemical synthesis, the invention specifically relates to a preparation method of 1-bromonaphthalene. The method includes: (1) dissolving naphthalene fully in dichloroethane, and adding hydrobromic acid under stirring to form a mixed solution; (2) adding sodium hypochlorite into the mixed solution dropwise at 25-30DEG C, and conducting heat preservation stirring; (3) carrying out standing layering, taking the oil phase layer, and performing washing; and (4) conducting drying, and performing reduced pressure fractionation, thus obtaining 1-bromonaphthalene. The synthesis process adopted by the invention has the advantages of simple reaction device, few by-product, high yield, high utilization rate of bromine compared with other processes, low pollution ofthree wastes, low production energy consumption, high safety of the reaction process, and conforms to the green chemistry concept.
Owner:SHANGHAI WOKAI BIOTECH
A kind of method for preparing vindesine from periwinkle
The invention discloses a method for preparing eldisine from Catharanthus roseus. The method comprises the following steps: (1) preparation of Catharanthus roseus total alkaloid; (2) preparation of vinblastine sulfate crude product; (3) preparation of vinblastine sulfate pure product; (4) preparation of vinblastine hydrazide; and (5) preparation of eldisine. The method is simple to technique and convenient to operate; and the production keypoints are easy to control, so the method is suitable for industrial production. The silica gel has the advantages of low cost and simple equipment requirements. The vinblastine sulfate refinement process after salification is simple and convenient; and the refined vinblastine sulfate has the advantages of high content and high yield. In the eldisine preparation process, the azidation reaction and hydrolysis reaction are carried out in one reactor, so the reaction time is short, and the amount of reaction byproducts is smaller. After the reaction, the eldisine has the advantages of higher content and high yield.
Owner:GUANGZHOU HANFANG PHARMA
A kind of preparation method of 2-chloro-n,n-dimethylnicotinamide
A preparation method of 2-chloro-N, N-dimethylnicotinamide, the method first disubstituted aminoacrolein and 2-cyano-N, N-dimethylacetamide under the conditions catalyzed by a catalyst Carrying out Knoevenagel reaction, the obtained target intermediate is reacted with hydrogen chloride gas to obtain 2-chloro-N,N-dimethylnicotinamide. The catalyst used in the present invention can greatly improve the yield of the Novenger reaction, the yield can reach more than 97%, and the content can reach more than 97%. The invention has easy-to-obtain raw materials, simple operation, improves the reaction yield, avoids the use of highly toxic reagents such as phosphorus oxychloride and thionyl chloride, has less discharge of three wastes, is environmentally friendly, saves production costs, and is suitable for industrial production.
Owner:江苏丰山生化科技有限公司
Cobalt-containing porous material and application thereof in preparation of phenol through benzene hydroxylation
InactiveCN102228833BHigh yieldMild reaction conditionsOrganic chemistryOrganic compound preparationBenzeneHigh activity
The invention relates to a cobalt-containing porous material and application thereof to preparation of phenol through benzene hydroxylation. The cobalt-containing porous material is characterized by being prepared by using a method comprising the steps of: dissolving an additive, an inorganic cobalt salt, a surfactant and a silicon source in deionized water according to a certain sequence, stirring and then regulating until a proper pH is reached to obtain sol, ageing under a hydrothermal condition, and then centrifuging, washing, drying and roasting to obtain the cobalt-containing porous material. The cobalt-containing porous material is used as a catalyst, and has high activity and high selectivity in preparation of the phenol through benzene direct hydroxylation.
Owner:NANJING TECH UNIV
A kind of double carbonylation preparation method of palladium catalyzed acetylene
ActiveCN107739309BIncrease productionAbundant resourcesOrganic chemistry methodsCarboxylic preparation from carbon monoxide reactionHydrogen halideButanedioic acid
The invention provides a double-carbonylation preparation method utilizing palladium to catalyze acetylene and relates to the field of preparing succinic acid by an acetylene method. According to thedouble-carbonylation preparation method, acetylene, carbon monoxide and water are utilized as raw materials to be catalyzed and synthesized into succinic acid (anhydride) under the conditions of a reaction temperature as 25 to 75 DEG C and a pressure as 0.1 to 5MPa. To the used catalyst, a palladium compound-lithium halide / hydrogen halide-nitrogenous / phosphine ligand is utilized as the catalyst, wherein n (palladium compound) to n (lithium halide / hydrogen hydride) is equal to 1 to (1 to 10), and n (phosphine / nitrogen ligand) to n (palladium compound) is equal to 1 to (1 to 10). Compared with the prior art, the double-carbonylation preparation method disclosed by the invention has the advantages of moderate reaction conditions, quick reaction speed, high selectivity, simpleness and safety in operation, ability in effectively reducing succinic acid production cost and very good industrial market prospect.
Owner:CHINA CHENGDA ENG +1
Preparation method of (S)-3-methylamino-1-(2-thienyl)-1-propanol
The invention relates to a preparation method of (S)-3-methylamino-1-(2-thienyl)-1-propanol. (S)-3-dimethylamino-1-(2-thienyl)-1-propanol is used as a raw material to be treated by the following stepsof (1) performing hydroxy group protective reaction on the (S)-3-dimethylamino-1-(2-thienyl)-1-propanol; (2) performing demethylation reaction; (3) performing hydroxy removal reaction protection. Thepreparation method has the advantages that the reaction byproducts are few; the purity is high; the reaction speed is high; the yield is high.
Owner:CHIRAL QUEST (SUZHOU) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com