Method for removing endotoxin in recombinant protein A solution
A recombinant protein and endotoxin technology, applied in the field of bioengineering, can solve the problems of high cost, low recovery rate of recombinant protein A, and poor endotoxin removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
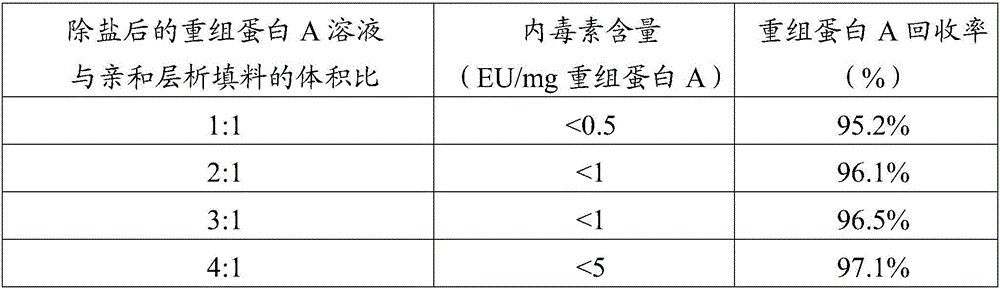
Image
Examples
Embodiment 1
[0060] A method for removing endotoxin in recombinant protein A solution, comprising the following steps:
[0061] (1) Take 10 g of cells containing recombinant protein A, add PBS buffer and mix the cells to obtain a cell suspension containing recombinant protein A, heat and stir in a water bath at 80° C. for 0.5 h to fully break the cells, the Recombinant protein A has a hexahistidine tag.
[0062] The PBS buffer solution is a mixed solution containing 10 mmol / L disodium hydrogen phosphate, 2 mmol / L sodium dihydrogen phosphate, and 150 mmol / L sodium chloride, and the pH value is adjusted by NaOH to 8;
[0063] The weight ratio of the thalline containing recombinant protein A to PBS buffer is 1:10;
[0064] (2) Add polyethyleneimine, after mixing evenly, centrifuge at 12000r / min, take the supernatant to obtain the supernatant containing recombinant protein A, the weight of the bacterial suspension containing recombinant protein A and polyethyleneimine The ratio is 10000:3; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com