Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
153 results about "Endotoxin removal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
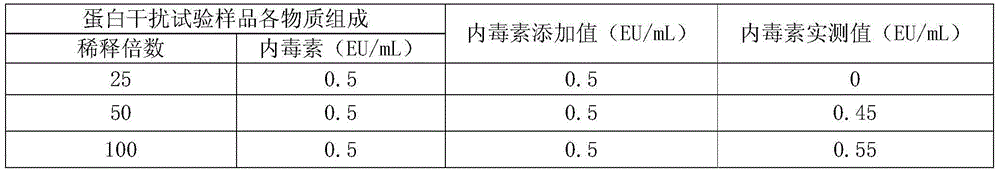
Endotoxin Removal Kit (Maxi) - This DNA purification kit is used for rapid removal of endotoxins from up to 1 mg of previously purified DNA. It can reduce endotoxin levels to 0.1 EU/µg DNA or less and can effectively remove endotoxins in as little as 30 minutes.
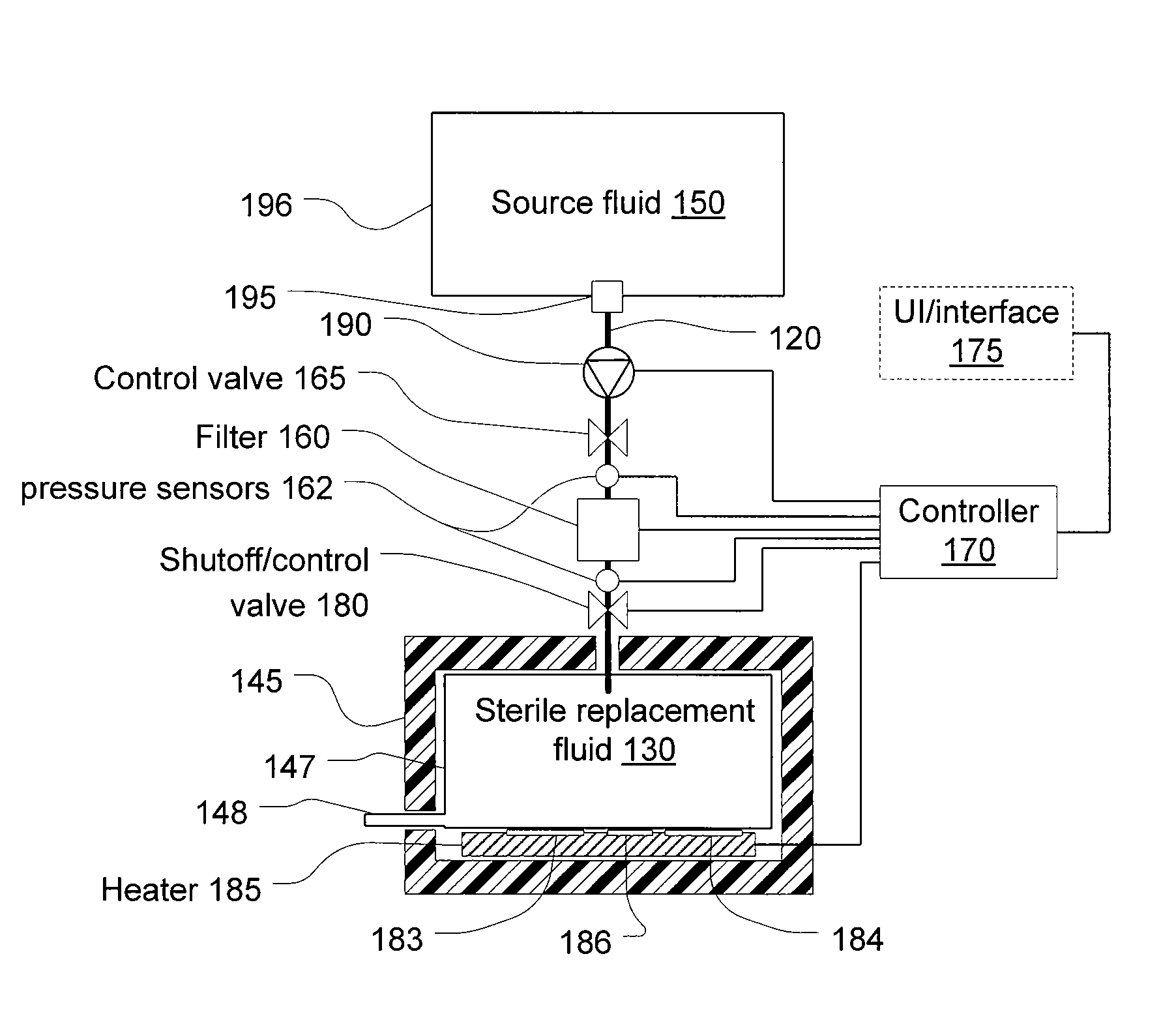
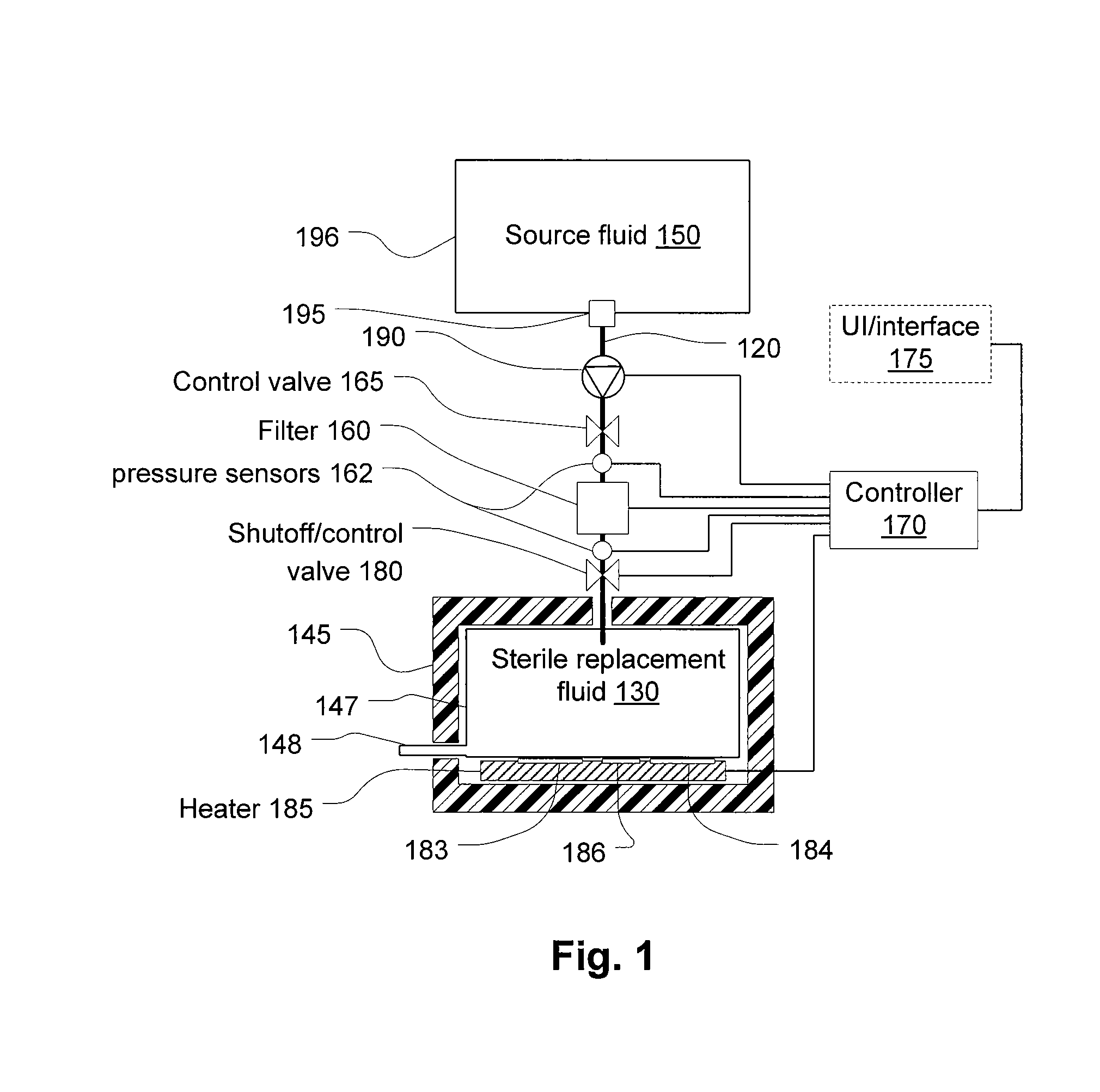
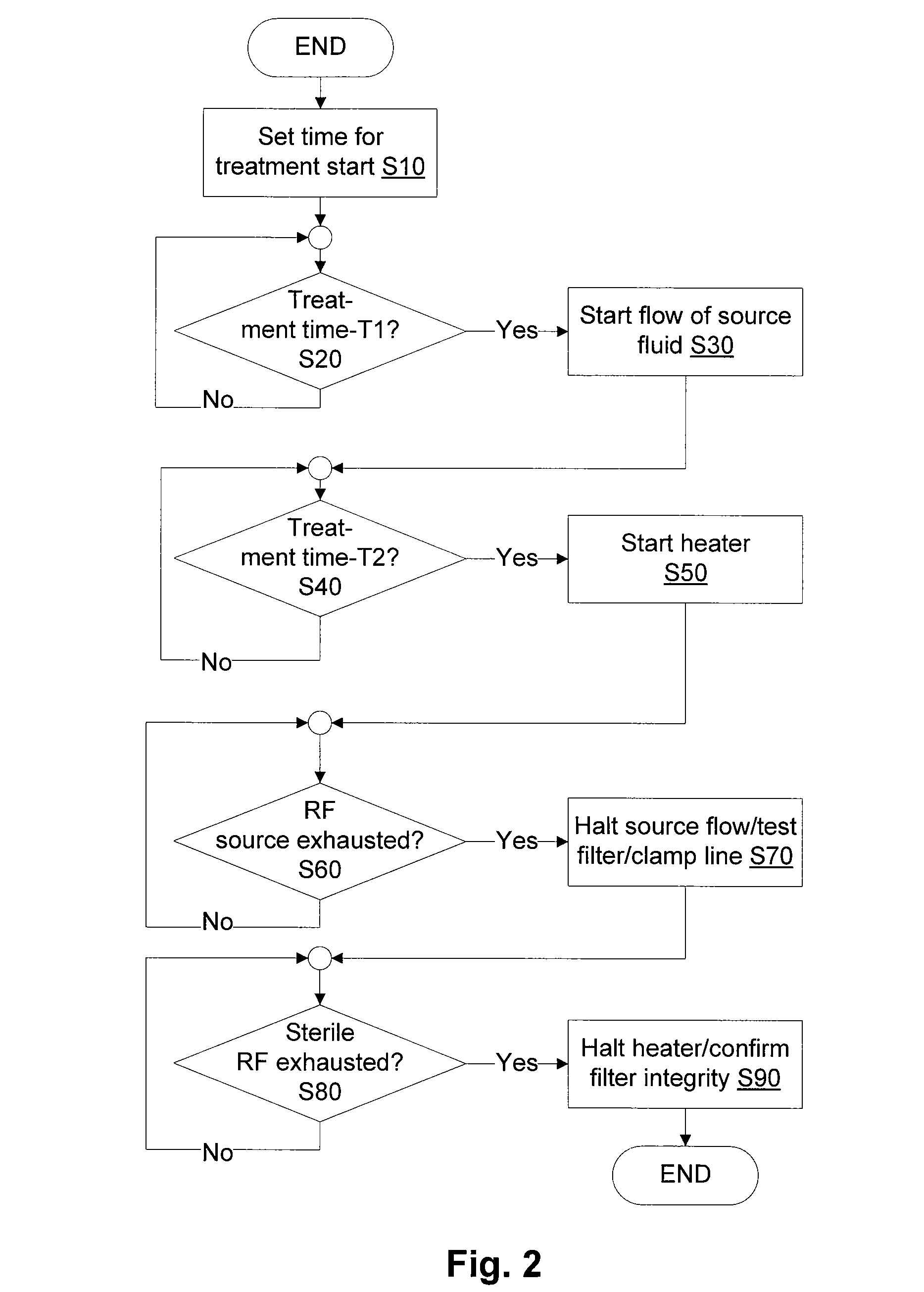
Last-chance quality check and/or air/pathogen filtger for infusion systems
ActiveUS20080203023A1Small apertureIncrease pressure dropSolvent extractionHaemofiltrationBlood treatmentsHigh rate
Blood treatment system and method for high rate hemofiltration ensures against pyrogenic patient reaction by providing various mechanisms for filtering replacement fluid to remove endotoxins and other safety features including detecting incorrect fluid administration.
Owner:NXSTAGE MEDICAL INC
Endotoxin adsorbent, and a method of removing endotoxin by using the same
This invention provides an adsorbent having a high ability to adsorb endotoxin selectively and a method of adsorbing endotoxin. The adsorbent comprises a basic substance bonded to a base material by means of a crosslinking agent.
Owner:JNC CORP
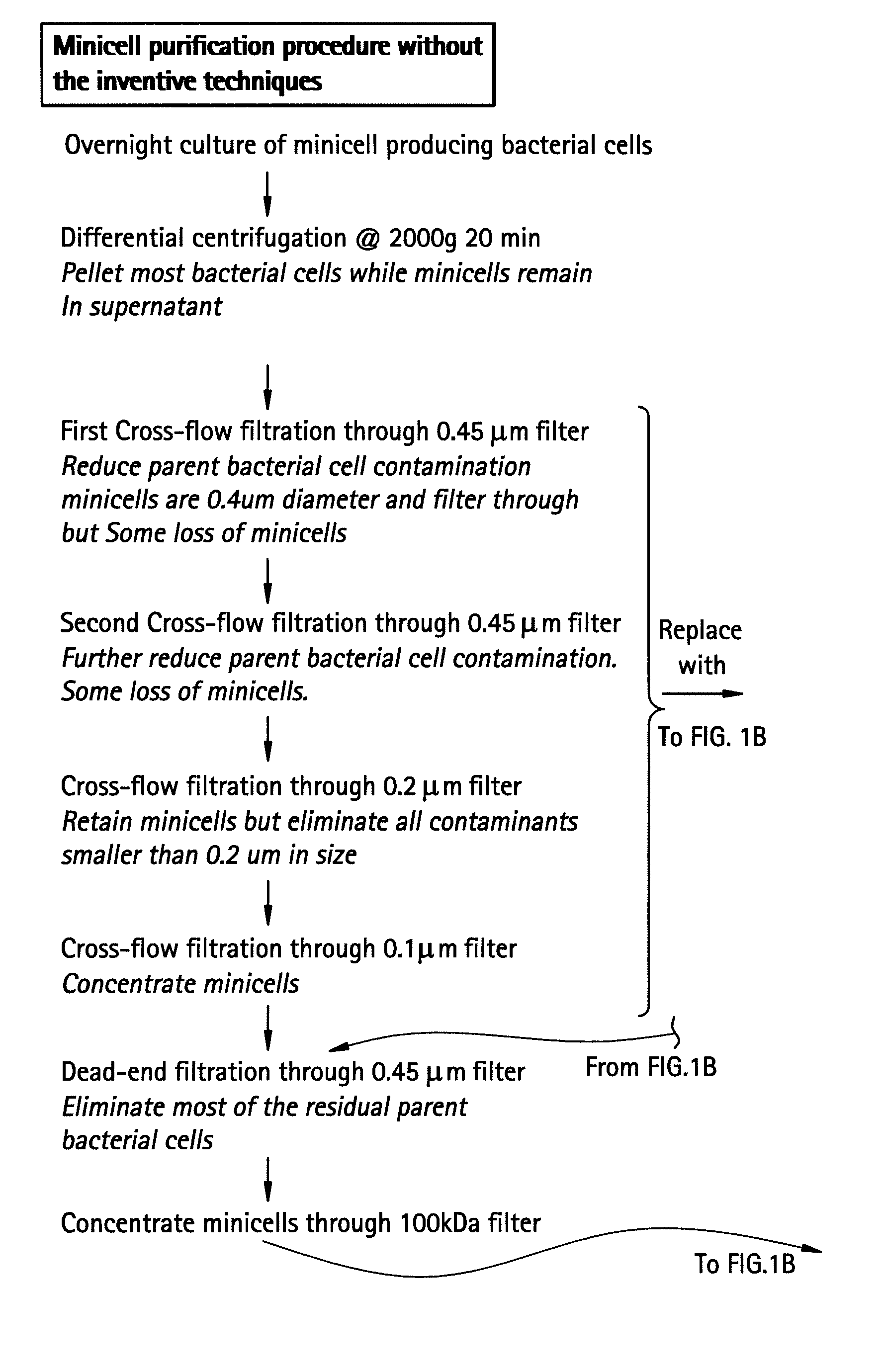
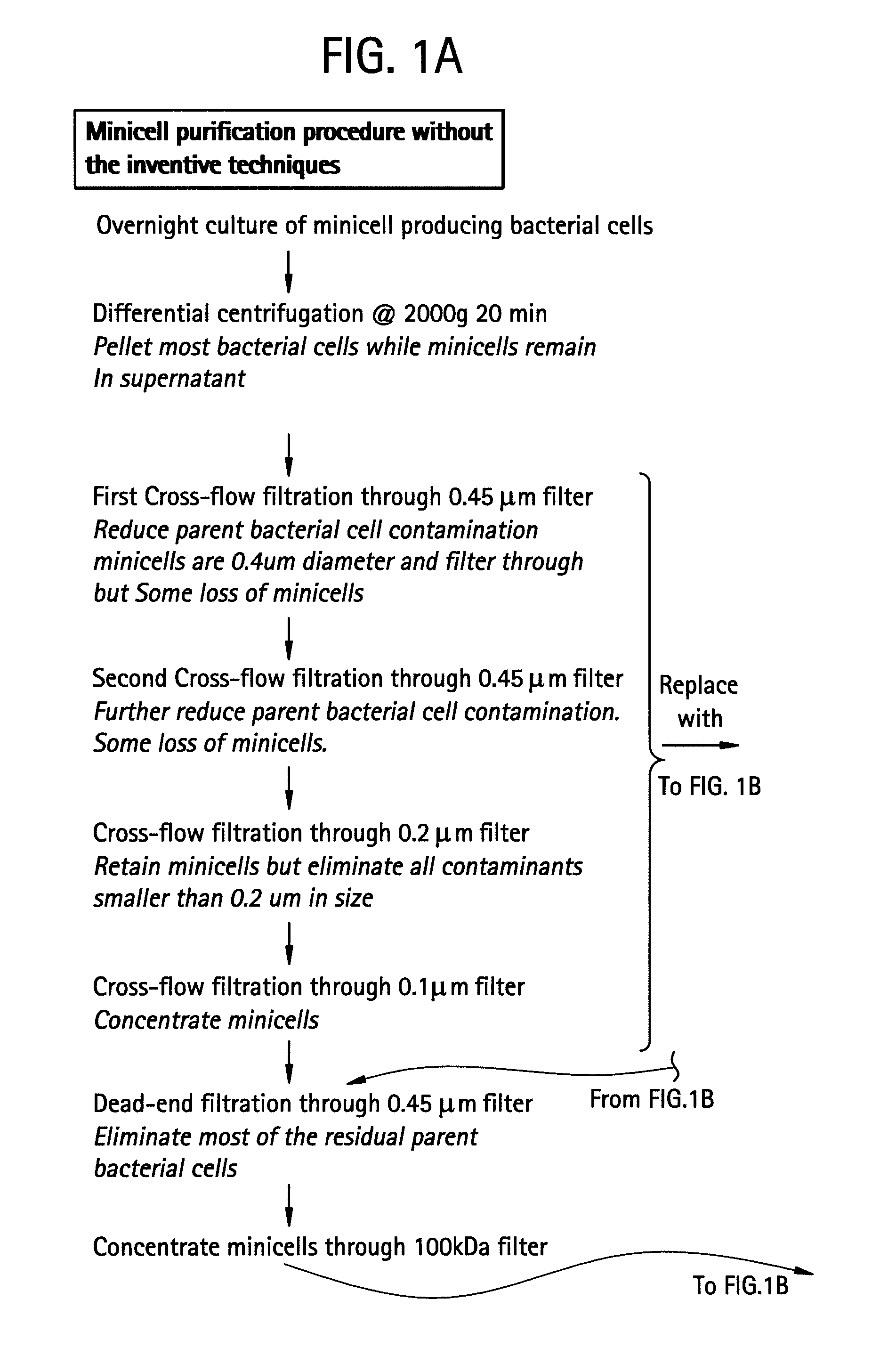
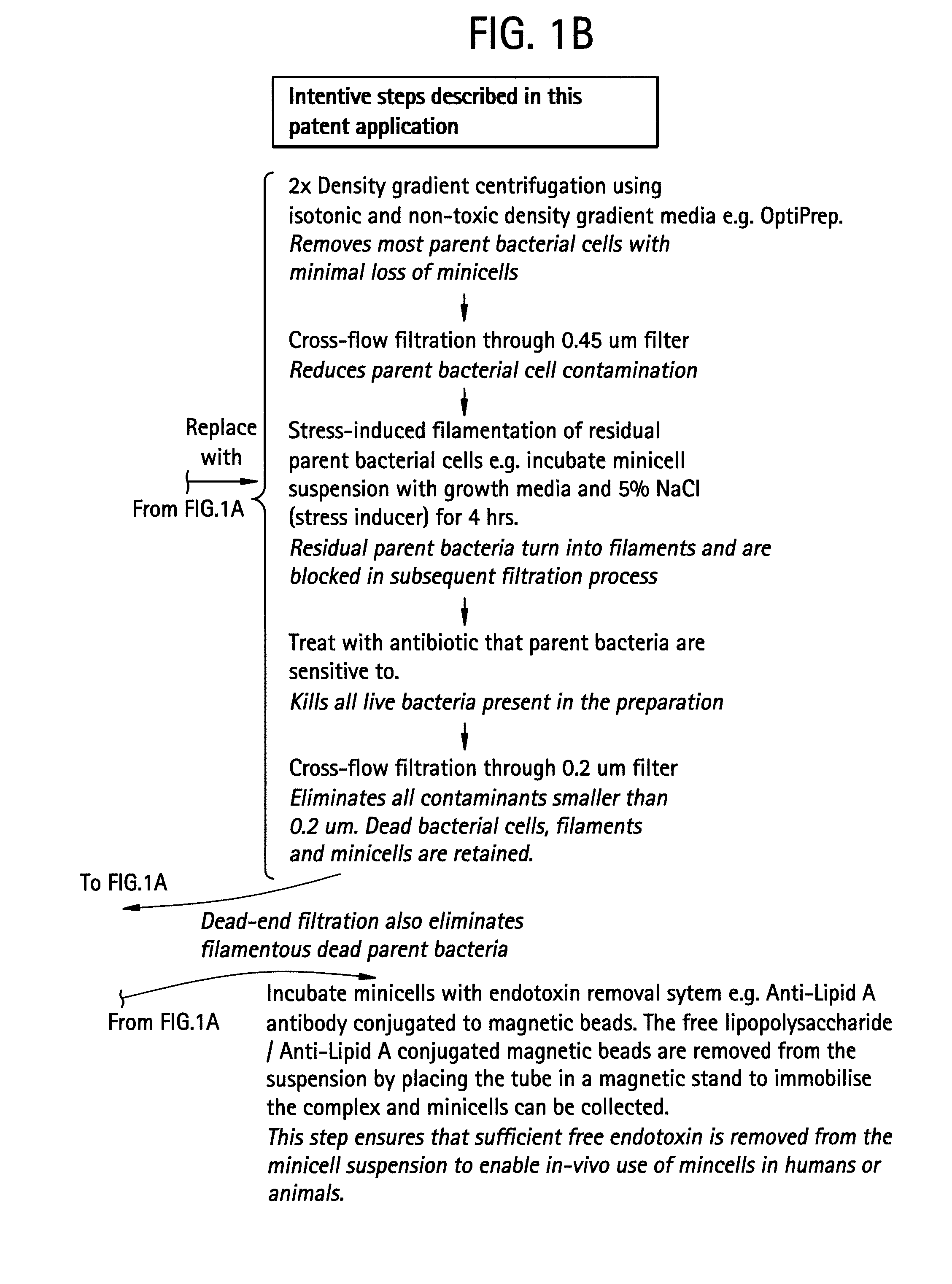
Pharmaceutically compatible method for purifying intact bacterial minicells
InactiveUS20070241067A1BiocideWater/sewage treatment by centrifugal separationFiltrationCentrifugation
The present invention provides a method for purifying bacterial minicells that involves subjecting a sample containing minicells to density gradient centrifugation in a biologically compatible medium. The method optionally includes a preliminary differential centrifugation step and one or more filtration steps. The invention also provides a method for purifying bacterial minicells in which a sample containing minicells is subjected to a condition that induces parent bacterial cells to adopt a filamentous form, followed by filtration of the sample to separate minicells from parent bacterial cells. The inventive methods optionally include one or more steps to remove endotoxin from purified minicell preparations, and / or treatment of purified minicell preparations with an antibiotic. Additionally, the invention provides purified minicell preparations, prepared according to the foregoing methods, and containing fewer than about 1 contaminating parent bacterial cell per 107, 108, 109, 1010, or 1011 minicells.
Owner:ENGENEIC MOLECULAR DELIVERY PTY LTD
Preparation method of endotoxin absorbent for blood perfusion
InactiveCN1864755AReduce non-specific adsorptionAvoid instabilityOther blood circulation devicesHaemofiltrationEndotoxin removalBiocompatibility Testing
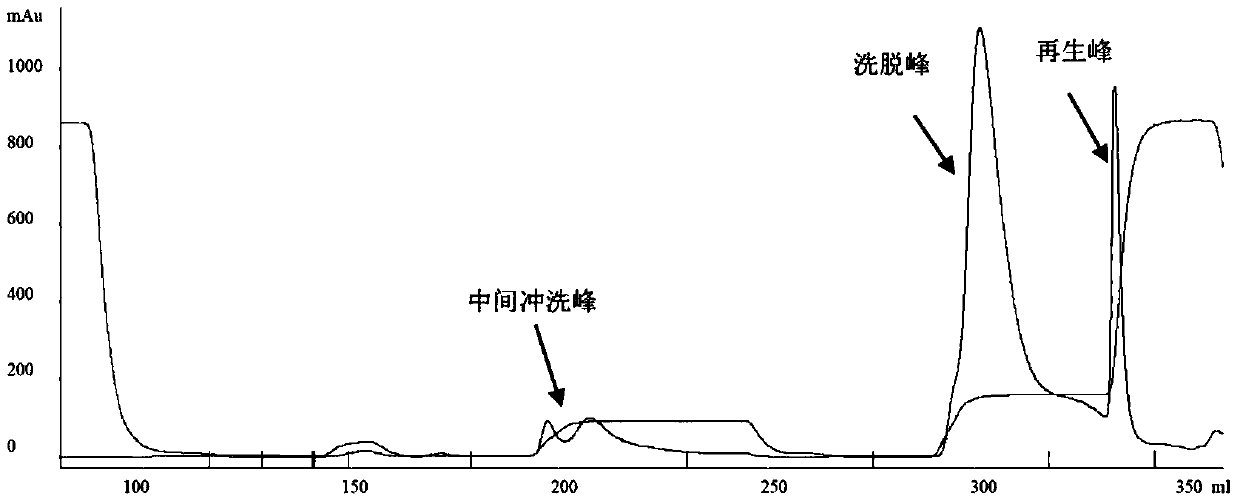
The invention relates to an endotoxin adsorbent, which in detail relates to a method of preparing endotoxin adsorbent for blood perfusion. The method employs spherical agarose gel with good biocompatibility as base material, employs epichlorohydrin, hexamethylene diamine, 1, 1'-carbonyldiimidazole as activating agent, and bonds polymyxin B for endotoxin removal. The invention is characterized in that it overcomes the toxicity of cyanogen bromide and instability of aglycone, improves the safty and reliability of operation, reducesnon-specific adsorption through bonding polymyxin B with 1, 1'-carbonyldiimidazole, and improves biocompatibility of adsorbent and suits for treating endotoxemia. The invention is also characterized by simple experimental procedure and high clearance for endotoxin.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Removal of bacterial endotoxin in a protein solution by immobilized metal affinity chromatography
InactiveUS6942802B2Ion-exchange process apparatusIon-exchanger regenerationProtein solutionBacteroides
The present invention relates to the purification of polypeptides and the removal of endotoxin via immobilized metal affinity chromatography (IMAC). More specifically, the invention relates to methods for removing bacterial endotoxin in a protein solution. In specific embodiments, the invention relates to the elimination of endotoxin from Moraxella catarrhalis outer membrane proteins.
Owner:WYETH HOLDINGS CORP
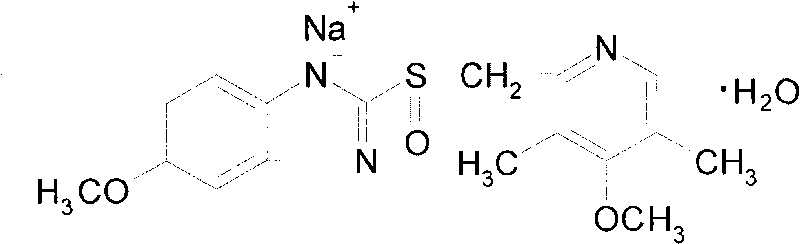
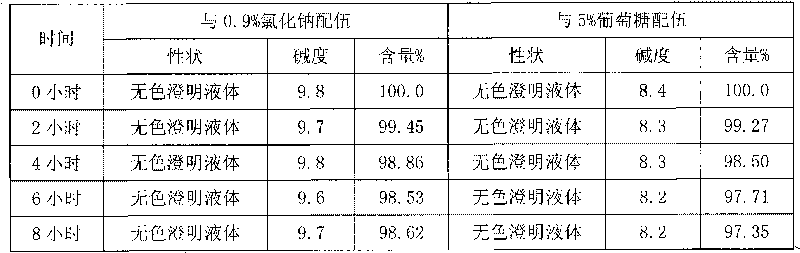
Omeprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN101703483AReduce the risk of adverse reactionsComply with the requirements of human intravenous injectionPowder deliveryOrganic active ingredientsOmeprazole SodiumFiltration
The invention discloses an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The omeprazole sodium freeze-dried powder injection contains an active ingredient, namely, omeprazole sodium monohydrate, and auxiliary materials, namely, calcium disodium edetate and sodium hydroxide. The preparation method of the omeprazole sodium freeze-dried powder injection is characterized by comprising the following steps: weighing the calcium disodium edetate of prescription amount and dissolving the calcium disodium edetate in water for injection, stirring, dissolving, and regulating pH value to 10.0-12.0 by using 10% of sodium hydroxide solution; weighing omeprazole sodium of the prescription amount and adding the omeprazole sodium in the mixture, stirring at room temperature for dissolution, supplementing and adding the water for injection to full amount; adding active carbon, stirring at room temperature for decoloration and endotoxin removal, conducting rough filtration to remove carbon firstly, and then conducting refining filtration by using a filter membrane of 0.22 Mum; taking refining filtrate to test intermediate, conducting encapsulation after meeting requirements; and freeze-drying and unboxing, thus obtaining the omeprazole sodium freeze-dried powder injection. The freeze-drying technology of the omeprazole sodium freeze-dried powder injection takes temperature below minus 40 DEG C as pre-freezing temperature; after pre-freezing for at least two hours, sublimation is started, wherein the sublimation temperature is 5-12 DEG C, the sublimation time is over 14 hours; and then drying is conducted for over 2 hours at the temperature of 20-35 DEG C. Unboxing is carried out after a stopper is added and a cover is put in place, thus obtaining the finished product of the omeprazole sodium freeze-dried powder injection.
Owner:HAINAN LEVTEC PHARMA
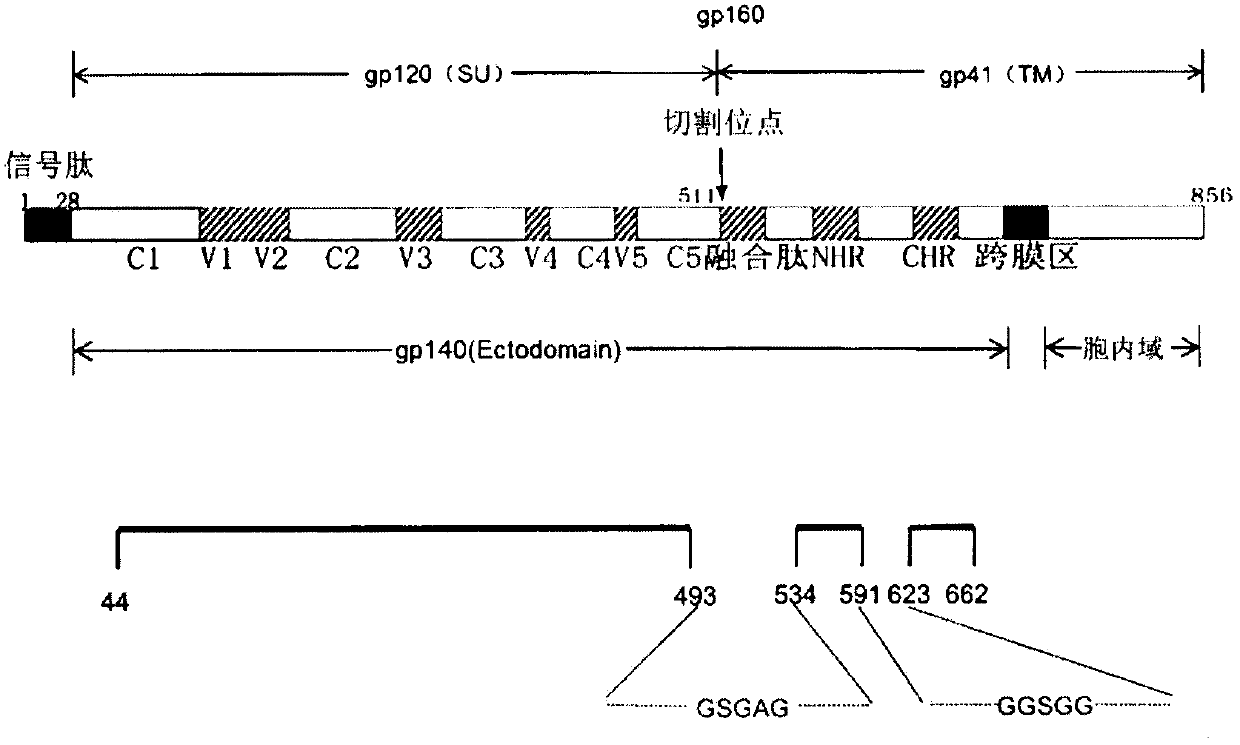
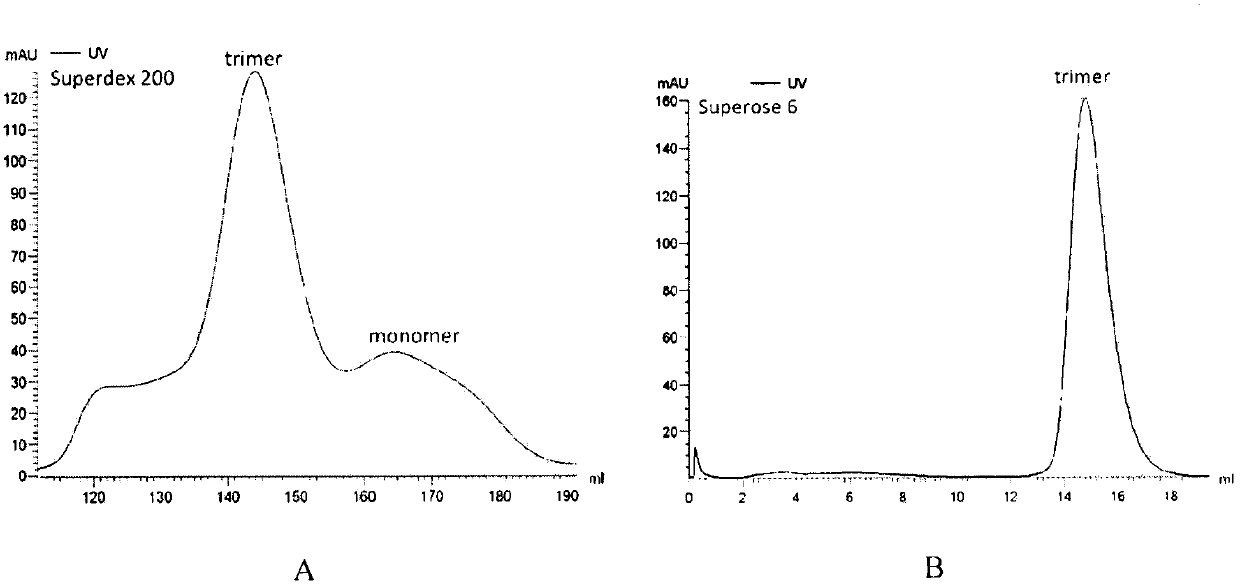
Potential preparation method for highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen
InactiveCN103992396AImprove uniformityHigh antigen reactivityVirus peptidesDepsipeptidesAntigenEndotoxin removal
The invention discloses a potential preparation method for a highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen. The immunogen is designed based on the structure of HIV-1 envelope protein crystals which have been published internationally and obtained in our lab. The specific method uses an overlap extension PCR technology to obtain gp140 gene segments, and comprises the steps that target genes are cloned into an eukaryotic expression vector pMT, endotoxin is removed through extraction of a large amount of plasmids, the gp140 gene segments and resistance screening plasmids pCoBlast are co-transfected into drosophila melanogaster Schneider2 (S2) cells together, blasticidin (Blasticidin S) is used for positive clone screening, S2 cell lines for stably and efficiently secreting and expressing gp140 are screened, and after enlarged cultivation and through two steps of purification of nickel column affinity chromatography and gel filtration chromatography, the gp140 with high purity can be obtained. A series of biochemical and biophysical technologies indicate that the gp140 is uniform in polymeric states, and high in antigenic reactivity, and is quite fittingly used as the immunogen for the research and the development of AIDS subunit vaccines or multivalent combined vaccines.
Owner:NANKAI UNIV
Heparin-phenylalanine adsorption material for blood purification method for removing endotoxin
InactiveCN103230781APrevent blood clottingSafe and non-toxicOther chemical processesDialysis systemsEthylenediamineEndotoxin removal
The invention relates to a heparin-phenylalanine adsorption material for a blood purification method for removing endotoxin. The preparation method of the heparin-phenylalanine adsorption material disclosed by the invention comprises the following steps: carrying out amination on chloromethylated polystyrene-diethylbenzene ene resin (merrifield resin) serving as a base material by using ethylenediamine to prepare the amino-methyl chloride resin, then utilizing 1-(3-dimethyl ami-nopropyl)-3-ethyl carbodiimide / N-hydroxysuccinimide (EDC / NHS) as a catalyst to connect spacer heparin, so as to prepare heparinized methyl chloride resin; and finally with the EDC / NHS as a catalyst, connecting phenylalanine to heparin, so as to prepare the heparin-phenylalanine adsorption material. The heparin-phenylalanine adsorption material disclosed by the invention can be effectively applied to the blood purification method for removing endotoxin, and is good in blood compatibility, and safe and nontoxic to a human body.
Owner:CHONGQING UNIV
Purification method of pegylated recombinant human granulocyte colony stimulating factor
ActiveCN102850450AIncrease lossReduce lossesPeptide preparation methodsDepsipeptidesPurification methodsEndotoxin removal
The present invention provides a purification method of pegylated recombinant human granulocyte colony stimulating factor. The inventor performed extensive researches of a variety of chromatographic methods, and found that high-purity pegylated recombinant human granulocyte colony stimulating factors can be obtained by using desalting chromatography, ion exchange tandem chromatography and desalting chromatography to perform sequential combined purification. The purification process of the present invention makes full use of the characteristics of the target protein in the surface charge and hydrophobicity, firstly uses an anion exchange chromatography column to effectively remove endotoxin and to increase controllability of the process; and makes full use of strong hydrophobicity of the target protein in the second-stage chromatography, realizes tandem chromatography, and combines removal of endotoxin and protein purification into a step. The process can not only effectively remove endotoxin, but also simplify operation steps, save production time, and improve production efficiency. The process is very suitable for large-scale industrial production.
Owner:QILU PHARMA
Purification method of human glucagon like peptide-1 (hGLP-1) analogue fusion proteins
ActiveCN110526982AImprove quality control standardsEfficient removalAntibody mimetics/scaffoldsPeptide preparation methodsPurification methodsLinear amplification
The invention provides a purification method of human glucagon like peptide-1 (hGLP-1) analogue fusion proteins (including dulaglutide). The purification method includes the step that a three-step chromatography method is adopted for purification, specifically, a sample is firstly subjected to a coarse purification step of Protein A affinity chromatography so as to effectively remove impurities such as HCP and endotoxin and maintain the high objective protein yield; and then anion exchange chromatography and hydrophobic chromatography are used for further fine purification so as to effectivelyremove charge isomers, residual HCP and other trace impurities in the sample, the content of all the impurities is controlled within the administration safety range, and the high objective protein yield and activity are maintained. According to the purification method, during separation and purification of an objective protein, good reproducibility and stability are achieved, and the purificationmethod is suitable for downstream purification process linear amplification of the hGLP-1 analogue fusion proteins so as to be used for production and preparation of drugs for treating type 2 diabetes or slimming drugs.
Owner:CHENGDU JINKAI BIOTECH CO LTD
Adsorbent used for blood or plasma perfusion to remove endotoxin and preparation method thereof
ActiveCN106334540AHigh mechanical strengthImprove mechanical stabilityAntibacterial agentsOrganic active ingredientsPolyvinyl alcoholSorbent
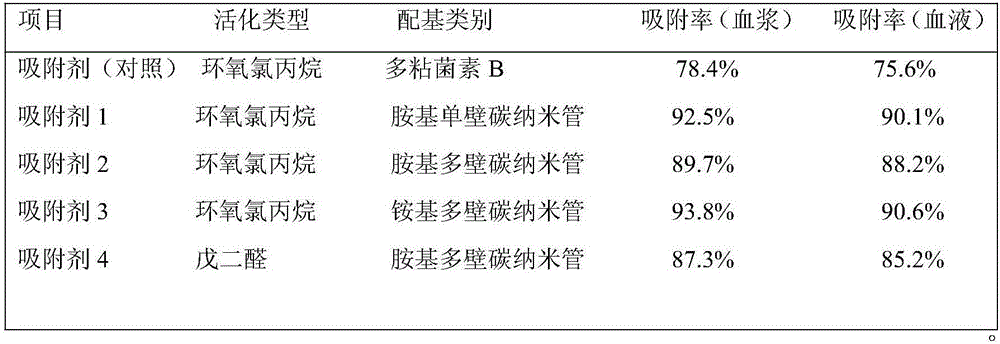
The invention relates to an adsorbent used for blood or plasma perfusion to remove endotoxin (lipopolysaccharide) and a preparation method thereof. The adsorbent is a nano-composite structure adsorbent. Large-aperture polyvinyl alcohol serves as a carrier, amino (ammonio) carbon nanotubes (single-walled or multi-walled) serve as a ligand, epichlorohydrin or glutaraldehyde, etc. serves as a crosslinking agent, and the ligand and the carrier microspheres undergo covalent coupling to obtain the adsorbent. The adsorbent is simple to prepare, and an endotoxin adsorption property is good. The adsorbent is suitable for blood or plasma perfusion to remove excessive endotoxin in the body of a patient.
Owner:NANKAI UNIV
Process for removing endotoxin in bacteria polysaccharide by using macroporous resin
ActiveCN1970780AGood removal effectImprove hydrophobicityUltrafiltrationMicroorganism based processesBacteroidesUltrafiltration
The invention discloses a removing method of endotoxin in the bacterial polysaccharide through large-hole resin in the biological technical domain, which comprises the following steps: fermenting bacteria through biological reactor; making rough sugar; extracting rough sugar through cooled phenol to remove protein; hyperfiltering through composite buffer of calcium ionic chelant-surface activator; removing endotoxin adsorbed by large-hole resin; obtaining bacterial polysaccharide with low endotoxin content.
Owner:YUXI WALVAX BIOTECH CO LTD
Method for removing endotoxin in recombinant protein A solution
InactiveCN106397552ALow adsorption capacityHigh recovery rateDepsipeptidesPeptide preparation methodsEndotoxin removalChromatography column
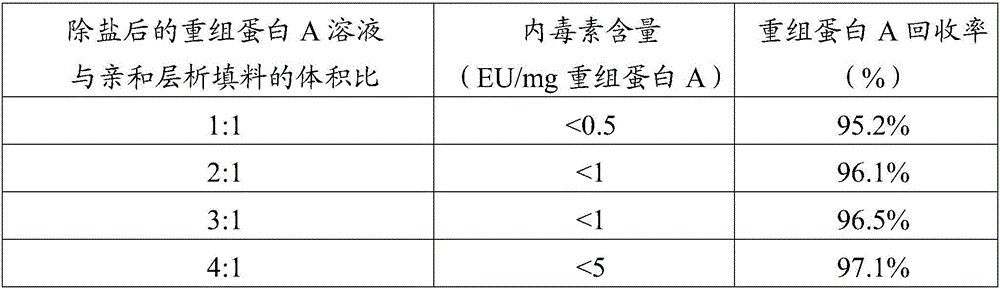
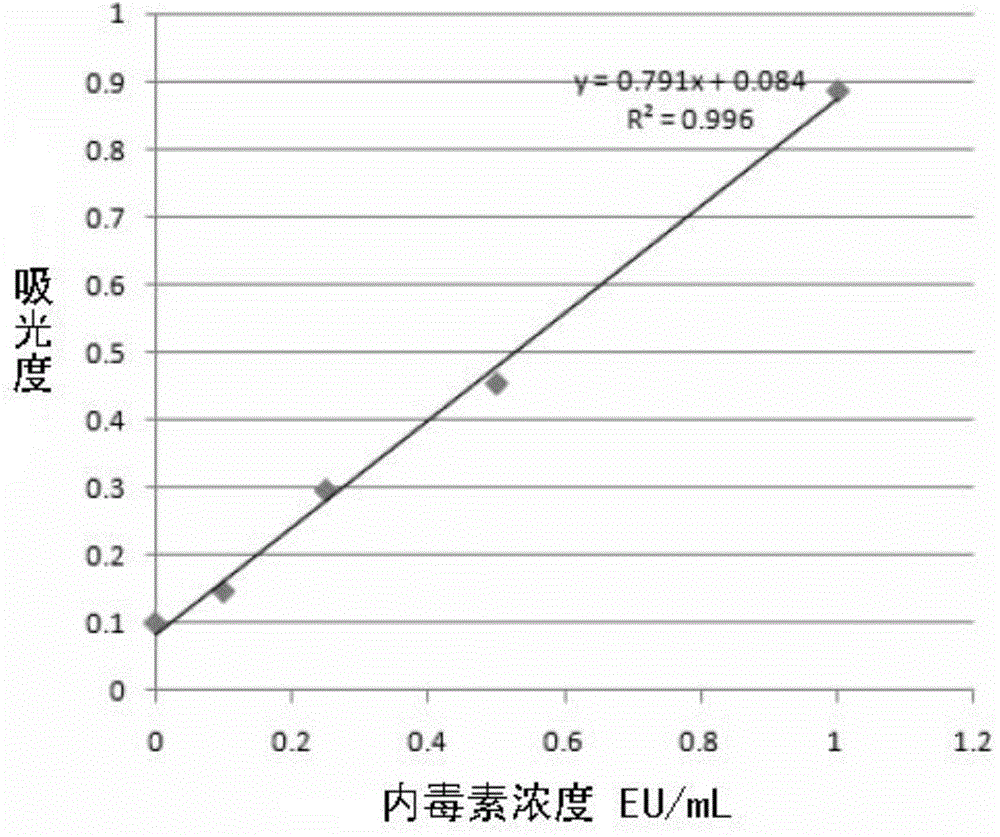
The invention provides a method for removing endotoxin in a recombinant protein A solution. The method is good in endotoxin removal effect, high in the recovery rate of recombinant protein A and greatly reduced in cost. The method comprises the following steps: (1) heating a bacterial suspension containing recombinant protein A with a histidine tag so as to fully fragment bacteria; (2) adding polyethyleneimine and successively carrying out uniform mixing and centrifugation so as to obtain supernatant containing the recombinant protein A, wherein a weight ratio of the bacterial suspension containing recombinant protein A to polyethyleneimine is 10000: 1 to 100: 1; (3) allowing the supernatant to pass through a metal ion chelated chromatography column and flushing a medium by using a PBS buffer solution so as to obtain a pretreated recombination protein A solution; (4) removing a salt so as to obtain a desalted recombination protein A solution; and (5) purifying the desalted recombination protein A solution with an affinity chromatographic filling material so as to obtain the recombinant protein A solution without endotoxin, wherein the affinity chromatographic filling material can specifically bind to endotoxin.
Owner:湖北中创医疗用品有限公司
Kit for removing bacterial endotoxin in biological product, method thereof, and preparation method of biological product
ActiveCN104370997AEasy to removeDoes not affect biological activitySerum albuminMicrobiological testing/measurementEndotoxin removalPotassium
The invention discloses a kit for removing bacterial endotoxin in a biological product, a method for removing the bacterial endotoxin in the biological product by using the kit, and a preparation method of the endotoxin removed biological product. The kit includes an anionic surfactant and a potassium salt. The methods commonly comprise the following steps: fully combining the anionic surfactant with the endotoxin in the biological product to form a conjugate, adding the potassium salt to precipitate the conjugate, filtering to remove the obtained precipitate in order to obtain an endotoxin removed biological product solution, and separating the biological product from the biological product solution. The kit can be used to simply and efficiently remove the endotoxin in the biological product and has a low cost, and the endotoxin removal method has the advantages of easy operation, no influences on the bioactivity of the biological product, and only use of compounds allowed in the medicine industry. The content of the endotoxin in the biological product prepared by using the methods accords with clinic pharmacy standards, and the loss of active substances is less.
Owner:陈辉
Method for removing endotoxin in biological medical preparations
The invention discloses a method for removing endotoxin in biological medical preparations. The method for removing the endotoxin in the biological medical preparations comprises the following steps of S1, pretreating; S2, detection of endotoxin; S3, filtering of raw liquid; S4, sample treatment; S5, balance treatment; S6, removing of endotoxin; S7, detection of endotoxin again; S8, storage of raw liquid. The method for removing the endotoxin in the biological medical preparations has the advantages that the external microorganisms and viruses can be primarily removed by pretreatment; by detecting the endotoxin, the content of the endotoxin can be detected; by adjusting the pH (potential of hydrogen) value, the endotoxin can be removed, and the convenience is realized; by heating in the endotoxin removal process, the removal rate of the endotoxin is accelerated.
Owner:青海七彩花生物科技有限公司
Method for removing endotoxin of biological product
ActiveCN104710527AEfficient removalHigh recovery ratePeptide preparation methodsImmunoglobulinsEndotoxin removalAnion-exchange chromatography
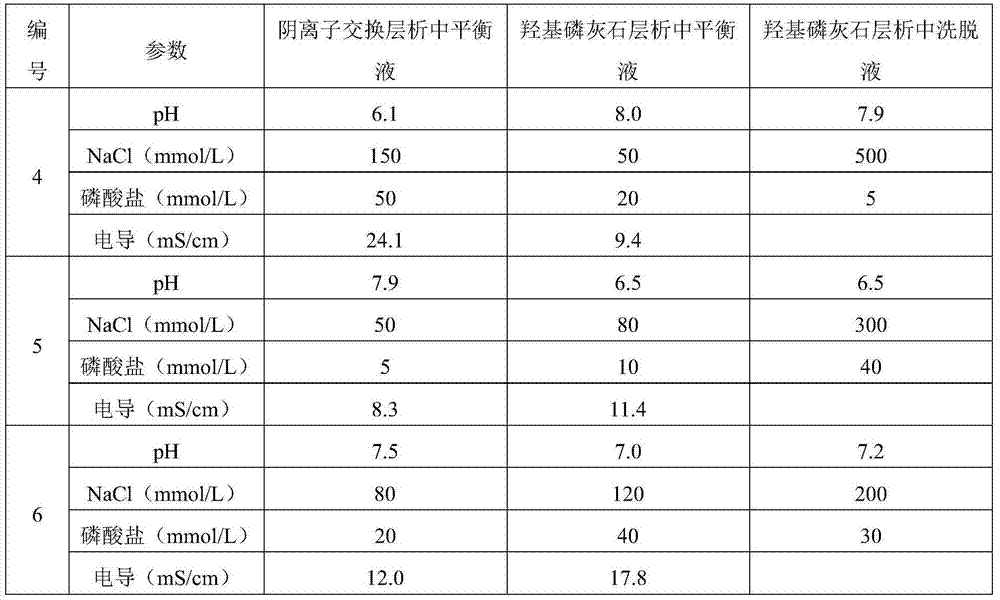
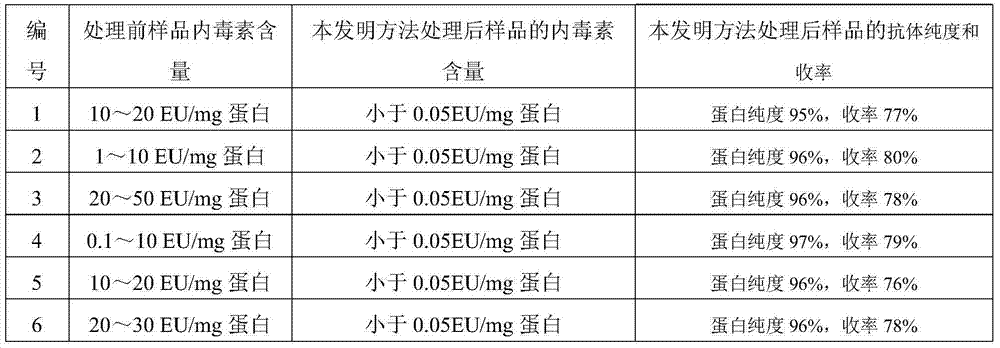
The invention discloses a method for removing endotoxin of a biological product. The method comprises the following steps: (1) performing anion exchange chromatography on a biological product to be treated; (2) performing hydroxyapatite chromatography, thereby removing endotoxin of the biological product. The invention further provides a biological product prepared by using the method. Due to the adoption of the method disclosed by the invention, endotoxin can be effectively removed, the protein recycling rate is high, no special antibody is needed to adsorb target protein, the treatment method is simple, the cost is low, the content of endotoxin of the biological product treated by using the method disclosed by the invention is smaller than 0.05EU / mg of protein, and the method is good in security and has relatively good application prospect.
Owner:信立泰(苏州)药业有限公司
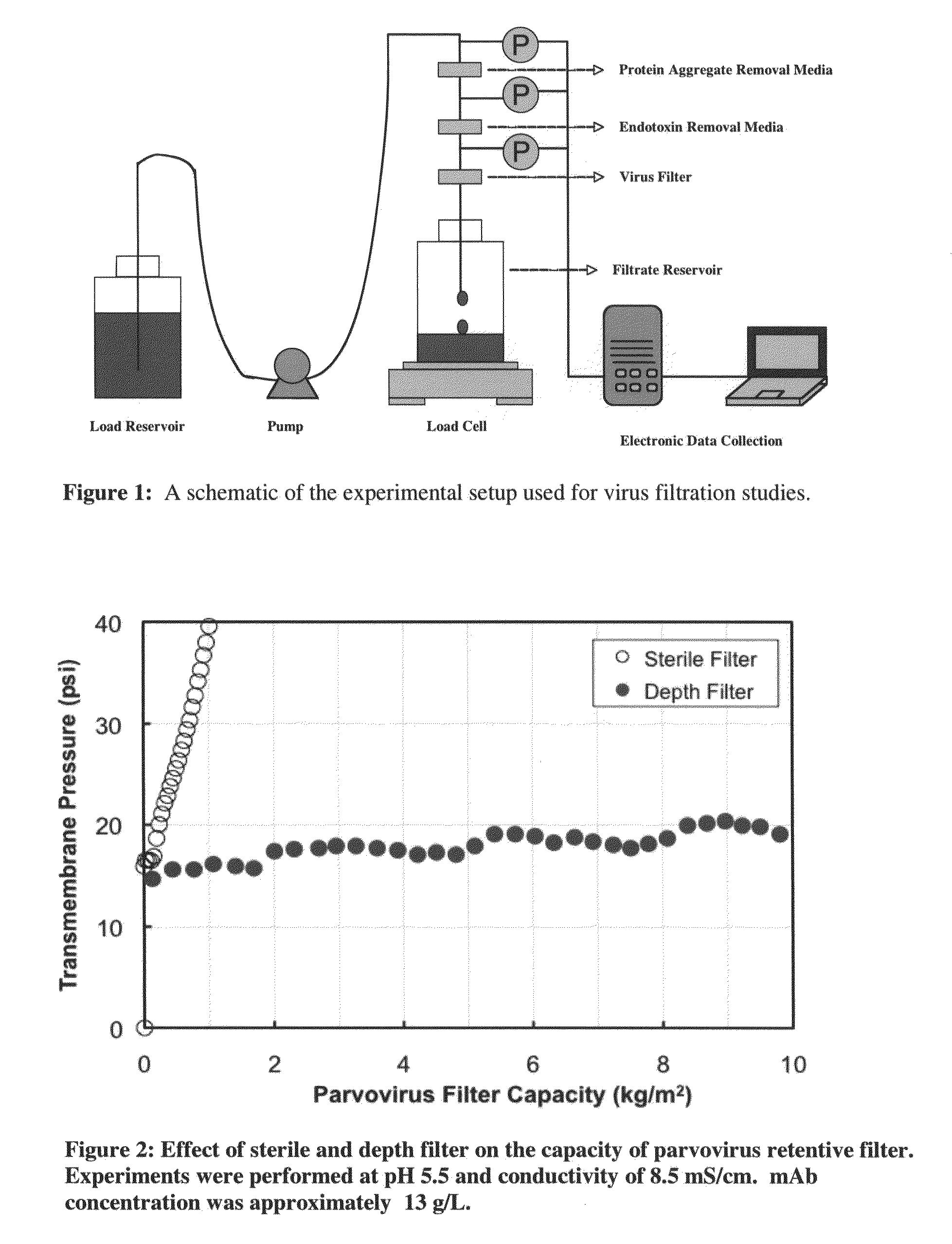
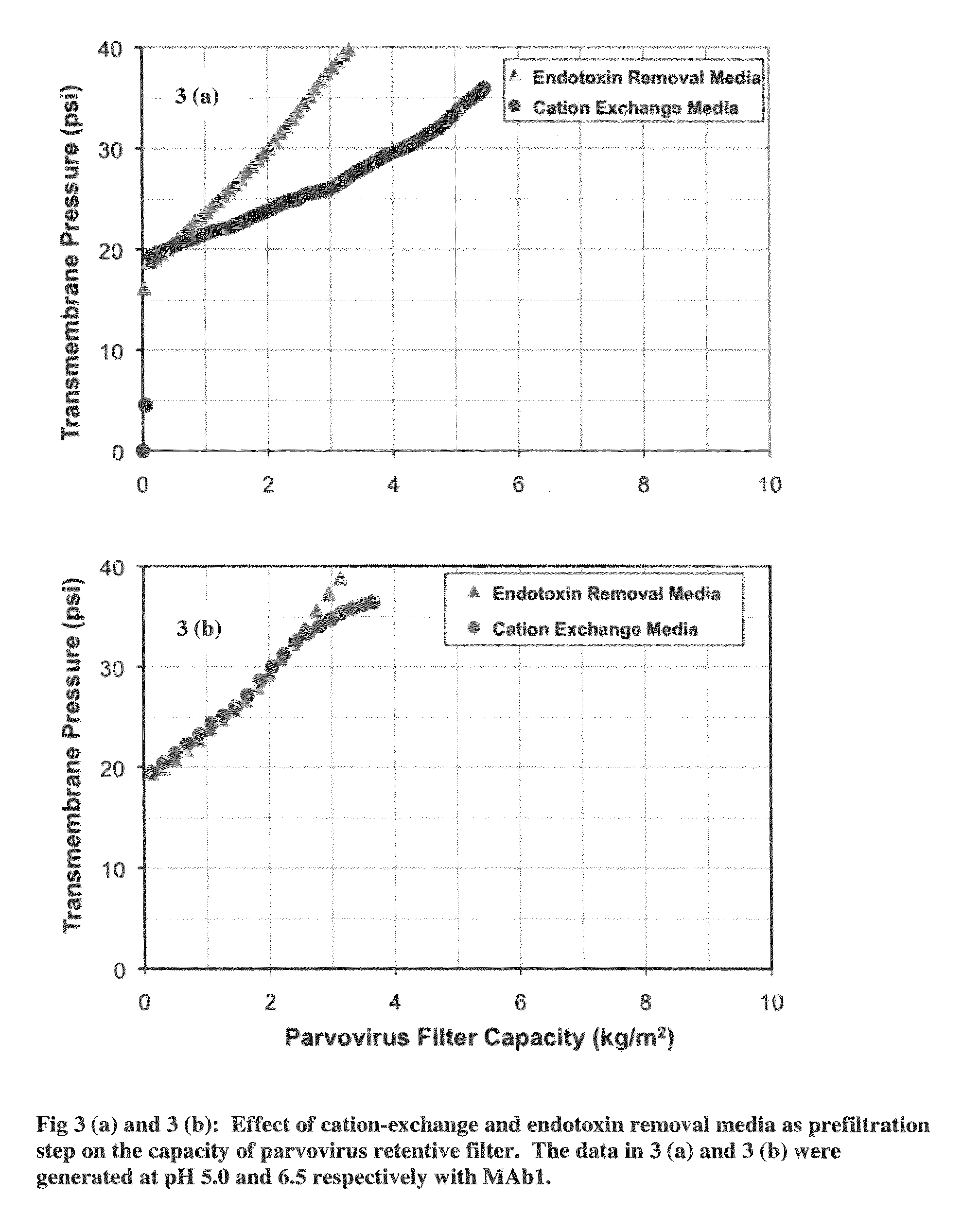
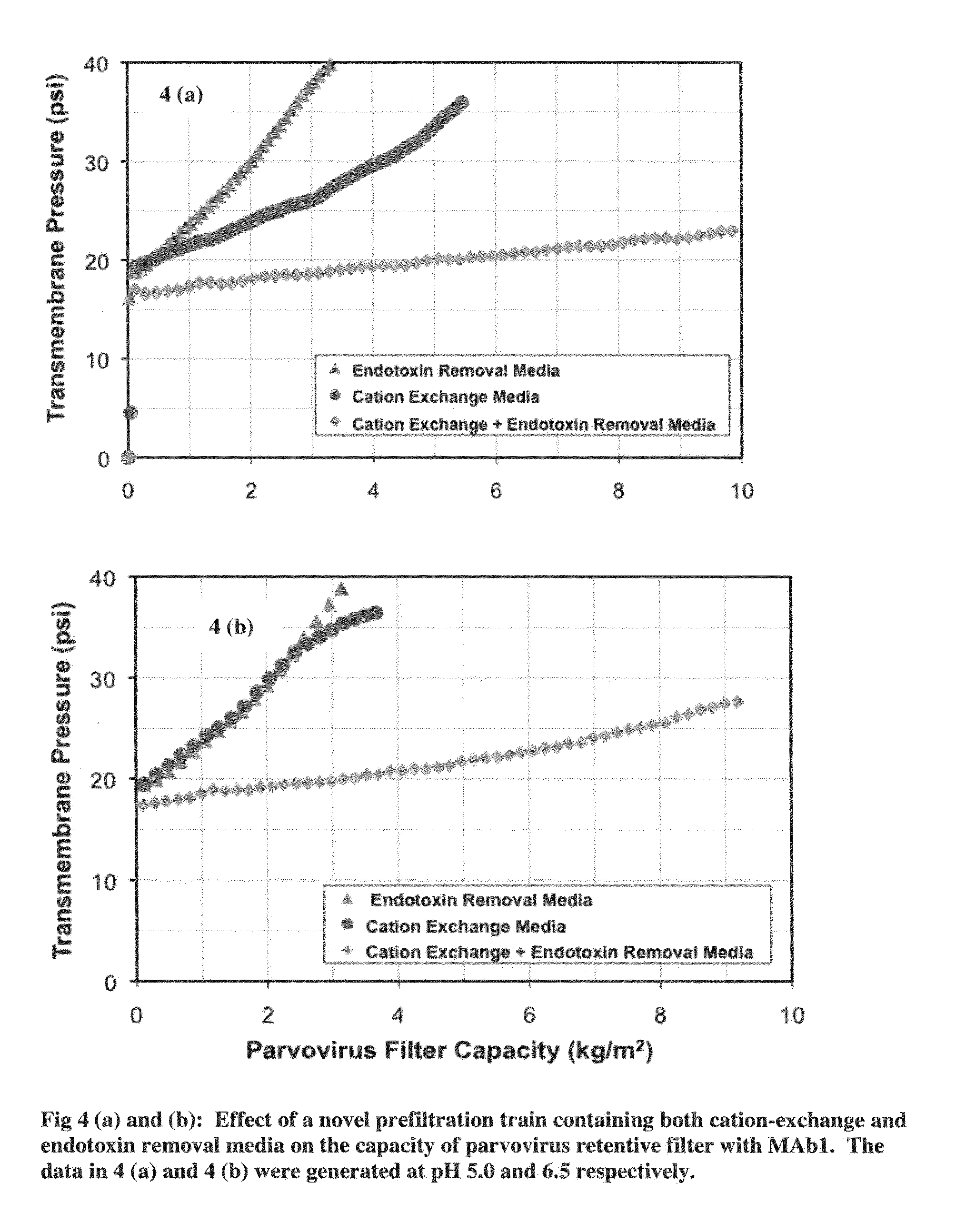
Virus filtration methods
ActiveUS20110034674A1Improve virus filtration capacityPerforms betterSerum immunoglobulinsImmunoglobulins against animals/humansEndotoxin removalFiltration
The present invention relates to the field of protein purification. In particular, the invention concerns methods for increasing the filtration capacity of virus filters, by combined use of endotoxin removal and cation-exchange media in the prefiltration process.
Owner:F HOFFMANN LA ROCHE & CO AG
Treatment of source water
ActiveUS10450212B1Avoid pollutionBiological treatment regulationSpecific water treatment objectivesEndotoxin removalHydraulic fracturing
There is provided herein a system and method for de-toxifying and de-scaling source water. In some embodiments, source water will be mixed with either an aluminum source or an iron source to separate endotoxins from acidic proteins and convert the naturally present bicarbonate in source water to carbon dioxide. Endotoxins and carbon dioxide will then be removed from source water by a stage of hydrophobic membranes to produce de-toxified and de-carbonated source water. Calcium hydroxide will be mixed with the de-toxified and de-carbonated source water to form precipitates comprising foulants and sulfate. A recoverable and reusable amine solvent will also be used to induce efficient precipitation. Possible reuse applications for the treated source water by the inventive methods that minimize excessive uses of potable water may include hydro-fracturing of shale and sand formations and heavy oil recovery by steam injection.
Owner:BADER MANSOUR S
Preparation method of low-endotoxin collagen
ActiveCN112778412AActivity is not destroyedReduce contentConnective tissue peptidesPeptide preparation methodsPharmaceutical industryEndotoxin removal
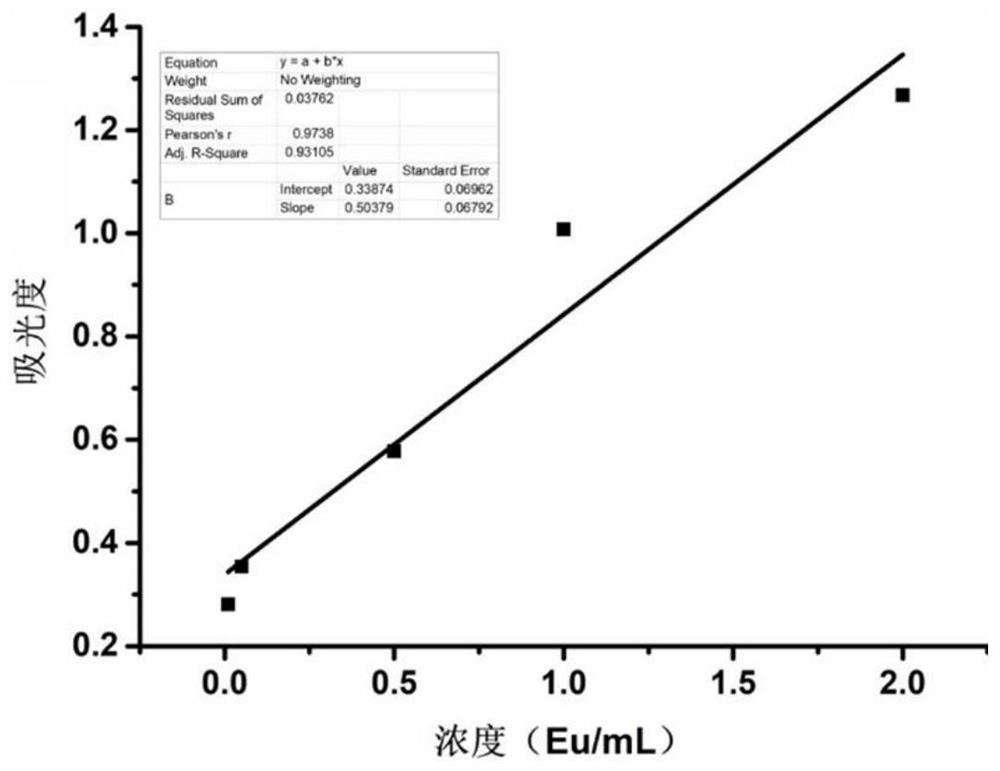
The invention belongs to the field of collagen preparation, and particularly relates to a preparation method of low-endotoxin collagen. According to the method, a procedure of low-temperature aqueous alkali treatment is added in a procedure of raw material treatment for collagen, so that the collagen with low endotoxin is prepared while ensuring undamaged activity of the collagen. The preparation method is high in endotoxin removal efficiency, and the endotoxin content of the prepared collagen is remarkably lower than 0.5 Eu / mL specified by the pharmaceutical industry standard (YY 0954-2015). The method provided by the invention is simple and convenient in operation, low in cost, and capable of removing endotoxin without special devices, so as to obtain the low-endotoxin collagen.
Owner:胶原蛋白(武汉)生物科技有限公司
Method for removing endotoxin in protein
InactiveCN105001299AEfficient removalEasy to operatePeptide preparation methodsUltrafiltrationFiltration
The invention provides a method for removing endotoxin in protein. The method adopts a sequential combination of chromatography, ultrafiltration and common filtration, and accords with a protein purification process flow. With the method, protein purification is realized while endotoxin is effectively removed, and the properties of protein is not influenced. The method is especially suitable to be used in treating various genetically engineered bacteria expressed intracellular and extracellular proteins. With optimized designs of the conditions of filtering mode, filtering pressure, membrane pore size and the like, operation steps and operation time are simplified, such that production cost is reduced, and processing capacity is improved. With the method provided by the invention, the endotoxin content can be reduced to an animal clinical application standard range, and the properties of the product is not influenced while the endotoxin is removed. The method is suitable for large-scale productions and applications.
Owner:TIANJIN RINGPU BIO TECH
Method for removing bacterial endotoxin in protein solution
ActiveCN104311628AReduce or prevent adsorptionImprove securityPeptide preparation methodsDepsipeptidesProtein solutionHigh concentration
The invention discloses a method for removing bacterial endotoxin in a protein solution. The method comprises the following steps of S1, preparing aluminum hydroxide gel, namely adding 2mol / L sodium hydroxide into 4.5% of aluminum chloride solution to obtain a solution with the pH value of 7.0-7.5, reacting at the temperature of 2-8 DEG C for 1-2 hours, and centrifugally collecting gel at the speed of 4000rpm; and S2, removing bacterial endotoxin in the protein solution, adding the aluminum hydroxide gel prepared in the step S1 into the protein solution, stirring at the temperature of 4 DEG C and the speed of 100rpm for 1.5-2.5 hours, and centrifugally collecting at the speed of 4000rpm to obtain a supernatant solution without endotoxin. According to the method, electrostatic adsorption is mainly used as the way of adsorbing proteins by using the aluminum hydroxide gel, the adsorption of aluminum hydroxide to the proteins can be reduced or avoided when phosphate radicals and high-concentration NaCl (contained in a PB buffer solution) exist, and 99% of bacterial endotoxin can be removed by using the buffer solution.
Owner:CHENGDU OLYMVAX BIOPHARM
Removal of bacterial endotoxin in a protein solution by immobilized metal affinity chromatography
InactiveUS20050186218A1Reduce concentrationProtein recovery is greatIon-exchange process apparatusBacterial antigen ingredientsProtein solutionEndotoxin removal
The present invention relates to the purification of polypeptides and the removal of endotoxin via immobilized metal affinity chromatography (IMAC). More specifically, the invention relates to methods for removing bacterial endotoxin in a protein solution. In specific embodiments, the invention relates to the elimination of endotoxin from Moraxella catarrhalis outer membrane proteins.
Owner:WYETH HOLDINGS CORP
Method for removing endotoxin in influenza virus stock solution
ActiveCN101503462AEfficient removalHigh removal rateVirus peptidesAntiviralsHemagglutininEndotoxin removal
The invention provides a method for removing toxin from flu virus stock solution, which comprises extraction separation and is characterized in that the medium-polyethylene glycol mono-octyl phenolic ether with the final concentration of 0.5-5% (volume percentage concentration) is added into the flu virus stock solution and used for extracting and separating protein and endotoxin in the flu virus stock solution. In the condition of without changing the former natures of the flu virus stock solution, the endotoxin in the flu virus stock solution can be effectively removed to cause that the contents of the endotoxin are reduced to be less than 0.25 EU / Ml and active ingredients in the flu virus stock solution are remained, thus achieving the effect of optimizing quality. The method has the advantages that the operation is simple, the removal rate of endotoxin is high, the recovery rate of the total protein can reach more than 85%, hemagglutination titer is constant, the recovery rate of hemagglutinin content reaches more than 99%, and the obtained flu virus stock solution is safe and reliable.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Factor c for treating gram-negative bacterial infection
InactiveUS20080085865A1Strong antibacterial effectSuppress LPS-induced cytokinesAntibacterial agentsBiocideEndotoxin removalClearance rate
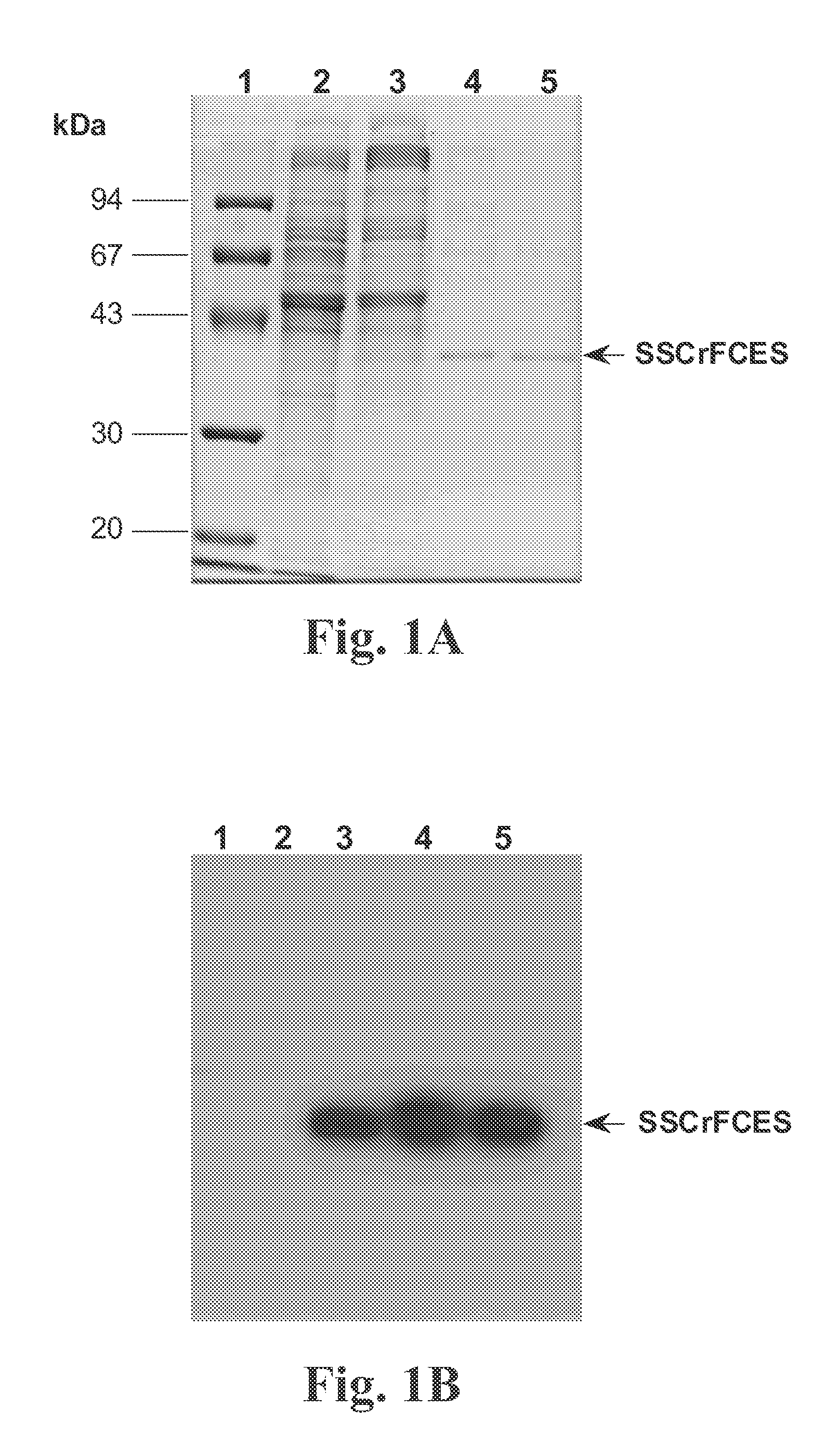
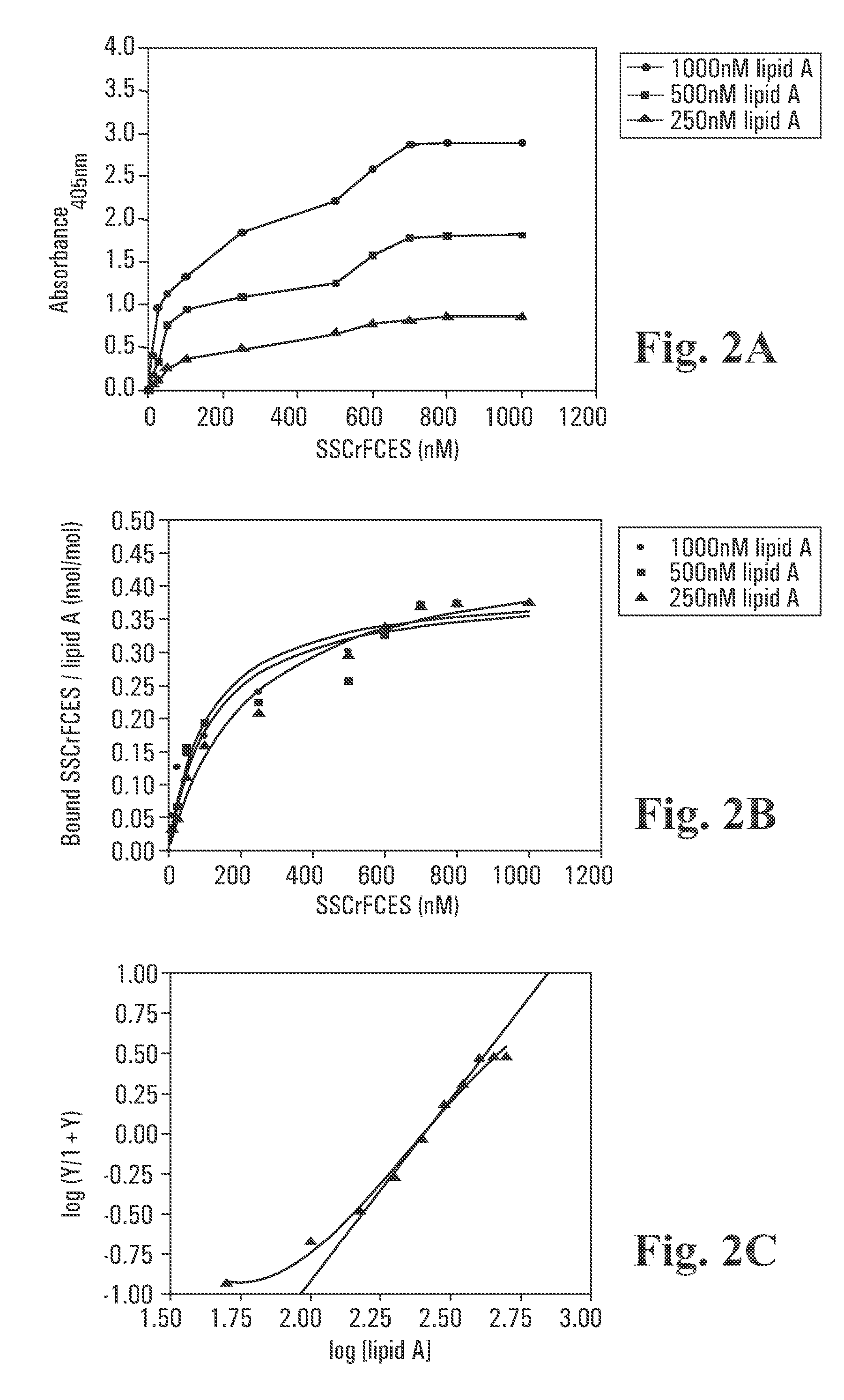
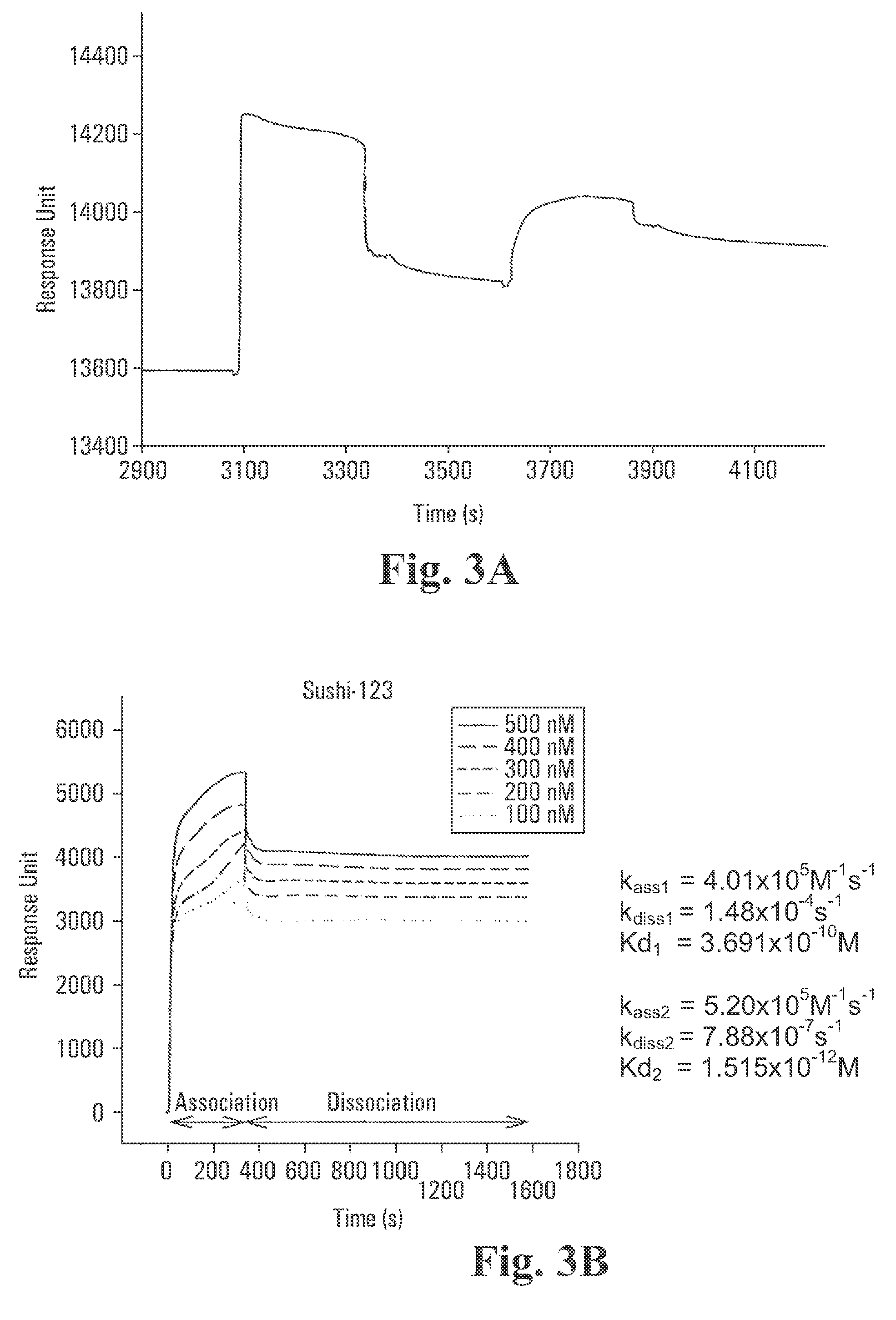
Recombinant fragments of Factor C are disclosed. These proteins and peptides show great potency in recognizing, binding to, neutralizing and removing endotoxin. These molecules can thus be used for anti-microbial, anti-endotoxin, and anti-sepsis therapy. SSCrFCES is a 38 kDa protein representing the LPS-binding domain of Factor C. The ability of SSCrFCES to bind lipid A was analyzed using an ELISA-based assay as well as surface plasmon resonance. Surface plasmon resonance similarly carried out for SSCrFC-sushi-1,2,3-GFP, SSCrFC-sushi-1GFP, and SSCrFC-sushi-3GFP confirmed their superior affinity for endotoxin. The 50% endotoxin-neutralizing concentration of SSCrFCES against 200 EU of endotoxin is 0.069 μM, suggesting that SSCrFCES is an effective inhibitor of LAL coagulation cascade. Although partially attenuated by human serum, as low as 1 μM of SSCrFCES inhibits the LPS-induced secretion of hTNF-α and hIL-8 by THP-1 and human pheripheral blood mononuclear cells with a potency more superior than polymyxin B. SSCrFCES is non-cytotoxic, with a clearance rate of 4.7 ml / minute. The LD90 of SSCrFCES for LPS lethality in mice is achieved at 2 μM. These results demonstrate the endotoxin-neutralizing capability of SSCrFCES in vitro and in vivo, as well as its potential for use in the treatment of endotoxin-induced septic shock. Also embodied in this application is the use of the sushi peptides and their mutant derivatives as potent antimicrobials.
Owner:NAT UNIV OF SINGAPORE
Method for Removing Endotoxin from Proteins
ActiveUS20090029921A1Reduce ionic strengthIncrease ionic strengthHydrolasesPeptide/protein ingredientsWound healingBiotechnology
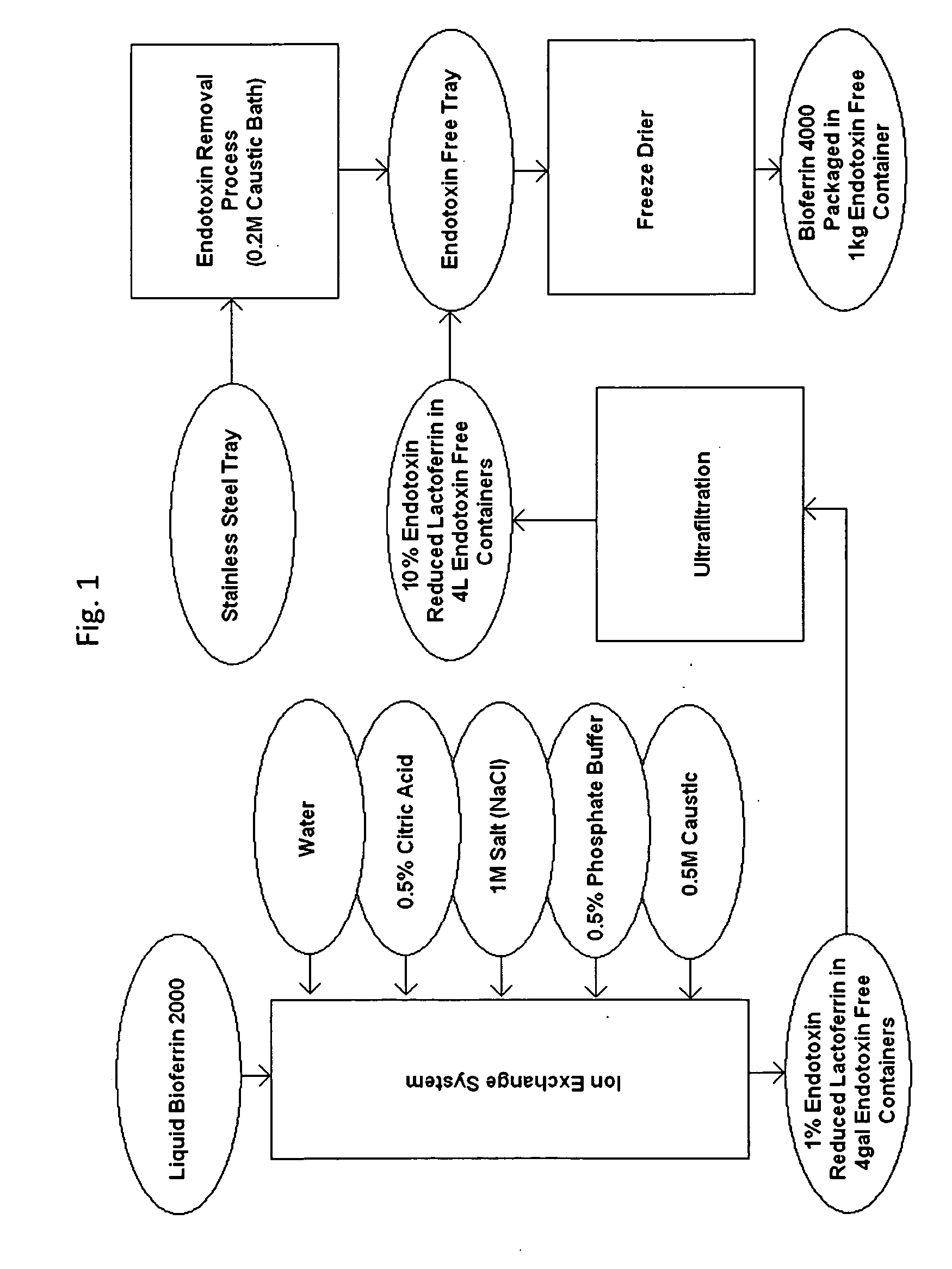
Disclosed is a method for removing endotoxin from proteins. Also disclosed are products made by using the method. The method may be used, for example, to produce endotoxin-free lactoferrin. Bovine milk-derived lactoferrin may be produced in commercial quantities by the method, and endotoxin-free bovine lactoferrin may be used for a variety of therapeutic uses, including improving wound healing.
Owner:GLANBIA NUTRITIONALS LTD
Materials and methods for removing endotoxins from protein preparations
InactiveCN105121457AIon-exchange process apparatusCation exchanger materialsEndotoxin removalAllantoin
A method includes (i) adding allantoin in a supersaturating amount to a protein preparation including a desired protein and at least one endotoxin as a contaminant, (ii) removing solids after the adding step to provide a sample for further purification by void exclusion chromatography on a packed particle bed of electropositive particles in a column, the packed particle bed having an interparticle volume, (iii) applying a sample volume to the packed particle bed, wherein the electropositive particles support void exclusion chromatography, and wherein the sample volume is not greater than the interparticle volume, and (iv) eluting a purified sample including the desired protein and a reduced amount of the endotoxin. The method is optionally carried out with only the allantoin treatment or only the void exclusion chromatography.
Owner:AGENCY FOR SCI TECH & RES
Method for removing endotoxin in porcin thrombin by gel molecular sieve
The invention discloses a method for removing endotoxin in porcin thrombin by a gel molecular sieve, which belongs to the field of deep processing of agricultural and sideline products. The method includes steps of centrifugally separation, column chromatography, enzymatic activation and separation by the molecular sieve so as to remove the endotoxin. The method has the advantages of fine separation effect and high safety in clinical use, and thrombin is high in activity, heat source substances and endotoxin in the thrombin are few.
Owner:JILIN UNIV
Novel sorbent for endotoxins
InactiveCN103221079AImprove abilitiesHigh speedOther chemical processesOther blood circulation devicesPorous substrateWater insoluble
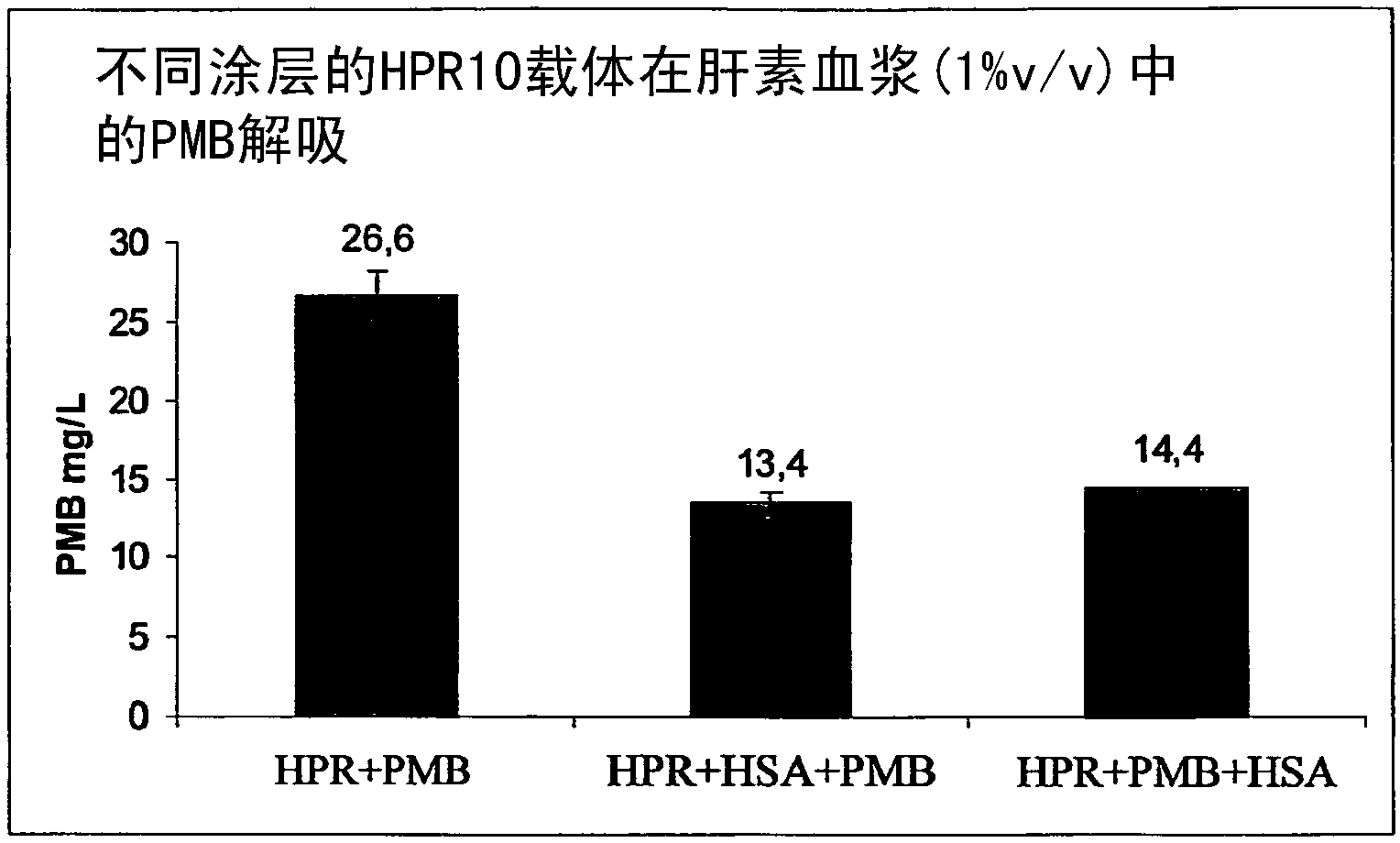
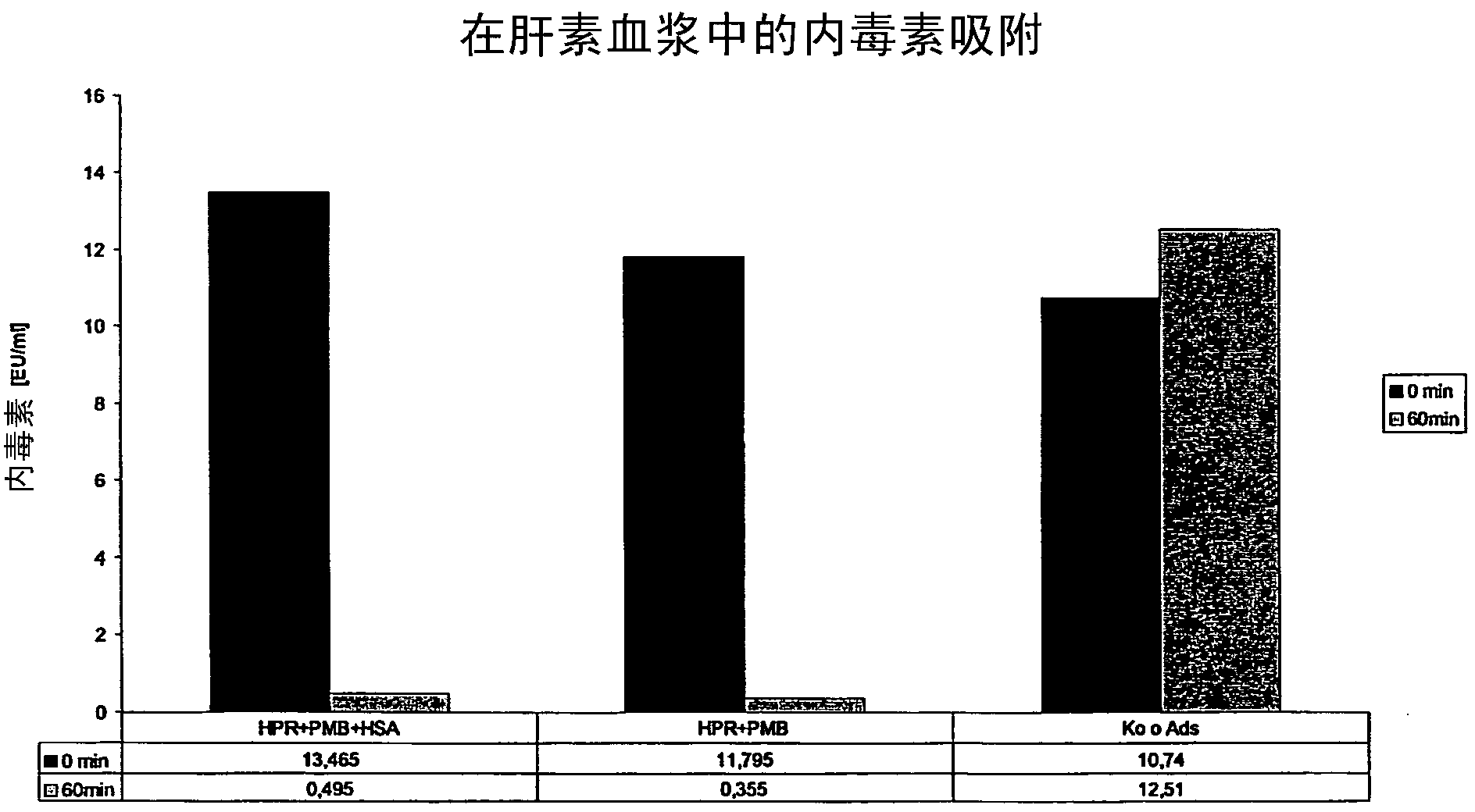
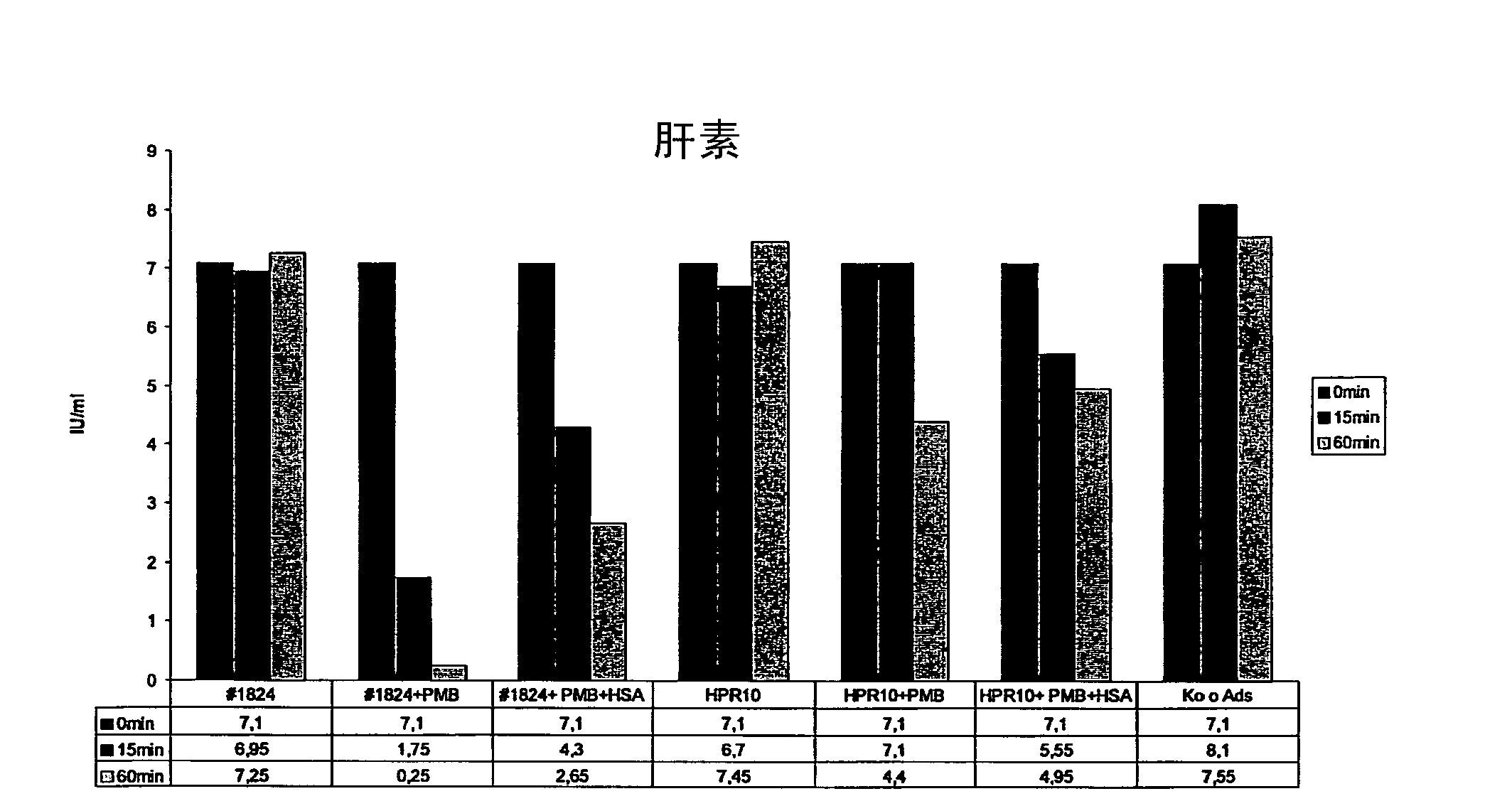
The invention relates to a sorbent for removing endotoxins from a biological liquid, comprising a water-insoluble, porous substrate having a neutral, hydrophobic surface, wherein the surface of the substrate has an adsorbent coating made of polymyxin and albumin, wherein polymyxin and albumin are bonded to the surface of the substrate in a noncovalent manner. The invention further relates to a method for producing such a sorbent. The sorbent is used in extracorporeal blood purification, in particular in order to treat individuals who have sepsis.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
Preparation method of bacterial cellulose capable of removing endotoxin
InactiveCN102977392AUnique three-dimensional porous network structureLarge specific surface areaWater bathsEndotoxin removal
The invention discloses a preparation method of bacterial cellulose capable of removing endotoxin. The preparation method comprises the following steps of: pretreating a bacterial cellulose membrane and preparing a PEG (polyethylene glycol) water solution or chitosan solution; putting the bacterial cellulose membrane in the PEG water solution or chitosan solution, heating to above 80 DEG C and maintaining at 80-100DEG C under a water bath for 0.5-1.5h; and washing the cooked bacterial cellulose membrane with purified water to obtain the bacterial cellulose. The method for removing the endotoxin is simple without considering the removal of the endotoxin in the bacterial cellulose culture process; and the preparation method is simple in preparation process and is suitable for industrial production of composite bacterial cellulose dressing.
Owner:SHANGHAI DIVINE MEDICAL TECH
Therapy for liver disease
ActiveUS20100025328A1Decrease endotoxin levelLower Level RequirementsSolvent extractionOther chemical processesToxinAlbumin
The invention provides an apparatus for use in the treatment of an individual suffering from liver disease, including: (a) means for selectively removing albumin from the blood of the individual; and (b) means for selectively removing endotoxin from the blood of the individual.
Owner:UCL BUSINESS PLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com