Purification method of human glucagon like peptide-1 (hGLP-1) analogue fusion proteins
A glucagon and fusion protein technology, applied in the preparation methods of glucagon and peptides, animal/human proteins, etc., can solve problems such as differences in physical and chemical properties, large differences in impurities, difficult antibody purification methods, etc., to achieve Improving the quality control standard and removing the effect of HCP content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1. A purification method of hGLP-1 analog fusion protein
[0084] 1. Clarify and filter cell culture harvest fluid
[0085] (1) For the 0-5L cell culture harvest liquid, centrifugation can be used to remove large particles of insoluble matter such as cells and debris, and then filtered with a 0.22 μm filter membrane to obtain a clarified filtrate containing hGLP-1 analogue fusion protein. The conditions for the centrifugation operation are: 3500g, 7-12min, 2-8°C.
[0086] (2) For the 50L and 250L cell culture harvest liquid, the DOHC and B1HC two-stage series connection method of Millipore Company can be used to perform depth filtration and filter with a 0.2 μm filter element to obtain a clarified filtrate.
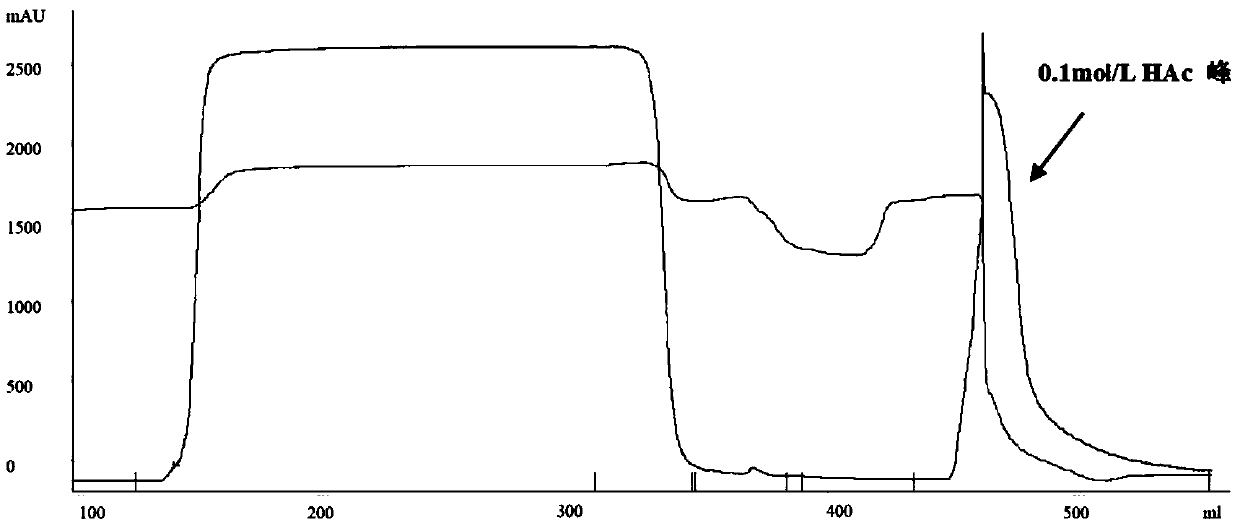
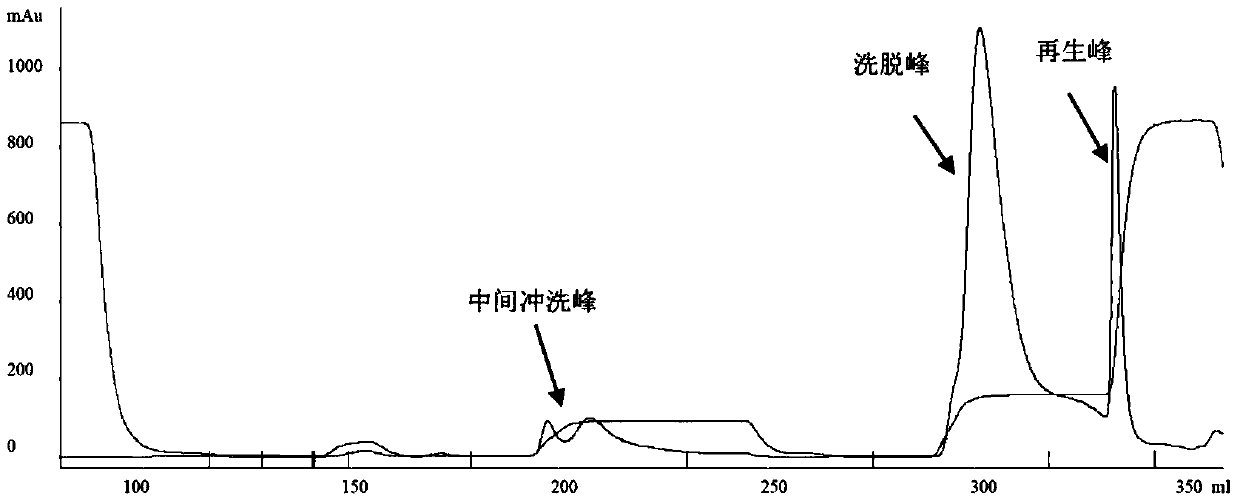
[0087] 2. Crude purification of Protein A affinity chromatography
[0088] The clarification filtrate that above-mentioned step 1 obtains is carried out the rough purification of Protein A affinity chromatography, and the step of Protein A affinity chroma...
Embodiment 2
[0130] Embodiment 2 A kind of purification method of hGLP-1 analogue fusion protein
[0131] 1. Clarify and filter cell culture harvest fluid
[0132] With embodiment 1 step 1 (1);
[0133] 2. Crude purification of Protein A affinity chromatography
[0134]Step is with embodiment 1 step 2, and concrete step parameter sees Table 2 for details;
[0135] 3. Low pH incubation inactivated virus
[0136] With embodiment 1 step 3;
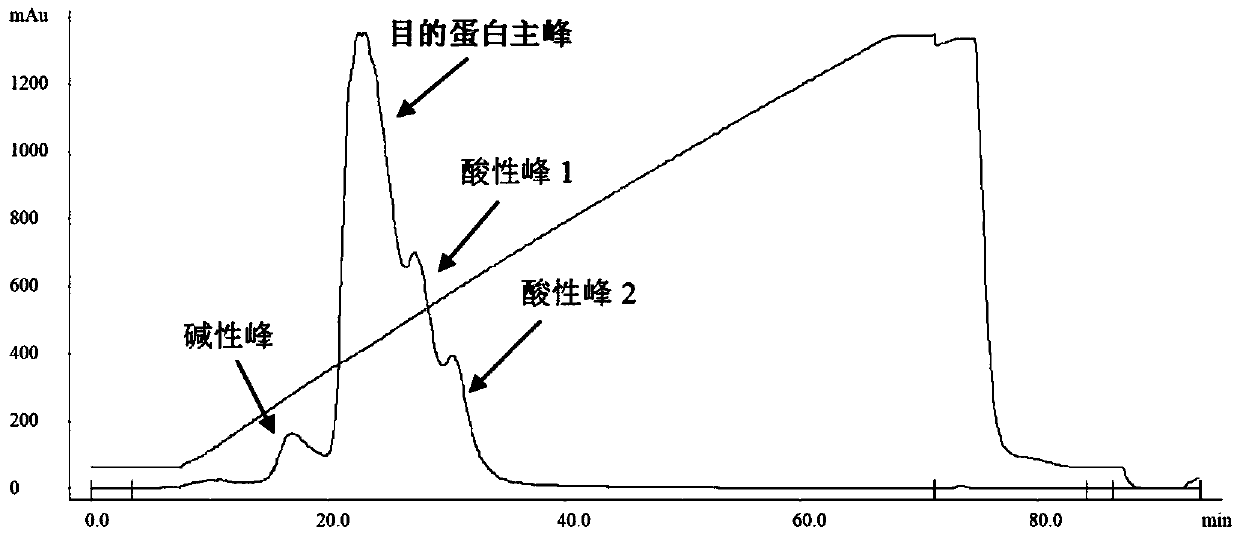
[0137] 4. Fine purification
[0138] Step is with embodiment 1 step 4, and concrete step parameter sees Table 2 for details;
[0139] 5. Prepare the target protein stock solution by filtration
[0140] Same as Step 5 of Example 1.
[0141] Table 2 crude purification and fine purification chromatography methods, steps and parameter conditions
[0142]
[0143] Using the purification method of this example, the HCP content in the target protein can also be reduced to below 100ppm, and charge variants and other trace impurities can be effectively ...
Embodiment 3
[0144] Embodiment 3 A kind of purification method of hGLP-1 analogue fusion protein
[0145] 1. Clarify and filter cell culture harvest fluid
[0146] With embodiment 1 step 1 (1);
[0147] 2. Crude purification of Protein A affinity chromatography
[0148] Step is the same as embodiment 1 step 2, and the specific step parameters are shown in Table 3;
[0149] 3. Low pH incubation inactivated virus
[0150] With embodiment 1 step 3;
[0151] 4. Fine purification
[0152] Step is with embodiment 1 step 4, and concrete step parameter sees Table 3 for details;
[0153] 5. Prepare the target protein stock solution by filtration
[0154] Same as Step 5 of Example 1.
[0155] Table 3 crude purification and fine purification chromatography methods, steps and parameter conditions
[0156]
[0157] Using the purification method of this example, the HCP content in the target protein can also be reduced to below 100ppm, and charge variants and other measured impurities can be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com