Synthesis method of alpha,gamma,gamma,gamma-tetrachlorobutyrate
A technology of tetrachlorobutyrate and synthesis method, which is applied in the field of synthesis of -α,γ,γ,γ-tetrachlorobutyrate, and can solve problems such as complex preparation methods, complex components of reaction by-products, and difficult post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
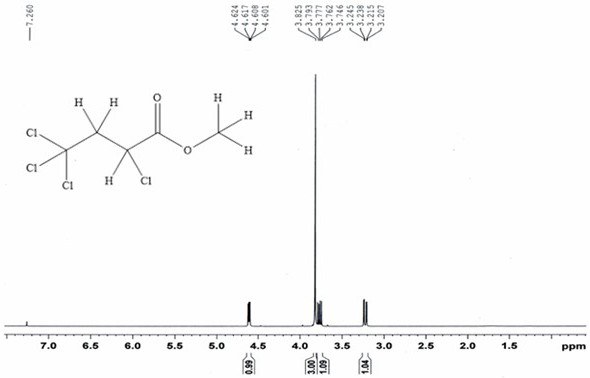
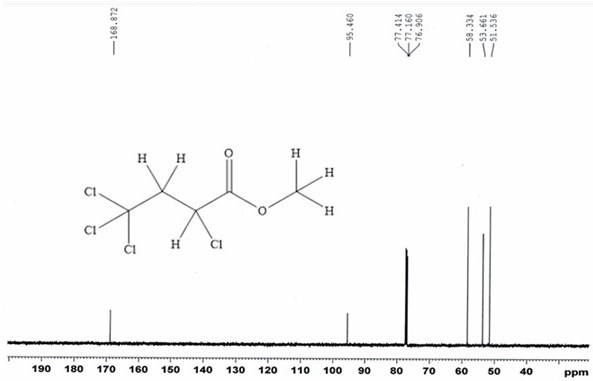
[0054] 13.0g (84.5mmol) carbon tetrachloride and 6.54g (76.0mmol) methyl acrylate, with 0.13g (0.76mmol) CuCl 2 • 2H 2 O main catalyst, 0.088g (0.76mmol) N,N,N',N'-tetramethylethylenediamine cocatalyst and 10mL of acetonitrile were placed in a stainless steel reactor with a polytetrafluoroethylene liner and stirred at room temperature After mixing for 20 minutes, close the reaction kettle, raise the temperature to 110°C, carry out the addition reaction for 12 hours, then cool to room temperature, filter, and distill the filtrate at 70°C for 2 hours under normal pressure to recover carbon tetrachloride. Under reduced pressure, rectify under reduced pressure for 2 hours to obtain 14.87 g of α, γ, γ, γ-tetrachlorobutyric acid methyl ester.
[0055] Through GC analysis, the purity of α, γ, γ, γ-methyl tetrachlorobutyrate obtained in the examples of the present invention is 95.6%, and the single-pass reaction yield calculated with methyl acrylate as the reference raw material is: ...
Embodiment 2
[0059] 23.4g (152.1mmol) of carbon tetrachloride and 6.54g (76.0mmol) of methyl acrylate were mixed with 0.95g (3.8mmol) of CuSO 4 • 5H 2 O main catalyst, 0.93g (15.2mmol) ethanolamine co-catalyst and 100mL propionitrile were placed in a stainless steel reaction kettle with a polytetrafluoroethylene liner, first stirred at room temperature for 40min after mixing, the reaction kettle was sealed, and the temperature was raised to 100°C. Addition reaction for 10 hours, then cooled to room temperature, filtered, the filtrate was distilled at 80°C for 2 hours at atmospheric pressure to recover carbon tetrachloride, and then rectified under reduced pressure at 90°C and 15kPa for 3 hours to obtain α, γ, γ , 15.49 g of methyl γ-tetrachlorobutyrate.
[0060]Through GC analysis, the purity of α, γ, γ, γ-methyl tetrachlorobutyrate obtained in the examples of the present invention is 96.5%, and the single-pass reaction yield calculated with methyl acrylate as the reference raw material i...
Embodiment 3
[0063] 2.59g (15.20mmol) CuCl 2 • 2H 2 O main catalyst and 2.50g (30.40mmol) N-methylimidazole cocatalyst in 100mL of methanol, at 70°C, reflux reaction for 2h, at 50°C, 10kPa, after vacuum distillation for 1.5h, wash with ether Disperse, filter, and vacuum-dry at 50°C and 10kPa for 8 hours to obtain 4.08g of catalyst complex 1.
[0064] Such as Figure 4 Shown, analyze the XRD spectrogram of the obtained catalyst complex cupric chloride of the embodiment of the present invention and N-methylimidazole, in 2 θ =14.25°, 15.81°, 17.58°, 20.90°, 24.22°, 26.47° and 26.67° all produce strong absorption peaks, which can be seen from the XRD spectrum of this series of absorption peaks and its standard card (PDF#32-1608), The catalyst complex obtained in the embodiment of the present invention is a single crystal formed by copper chloride and N-methylimidazole in a molar ratio of 1:2.
[0065] 46.8g (304.2mmol) carbon tetrachloride and 6.54g (76.0mmol) methyl acrylate, and 0.23g c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com