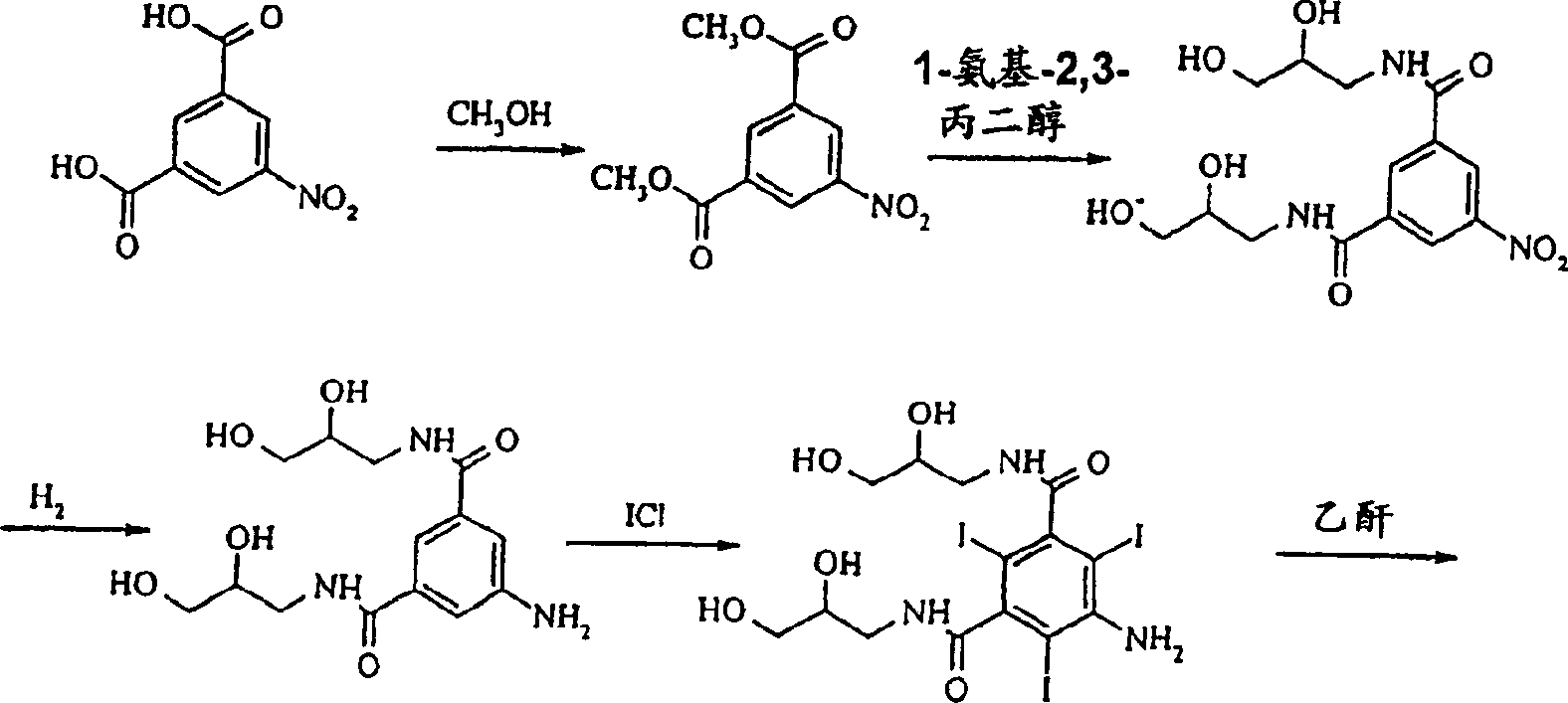
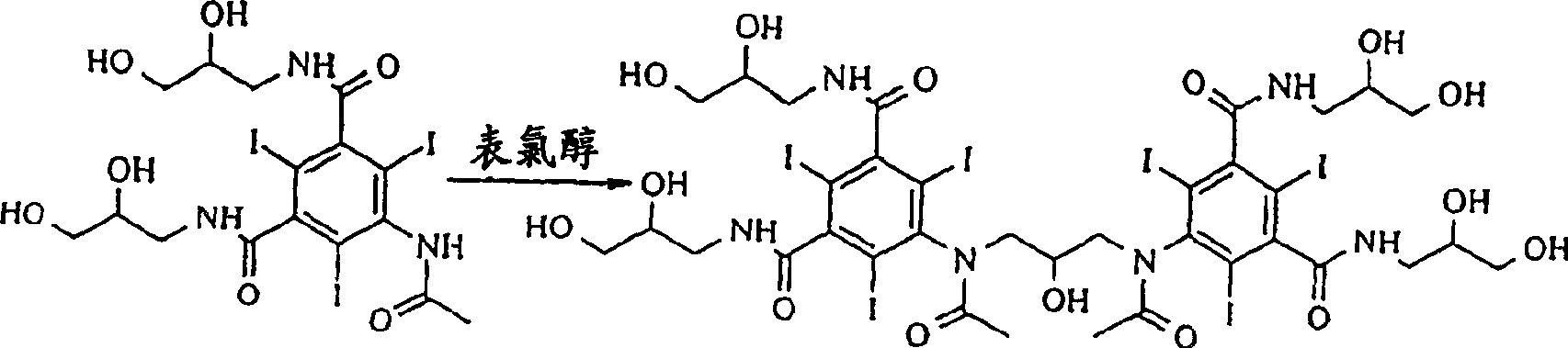
Preparation of iodixanol
A technology of iodixanol and epichlorohydrin, applied in the field of preparation of iodixanol, can solve problems such as lack of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Compound A (366g) was dissolved in 2-methoxyethanol solution (360ml) containing NaOH (23g) at 50°C, when all solids were dissolved, the temperature was lowered to 15°C, and concentrated hydrochloric acid (28g) was added to the solution . Epichlorohydrin (13 g) was added in one portion and the reaction was monitored by HPLC. After 46 hours the iodixanol content of the reaction mixture was 49.6%. Water (575ml) was added and the temperature was raised to 19°C. The solution was clear at this point, so no further addition of sodium hydroxide was necessary. The pH value was adjusted to 10.8 with 18% hydrochloric acid, and 1 g of compound A was used as a seed crystal to add to the solution. The resulting suspension was again adjusted to pH 4.0 with 18% hydrochloric acid. The suspension was stirred overnight and filtered, washing the filter with water (60ml). The filtrate is further desalted and crystallized in the usual way to obtain iodixanol which is suitable for pharma...
example 2
[0021] Compound A recovered from Example 1 was directly taken out from the filter and was completely dissolved in water (440ml) and 50% NaOH aqueous solution (15ml) without drying, and the solution was filtered through a 3 μm filter to remove trace insolubles. A little more water (50ml) was added to the filtrate. Methanol (95ml) was added to the solution and the temperature was raised to 60°C. The pH value was adjusted from 11.5 to 9.8 with 18% hydrochloric acid, and 0.8 g of compound A was added as a seed crystal. After 30 minutes, the pH was further lowered to 6 with 18% hydrochloric acid. The temperature was gradually lowered to 15°C and the precipitated material was filtered off, washed with methanol (140ml) and dried in vacuo at 60°C. The yield of pure compound A (>99% by HPLC analysis) was 118 g, corresponding to 32% of the compound initially employed in Example 1.
[0022] The recovered Compound A (118 g) was mixed with fresh Compound A (248 g) for a new dimerization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com