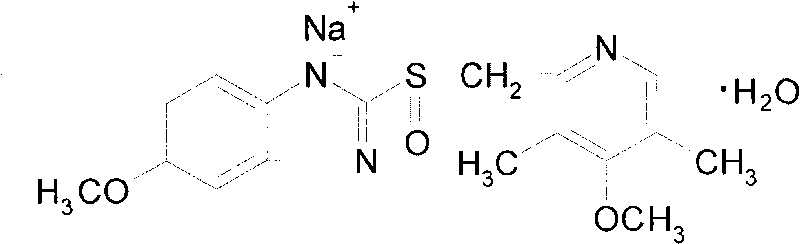
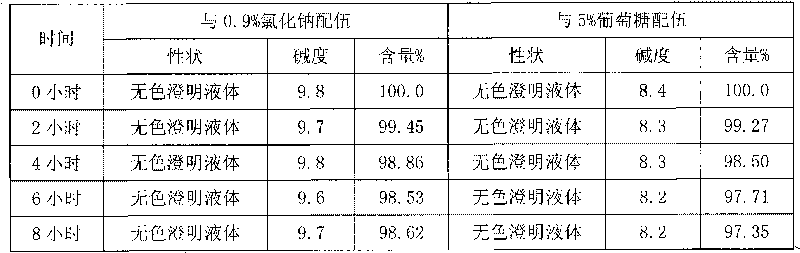
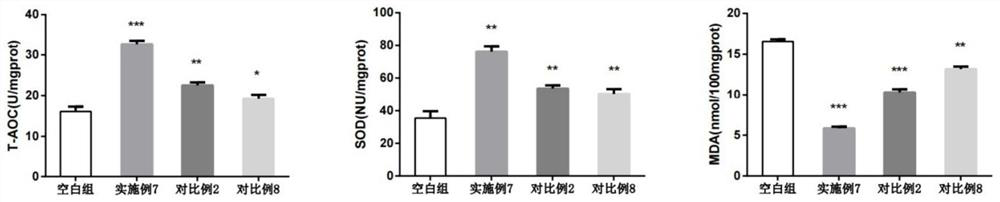
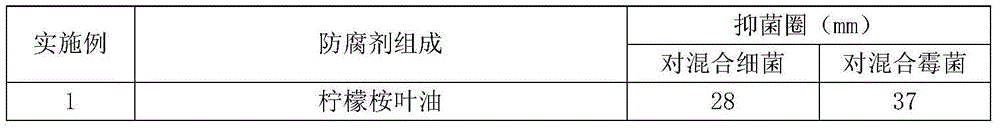
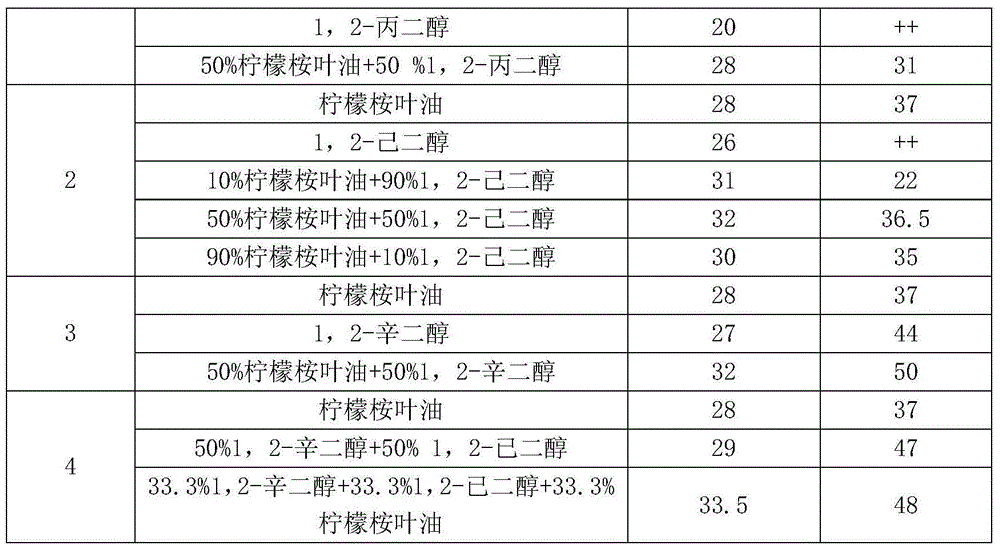
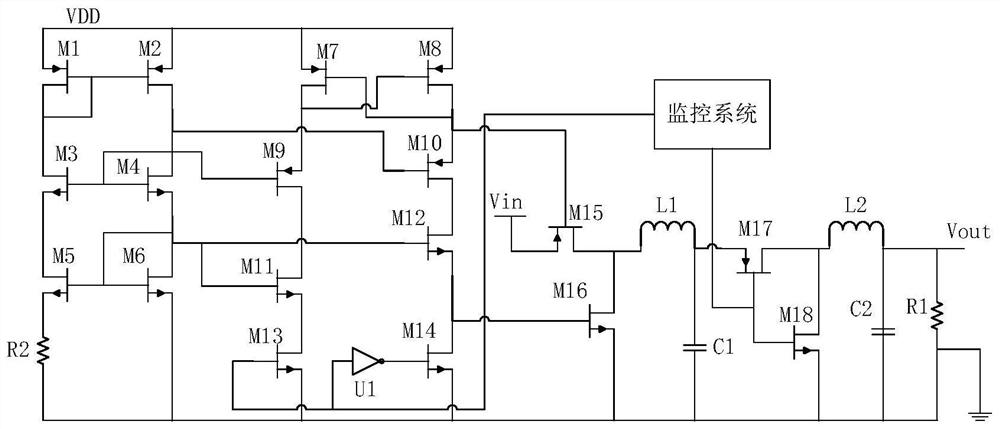
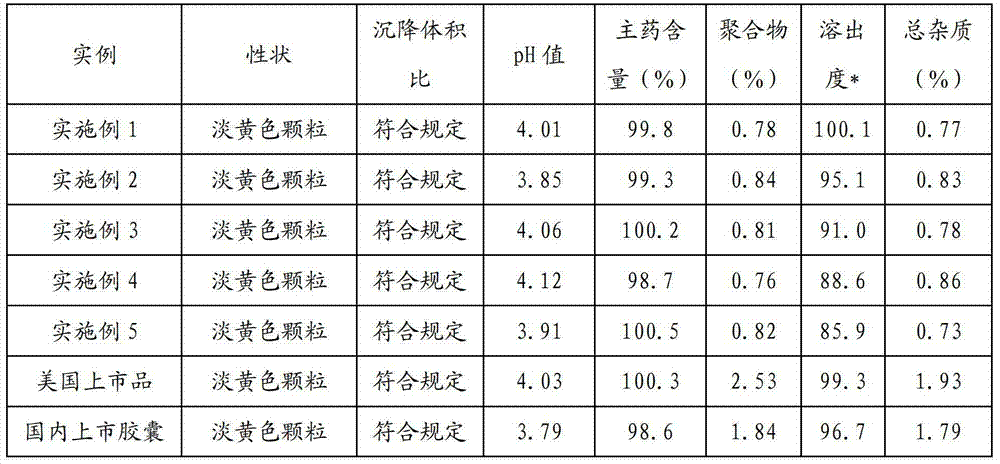
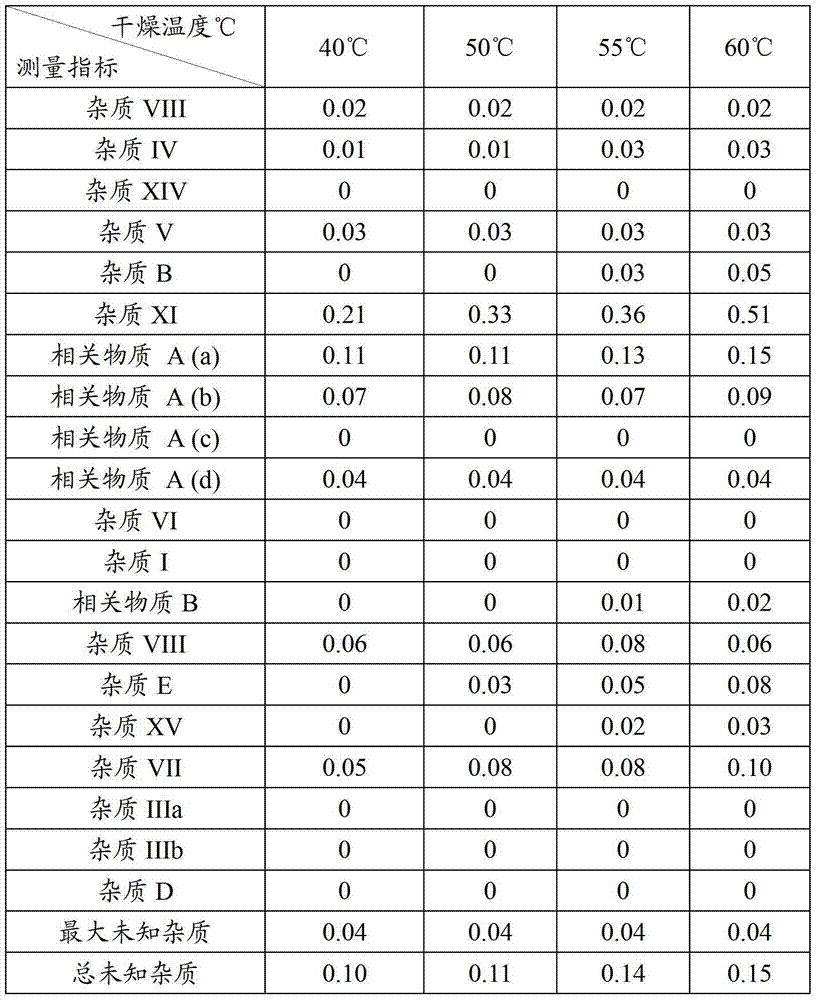
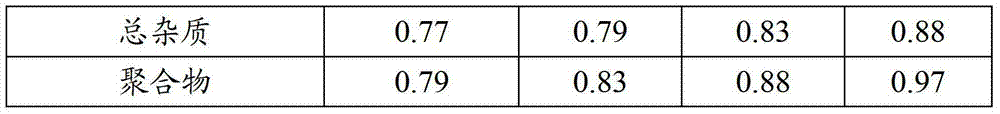
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69results about How to "Reduce the risk of adverse reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Omeprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN101703483AReduce the risk of adverse reactionsComply with the requirements of human intravenous injectionPowder deliveryOrganic active ingredientsOmeprazole SodiumFiltration
The invention discloses an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The omeprazole sodium freeze-dried powder injection contains an active ingredient, namely, omeprazole sodium monohydrate, and auxiliary materials, namely, calcium disodium edetate and sodium hydroxide. The preparation method of the omeprazole sodium freeze-dried powder injection is characterized by comprising the following steps: weighing the calcium disodium edetate of prescription amount and dissolving the calcium disodium edetate in water for injection, stirring, dissolving, and regulating pH value to 10.0-12.0 by using 10% of sodium hydroxide solution; weighing omeprazole sodium of the prescription amount and adding the omeprazole sodium in the mixture, stirring at room temperature for dissolution, supplementing and adding the water for injection to full amount; adding active carbon, stirring at room temperature for decoloration and endotoxin removal, conducting rough filtration to remove carbon firstly, and then conducting refining filtration by using a filter membrane of 0.22 Mum; taking refining filtrate to test intermediate, conducting encapsulation after meeting requirements; and freeze-drying and unboxing, thus obtaining the omeprazole sodium freeze-dried powder injection. The freeze-drying technology of the omeprazole sodium freeze-dried powder injection takes temperature below minus 40 DEG C as pre-freezing temperature; after pre-freezing for at least two hours, sublimation is started, wherein the sublimation temperature is 5-12 DEG C, the sublimation time is over 14 hours; and then drying is conducted for over 2 hours at the temperature of 20-35 DEG C. Unboxing is carried out after a stopper is added and a cover is put in place, thus obtaining the finished product of the omeprazole sodium freeze-dried powder injection.
Owner:HAINAN LEVTEC PHARMA
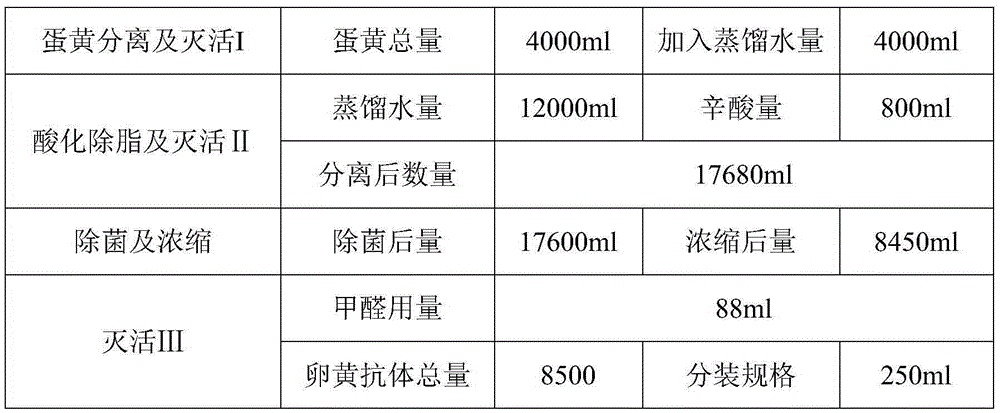
Preparation method of I-type duck hepatitis refined yolk antibodies
InactiveCN105367654AReduce contentReduce the risk of adverse reactionsEgg immunoglobulinsImmunoglobulins against virusesDuck hepatitis A virusYolk
The invention provides a preparation method of I-type duck hepatitis refined yolk antibodies. The preparation method comprises following steps: I-type duck hepatitis virus hyper-immune egg yolk is collected, is subjected to primary inactivation, is subjected to primary acidification extraction with an acetate buffer solution, and then is subjected to secondary inactivation taking octanoic acid as the inactivator and extraction agent; filtering clarification, filtering sterilization, and ultrafiltration concentration are carried out; and then formaldehyde is used for a third time of inactivation so as to obtain a yolk antibody solution. According to the preparation method, content of lipid in the finished product is reduced effectively via acidification water-octanoic acid method, occurrence rate of adverse reaction is reduced, the step of ultrafiltration concentration is beneficial for ensuring product antibody concentration, and increasing protection rate, in application, dilution ratio of the finished product is relatively high because of the high antibody concentration, so that impurity content is reduced indirectly, and safety is improved.
Owner:TIANJIN RINGPU BIO TECH
Egg yolk antibody for preventing novel goose astrovirus and preparation method thereof
ActiveCN108558995AImproving immunogenicityStable passageEgg immunoglobulinsViral antigen ingredientsAnimal scienceWhole body
The invention discloses an egg yolk antibody for preventing novel goose astrovirus and a preparation method thereof. The egg yolk antibody contains a goose astrovirus strain resistance antibody; the preservation number of the goose astrovirus strain is CCTCC NO:V201808. The prepared egg yolk antibody is good in safety, and no any partial or whole adverse reaction is caused by the egg yolk antibody; infections caused by novel goose astrovirus can be effectively prevented and / or treated, and the good commercialization development prospect is achieved.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
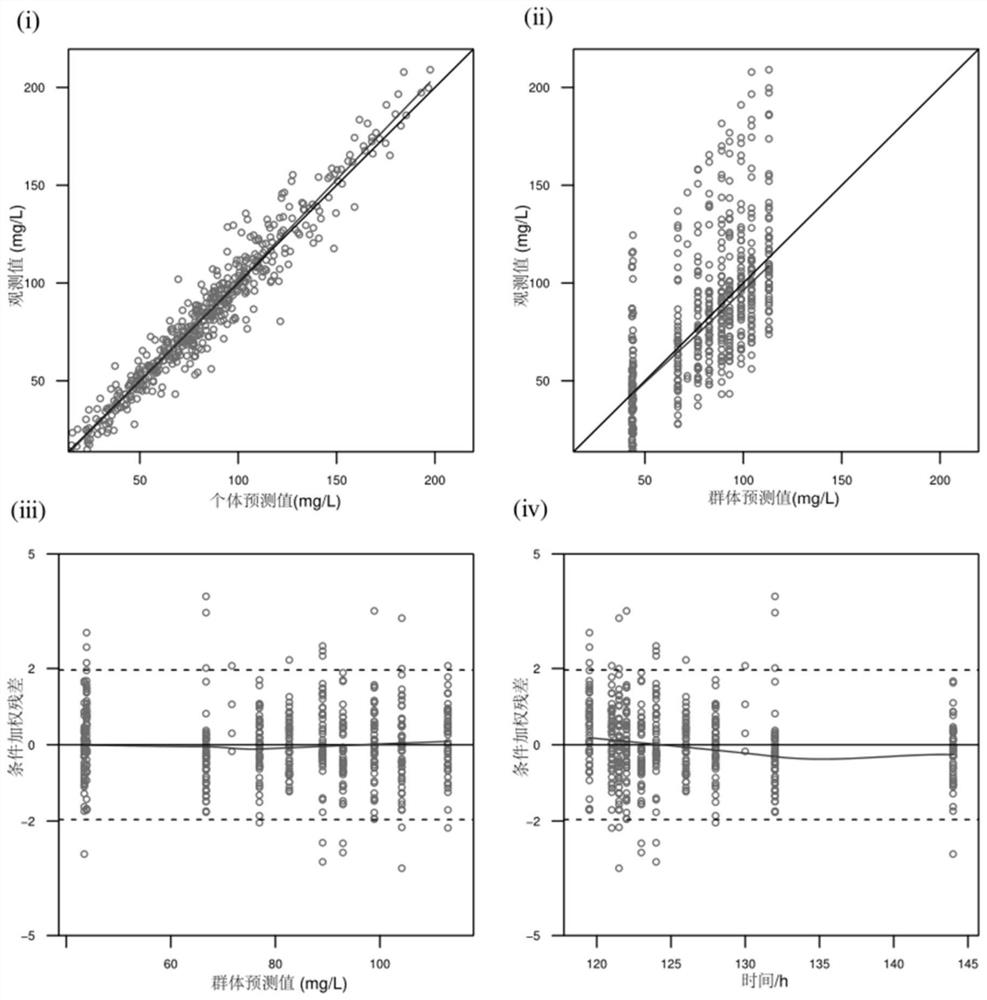
Construction method of kidney transplantation anti-infection drug dosage prediction model
ActiveCN113035369ARapid MedicationAccurate MedicationMolecular designDrug referencesBlood drug concentrationPharmacology
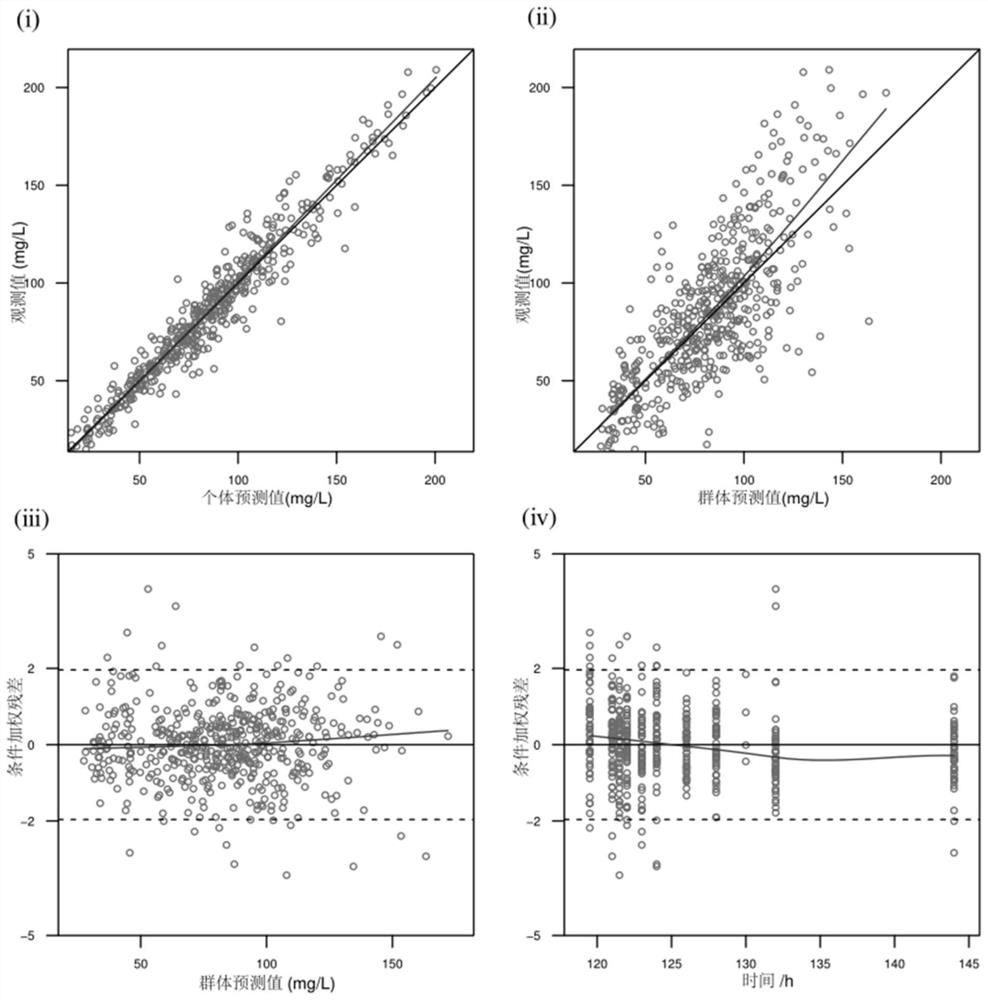
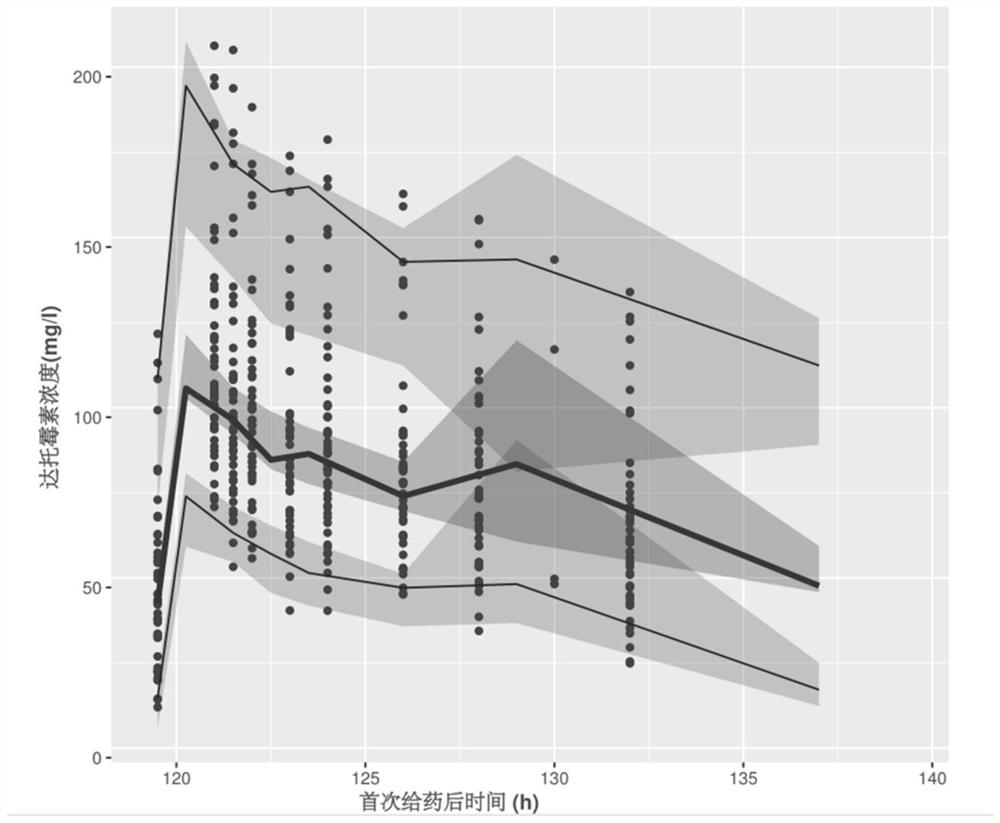
The invention provides a construction method of a kidney transplantation anti-infection drug dosage prediction model. The method comprises the following steps: establishing a group pharmacokinetic model of an anti-infective drug by adopting a nonlinear mixed effect model, a two-atrioventricular model and a mixed residual model through plasma concentration analysis, and calculating pharmacokinetic parameters of the corresponding model; and carrying out related hypothesis testing and estimation on the obtained model pharmacokinetic parameters based on a bootstrap method, visual prediction testing and normalized prediction distribution errors, and completing the steps of stability and prediction capability evaluation and the like. According to the established pharmacokinetic model of the kidney transplantation patient population, the reasonable judgment on the medication scheme information is accurately realized, the reference of the optimal dosage is expected to be provided in the infection prevention and treatment process, the adverse reaction risk is reduced, the curative effect is improved, and finally the individualized administration of the anti-infection medicine of the kidney transplantation patient is realized.
Owner:ZHEJIANG UNIV
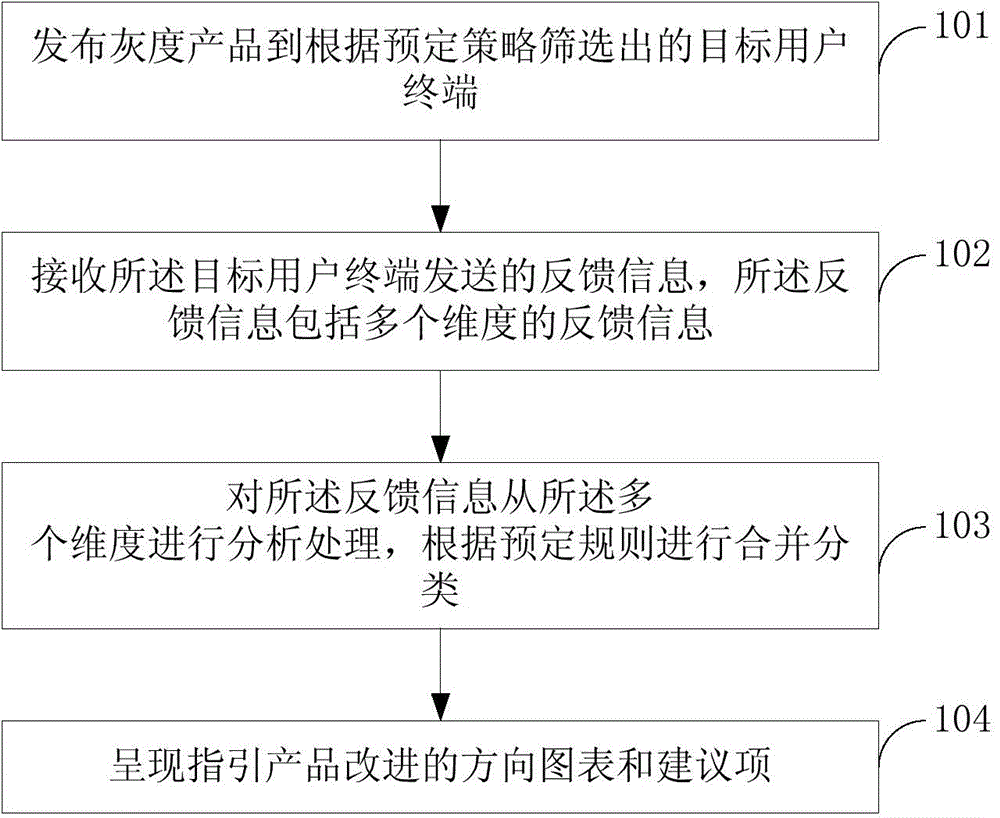
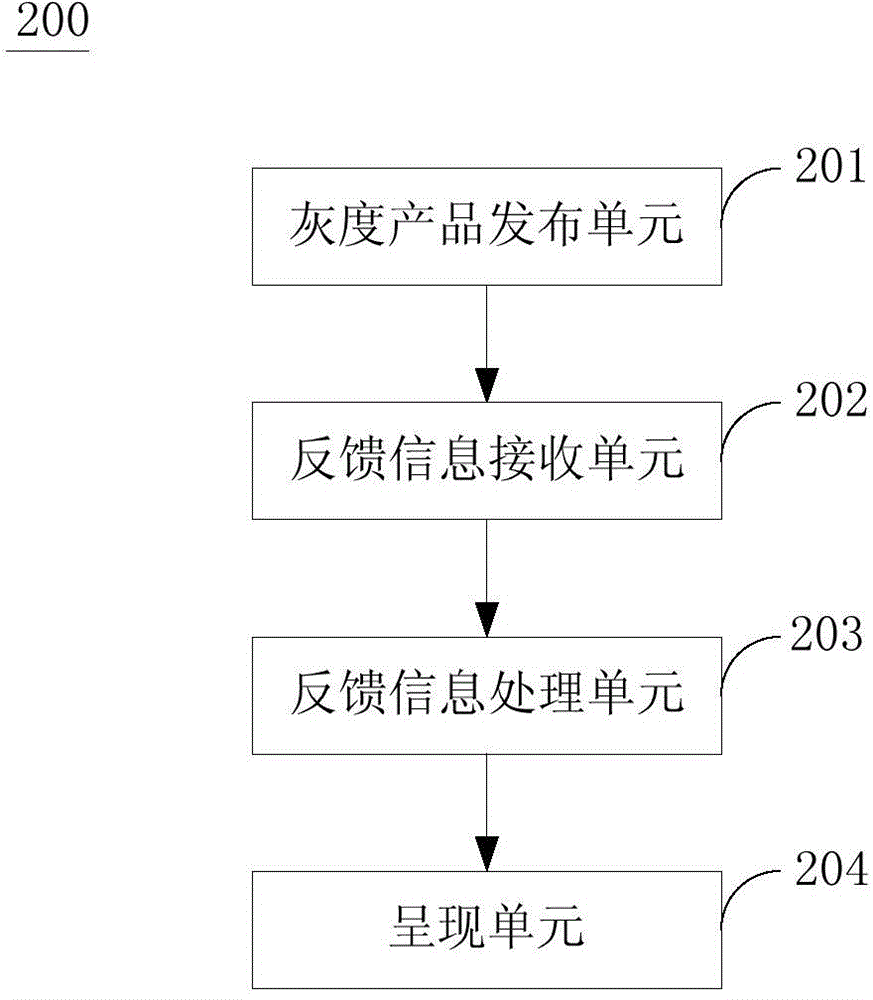
Method, device and system for guiding product improvement based on gray release
InactiveCN104881734AReduce the risk of adverse reactionsAccurate and detailed informationResourcesMarketingComputer terminalComputer science
The invention discloses a method, device and system for guiding product improvement based on gray release. The method for guiding product improvement based on gray release comprises: releasing a gray product to a target user terminal which is screened according to predetermined pre-determined strategy; receiving feedback information sent by the target user terminal, wherein the feedback information comprises feedback information with multiple dimensions; analyzing and processing the feedback information in multiple dimensions, and performing merging and sorting according to a predetermined rule; and showing a directional chart and suggestion items guiding product improvement. A gray product is released to the target user terminal which is screened according to the predetermined pre-determined strategy for performing typical gray release. The feedback information in multiple dimensions is quickly obtained for guiding the product to improve and to be updated; a risk of bad reflection is reduced; and accurate detail information is obtained.
Owner:GUANGDONG XIAOTIANCAI TECH CO LTD
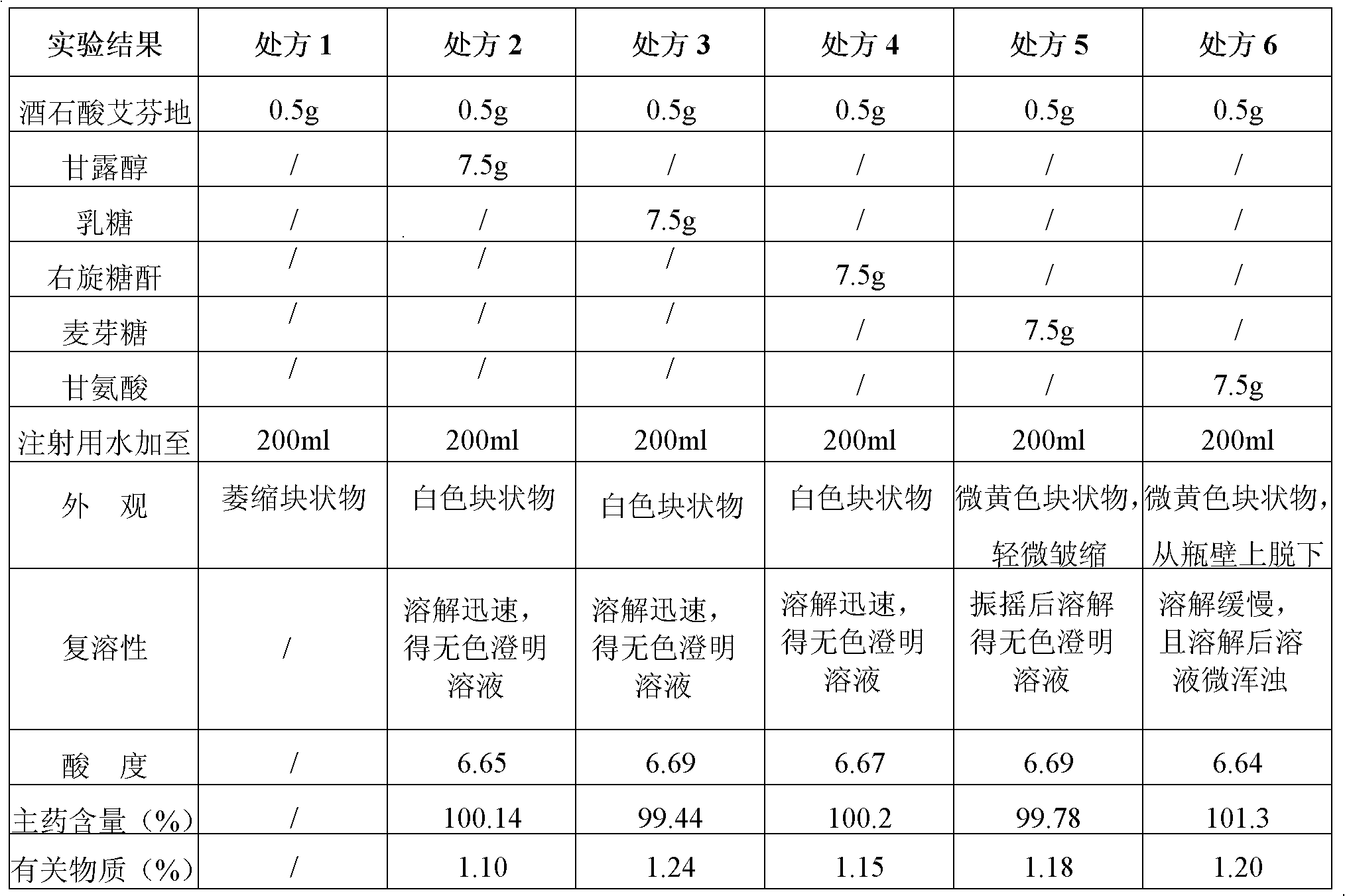
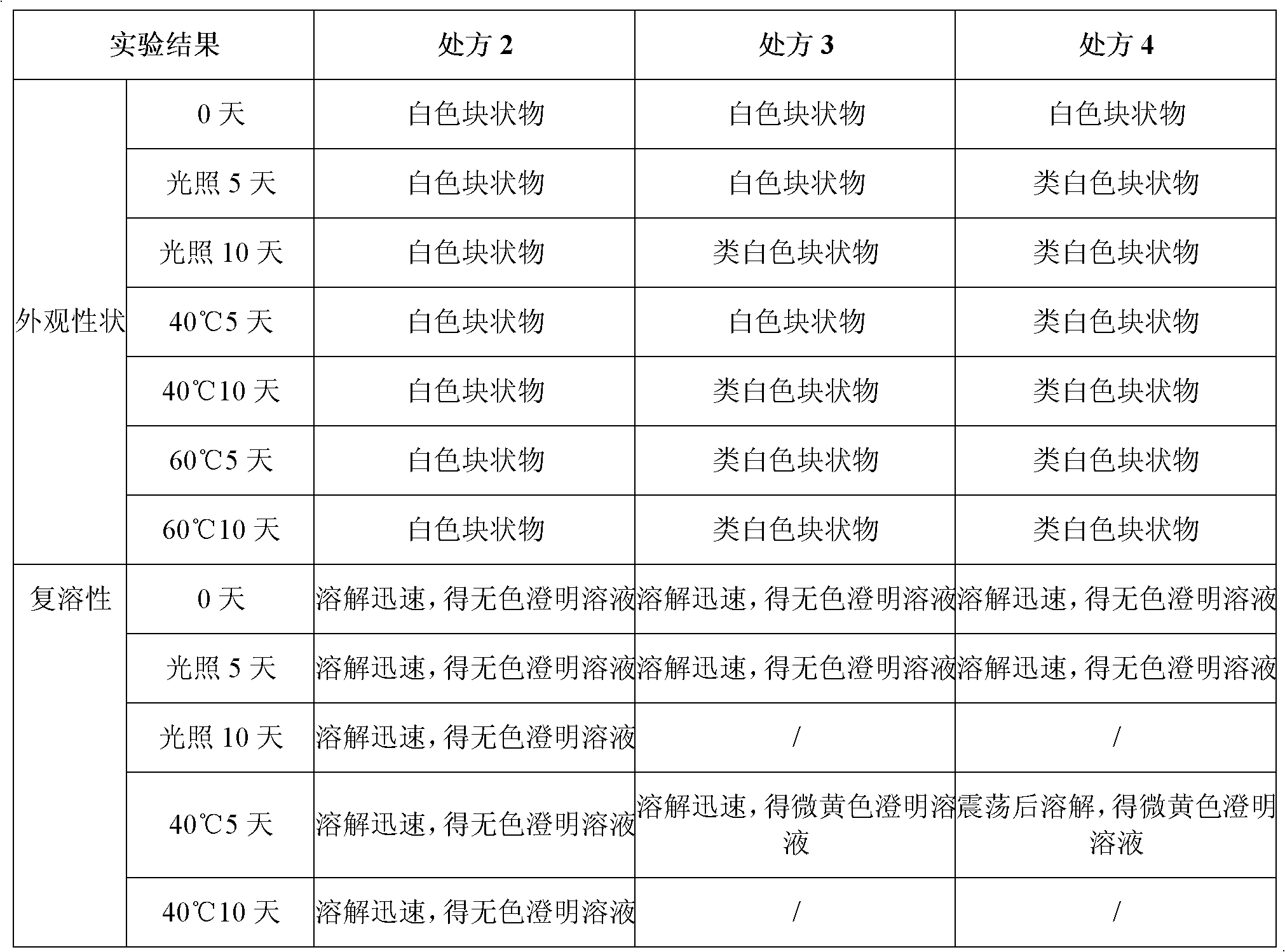
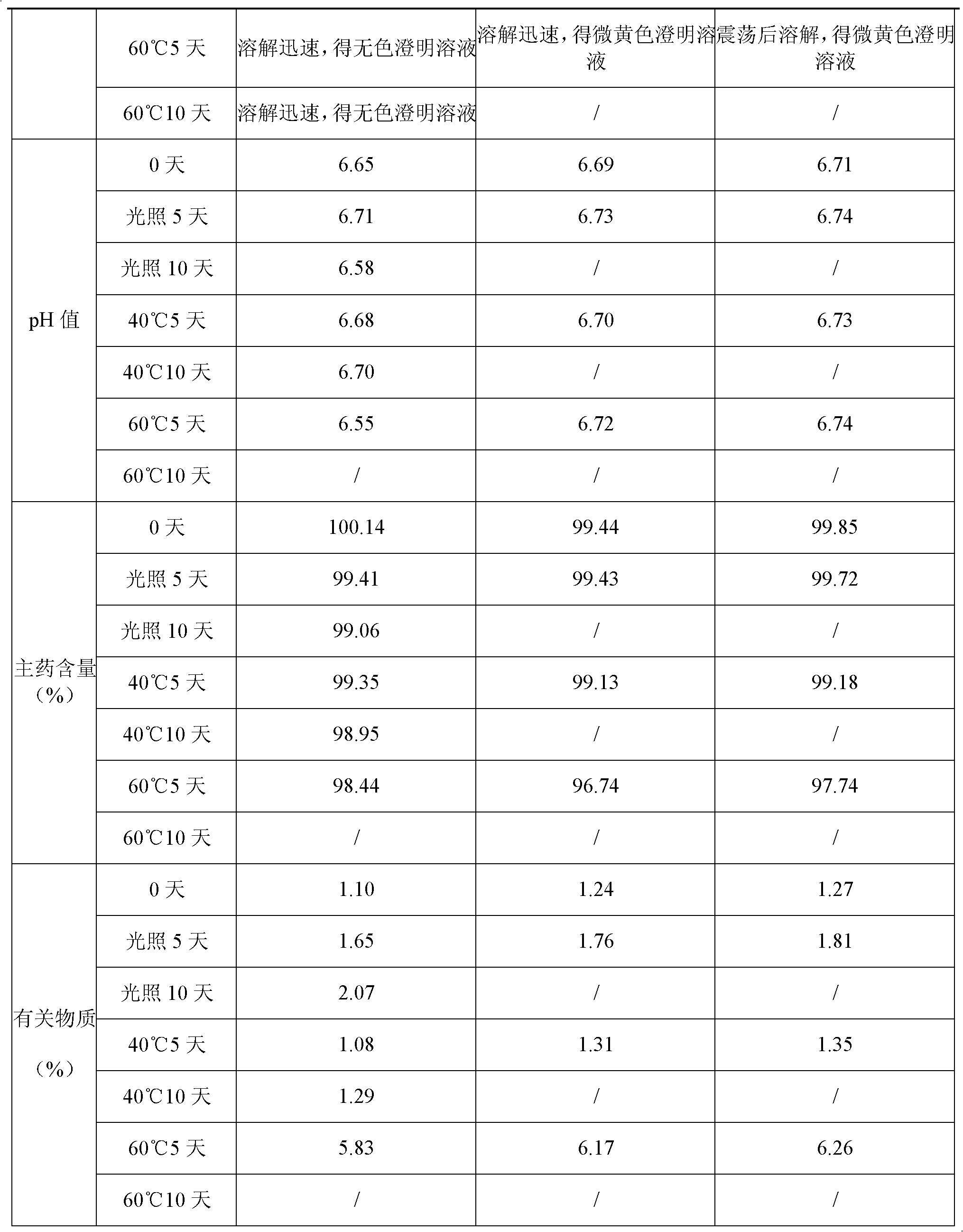
Stable tartaric acid ifenprodil injection and method of preparing the same
InactiveCN101347429AEasy to solveLow costNervous disorderInorganic non-active ingredientsOxygenInjection solution
The invention discloses a stable ifenprodil tartrate injection which contains ifenprodil tartrate, an osmotic pressure regulator, a pH regulator and water for injection. The invention also discloses a preparation method of the stable injection. The stable ifenprodil tartrate injection is obtained by controlling the range of the pH value of the solution and insulating oxygen, thus avoiding use of an antioxidant and possible adverse reactions thereof. Sterility tests, accelerated tests and long duration tests prove the stable ifenprodil tartrate injection.
Owner:重庆人本药物研发有限责任公司 +1
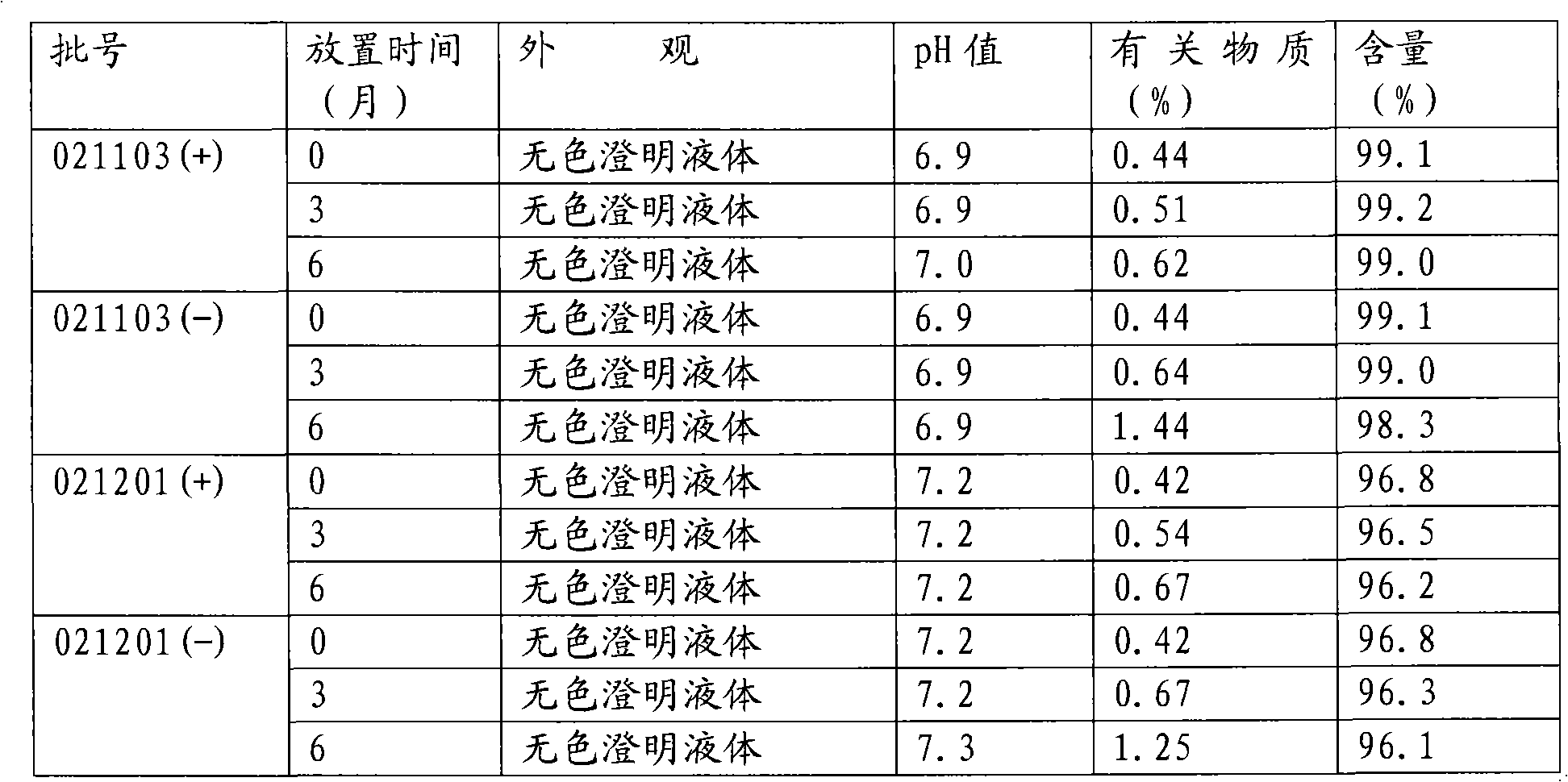
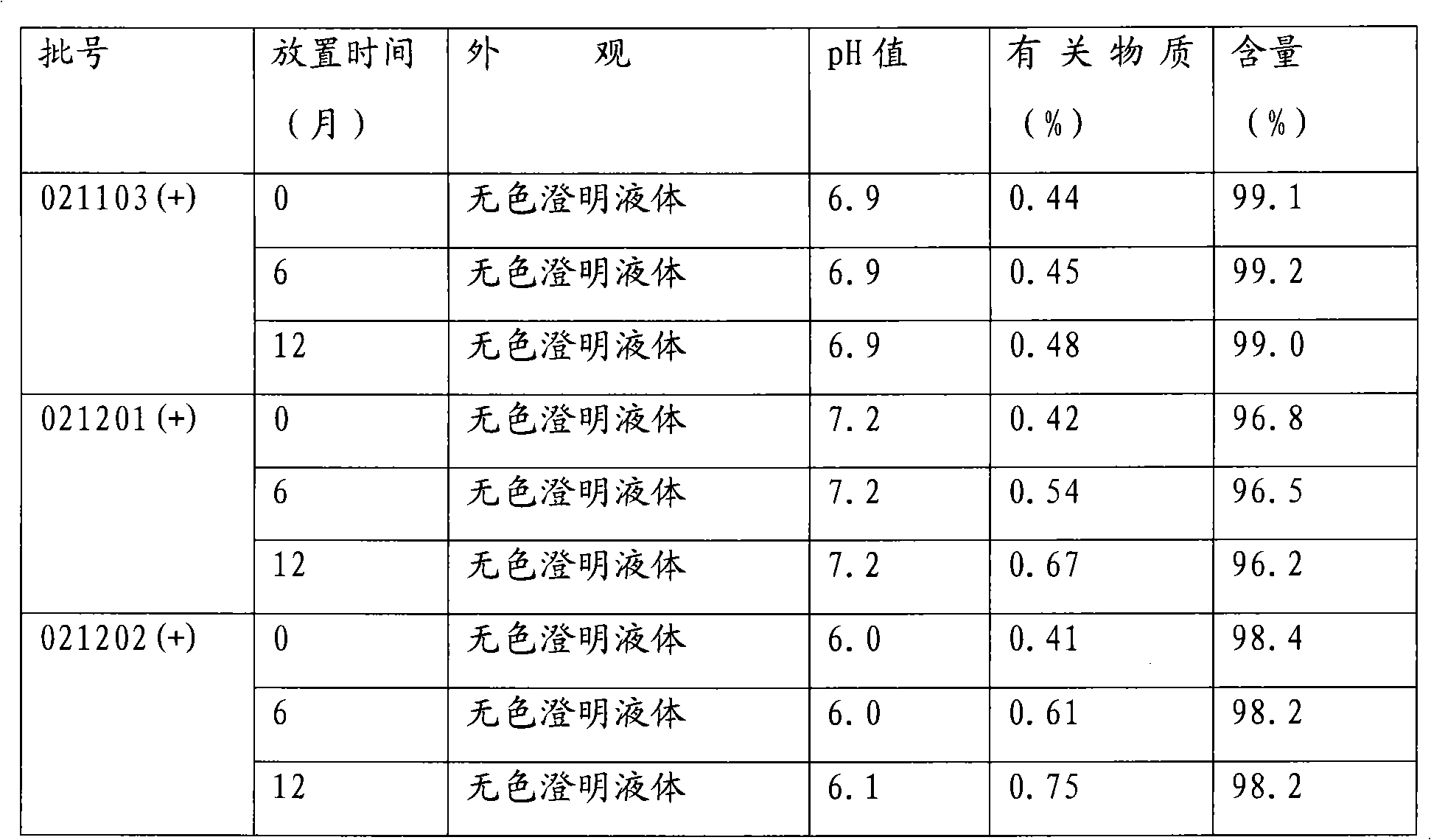
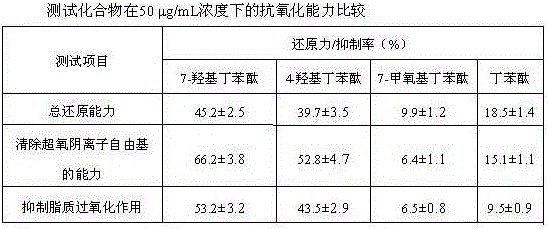
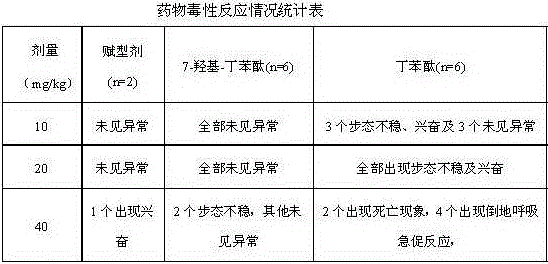
Medical use of 7-hydroxy-butylphthalide
ActiveCN106214674AImprove protectionStrong medicinal effectOrganic active ingredientsAntinoxious agentsVascular diseaseButylphthalide
The invention belongs to the technical field of pharmaceutical chemistry and particularly relates to medical use of 7-hydroxy-butylphthalide in preparation of drugs for preventing and / or treating cardiac and cerebral vascular diseases.
Owner:NCPC NEW DRUG RES & DEV
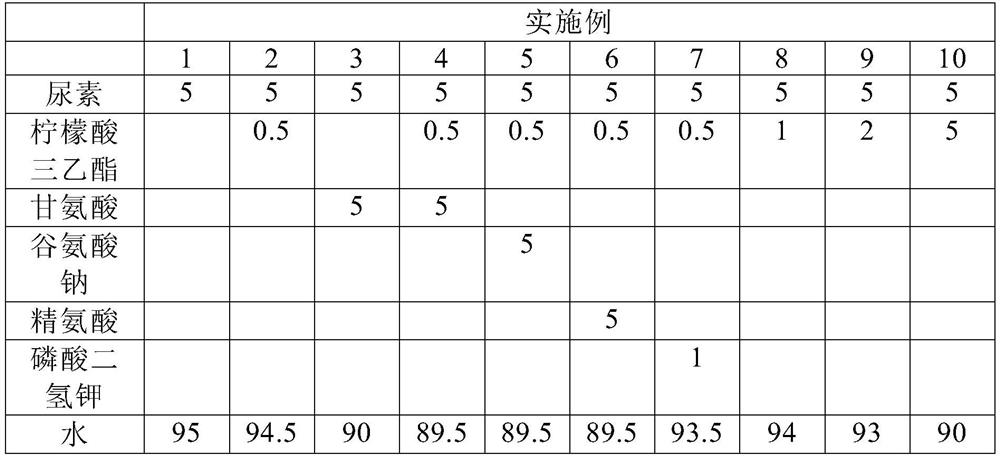
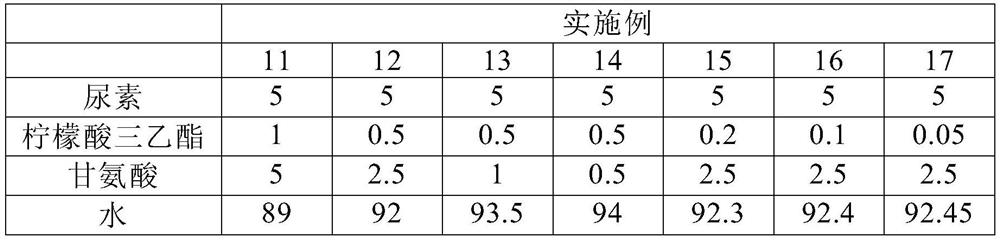
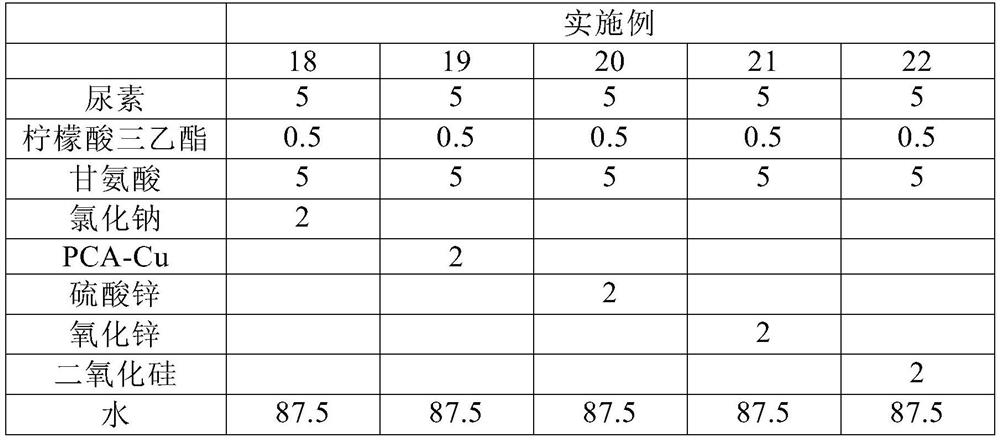
Stabilizing composition containing triethyl citrate and glycine
ActiveCN112515992AReduce dosageImproves pH stabilityCosmetic preparationsToilet preparationsGlycineActive agent
The invention provides a stabilizing composition containing triethyl citrate and glycine. The composition is an aqueous solution, and the aqueous solution further comprises an active agent capable ofproducing ammonia by hydrolysis, wherein the weight ratio of the active agent to the triethyl citrate is from 1: 1 to 100: 1, and the weight ratio of the active agent to the glycine is from 1: 1 to 10: 1. The invention also relates to a method of stabilizing the aqueous solution containing the active agent capable of producing ammonia by hydrolysis, and relates to application of the stabilizing composition in a skin preparation for external use.
Owner:SHANGHAI JAHWA UNITED
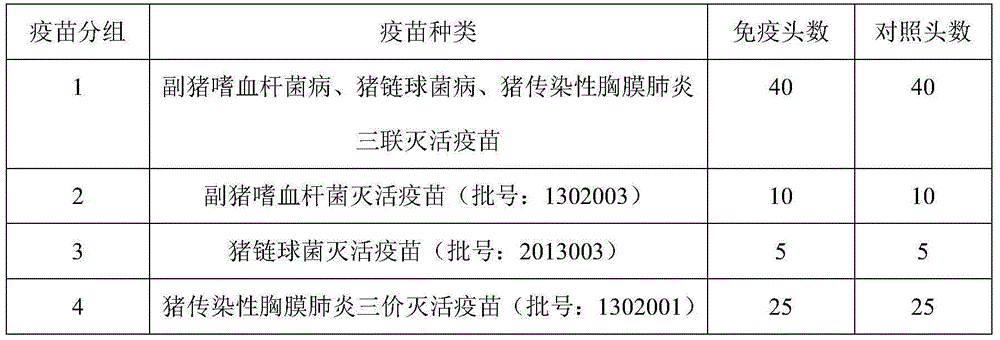
Preparation method of triple inactivated vaccine for pigs
InactiveCN104998256AImprove immunityHighlight immune functionAntibacterial agentsBacterial antigen ingredientsAntigenProtective antigen
The invention provides a preparation method of a triple inactivated vaccine for pigs. The method determines an antigen composition with excellent immunization effects by selection of the antigen. The prepared polyvalent vaccine has outstanding immunization effects. The prepared vaccine contains a PCP immunization protective antigen exotoxin (Aps), has cross immunization protection effects better than those of a whole cell inactivated vaccine, greatly reduces side reaction, and can simultaneously prevent haemophilus parasuis, swine streptococcosis and actinobacillus pleuropneumonia by combined immunization with inactivated haemophilus parasuis and streptococcus suis. Compared with haemophilus parasuis and streptococcus suis inactivated vaccines sold on the market, the triple inactivated vaccine has the same corresponding pathogen immune protection force. Compared with the actinobacillus pleuropneumonia inactivated vaccine sold on the market, the triple inactivated vaccine has a cross immunization protecting force on diseased pigs with different serotypes and realizes multiple protection purposes.
Owner:TIANJIN RINGPU BIO TECH
Egg yolk antibody for preventing and treating novel duck reovirus and preparation method of egg yolk antibody
ActiveCN108676092AImproving immunogenicityStable passageEgg immunoglobulinsImmunoglobulins against virusesAnimal scienceWhole body
The invention discloses an egg yolk antibody for preventing and treating novel duck reoviruses and a preparation method of the egg yolk antibody. The egg yolk antibody comprises an anti-duck reoviru strain antibody, and a duck reoviru strain has a preservation number of CCTCC NO: V201818. The egg yolk antibody disclosed by the invention is good in safety, free of partial or whole-body adverse reaction caused by the egg yolk antibody, is capable of effectively preventing and / or treating infection of the novel duck reoviruses, and has a very good commercial development prospect.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Recombinant collagen hydrogel for injection and preparation method thereof
ActiveCN114259602AGood biocompatibilityNon-immunogenic and low risk of adverse reactionsProsthesisSkin damageMolecular biology
The invention discloses recombinant collagen hydrogel for injection and a preparation method of the recombinant collagen hydrogel. The method comprises the following steps: dissolving recombinant collagen and tea polyphenol in an alkaline solution to obtain a premixed solution with the pH value of 9-13, adding a cross-linking agent, heating the mixed solution in a water bath, carrying out a cross-linking reaction to obtain a cross-linked recombinant collagen hydrogel, cutting the cross-linked recombinant collagen hydrogel into blocks, cleaning, homogenizing, filling, and sterilizing to obtain the recombinant collagen hydrogel. The recombinant collagen hydrogel for injection is obtained. The recombinant collagen hydrogel provided by the invention has excellent mechanical properties, can be used by injection, and improves skin injury caused by photoaging.
Owner:JIANGSU JLAND BIOTECH CO LTD
Recombinant human interferon beta-1b freeze-dried preparation and preparing method thereof
InactiveCN104415325AReduce the risk of adverse reactionsDoes not affect activityPowder deliveryPeptide/protein ingredientsFreeze-dryingRecombinant Human Interferon beta
The invention provides a stable recombinant human interferon beta-1b freeze-dried preparation and a preparing method thereof. The freeze-dried preparation is prepared from recombinant human interferon beta-1b, human blood albumin, a phosphate buffer solution and the like according to a certain proportion and through a vacuum freeze-drying technology; and at the same time, the preparing method of the preparation can ensure the preparation has good stability and activity after redissolving.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Bacteriostatic preservative composition and cosmetic composition comprising same
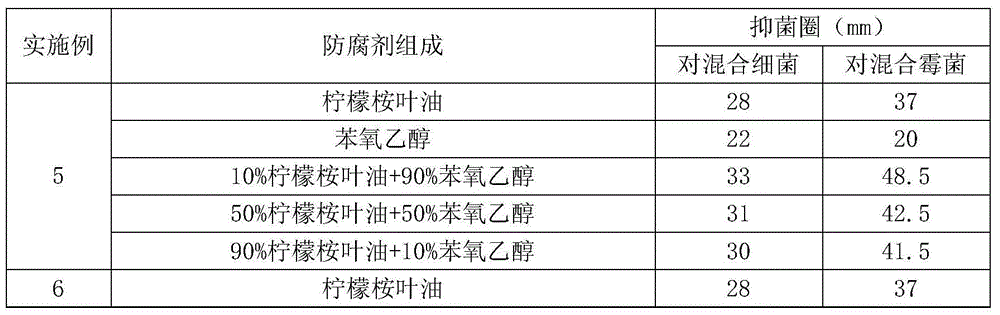
ActiveCN105310903AReduce the risk of adverse reactionsAvoid or reduce the concentrationCosmetic preparationsHair cosmeticsCarbon numberBenzoic acid
The invention discloses a bacteriostatic preservative composition and a cosmetic composition comprising same. The bacteriostatic preservative composition includes oil of eucalyptus citriodora leaves and at least one additive selected from the following components: 1,2-alkylglycol being 3-10 in carbon number, phenoxyethanol, benzyl alcohol, chlorphenesin, ethylhexyl glycerin, p-hydroxybenzoic acid and salts and esters thereof, benzoic acid and salts and esters thereof, sorbic acid and salts thereof, iodopropynyl butylcarbamate, imidazolidinyl urea and bis(hydroxymethyl)imidazolidinyl urea, polyaminopropyl biguanide, DMDM hydantoin, 2-bromo-2-nitropropane-1,3-diol, methyl isothiazolinone, methyl chloroisothiazolinone or a mixture of the components. The bacteriostatic preservative composition significantly improves mildew-proof and anti-bacterial performance.
Owner:SHANGHAI LIGHT IND RES INST
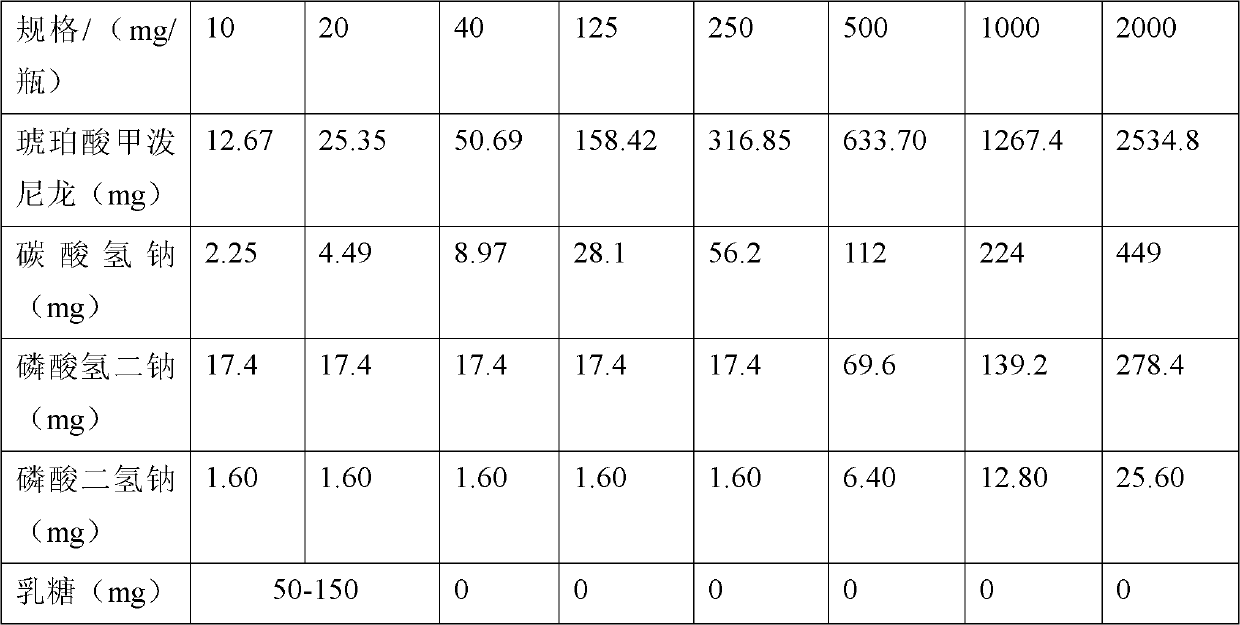
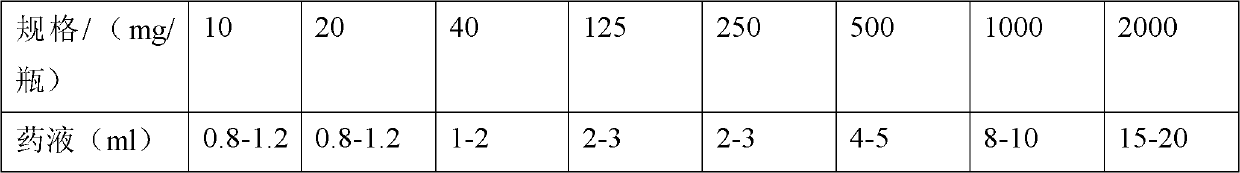
Methylprednisolone sodium succinate freeze-dried powder injection
ActiveCN103371979AChange physical propertiesGood storage stabilityOrganic active ingredientsPowder deliveryFreeze-dryingSuccinic acid
The invention relates to a methylprednisolone sodium succinate freeze-dried powder injection. Every bottle of freeze-dried powder injection is prepared from 12-2550mg of methylprednisolone succinic acid, alkaline auxiliary material in which the sodium ion:succinic acid methylprednisolone mol ratio is 1:1, 0-200mg of excipient and pH value regulator. The powder injection is prepared by dissolving the components in the formula and carrying out freeze-drying. The invention is characterized in that the solvent is ethanol-containing water for injection, and the liquor prepared by dissolving the components in the formula in the solvent contains 5-10 vol% of ethanol.
Owner:TIANJIN JINYAO GRP
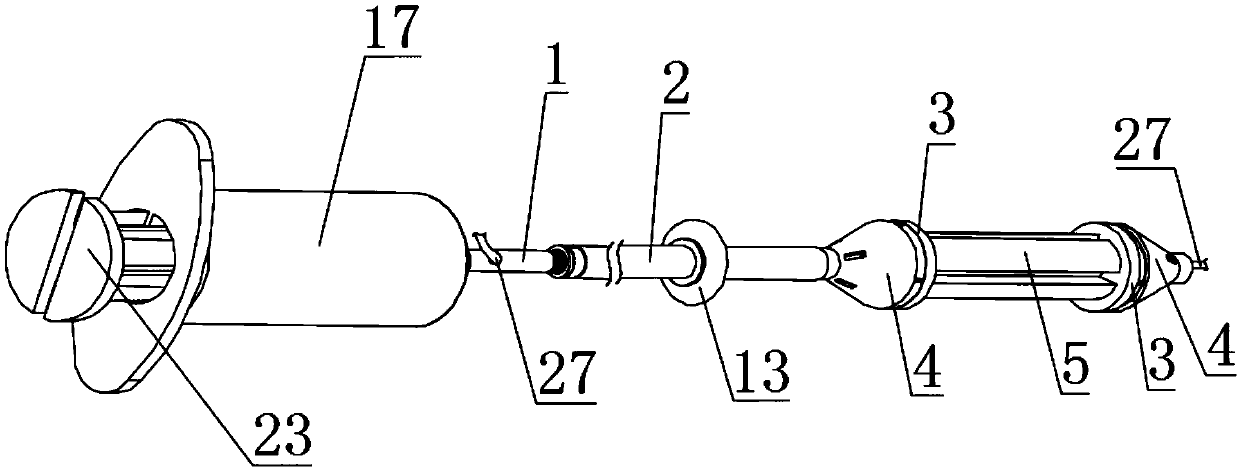
Interventional therapy apparatus used for cardiovascular diseases
PendingCN108670374AImprove efficacySpeed up recoveryInfusion syringesSurgical needlesDual injectionInterventional therapy
The invention discloses an interventional therapy apparatus used for cardiovascular diseases. The interventional therapy apparatus comprises an inner tube. The interior of the inner tube is equipped with a through hole and a wire penetrating hole. A guide wire is arranged in the interior of the wire penetrating hole. Tapered sleeves are connected through connection columns. The interiors of the connection columns are all equipped with drainage chambers. One end of the inner tube is movably connected with a first needle tube. A cavity is arranged in the interior of the first needle tube. An annular groove is arranged in the cavity. By the pulling the inner pipe, two sealing rings are supported by extrusion. Therefore, heart and blood vessels at the connection columns are separated from those at other parts. At this time, blood at a cardiovascular diseased position remains stationary. Then, medicine is injected to blood at cardiovascular diseased position through the through hole. The medicine stays at the cardiovascular diseased position, thereby effectively reducing the medicine applying amount. The risk due to excessively great amount of medicine application is minimized. Additionally, in the manner of dual injection cavities, pressure build-up caused by medicine injection is effectively prevented.
Owner:NANHUA UNIV
Full-automatic multifunctional cell treatment system
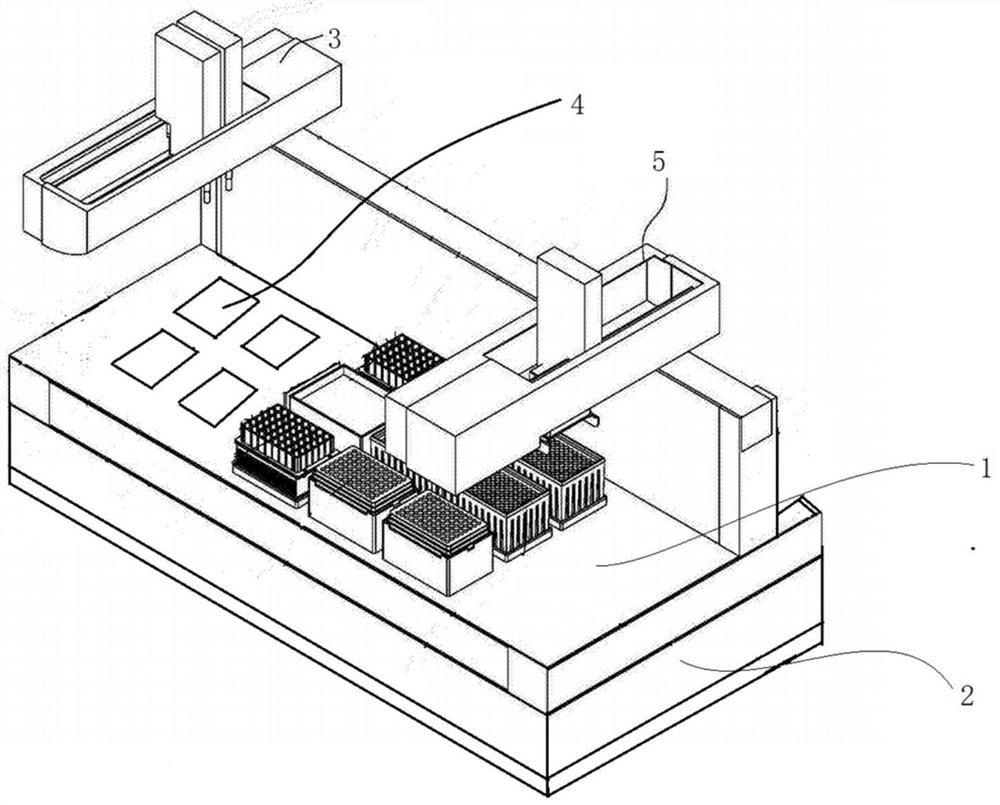
PendingCN113106022AExquisite structureImprove securityBioreactor/fermenter combinationsBiological substance pretreatmentsPhysicsQuality control
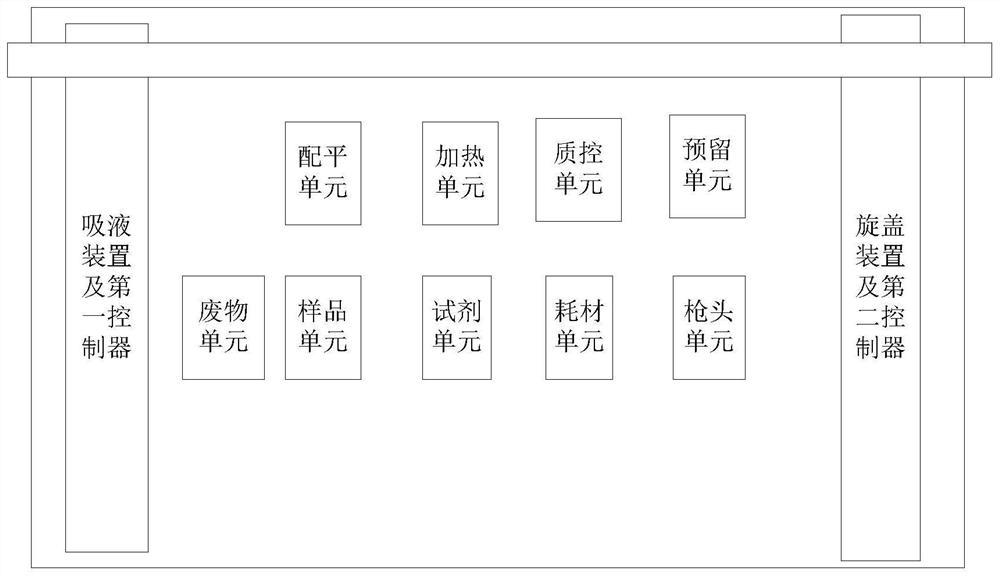
The invention relates to a full-automatic multifunctional cell treatment system which comprises a biosafety cabinet and a workbench, and the workbench comprises an on-table part and an off-table part; wherein the on-table part of the workbench comprises a balancing unit, a heating unit, a quality control unit, a connection unit, a sample unit, a reagent unit, a consumable unit, a gun head unit, a waste unit, a liquid suction device, a first controller, a cap screwing device with recognition and clamping functions, and a second controller; the off-table part of the workbench comprises a centrifugal machine and a power supply control module. The biosafety cabinet is arranged outside the workbench, and a switch window is arranged on the biosafety cabinet.
Owner:TIANJIN CITY THIRD CENT HOSPITAL
Cefdinir, citric acid and sodium citrate dry suspension composition
ActiveCN103239411AFully absorbedSmall particle sizeAntibacterial agentsOrganic active ingredientsSodium citrateBioavailability
The invention provides a cefdinir (C14H13N5O5S2), citric acid and sodium citrate dry suspension composition and a preparation method thereof. The cefdinir, citric acid and sodium citrate dry suspension composition comprises the following components in parts by weight: 100 parts of cefdinir, 1900-2100 parts of a diluent, 120-200 parts of a flocculant, 28-35 parts of a corrigent, 185-195 parts of an adhesive, 15-30 parts of a suspending aid, 8-12 parts of a flow aid and 1.5-2.5 parts of a lubricant. The cefdinir, citric acid and sodium citrate dry suspension composition disclosed by the invention is convenient for being taken by patients, and has high bioavailability and stability.
Owner:XIAN ENCI PHARMA CO LTD
Composition for inhibiting methicillin-resistant staphylococcus aureus biofilm
InactiveCN108272792AIncreased mortalityIncrease doseAntibacterial agentsOrganic active ingredientsMethicillin resistant StaphylococcusBaicalein
The invention discloses a composition for inhibiting methicillin-resistant staphylococcus aureus biofilm, belongs to the technical field of microorganism. The composition is prepared from baicalein and an antibacterial medicine, wherein the antibacterial medicine is linezolid. Due to the synergistic effect of the composition provided by the invention, not only is the antibacterial effect of the medicine against methicillin-resistant staphylococcus aureus improved, but the dosage of the antibacterial medicine is also reduced, and the toxic and side effects are further reduced. The composition is a more ideal pharmaceutical composition for interfering with methicillin-resistant staphylococcus aureus biofilm associated infection.
Owner:GUANGXI MEDICAL UNIVERSITY
Nanofiber reinforced absorbable intravascular stent and preparation method thereof
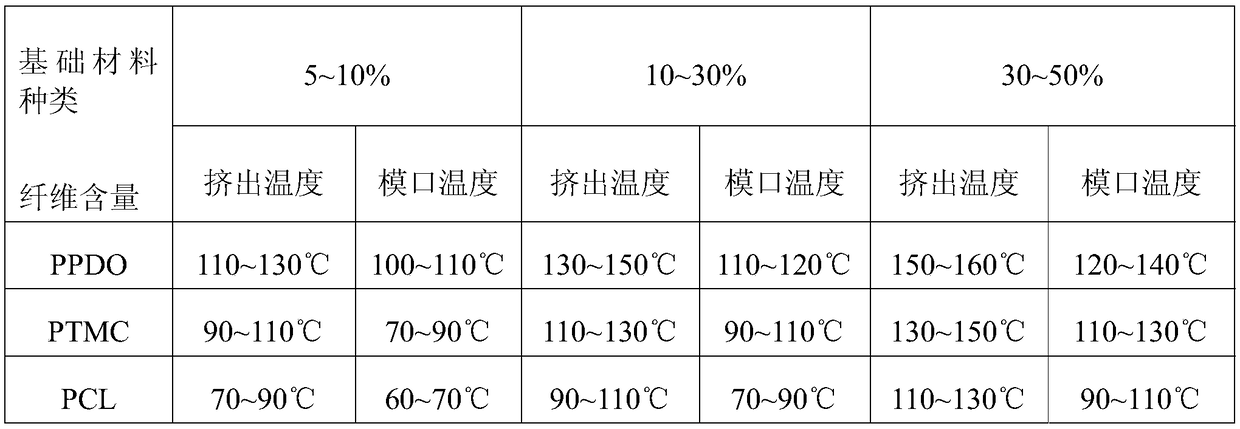
InactiveCN109453437AImprove support strengthImprove stent elasticitySurgeryPharmaceutical delivery mechanismCrystal morphologyMicrometer
The invention discloses a nanofiber reinforced absorbable intravascular stent which is made of a medical nanofiber reinforced composite material; the medical nanofiber reinforced composite material comprises a basic material and nano short-staple, and the nano short-staple keeping at a crystal morphology disperses in the basis material. According to the nanofiber reinforced absorbable intravascular stent, the wall thickness thereof is reduced to be about 100 micrometers, that is reduced by about 30% as compared with the wall thickness of the existing stent, and thus, influence to blood vesselsfrom the stent is reduced, quantity of polymer and acid degradation products is decreased, and risk of thrombus is reduced; as the basic material of the nanofiber reinforced material contains fewer ester bonds, in other words, there is one ester bond every five to six carbon atoms, content of carboxyl is reduced and acidity is decreased greatly as compared with that of Poly-L-lactide after degradation, risk of adverse reaction is much lower, and safety performance is higher.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Ifenprodil tartrate freeze-dried powder injection and preparation method thereof
InactiveCN102133198AGood qualityGood stabilityPowder deliveryNervous disorderFreeze dryIfenprodil tartrate
The invention discloses an ifenprodil tartrate freeze-dried powder injection, and belongs to the technical field of medicine. The ifenprodil tartrate freeze-dried powder injection comprises the following raw materials: 2 to 10 weight parts of ifenprodil tartrate (C21H27NO2)2.C4H6O6 and 20 to 200 weight parts of excipients. The invention also discloses a preparation method of the freeze-dried powder injection. The ifenprodil tartrate freeze-dried powder injection prepared by the method only contains the excipients required by the freeze-dried powder injection, so that possible adverse reaction caused by the addition of other auxiliary materials is avoided. Besides, the ifenprodil tartrate freeze-dried powder injection is high in stability and convenient to store and transport.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
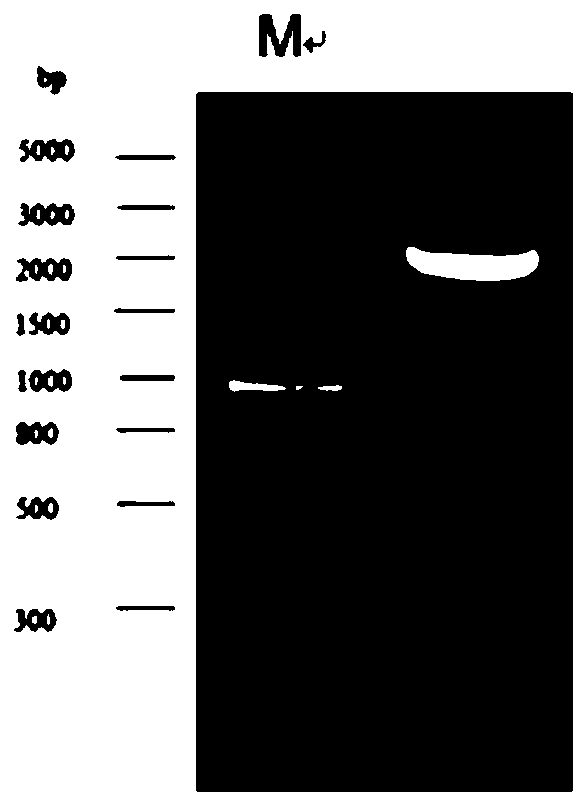
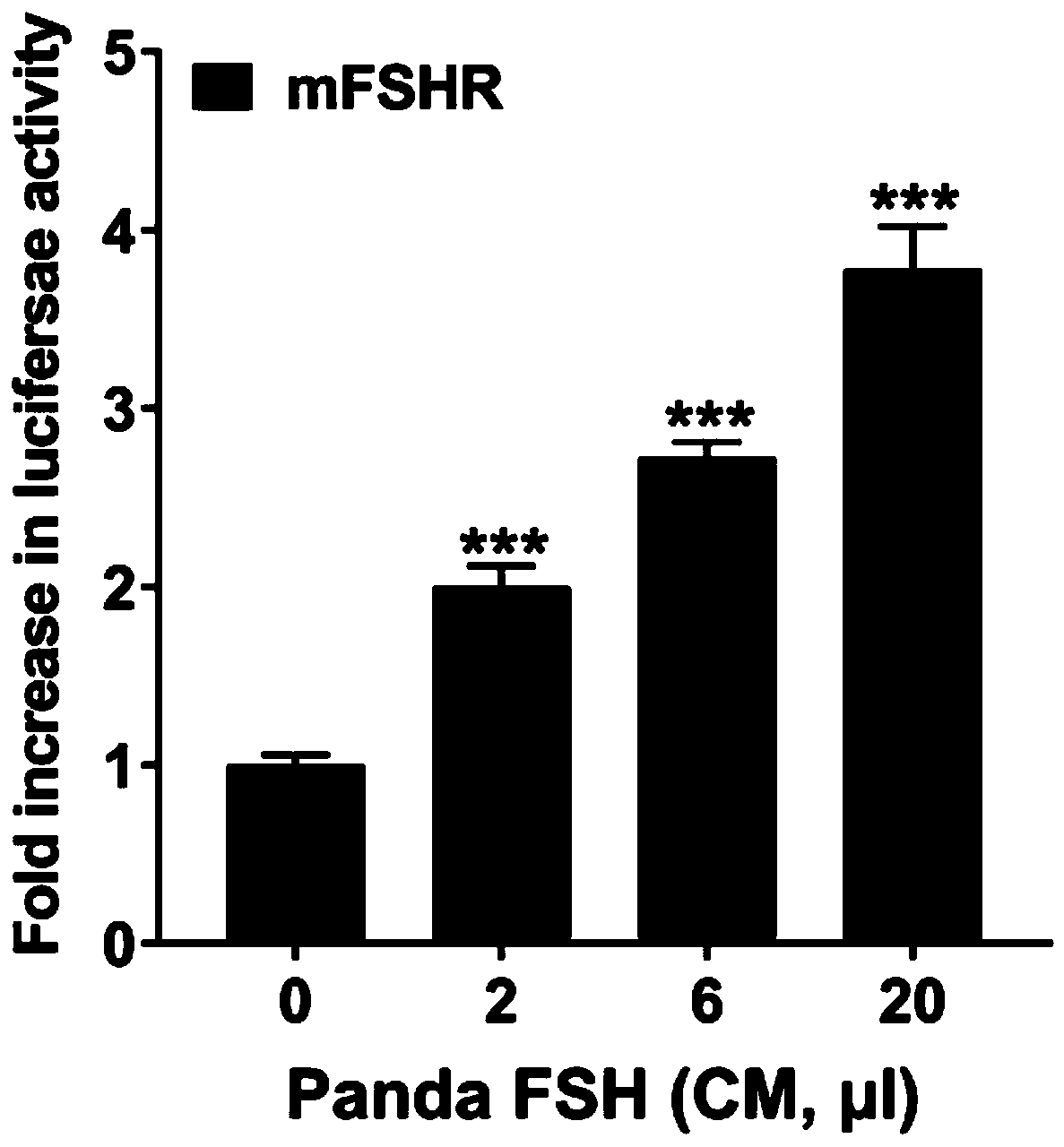
Vector for recombinant expression of panda follicle-stimulating hormone, expression system and preparation method thereof
ActiveCN110938656AReduce the risk of adverse reactionsConducive to breed selectionDepsipeptidesNucleic acid vectorPANDASPhysiology
The invention discloses a vector for recombinant expression of panda follicle-stimulating hormone, an expression system and a preparation method thereof, which relate to the technical field of gene engineering. The vector comprises a first gene for encoding a panda FSH alpha subunit and a second gene for encoding a panda FSH beta subunit, the sequence of the first gene is shown as SEQ ID NO.1, andthe sequence of the second gene is shown as SEQ ID NO.2. By constructing the vector and the expression system for recombinant expression of the panda follicle-stimulating hormone, stable and continuous active expression of the panda FSH alpha subunit, the FSH beta subunit and subunit compounds thereof can be realized. By using the panda-derived follicle-stimulating hormone, the risk of adverse reaction of organisms is reduced, and the breeding and propagation of varieties of rare endangered felines in China, such as giant pandas, small pandas and the like, are facilitated. In addition, the recombinant preparation method is simple and low in cost.
Owner:CHINA CONSERVATION & RES CENT FOR THE GIANT PANDA SICHUAN
Asarone drug composition for injection or inhalation
InactiveCN105997899ASimilar curative effectSimple prescriptionPowder deliveryDispersion deliveryFreeze-dryingTurbidity
The invention discloses an asarone drug composition for injection or inhalation. The asarone drug composition for injection or inhalation is prepared from a composite solvent prepared from ethanol, polyethylene glycol 400 and / or propylene glycol and water and an active ingredient asarone. When the asarone drug composition is prepared into an injection, a pH value is regulated with buffer salt, freeze-dried powder can be prepared by adding mannitol, sorbitol and other injectable auxiliary materials as excipients. The problem of unqualified clarity caused by poor devitrification, turbidity and redissolution when the asarone drug composition is prepared into the injection is solved, accordingly the quality and stability of drugs are ensured, the quality of the product stored for 2 years under the shading room temperature condition meets the requirements, the using requirements for industrialized mass production and clinical use can be met, and the injection can be atomized for inhalation medication.
Owner:HAINAN LEVTEC PHARMA
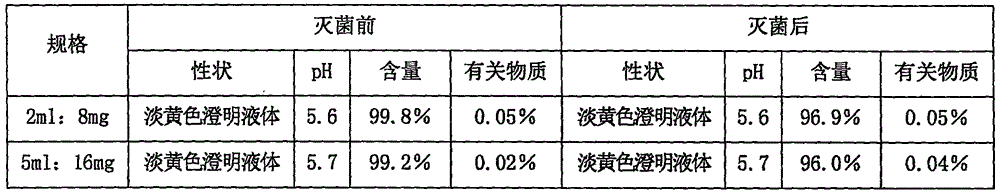
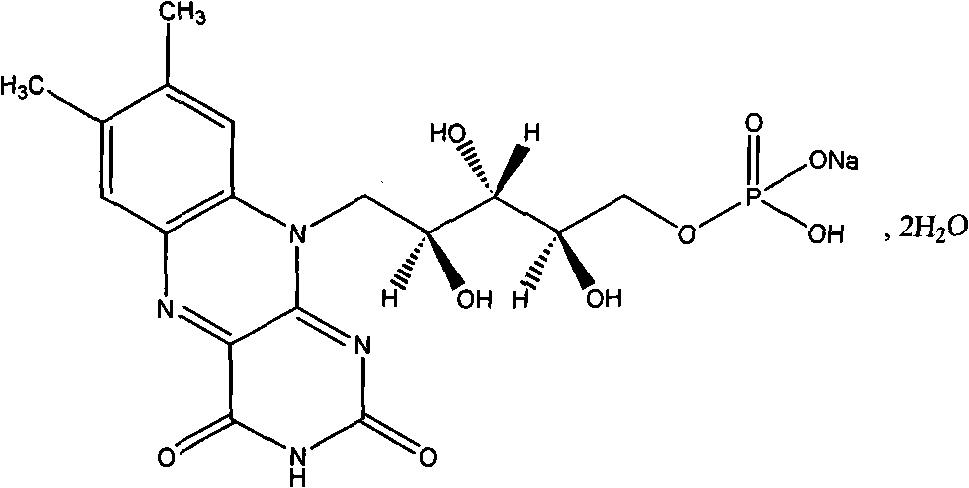
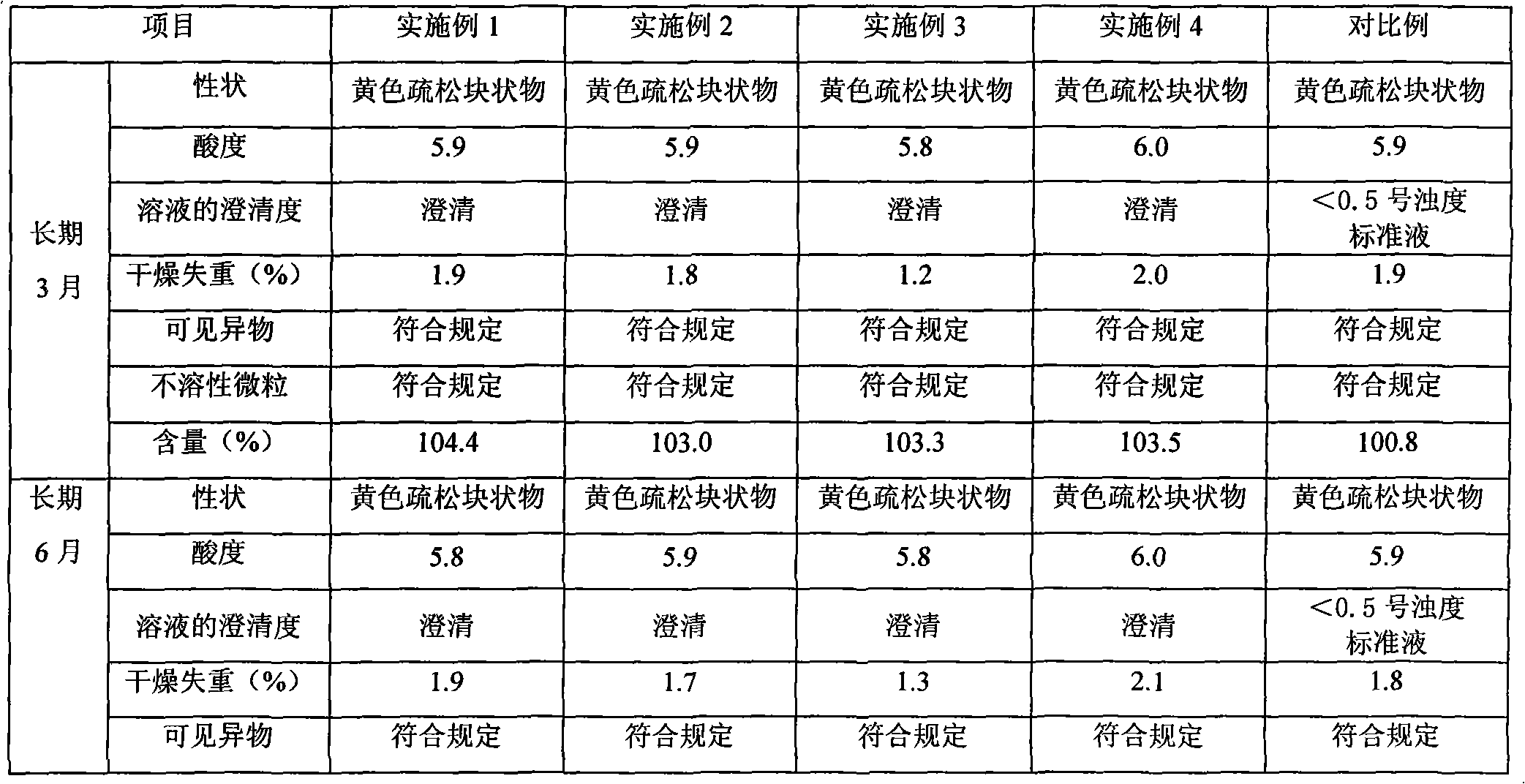
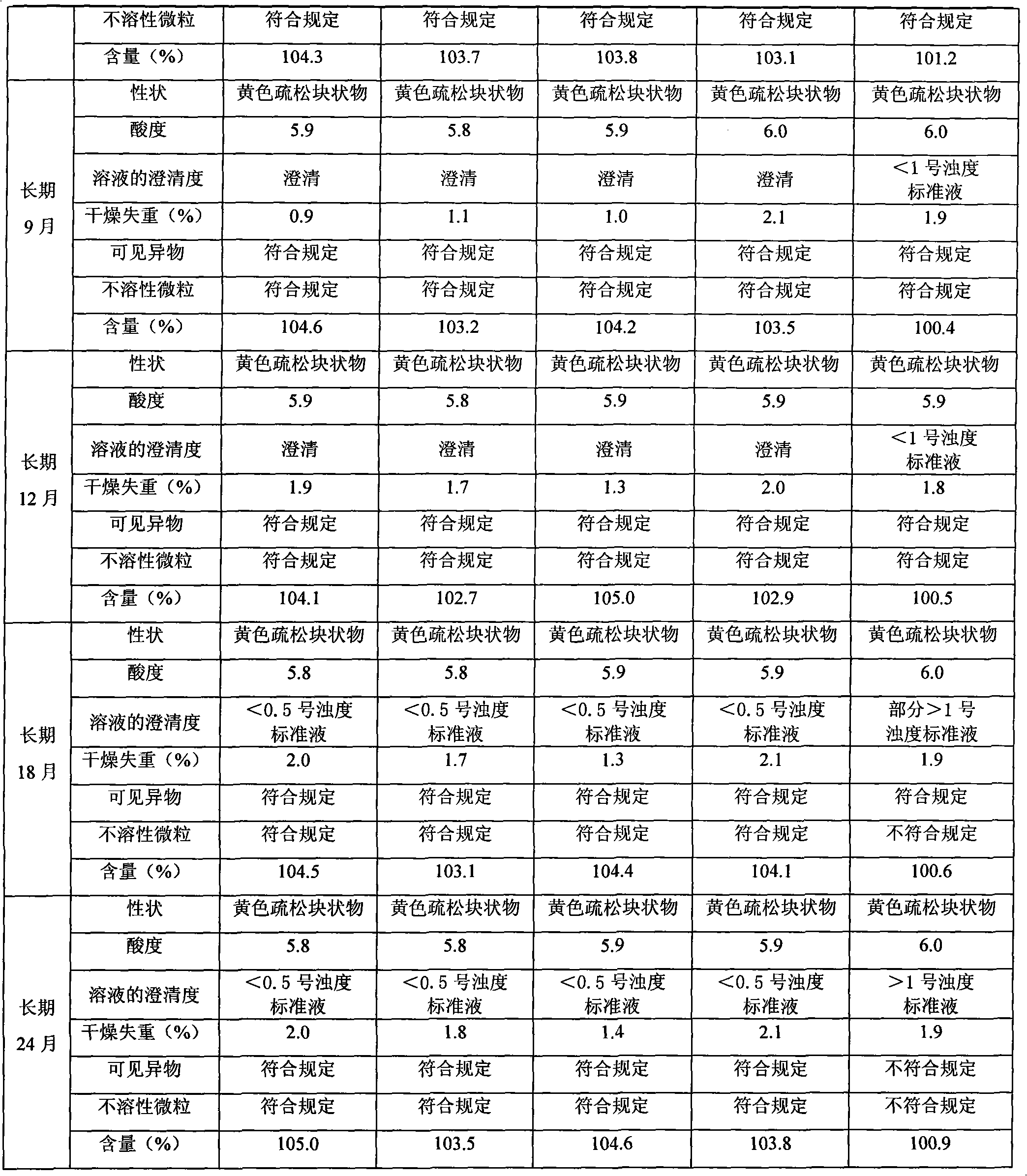
Riboflavin sodium phosphate freeze-dried powder injection and preparation method thereof
ActiveCN101874787AMeet the requirements for intravenous drug useReduce the risk of adverse reactionsOrganic active ingredientsPowder deliveryHaemolysisFreeze-drying
The invention relates to a riboflavin sodium phosphate freeze-dried powder injection and a preparation method thereof. The injection is characterized by comprising a riboflavin sodium phosphate dehydrate, mannitol and / or ammonia water. The injection is sensitive to light, so a brown amoxicillin bottle is used as the inner package of the injection. The riboflavin sodium phosphate freeze-dried powder injection has the following advantages: (1) single and clear auxiliary materials in the formula can meet the requirement of industrialized batch production; (2) medicinal injection grade raw and auxiliary materials accord with the medication requirement of human intravenous injection, so that potential adverse reaction risk in clinical application can be greatly reduced; (3) long-term stability tests prove that the preparation can be preserved for 2 years at the temperature below 30 DEG C, so that stability of the medicament in circulation and safety of clinical medication can be ensured; and (4) special safety tests, such as irritability, haemolysis, vascular stimulation and the like, prove that haemolysis, agglutination, stimulation and anaphylaxis reactions are not found.
Owner:HAINAN LEVTEC PHARMA
Juno<TM>-based safety medication detection kit for children and chip
InactiveCN108728524AGood curative effectReduce the risk of adverse reactionsMicrobiological testing/measurementDNA/RNA fragmentationHLA-BCYP2C9
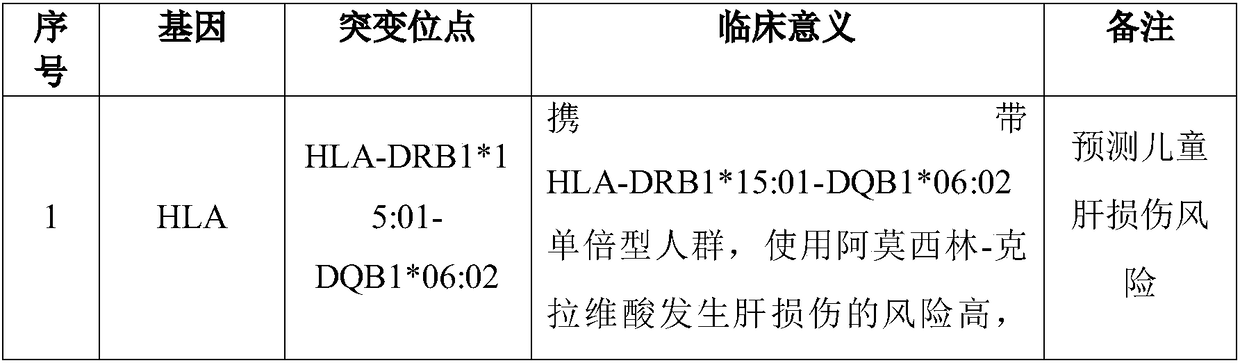
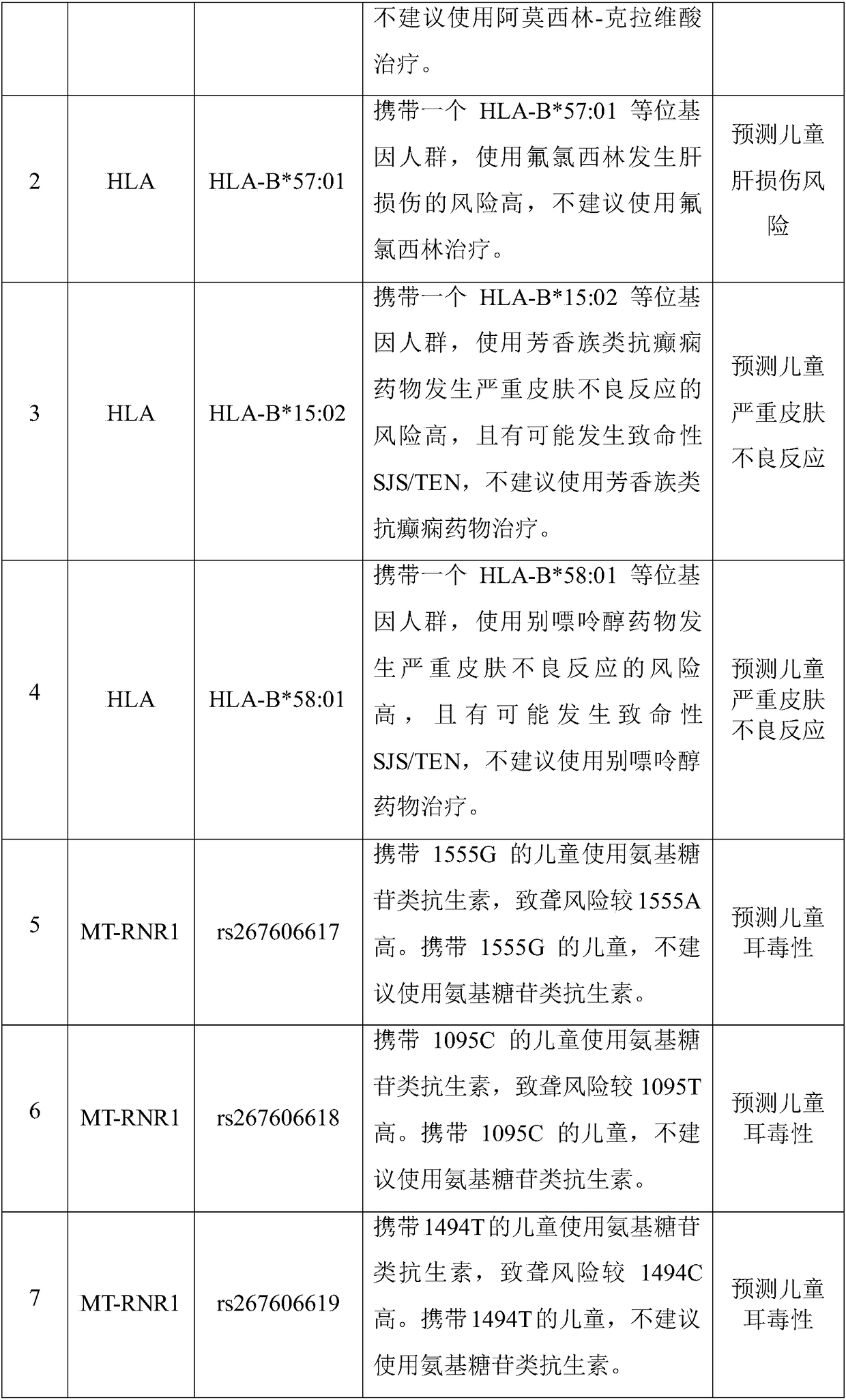
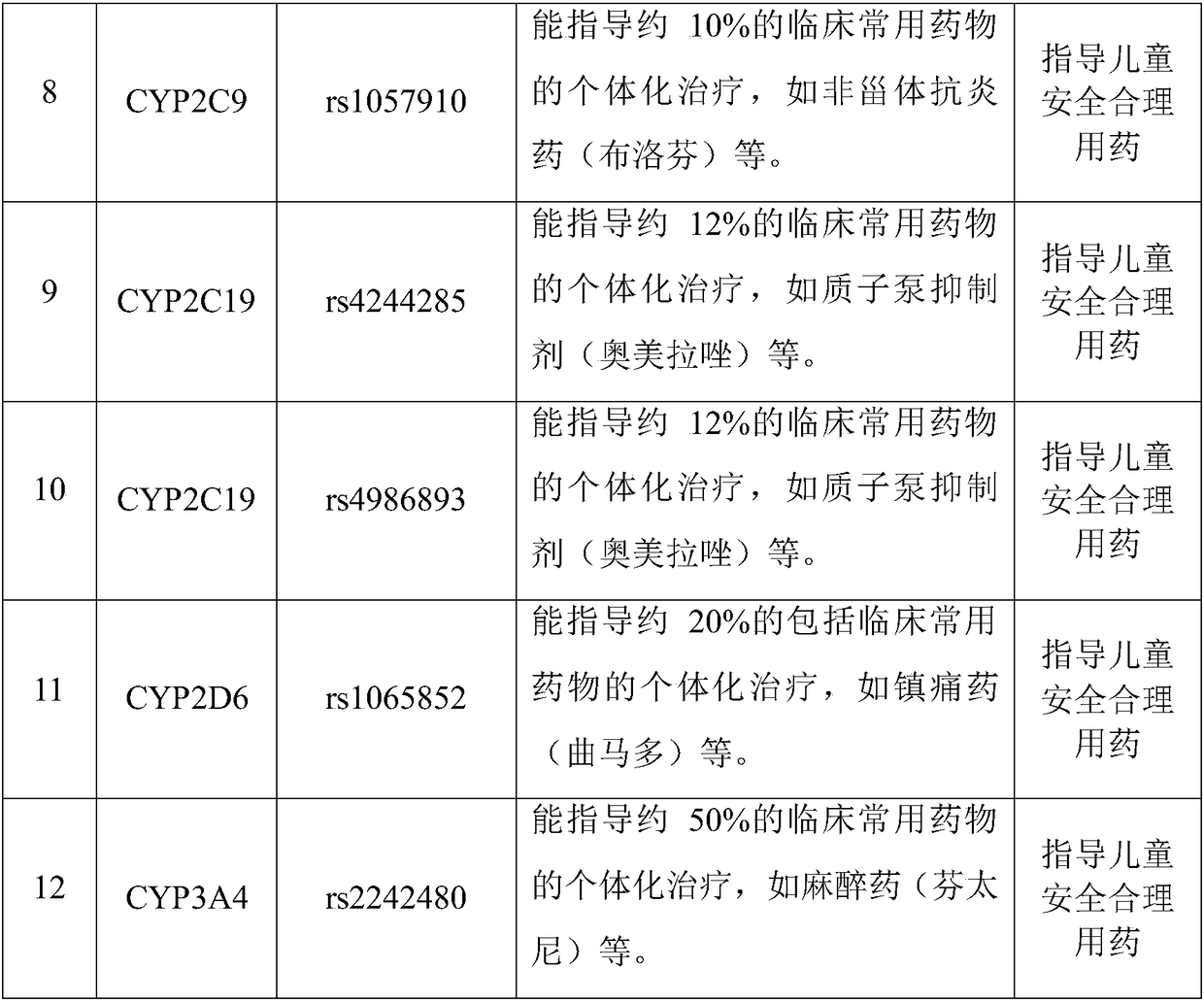
The invention discloses a Juno<TM>-based safety medication detection kit for children and a chip. The kit and the chip comprise primers at the following loci: HLA-DRB1*15:01-DQB1*06:02 loci of an HLAgene, an HLA-B*57:01 locus of the HLA gene, an HLA-B*15:02 locus of the HLA gene, an HLA-B*58:01 locus of the HLA gene, a rs267606617 locus of an MT-RNR1 gene,a rs267606618 locus of the MT-RNR1 gene,a rs267606619 locus of the MT-RNR1 gene, a rs1057910 locus of a CYP2C9 gene, a rs4244285 locus of a CYP2C19 gene, a rs4986893 locus of the CYP2C19 gene, a rs1065852 locus of a CYP2D6 gene, and a rs2242480 locus of a CYP3A4 gene.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV +1
Process for preparing bupleurum chinense injection
ActiveCN101884654BCause oxidative deteriorationThe basis of effective medicinal substances does not changePowder deliveryAntiviralsPharmaceutical drugPharmaceutical Aids
Owner:SICHUAN DE PEI YUAN TRADITIONAL CHINESE MEDICINE SCI & TECH DEV CO LTD
Medicinal composition for treating diabetes, and its application
InactiveCN103263405AReduce the dosing concentrationEasy to useOrganic active ingredientsMetabolism disorderDiabetes mellitusGlycine
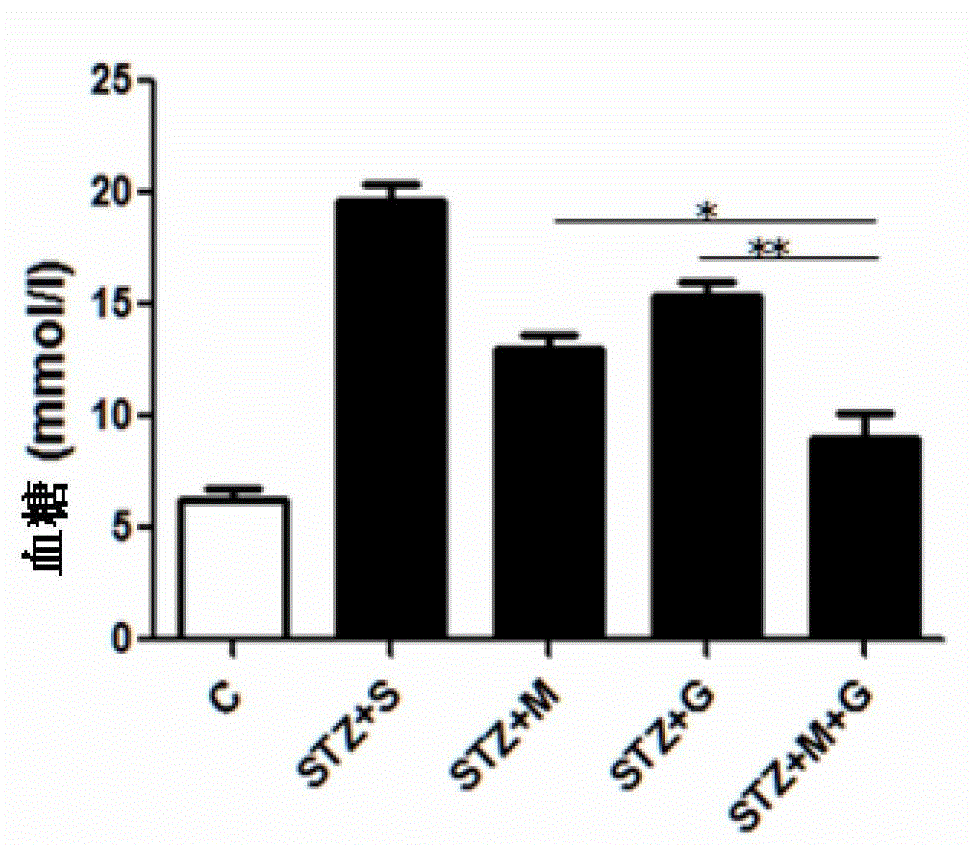
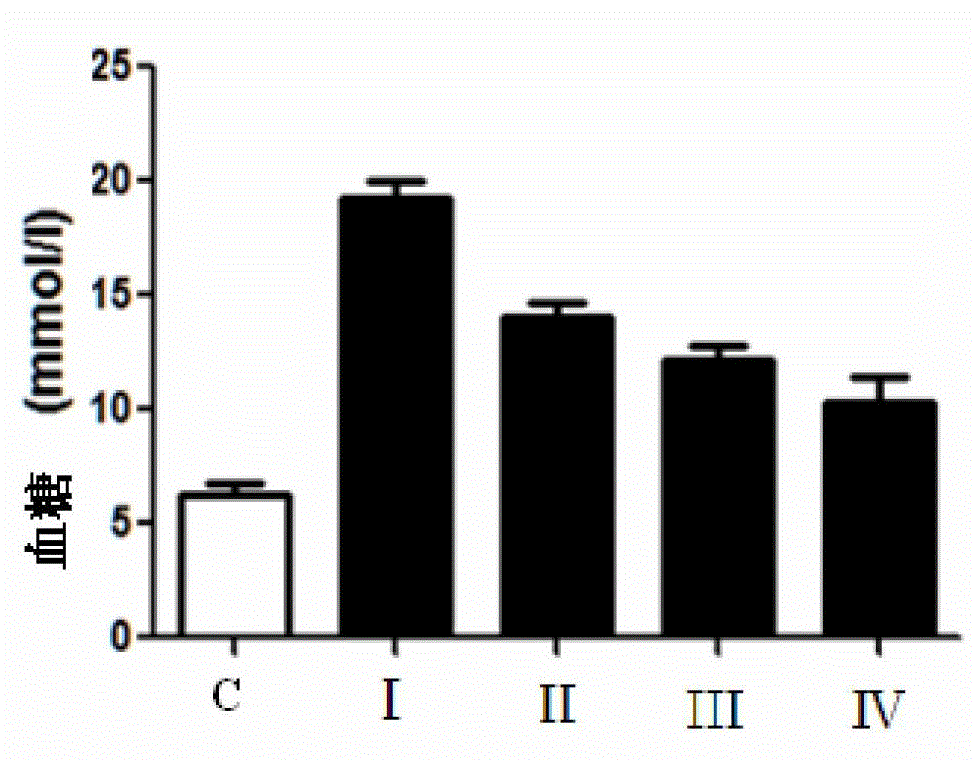
The invention relates to a medicinal composition for treating diabetes, and its application in the preparation of medicines for treating the diabetes. The medicinal composition comprises glycine and metformin hydrochloride, and the best mass ratio of glycine to metformin hydrochloride is 3:1, 4:1 or 5:1. The cooperation of glycine and metformin hydrochloride realizes a reinforced effect or a synergistic effect, so the use effect of the medicinal composition can be improved. The administration concentrations of glycine and metformin hydrochloride are reduced on the premise that a same treatment effect is obtained, so the generation risk of adverse reactions is reduced.
Owner:NANJING MEDICAL UNIV
Cosmetic preservative removing device
ActiveCN112221199AReduce the risk of adverse reactionsLow in preservativesOther chemical processesSolid sorbent liquid separationSpecific adsorptionBiomedical engineering
The invention relates to the technical field of cosmetic packaging, and discloses a cosmetic preservative removing device. The cosmetic preservative removing device comprises a preservative specific adsorbent and a screen device used for fixing the preservative specific adsorbent. The screen device comprises two layers of screens, and the preservative specific adsorbent is arranged between the twolayers of screens. The cosmetic preservative removing device is connected and formed with a cosmetic package. The device has a good specific adsorption effect on preservatives in cosmetics on the basis of not adsorbing effective components of the cosmetics, so that the purpose of reducing the content of the preservatives in cosmetic extrudate is achieved.
Owner:NANTONG UNIVERSITY
Celecoxib lyophilized orally disintegrating tablets with high bioavailability and preparation method thereof
ActiveCN110840850AQuick effectClear therapeutic advantageOrganic active ingredientsAntipyreticOrally disintegrating tabletGastrointestinal absorption
The invention relates to celecoxib lyophilized orally disintegrating tablets with high bioavailability and a preparation method thereof. The celecoxib lyophilized orally disintegrating tablets comprise, by weight, 30-75% of celecoxib, 10-50% of a filler, 10-50% of a stabilizer, and 0.01-10% of a flavoring agent, adding up to 100% by weight, wherein the drug particle size of celecoxib is 10-1000 nm. The drug in the orally disintegrating tablets is subjected to nano-micronization treatment to prepare a nano drug delivery system with the particle size of 10-1000 nm, so that the solubility and dissolution speed of the drug can be obviously increased, the gastrointestinal absorption speed and the bioavailability can be increased, the same treatment effect can be achieved with lower dosage, andthe risk of adverse reactions can be reduced.
Owner:烟台药物研究所
Full-automatic cell treatment pulse type workstation
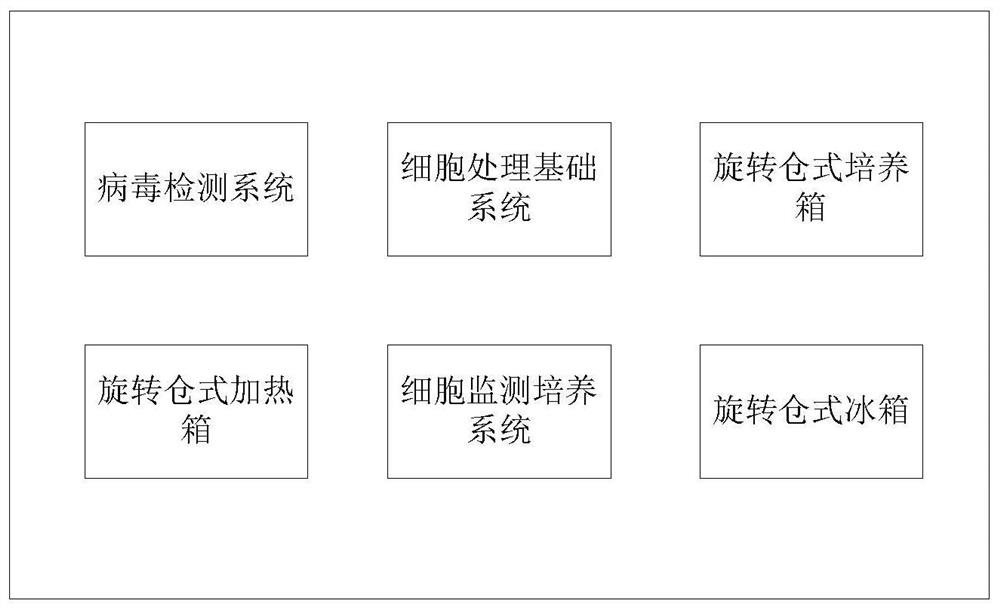
PendingCN113088433AExquisite structureImprove securityBioreactor/fermenter combinationsBiological substance pretreatmentsStem like cellVirus detection
The invention relates to a full-automatic cell treatment pulsating type workstation and a control method. The full-automatic cell treatment pulsating type workstation comprises a plurality of virus detection systems, a cell treatment basic system, a plurality of rotary bin type incubators, a plurality of rotary bin type refrigerators, a plurality of rotary bin type heating boxes and a stem cell monitoring culture system, the cell treatment basic system comprises a biological safety cabinet and a worktable, and the worktable comprises an on-table part and an under-table part; a connection unit, a quality control unit, a film pasting unit, a device with recognition and clamping functions, a controller and a monitoring system are arranged on the on-table part of the worktable, and the under-table part comprises a butt joint interface.
Owner:TIANJIN CITY THIRD CENT HOSPITAL
Medicinal composition for treating diabetes, and its application
InactiveCN103263405BReduce the dosing concentrationEasy to useOrganic active ingredientsMetabolism disorderDiabetes mellitusGlycine
Owner:NANJING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com