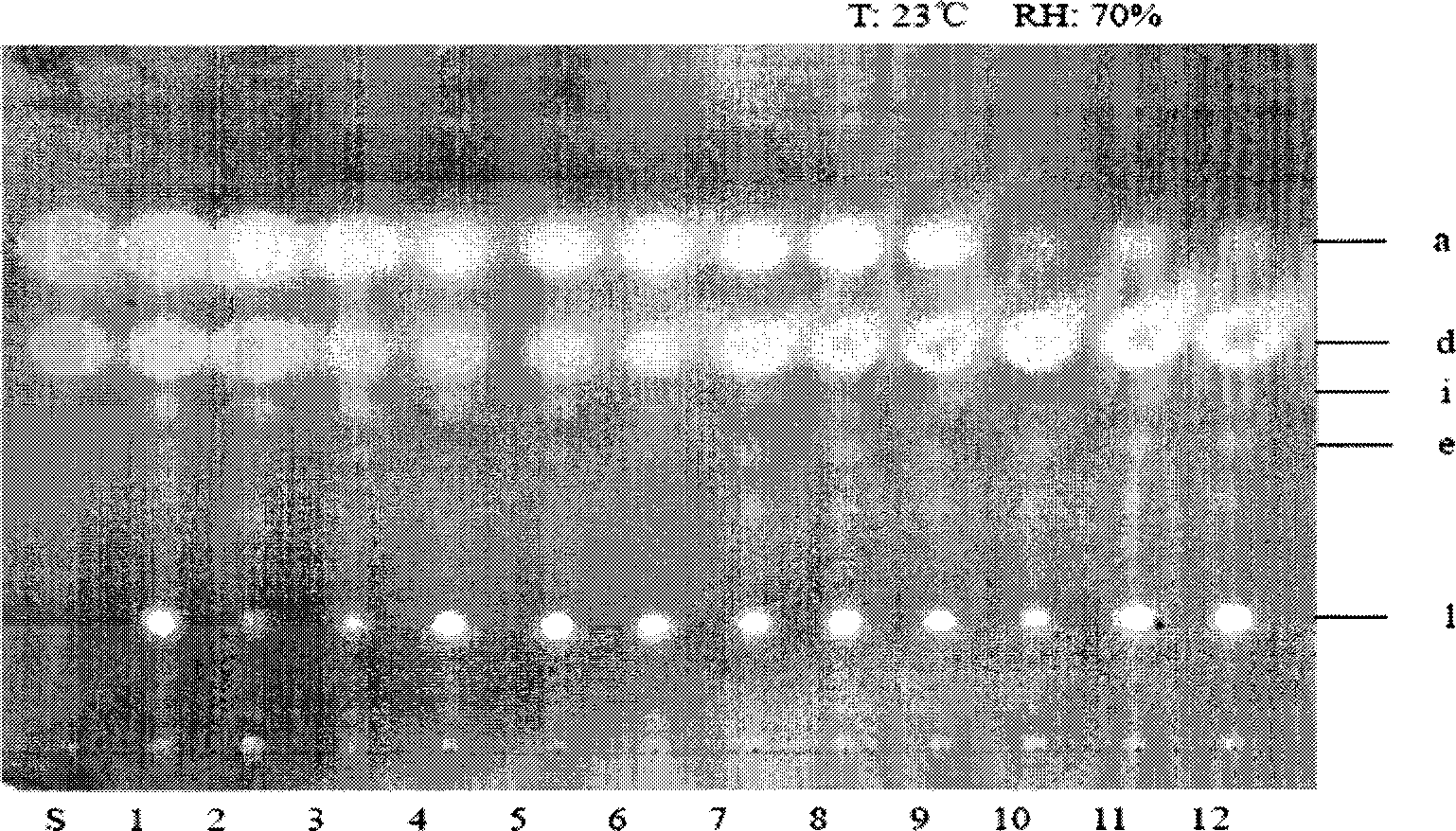
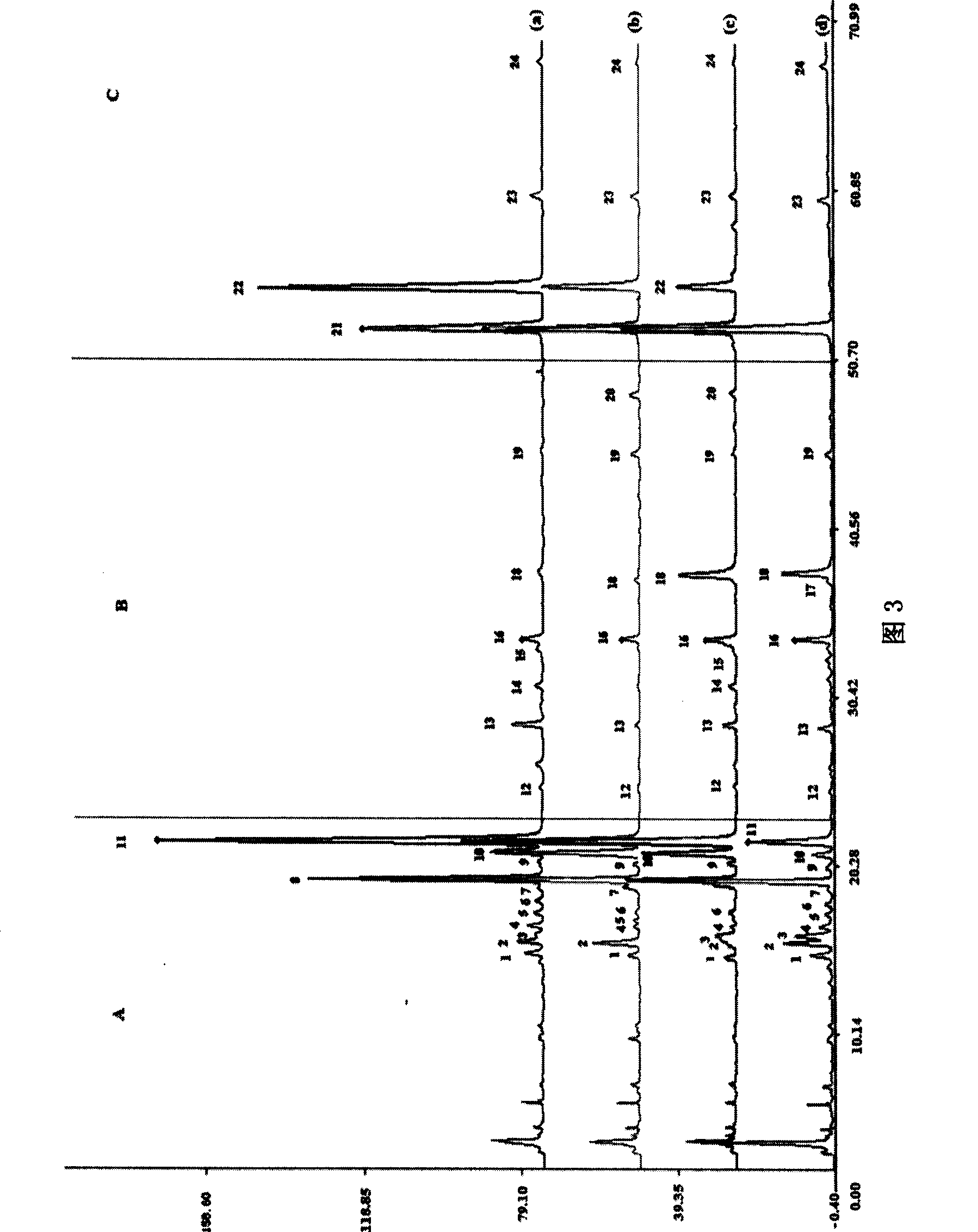
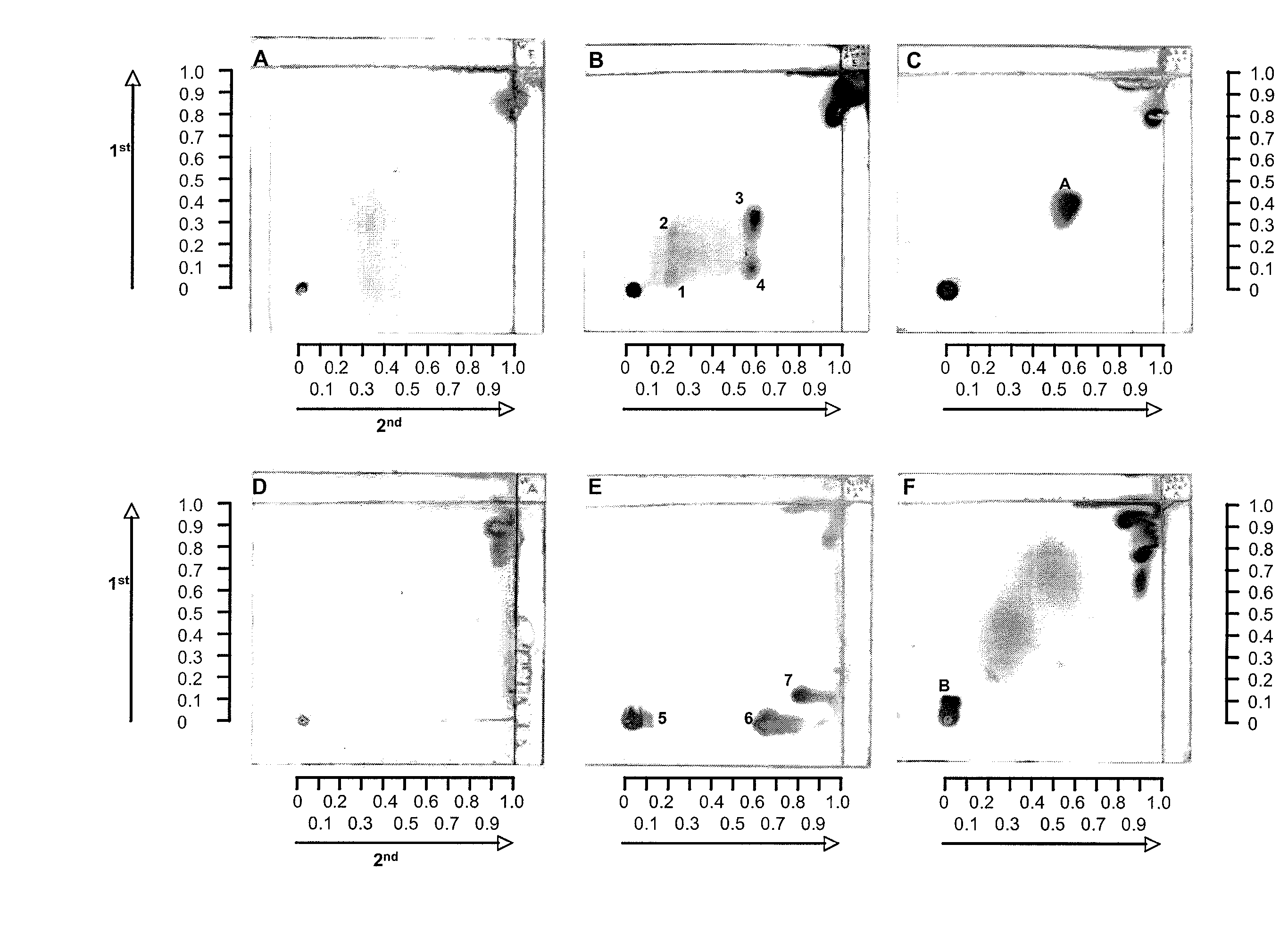
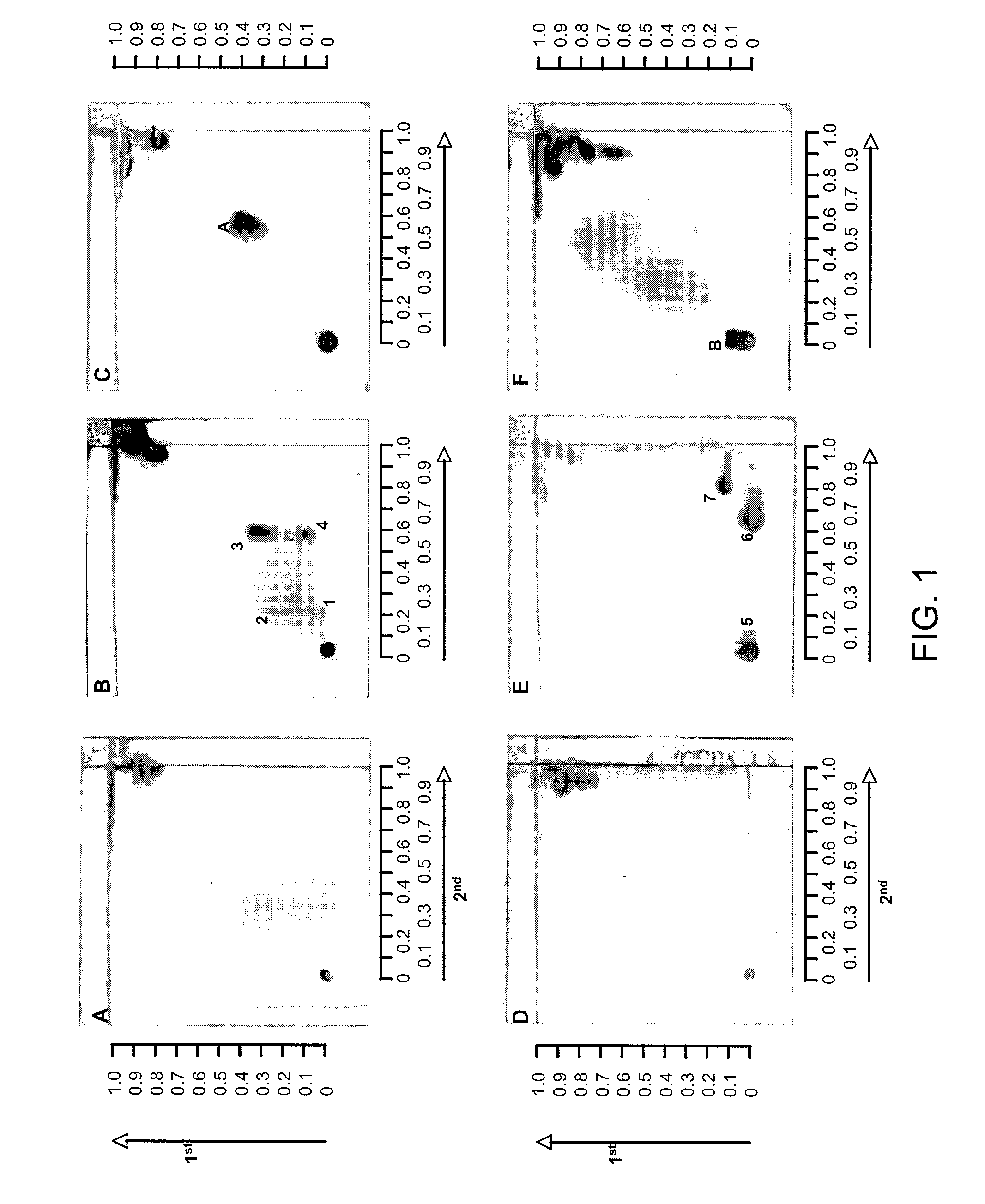
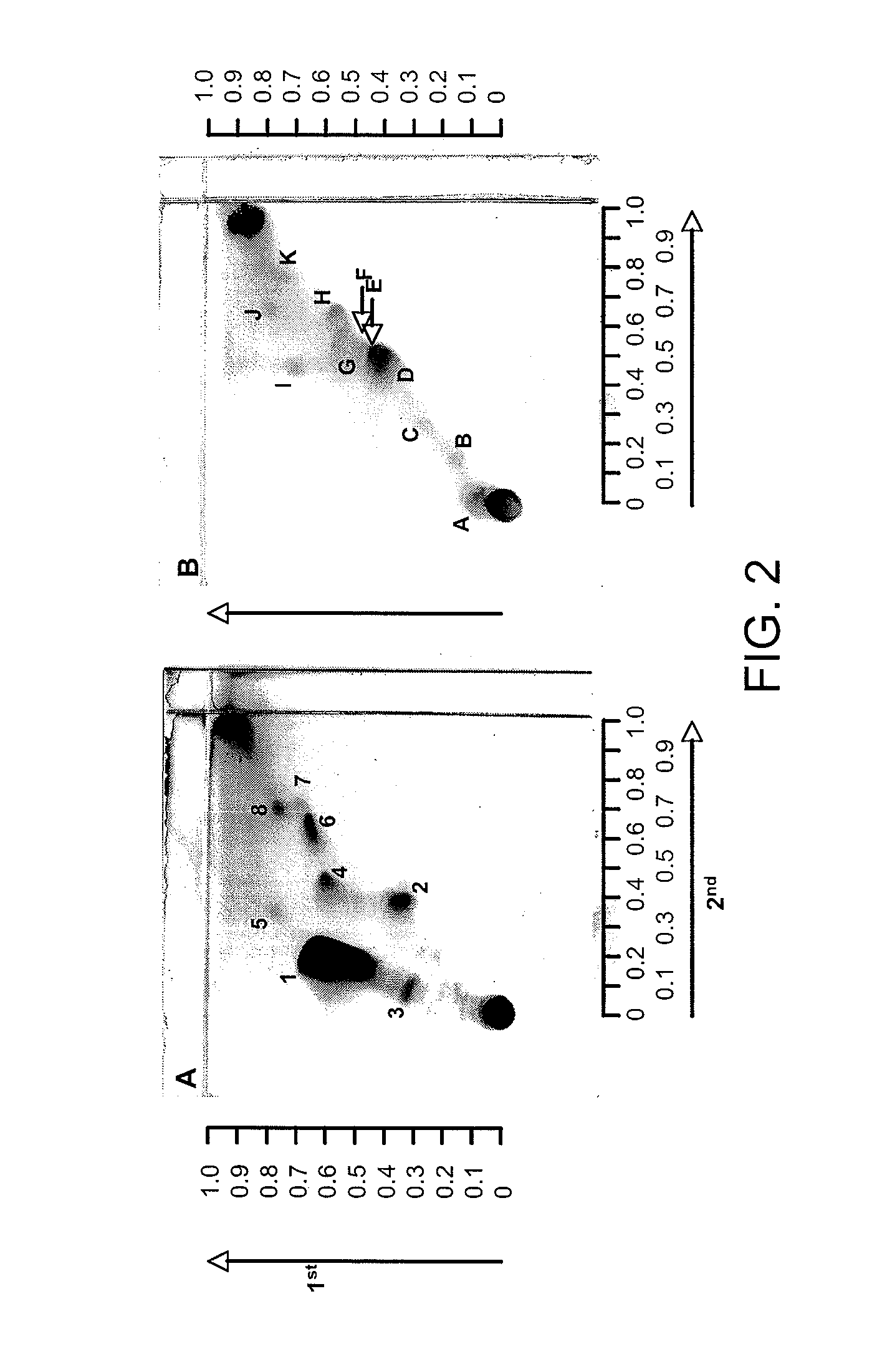
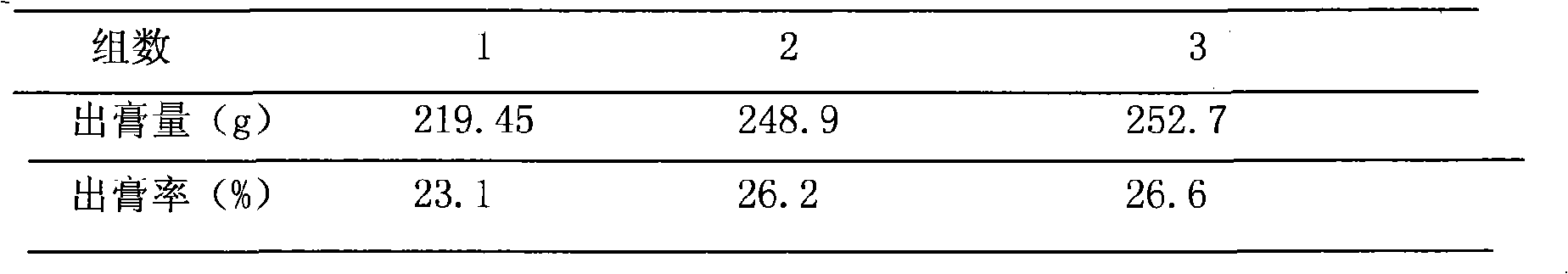
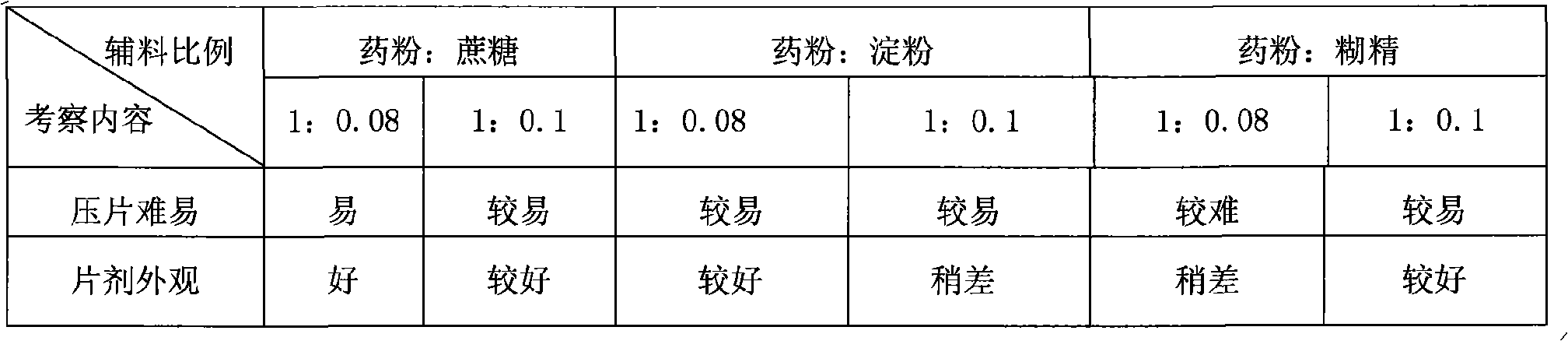
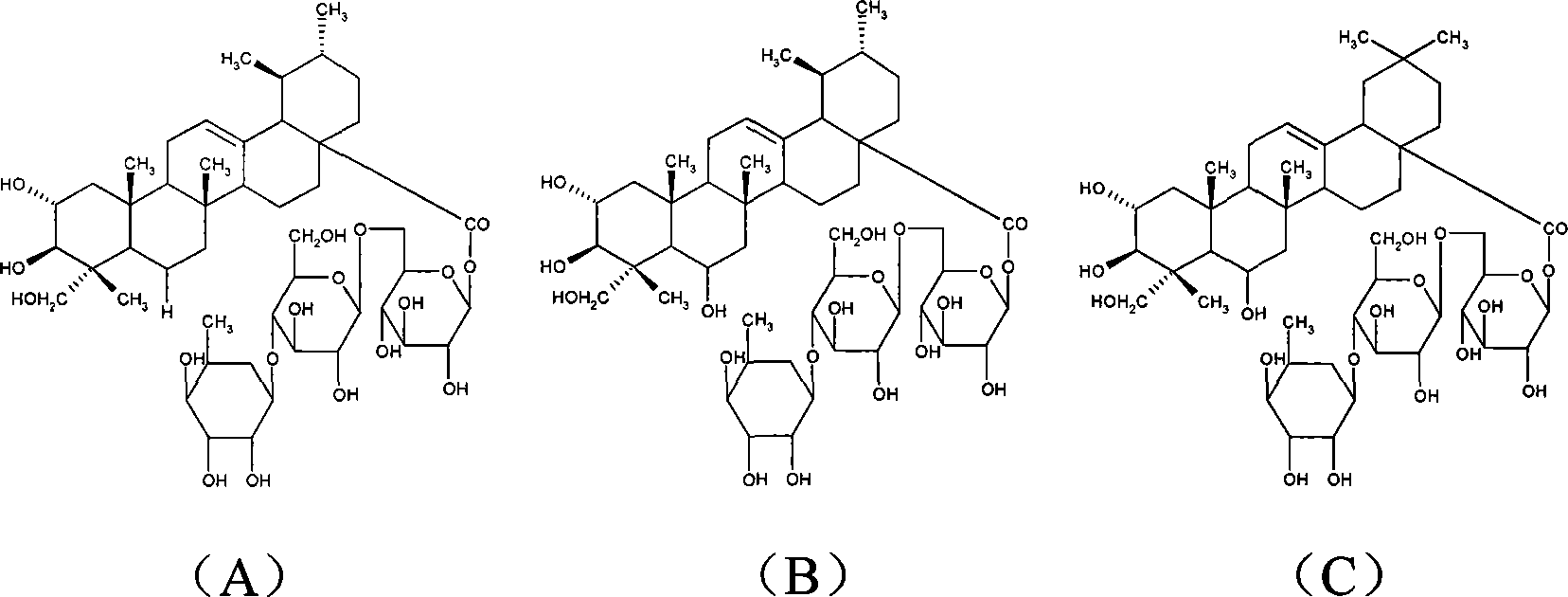
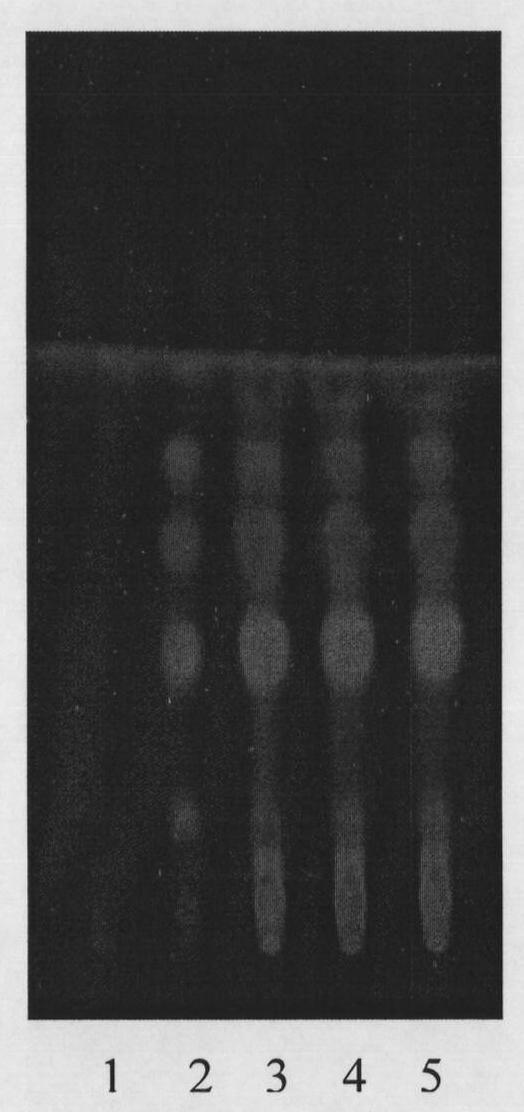
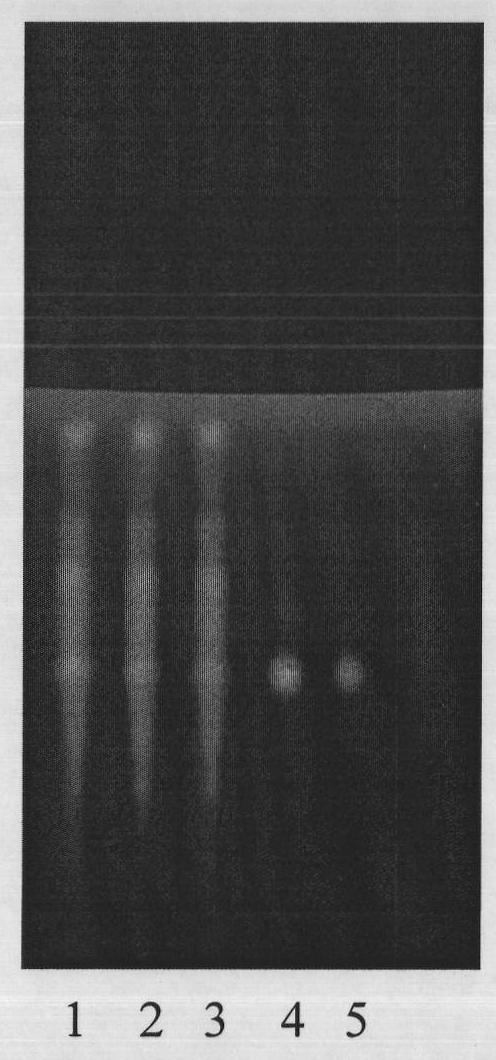
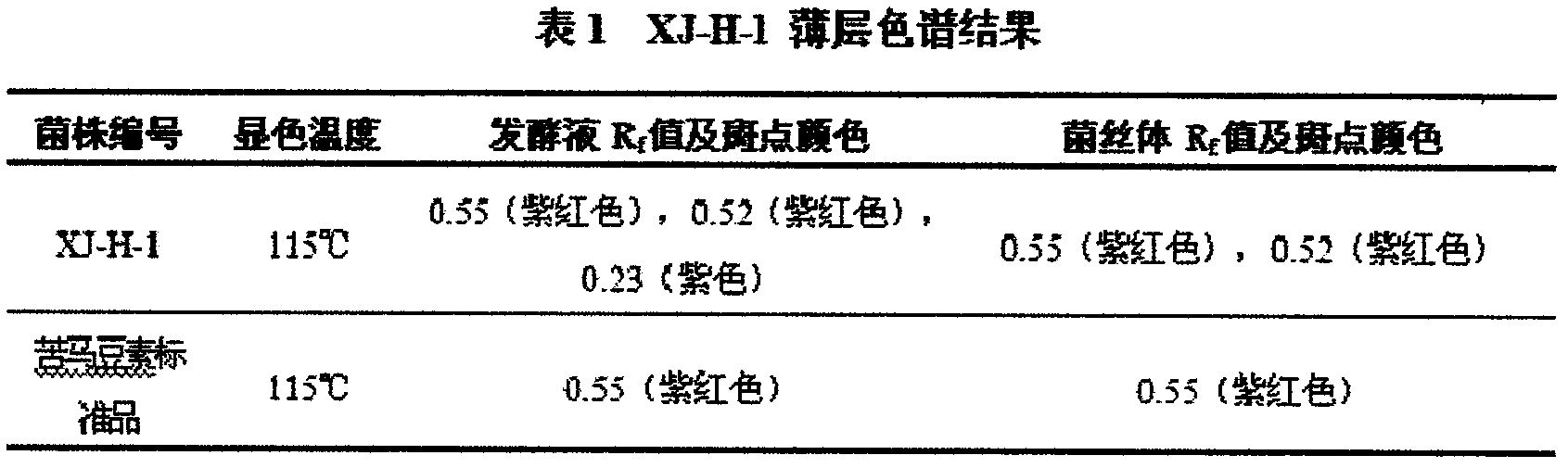
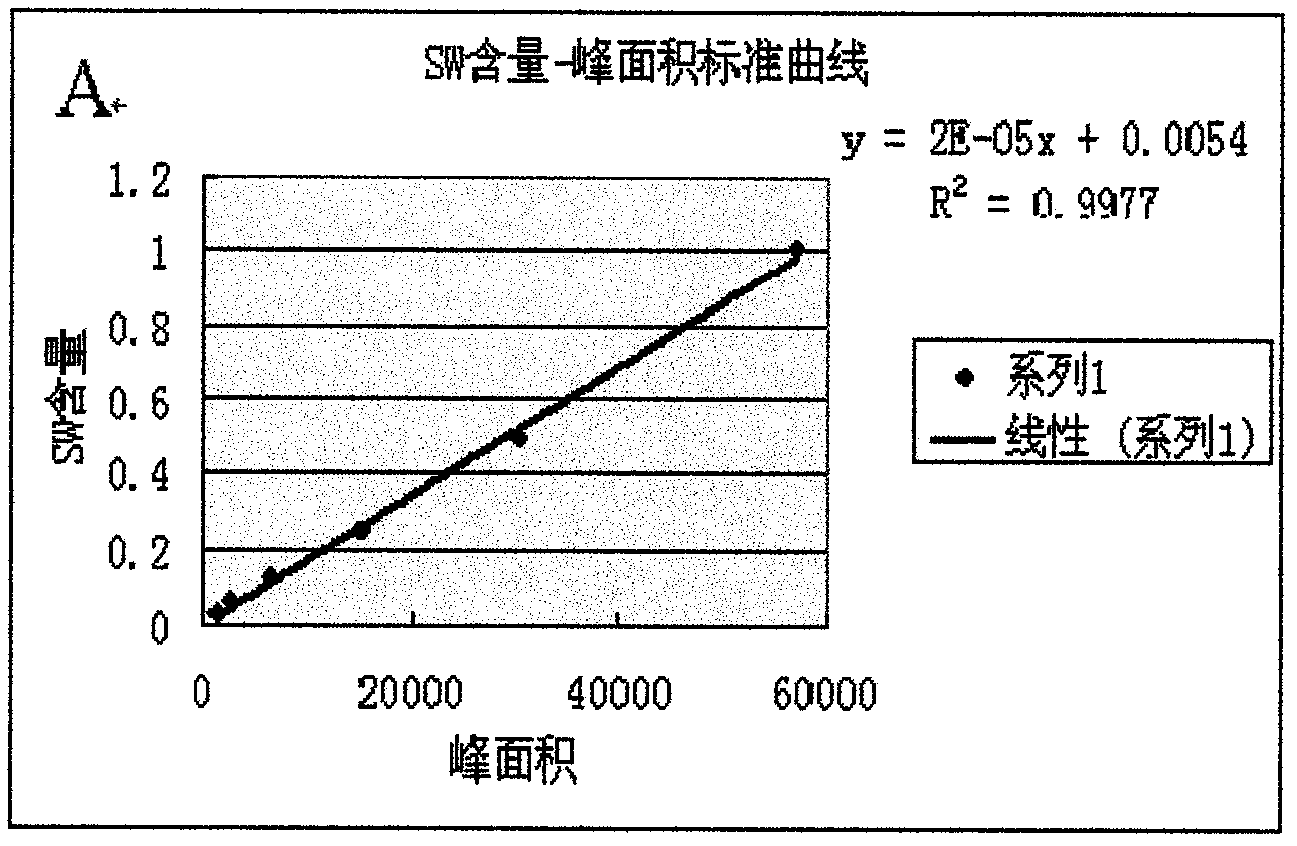
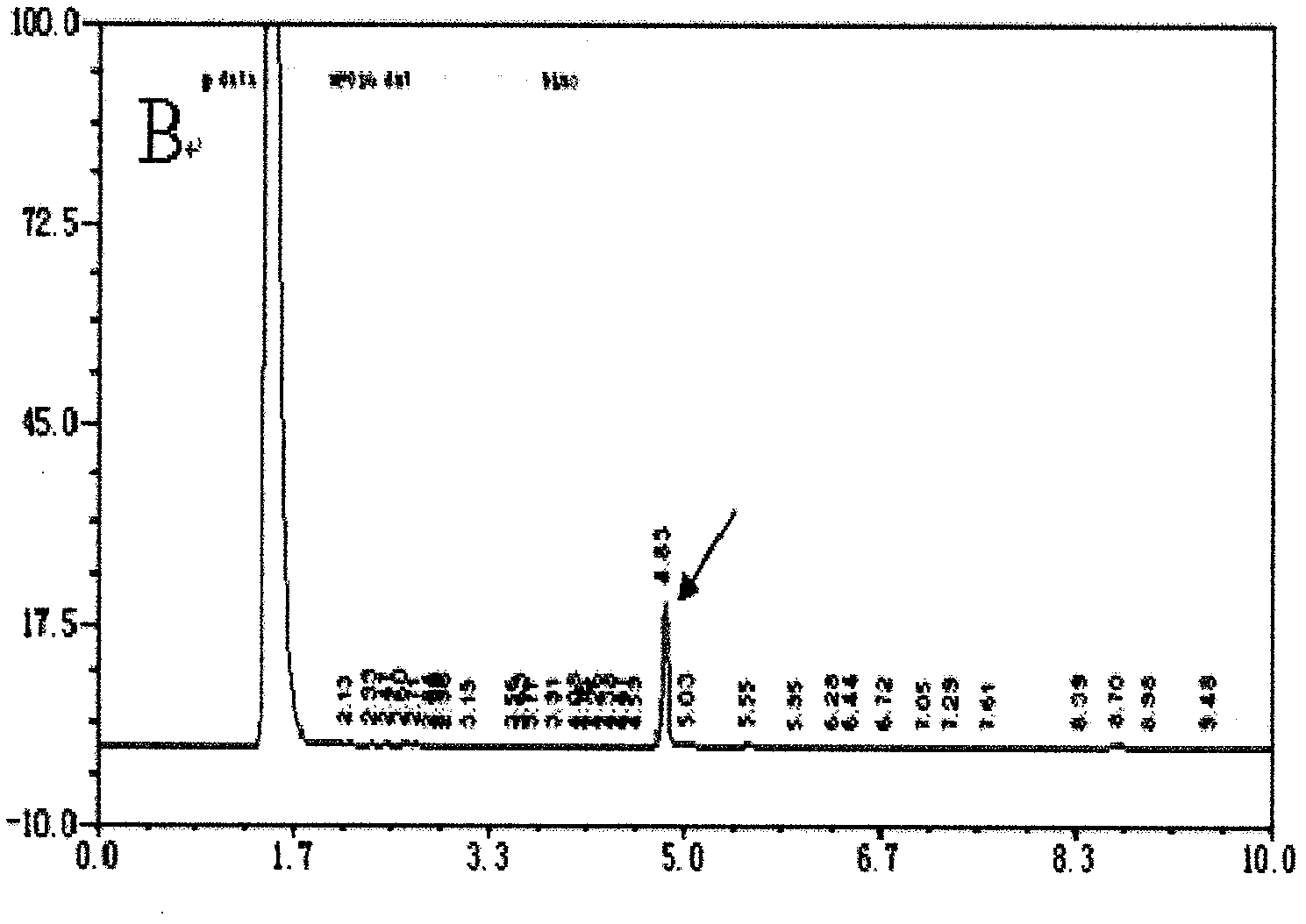
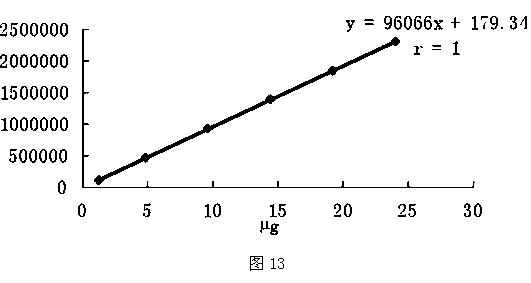
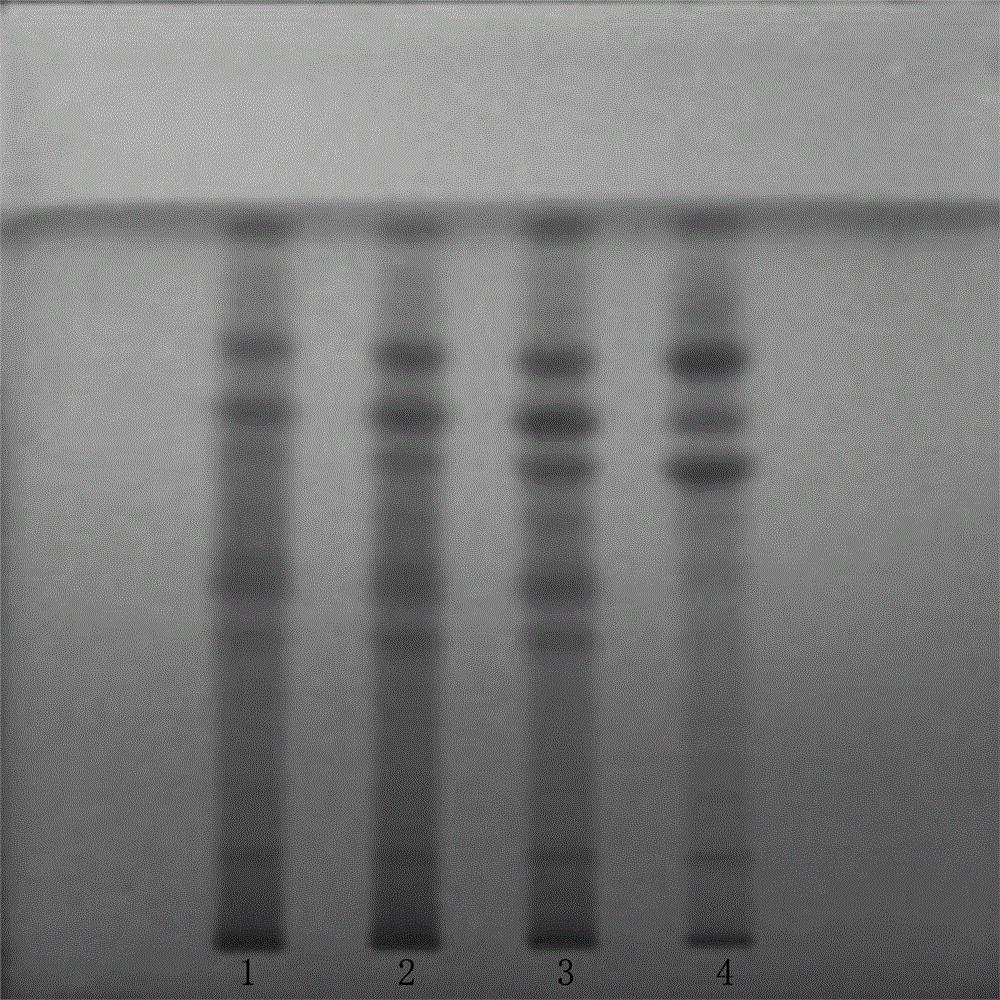
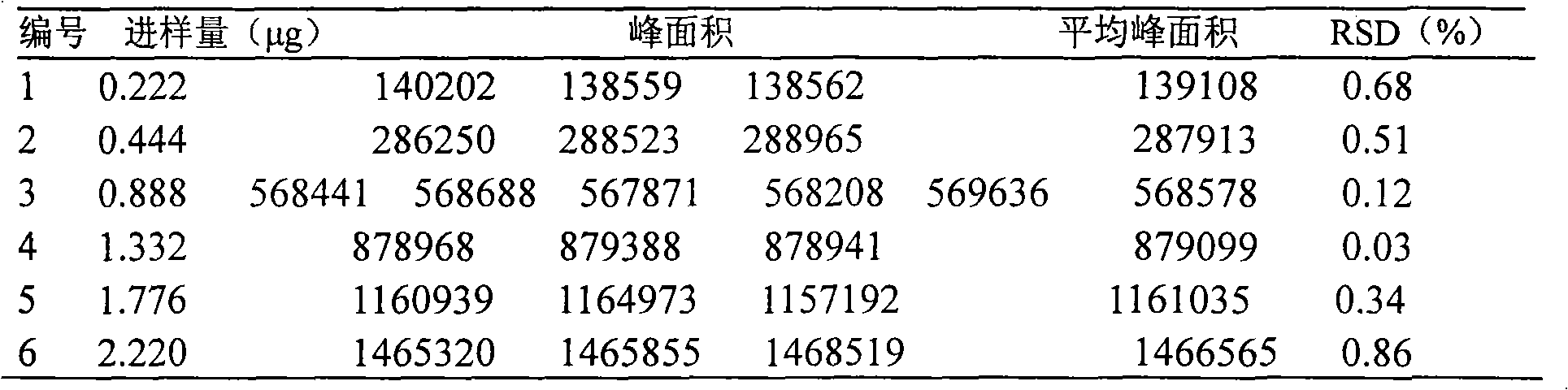
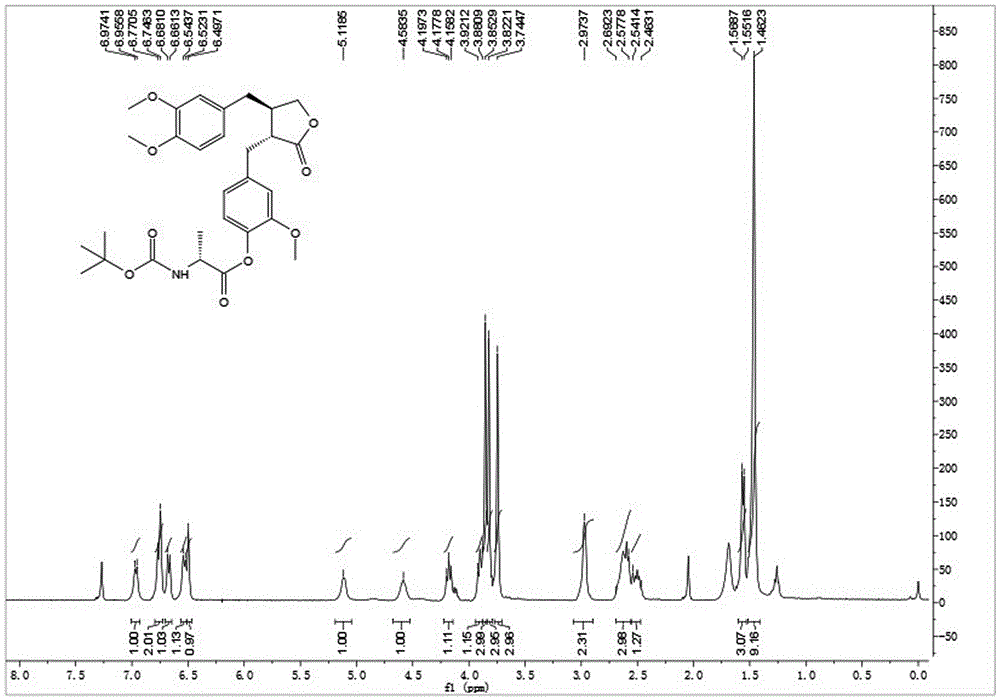
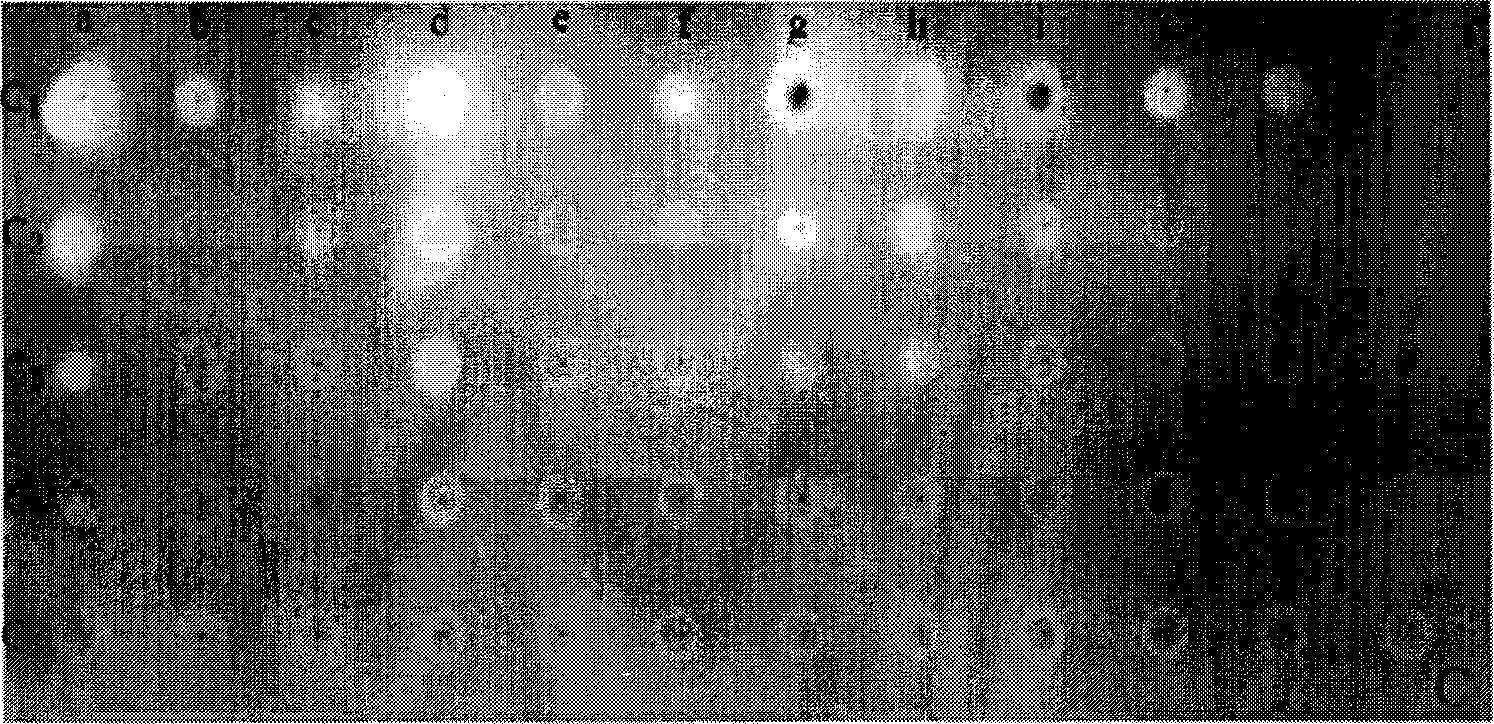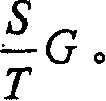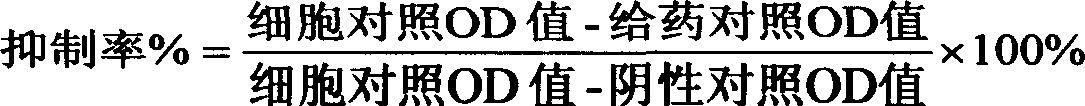Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
558 results about "Thin layer chromatographic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Total alkaloid extract of seeds of harmel genus and effective monomer component thereof, and preparation and use thereof
The invention discloses preparation and application of total alkaloids extract and effective components in peganum plant seeds. The peganum plant (comprising harmel, multisectum peganum and peganum nigellastrum) seeds are heated, refluxed and extracted by ethanol, dissolved along with heat, acidulated by added 2 to 10 percent hydrochloric acid, and basified by ammonia water to separate out sediment; and the sediment is dried to obtain the total alkaloids extract mainly containing dehydrogenized peganine and peganine according to a proportion of between 0.18 to 1 and 4.30 to 1, wherein the content of the total alkaloids is more than 50 percent. The total alkaloids extract is separated by chromatography to obtain ten effective monomer components such as the dehydrogenized peganine, the peganine, and the like. Through thin layer chromatography and biological self-developing analysis, the total alkaloids extract and the effective monomer components thereof have the effect of resisting activity of acetylcholine esterase, and can be used for preparing medicament for resisting the activity of the acetylcholine esterase and medicament for treating neurodegenerative diseases.
Owner:SHANGHAI UNIV OF T C M +1
Identification of bacterial species and subspecies using lipids
InactiveUS20080108104A1Reduce in quantityMicrobiological testing/measurementBiological material analysisLipid formationParatuberculosis
The use of free, extractable lipids found in bacteria for identification of bacterial species and subspecies is described. Bacteria have been found to differ sufficiently in their extracted lipid compositions to effect identification using thin layer chromatographic techniques. Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei have been distinguished in this manner. Lipopeptides specific to Mycobacterium avium subspecies paratuberculosis, but not to the closely related bacterium Mycobacterium avium subspecies avium have also been used as a basis for bacterial subspecies identification using mass spectrometry and seroreactivity. Mass spectrometric analysis of total bacterial lipids of Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei, and mass spectrometric analysis of total bacterial lipids for Mycobacterium avium subspecies paratuberculosis and Mycobacterium avium subspecies avium, without further lipid separation, has shown that species and subspecies of bacteria may be identified using such analysis.
Owner:COLORADO STATE UNIVERSITY
Traditional Chinese medicine composition for treating chronic pharyngitis and preparation method and quality control method thereof
The invention relates to a pharmaceutical composition for treating chronic pharyngitis, in particular to a traditional Chinese medicinal composition for treating chronic pharyngitis, as well as a preparation method and a quality control method thereof. The pharmaceutical composition comprises figwort roots, radix asteris, radix asparagi, cortex moutan radicis, ophiopogon roots and other raw medicinal materials, and is prepared into various preparations suitable for clinical application according to traditional Chinese medicinal routine techniques, including tablets, capsules, orally taken liquid preparations, dropping pills, granules, and the like. The quality control method comprises qualitative identification for thin-layer chromatography, content determination of high performance liquid chromatography and other methods. The pharmaceutical composition has good effects of treating throat dryness, throat itch, irritable cough and other symptoms caused by chronic pharyngitis.
Owner:BEIJING ASIA EAST BIO PHARMA CO LTD
Detection method of Chinese patent medicines containing at least two kinds of Radix Paeoniae Alba, Ginseng, Danshen, Artemisia annua, Licorice, Chuanxiong and Angelica
InactiveCN101991661AThe identification result is accurateChange processing methodAnthropod material medical ingredientsComponent separationSalvia miltiorrhizaDrugs solution
The invention provides a method for detecting a Chinese patent drug containing at least two of white paeony root, ginseng, salvia miltiorrhiza, sweet wormwood, liquorice and angelica sinensis, comprising the steps as follows: preparing a sample solution and a comparison product drug solution, adopting acetonitrile and water as mobile phases, and performing high performance liquid chromatography (HPLC) measurement, wherein the detection wavelength is 201-290nm; and detecting whether a corresponding chromatographic peak exists at a position, which has the same reservation time with the chromatograph of a comparison drug, in the chromatography of a sample. Compared with the traditional thin-layer chromatography identification method, the detection method in the invention has the advantages that the mode of processing a sample containing various drugs in a liquid phase chromatograph is changed, the mixed peak in the liquid phase chromatography except the components to be detected in the Chinese patent drug is successfully eliminated, and results are more accurate and stable.
Owner:NAT INST FOR FOOD & DRUG CONTROL
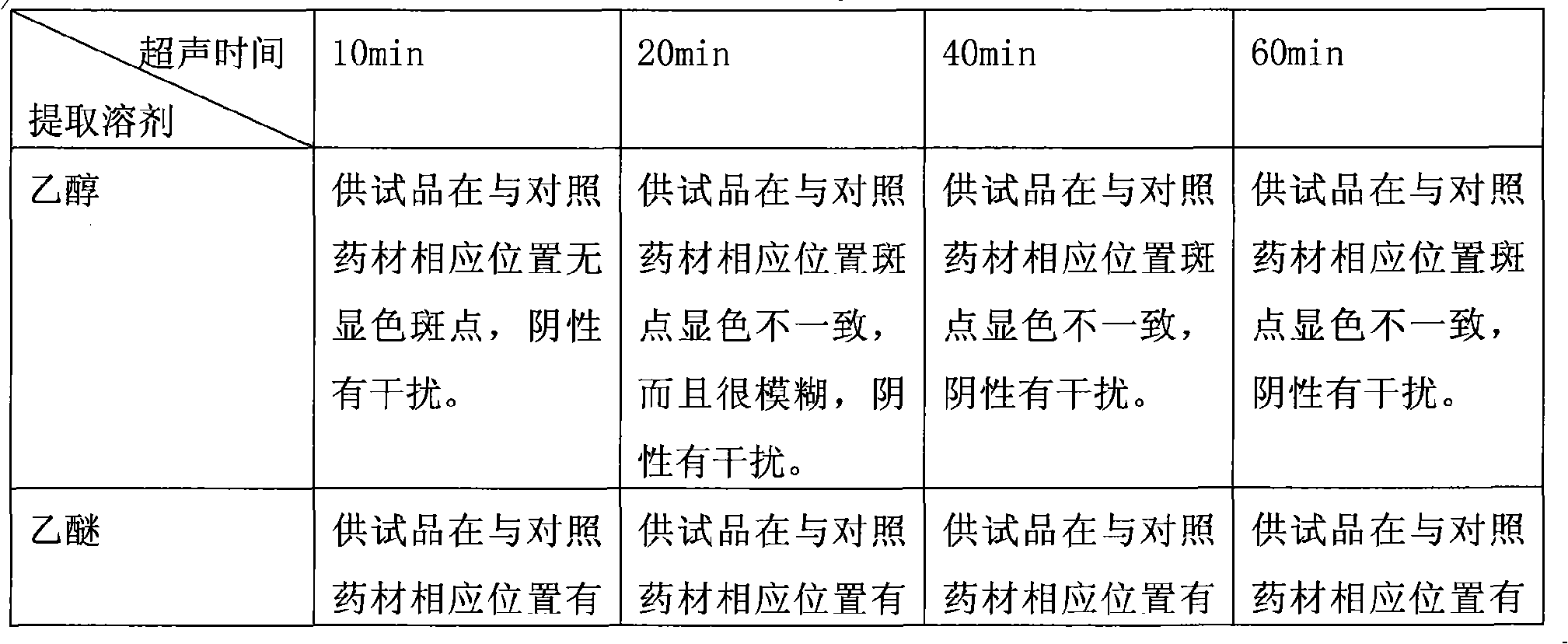
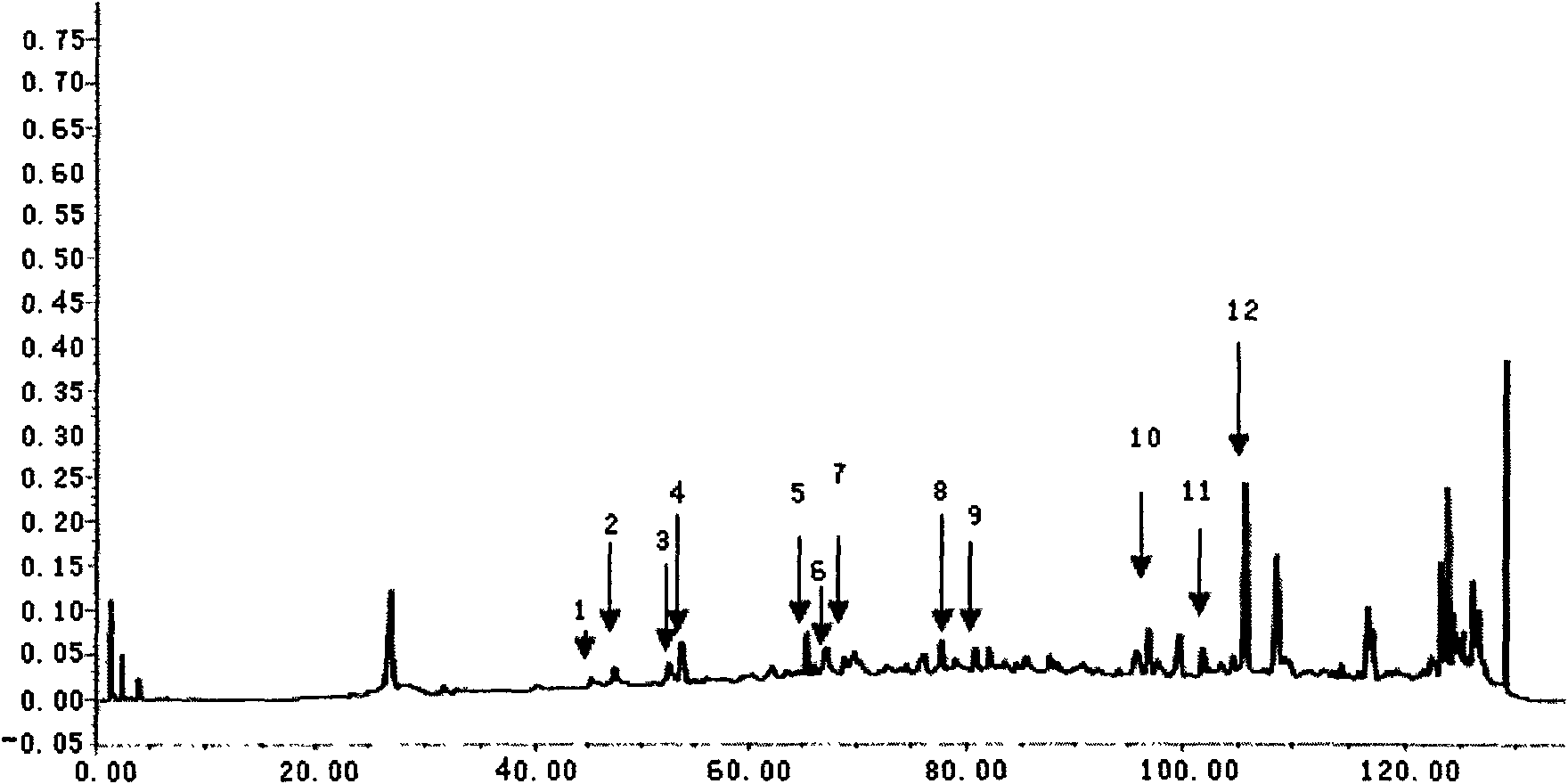
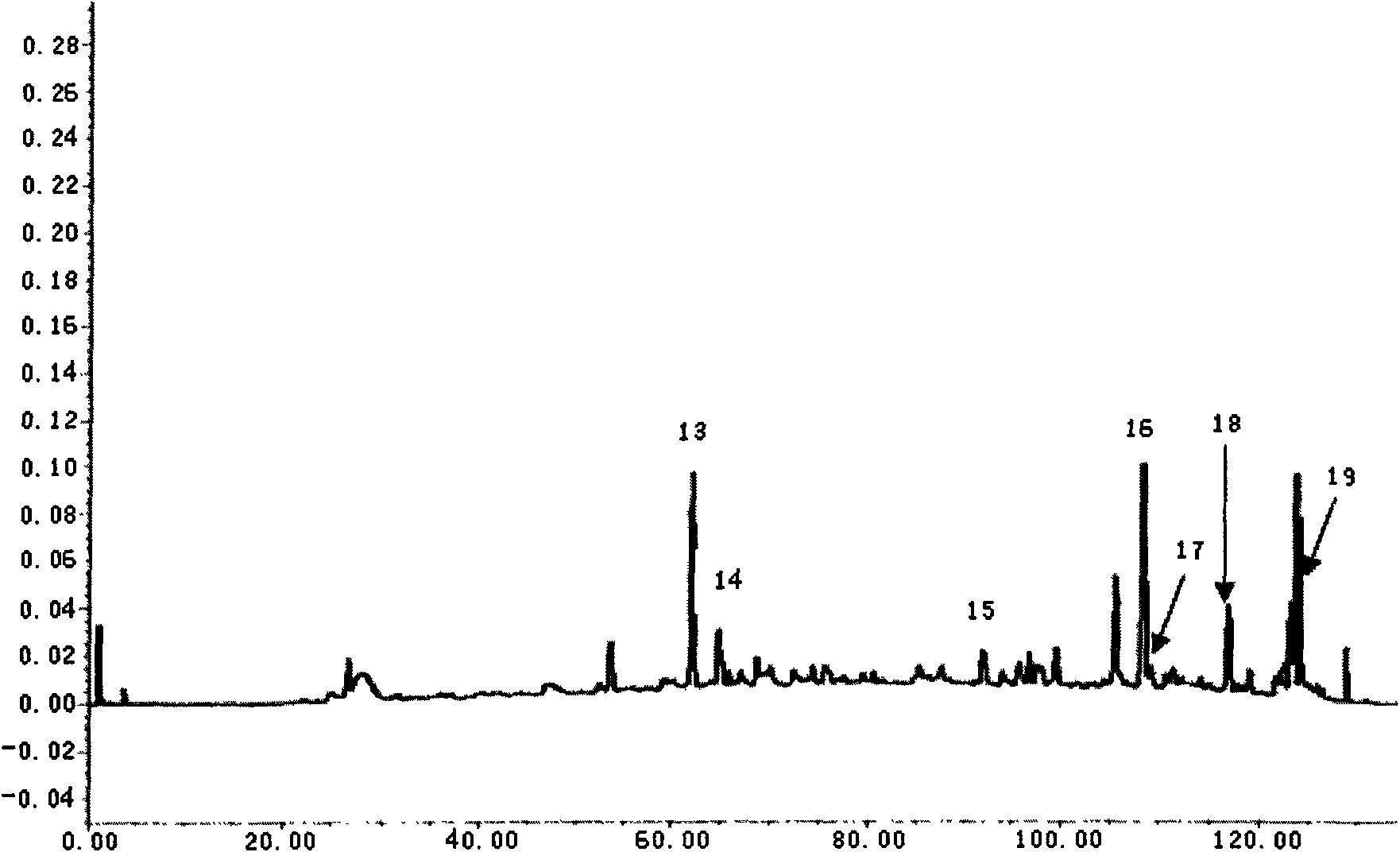
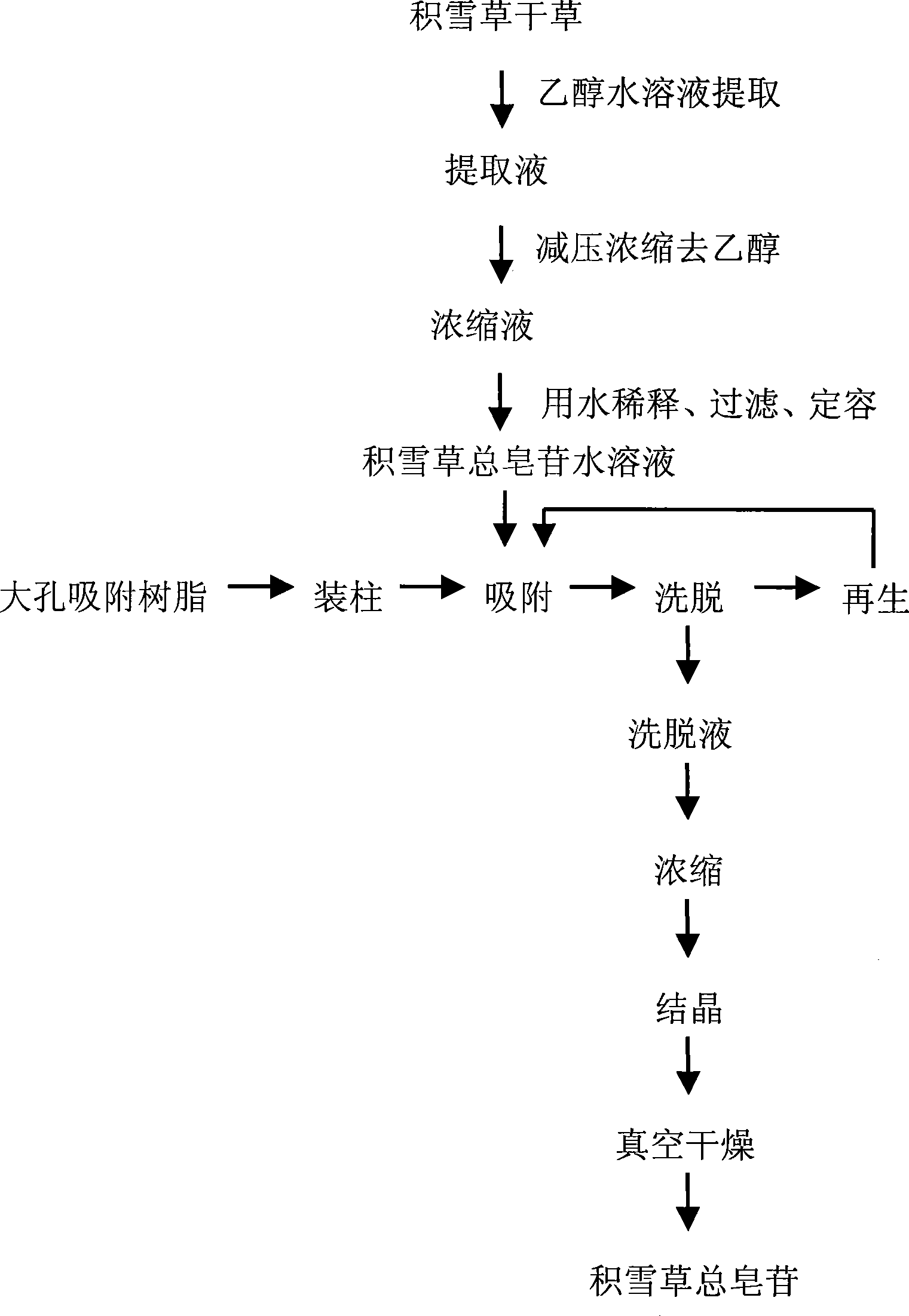
Method for preparing asiatic centella total saponins by using macroporous adsorption resin
InactiveCN101200487ALarge adsorption capacityEasy to desorbSteroids preparationPlant ingredientsAbsorption capacityHigh absorption
The invention discloses a method for utilizing the macroporous adsorption resin to prepare the total saponins of the herba centellae. The operation procedures of the method is carried out as follows, 1) aqueous ethanol solution is added into the dry herba centellae for immersion and extraction, the extracting solution is mixed and removed ethanol by the vacuum concentraction, and the concentrated solution is diluted by water and filtered to obtain the aqueous total saponins of the herba centellae solution. 2), the pretreated macroporous adsorption resin is filled into the chromatography column. 3), the aqueous total saponins solution is introduced to flow through the resin column till penetration of the total saponins in the effluent. 4), the resin column is washed by water and eluted by the organic solvent-water mixture, the eluent is mixed after the thin layer chromatographic analysis, then is concentrated, crystallized and vacuum dried to obtain the total saponins product. 5), the resin column is washed and regenerated by the organic solvent-water mixture and water for reuse. The invention has the advantages of the higher absorption capacity of the total saponins of the herba centellae by the macroporous adsorption resin, easy desorption, higher recovery rate and higher contents of the total saponins of the herba centellae, simple method, strong operability, higher yield of the total saponins of the herba centellae and lower cost.
Owner:ZHEJIANG UNIV
Process for extracting indoline amide alkaloid from purslane and detection methods for indoline amide alkaloid
ActiveCN102973619ASimple preparation processQualitative detection method is simple and easyNervous disorderComponent separationReflux extractionSephadex
The invention discloses a process for extracting indoline amide alkaloid from purslane. The process comprises the following steps: (1) taking the purslane and carrying out reflux extraction by ethanol; (2) adding an extracting solution onto macroporous resin, eluting by ethanol, decompressing and concentrating; (3) adding a concentrated solution onto a polyamide column, eluting by ammonia water, decompressing, concentrating and drying; and (4) dissolving ammonia water eluate by a solvent, centrifuging, filtering, adding a filtered solution onto sephadex LH-20, eluting by the ethanol, collecting a yellow stripe part, decompressing, concentrating and drying to obtain an indoline amide alkaloid extract, wherein the yield of the extract is 2 mg / g in medicinal materials and the extract mainly contains purslane amides A and B. The invention further discloses a thin-layer chromatography detection method and an HPLC (high performance liquid chromatography) detection method for the indoline amide alkaloid extract.
Owner:广州壹木新材料科技有限公司
Thin layer chromatography quantitative analysis method based on image processing technology
InactiveCN1766607AComponent separationCharacter and pattern recognitionThin-layer chromatographyDistortion correction
The invention belongs to digital image processing and chemical analyzing domain, which is characterized in that it uses digital camera or video camera to sight the lathe on the light condition with different wavelength; it dose lens distortion correction and noise reduction process to the image; it uses the character of point sampling time point equidistribution and the rule of vertical distance five equilibrium to ascertain the control point; it uses bracketing method to construct image background by the control point; it exorcizes the background image from the adjusted thin layer image and dose brightness normalization to each image element point of the constructed background image; it divides the thin layer image and dose monomer to the grey of each area by the dividing result.
Owner:TSINGHUA UNIV
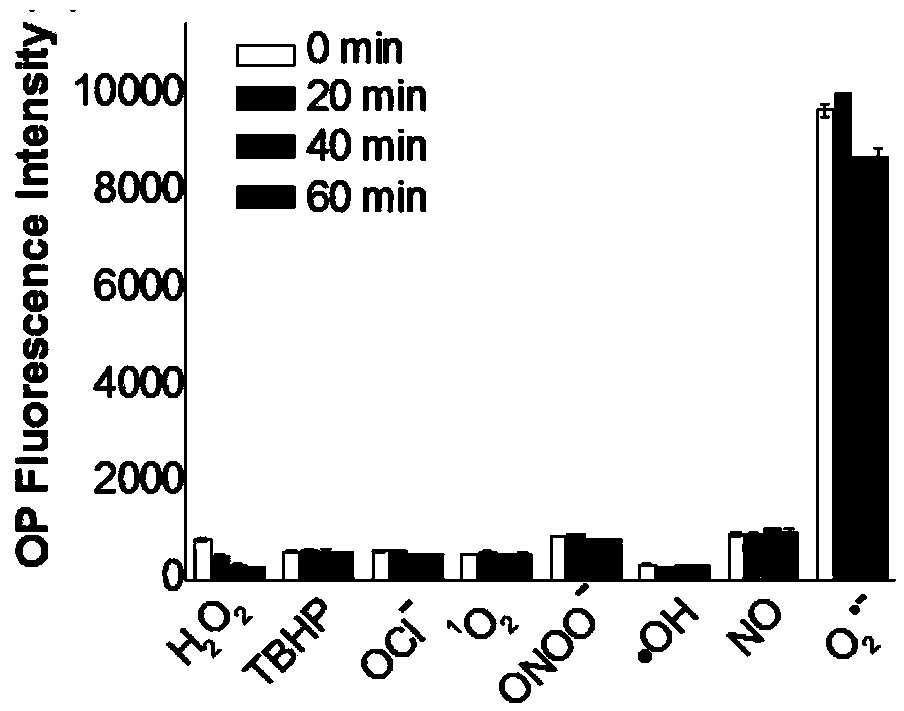
Fluorescent probe and preparation method thereof, and application in detecting superoxide anions
InactiveCN104371707AGood chemical stabilityShort response timeOrganic chemistryFluorescence/phosphorescenceN dimethylformamideSuperoxide
The invention discloses a fluorescent probe and a preparation method thereof, and application in detecting superoxide anions. The structural formula of the fluorescent probe is disclosed in the specification. The preparation method comprises the following steps: in an inert gas protective atmosphere, dissolving melamine and caffeic acid in a DMF (N,N-dimethylformamide) and CH2Cl2 mixed solution, adding HOBT (N-hydroxybenzotriazole) and EDC.HCL (1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride), and stirring to react at room temperature for 8-16 hours; after the reaction, concentrating to remove the solvent, thereby obtaining the fluorescent probe crude product; and separating and purifying the fluorescent probe crude product with a silica gel thin-layer chromatographic sheet to obtain the fluorescent probe pure product. The fluorescent probe has the advantages of novel structure, high sensitivity, high selectivity and high light stability.
Owner:SHANDONG NORMAL UNIV
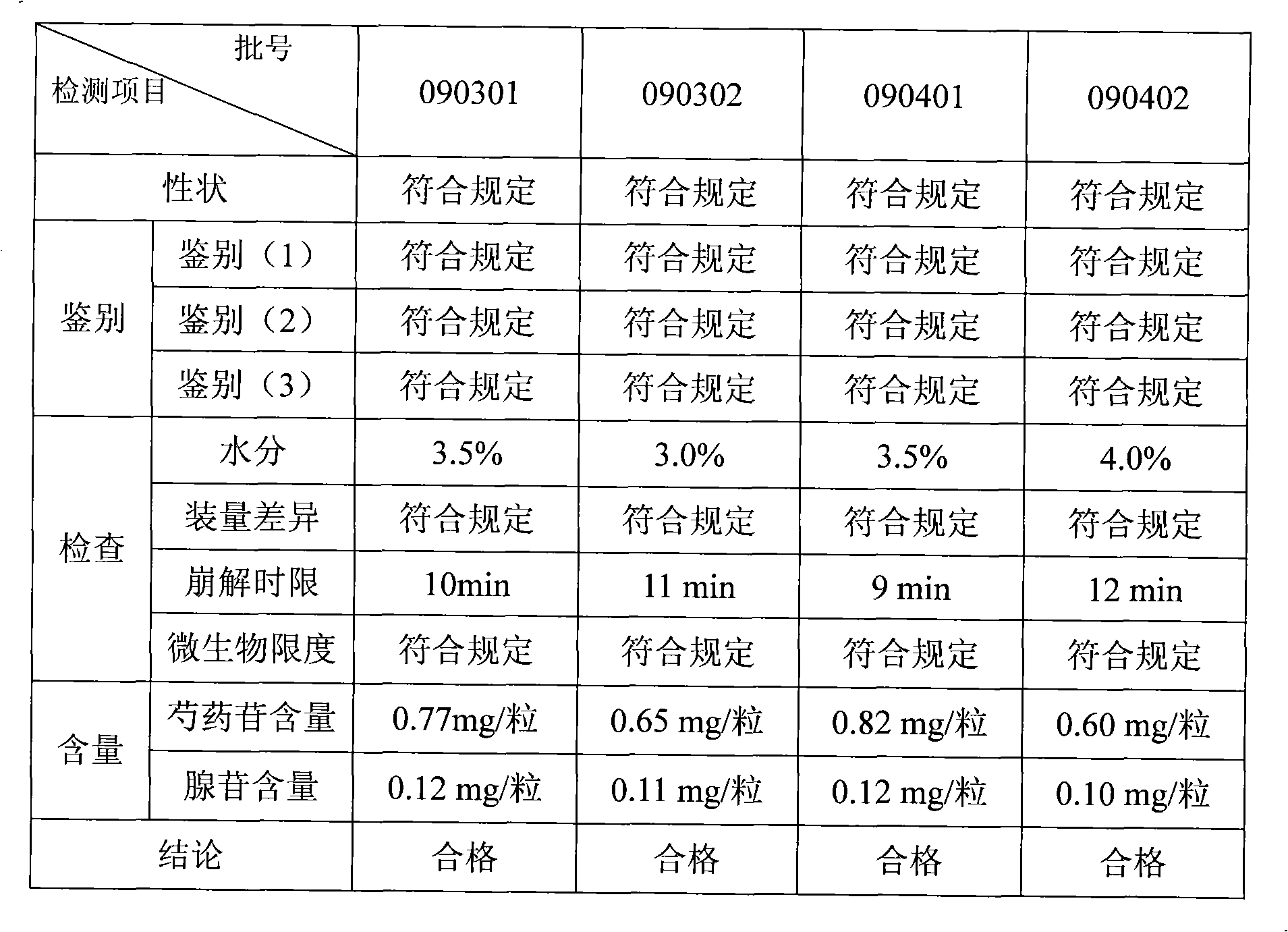
Quality detection method for houtou jianweiling Chinese medicament
The invention discloses a quality detection method for a houtou jianweiling Chinese medicament, which comprises the following steps: performing thin layer chromatography identification on rhizoma corydalis; and measuring that the content of paeoniflorin in radix paeoniae alba is not less than 0.40 mg by a high performance liquid chromatography. The method also relates to the following detection steps: a, identifying the thin layer chromatography of rhizoma cyperi; b, identifying the thin layer chromatography of the radix paeoniae alba and liquorice; and c, measuring that the content of adenosine C10H13N5O4 is not less than 0.070 mg by the high performance liquid chromatography. The method can effectively improve the quality standard of the houtou jianweiling Chinese medicament; and the detection method can come up with new related detection indexes, adds detection indexes, strictly controls the product quality, and ensures the efficacies.
Owner:HUNAN XINHUI PHARMA
Method for enriching and purifying veralkcohol from peanut root by macporous adsorptive resin
InactiveCN101074188AImprove adsorption capacityHigh adsorption selectivityOrganic chemistryOrganic compound preparationThin layer chromatographicSolvent
A method for enriching and purifying resveratrol from peanut root by macro-porous adsorptive resin is carried out by crushing dried peanut root, extracting by organic solvent, extraction filtering for extract, recovering solvent to obtain first extract, heating by water, dissolving, adding into acetic ether, extracting, regulating pH value to 7.0-11.0, laying aside, extraction filtering, decompressing, concentrating to obtain resveratrol coarse extract, adsorbing by macro-porous adsorptive resin, eluting by alcohol, tracking while inspecting by thin-layer chromatographic method, concentrating while recovering alcohol to obtain half-finished product with content above 35%, coating silica gel by chromatographic column, gradient eluting by different proportion acetic ether and chloroform, tracking while inspecting by thin-layer chromatographic method, de-coloring by active carbon and re-crystallizing to obtain final product with content above 98%. It's fast, cheap and convenient, has high purity and various raw materials.
Owner:河南省农科院农副产品加工研究所
Method for controlling quality of Longdanxiegan Capsule
ActiveCN101537088AScientific and perfect quality controlQuality improvementComponent separationDigestive systemAdjuvantGentiakochianin
The invention discloses a method for controlling the quality of the Longdanxiegan Capsule. The invention adopts the combined methods of character and thin layer chromatography discrimination and high efficiency liquid chromatography content measurement so as to control the quality of the Longdanxiegan Capsule, wherein during the operation of thin layer chromatography discrimination, characteristic spots of bupleurum, radix scutellariae, scutelloside, ferulic acid, glycyrrhiza and ammonium glycyrrhetate are respectively detected, the content of gentiamarin and geniposide is detected simultaneously by the high efficiency liquid chromatography under the condition of identical chromatogram through the mode of gradient elution, the content of the gentianella of each capsule is more than 0.07 mg according to gentiamarin, and the content of the gardenia of each capsule is more than 1.0 mg according to geniposide. The method respectively carries out the operation of thin layer chromatography discrimination on the characteristic components of the Longdanxiegan Capsule, can scientifically and comprehensively reflect the existence of monarch drugs, ministerial drugs, adjuvant and conductant drugs in the Longdanxiegan Capsule, simultaneously detects the content of the characteristic components of the gentianella and the gardenia; moreover, the method is simple, scientific and easy to operate, and is conducive to the comprehensive quality control of the Longdanxiegan Capsule.
Owner:成都尚科药业有限公司
Polypeptide of ascidicea and preparation thereof
Ascidian polypeptide and its production are disclosed. The procedure is carried out by immersion depressing at normal temperature by alcohol, recovering alcohol to obtain extract, extracting by organic solvent, concentrating, separation purifying by macroporous resin chromatography, silica-gel chromatography and Sephadex LH20 chromatography, gradient eluting by eluent in proportion of CHCl3: MeOH: H2O=7:3:0.5, and collecting concentrated eluent with thin-layer chromatographic ultraviolet verification Rf=0.5~0.6. The molecular weight of ascidian polypeptide is 711.4. It is simple, has less investment, convenient control for production and various raw materials.
Owner:SOUTHERN MEDICAL UNIVERSITY
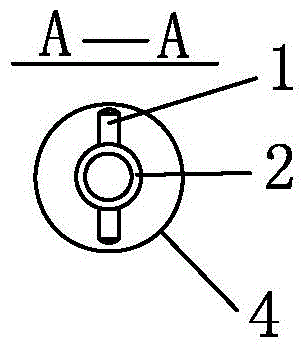
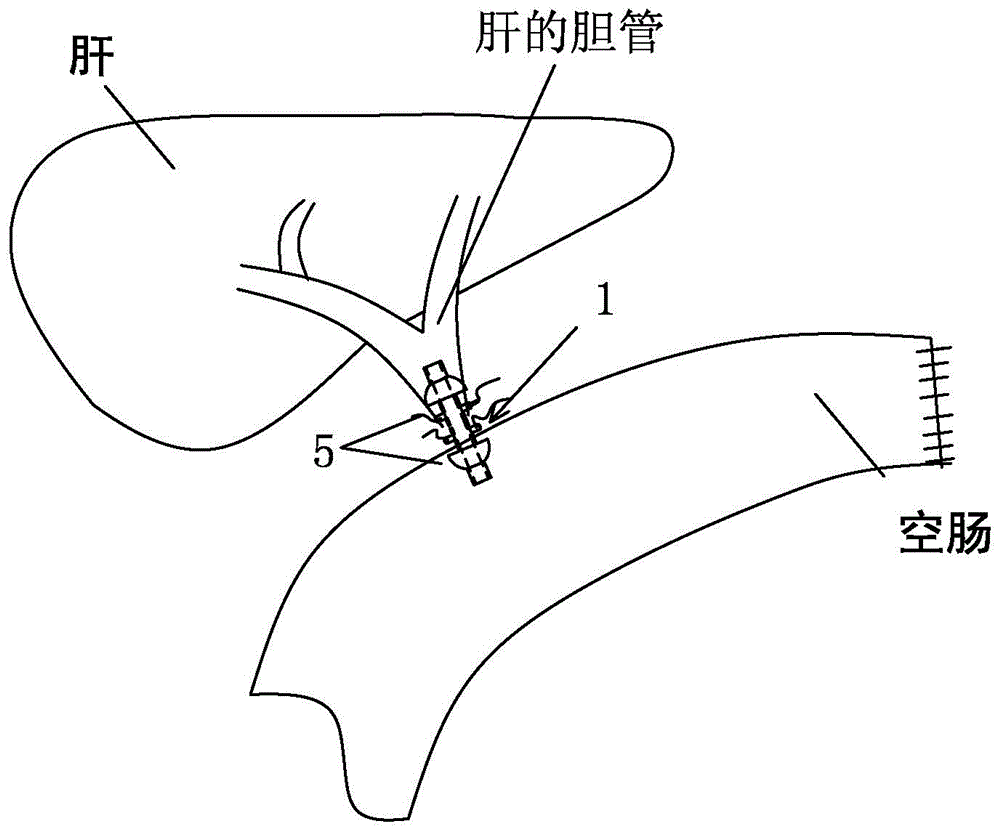
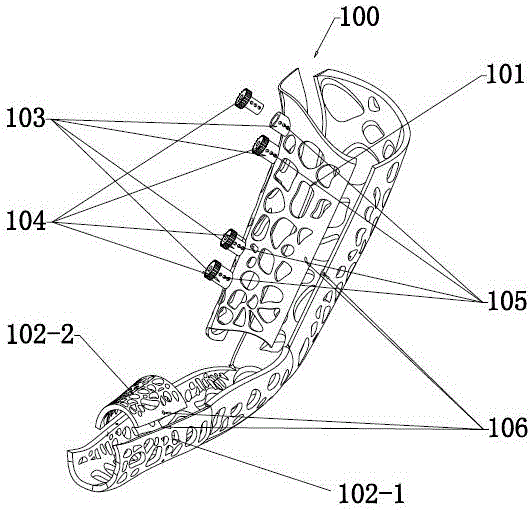
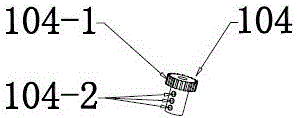
Suture-free bilioenteric anastomosis stent manufactured through 3D (three-dimensional) printing and manufacturing method thereof
InactiveCN104873240AAvoid direct actionAdequate blood supplySurgeryThin layer chromatographicBilioenteric anastomosis
The invention provides a suture-free bilioenteric anastomosis stent manufactured through 3D (three-dimensional) printing. The bilioenteric anastomosis stent comprises a stent pipe and bulging columns bulging out of the outer wall of the stent pipe, and bulging heads are integrally connected at two ends of the stent pipe. The bilioenteric anastomosis stent is made from a degradable biological material through a 3D printing technique. A manufacturing method comprises steps as follows: (1), the diameter of the bile duct of a patient requiring gallbladder-pancreas reconstructive surgery is tested through CT (computed tomography); (2), required 3D printing data are acquired through algorithm conversion and image processing according to a bile duct CT thin-layer chromatographic scanning result, and the bulging columns and the bulging heads are designed on fit contact surfaces; (3), a 3D printer which is most precise currently is used, the biocompatible degradable material is used, the 3D bilioenteric anastomosis stent is printed and manufactured, and the bilioenteric anastomosis stent is applied to suture-free bilioenteric anastomosis. The suture-free bilioenteric anastomosis stent has the benefits of simple structure, convenience in use, remarkable effect, capability of realizing suture-free bilioenteric anastomosis, the risks of anastomotic leakage and stenosis are reduced, and the operation difficulty and risks are greatly reduced.
Owner:洪德飞 +2
Quality control method for rhizoma gastrodiae capsule
ActiveCN104306745AToxicEasy to emulsifySenses disorderNervous disorderMedicineThin layer chromatographic
The invention relates to a quality control method for a rhizoma gastrodiae capsule and belongs to the technical field of biomedicine. The quality control method comprises the steps of raw material control, preparation method control, an identification method and a content measurement method. Modern drug production management is integrated in quality control. The quality of drugs and solvents used in production is examined from the source. Product quality control in the production process is established, perfect product quality standards are established, the thin-layer chromatographic detection ratio is higher than national requirements, the gastrodin content is greatly increased, the increase degree reaches 28.3%, and the quality and the therapeutic effect of the drug product are ensured.
Owner:YUNNAN YONGZITANG PHARMA
Intelligent individual exoskeleton device for fracture treatment
ActiveCN106420022AReduce difficultyShorten operation timeAdditive manufacturing apparatusExternal osteosynthesisPersonalizationBiomechanics
The invention relates to an intelligent individual exoskeleton device for facture treatment. The intelligent individual exoskeleton device is characterized by comprising an intelligent individual exoskeleton and an installation assisting device. The installation assisting device comprises a steel needle with scale marks, a skeleton reset mechanism and a reference fixing plate. The steel needle with scale marks and the skeleton rest mechanism are installed at a focus position corresponding to the intelligent individual exoskeleton in a matched mode through the reference fixing plate. The device is designed by reserving advantages of internal and external fixation and overcoming defects of internal and external fixation. The exoskeleton is obtained through 3D printing; according to the design of the exoskeleton, a series of technologies of precision needle setting, CT thin-layer chromatographic scanning, computer virtual resetting, biomechanics design and the like are included, and the intelligent individual customized solution for fracture treatment is obtained. The purposes of minimal invasion, precision resetting, firmness, fixation and early recovery of limbs can be achieved.
Owner:杭州首时科技有限公司
Quality detection method for chinarue cough particle
ActiveCN102133363AImprove quality inspection standardsEasy to operateComponent separationRespiratory disorderAsparagus cochinchinensisLaggera pterodonta
The invention relates to a quality detection method for chinarue cough particles. In the formula, the chinarue cough particles include following materials: 156g of pericarpium citri reticulatae, 96g of rhizoma acori graminei, 312g of polygonum cuspidatum, 188g of asparagus cochinchinensis, 375g of chinarue, 96g of radix stemonae, 750g of marsdenia tenacissima, 375g of laggera pterodonta, 96g of semen armeniacae amarae, 375g of houttuynia cordata and 188g of cortex mori radicis, and the materials are prepared into particles. On the basis of the original standard, the thin-layer chromatography identification of the chrinarue is added, and the chinaure is one of main ingredients. After the thin-layer indentification of the chinaure is added on the basis of the original standard, the scientific basis is provided for qualitative control of the chinaure in the preparation, the quality detection standard of the chinaure cough particles is perfected, and the scientific basis is provided for the judgment of true and false and quality control of the product. In the method, the test and the operation of samples are simple and convenient, and by the continuous verification test of negative interference and the like, free interfered negativeness, good repeatability, and strong specificity are verified and tested. The quality test standard is perfected, and the product quality control capability is promoted.
Owner:唐秋海
Quality control method for mulberry chrysanthemum granules
ActiveCN101862373AGuaranteed validityPrevent medicineComponent separationAntiinfectivesQuality controlPrunella vulgaris
The invention discloses a quality control method for mulberry chrysanthemum granules. The method comprises a step of measuring rosmarinic acid characteristic component content of self-heal by adopting high performance liquid chromatography. The established thin-layer chromatography and high performance liquid chromatography method is simple and convenient and has good practicability; an established fingerprint map for the mulberry chrysanthemum granules is favorable for comprehensively grasping the quality of the mulberry chrysanthemum granules; and meanwhile, the method identifies the main chromatographic peak, makes up the fuzziness of the fingerprint map of the mulberry chrysanthemum granules, jointly forms a quality standard system of the mulberry chrysanthemum granules, and fulfills the purpose of effectively controlling the quality of the mulberry chrysanthemum granules.
Owner:GUANGZHOU XINGQUN PHARMA
Method for detecting quality of bezoar snake bile bulbus fritilariae liquid
The invention discloses a method for detecting the quality of bezoar snake bile bulbus fritilariae liquid which consists of the following raw medicament components in part by weight: 1.0 to 2.0 parts of artificial bezoar, 40 to 60 parts of unibract fritillary bulb, 6.0 to 10 parts of snake bile and 0.03 to 0.05 part of menthol. The method comprises the following steps of chemical identification of the unibract fritillary bulb, thin layer chromatography identification of the artificial bezoar, thin layer chromatography identification of the snake bile, and efficient liquid-phase method content determination of bile acid. The invention also discloses a preparation process and preferential raw material components of the bezoar snake bile bulbus fritilariae liquid. The method for detecting the quality can reflect the preparation quality of the bezoar snake bile bulbus fritilariae liquid from a plurality of aspects so as to ensure the curative effect and the medication safety.
Owner:江西南昌桑海制药有限责任公司
Separation method of swainsonine-producing endophytic fungi in glabrous crazyweed
A separation method of swainsonine-producing endophytic fungi in glabrous crazyweed comprises the following steps of: acquiring mycelium of the strain and a broth through a bio-fermentation method, carrying out ultrasonic extraction, quantitatively and qualitatively detecting swainsonine by thin-layer chromatography and gas chromatography technologies after solvent extraction, and identifying swainsonine-producing endophytic fungi through morphological and molecular biology methods. The result shows that one out of six separated strains of endophytic fungi can generate swainsonine and the output is 16.7338 mg / L. After identification, the strain is fusarium proliferatum. In comparison with present strains for the production of swainsonine, swainsonine provided by the invention has high output. The bio-fermentation method for the production of swainsonine doesn't influence the meadow ecological environment. The separation method provided by the invention solves the problem of swainsonine sources and provides strain supports, thus establishing a theoretical foundation for future industrial production of swainsonine. The invention has a wide development prospect.
Owner:鄂尔多斯市普众生物技术有限公司
Method for detecting medicine for treating irritable bowel syndrome
ActiveCN104306500AEffective quality controlHigh sensitivityComponent separationDigestive systemThin layer chromatographicLicorice roots
The invention discloses a method for detecting a medicinal oral preparation for treating irritable bowel syndrome. The medicinal oral preparation is mainly prepared from radix bupleuri, fried radix paeoniae alba, roasted rhizoma atractylodis macrocephalae, saposhnicovia divaricata, roasted fructus aurantii immaturus, roasted pericarpium citri reticulatae, fructus mume, coptis chinensis and honey-fried licorice root, as well as auxiliaries. The detection method comprises the following steps: carrying out thin-layer chromatography identification on roasted rhizoma atractylodis macrocephalae, roasted pericarpium citri reticulatae, roasted fructus aurantii immaturus, fructus mume and honey-fried licorice root in the oral preparation and detecting the content of paeoniflorin and berberine hydrochloride. The detection method is accurate and has high sensitivity, good repeatability and stable detection result, the quality of the medicinal oral preparation for treating irritable bowel syndrome can be effectively detected, and the standard of each detection item can guarantee the curative effect of the medicine.
Owner:叶祖光
Thin-layer chromatography identification method of carbon traditional Chinese medicine formula granules
InactiveCN105241997AMeet the requirements of the rapid inspectionSimple and fast operationComponent separationMedicinal herbsBenzoic acid
The invention discloses a thin-layer chromatography identification method of carbon traditional Chinese medicine formula granules. The method comprises the steps that 1, sample powder is taken and dissolved through water, hydrochloric acid is added, heating is conducted for hydrolysis, filtration is conducted, filtrate is extracted through ethyl acetate or ethyl ether, extract liquor is evaporated to dryness, and residues are dissolved through methyl alcohol to serve as a test sample solution; 2, reference medicinal materials are taken, decocted through water and filtered, filtrate is concentrated, and then a reference medicinal material solution is prepared through the method for preparing a test sample; 3, the test sample solution and the reference medicinal material solution are sucked and dripped on a same silica gel GF254 thin-layer plate, and developing is conducted by taking a mixture of methylbenzene, one or more of ethyl formate or ethyl acetate or butyl acetate and formic acid as developing solvents according to the volume ratio of 2-8 to 2-8 to 0.1-1; 4, the thin-layer plate is viewed under a 254 nm ultraviolet lamp, and principal spots with the same color are showed on the positions, corresponding to the chromatography of the reference medicinal materials, in the test sample chromatography. The method is rapid, comprehensive and high in specificity, and the requirements of carbonized herbs and the carbonizing drug characteristic of the formula granules thereof can be embodied.
Owner:GUANGDONG YIFANG PHARMA
Quality control method of Gongyankang grannule
The invention discloses a method for controlling the quality of the Gongyankang granules, revises and enlarges the thin layer chromatography (TLC) identification of bupleurum, semen plantaginis and baked ginger, purifies the method for extracting the sample of the content determination and the TLC identification of paeoniflorin which is a characteristic component of red paeony root, and revises and enlarges the method for examining the microorganism as well. The method for preparing the sample solution of the TLC identification of semen plantaginis and red paeony root in the quality control method is consistent with that of bupleurum, while the method for preparing the sample solution of the TLC identification of rhizoma corydalis is consistent with that of baked ginger, therefore, the quality control method has the advantages of more comprehensive detection items, higher resolution of content determination method, clearer TLC identification of red paeony root, more scientific and more reasonable quality control index, more simplified operation and further confirmed medicament curative effect.
Owner:广西厚德药业有限公司
Manufacturing method of individual hip joint percutaneous puncture guide plate
InactiveCN105796160AReduce radiation doseAccurate guidanceAdditive manufacturing apparatusSurgical needlesSkin surfaceModeling software
The invention discloses a manufacturing method of an individual hip joint percutaneous puncture guide plate. The manufacturing method comprises the following steps that 1, a three-dimensional geometric model is built, wherein thin-layer chromatographic scanning data of the hip joint of a patient is obtained, and three-dimensional models of skeleton, soft tissue, skin and important blood vessels are built and exported and stored in a stl format; 2, a puncture channel is determined, wherein in modeling software, a puncture target point and the needle entering direction and angle are determined, the puncturing needle form is extracted, a puncture channel is formed through fitting, and then the puncture channel is reversely prolonged to puncture out of skin to form a body surface marker to be exported and stored in an stl format; 3, the guide plate is designed, wherein in software, mesh optimization is carried out on the three-dimensional models of skeleton, soft tissue, skin and important blood vessels, a skin surface mesh is extracted, reverse offset thickening is performed to form a guide plate panel, a bone mark is marked, and the guide plate panel is in fit with the reverse puncture channel to obtain a guide plate model; 4, a guide plate entity is obtained through 3D printing. The manufacturing method has the advantages of being individual, accurate, rapid, low in cost and efficient.
Owner:GUIZHOU NORMAL UNIVERSITY +1
Arctigenin amino-acid ester derivatives, and preparation method and application thereof
ActiveCN105541765ASignificant neuroprotective effectReasonable designOrganic active ingredientsNervous disorderChemical structureSilica gel
The invention belongs to the technical field of drug synthesis, and particularly relates to novel arctigenin amino-acid ester derivatives, and a preparation method and application thereof. The structure of the arctigenin amino-acid ester derivatives is disclosed as General Formula I. The preparation method comprises the following steps: dissolving amino acids and arctigenin in a solvent, adding a condensing agent in an ice bath, stirring at room temperature or in a heating state for 1-24 hours, and tracking the reaction by thin-layer chromatography; and after the reaction finishes, evaporating to remove the solvent, and purifying by silica gel column chromatography to obtain the arctigenin amino-acid ester derivatives. The preparation method is reasonable in design and easy for synthesis. The synthesized compounds have a novel chemical structure; the in-vitro cell activity experiment proves that the most synthesized amino-acid ester derivatives have an obvious neural protection action; and the compounds I-2, I-5 and I-6 have higher activity than arctigenin, and thus, provide new therapeutic drugs for preventing and treating parkinsonism and other neurodegenerative diseases.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Flavonoid glycoside compound in flos elaeagni angustifoliae, and preparation method thereof
The invention relates to a flavonoid glycoside compound in flos elaeagni angustifoliae, and a preparation method thereof. The method comprises the following steps: taking flos elaeagni angustifoliae medicines as raw materials, extracting by solvent, extracting by solvent, carrying out separation through two or three ways including positive phase and reverse phase silica gel column chromatography,or glucan gel LH-20 column chromatography and a semi-preparation high-performance liquid chromatography, adopting thin-layer chromatography and the high-performance liquid chromatography to detect andanalyze to obtain two new flavonoid glycoside compounds, wherein the first compound is flos elaeagni angustifoliae glycoside A, and the second compound is flos elaeagni angustifoliae glycoside B. Through the measurement of in vitro inhibition of COX (cyclo-oxygense) enzyme activity, an experiment result indicates that the flavonoid glycoside compound separated from the flos elaeagni angustifoliaecan inhibits the COX enzyme activity in different degrees, and the flavonoid glycoside compound in flos elaeagni angustifoliae can be used for preparing anti-inflammatory medicines.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
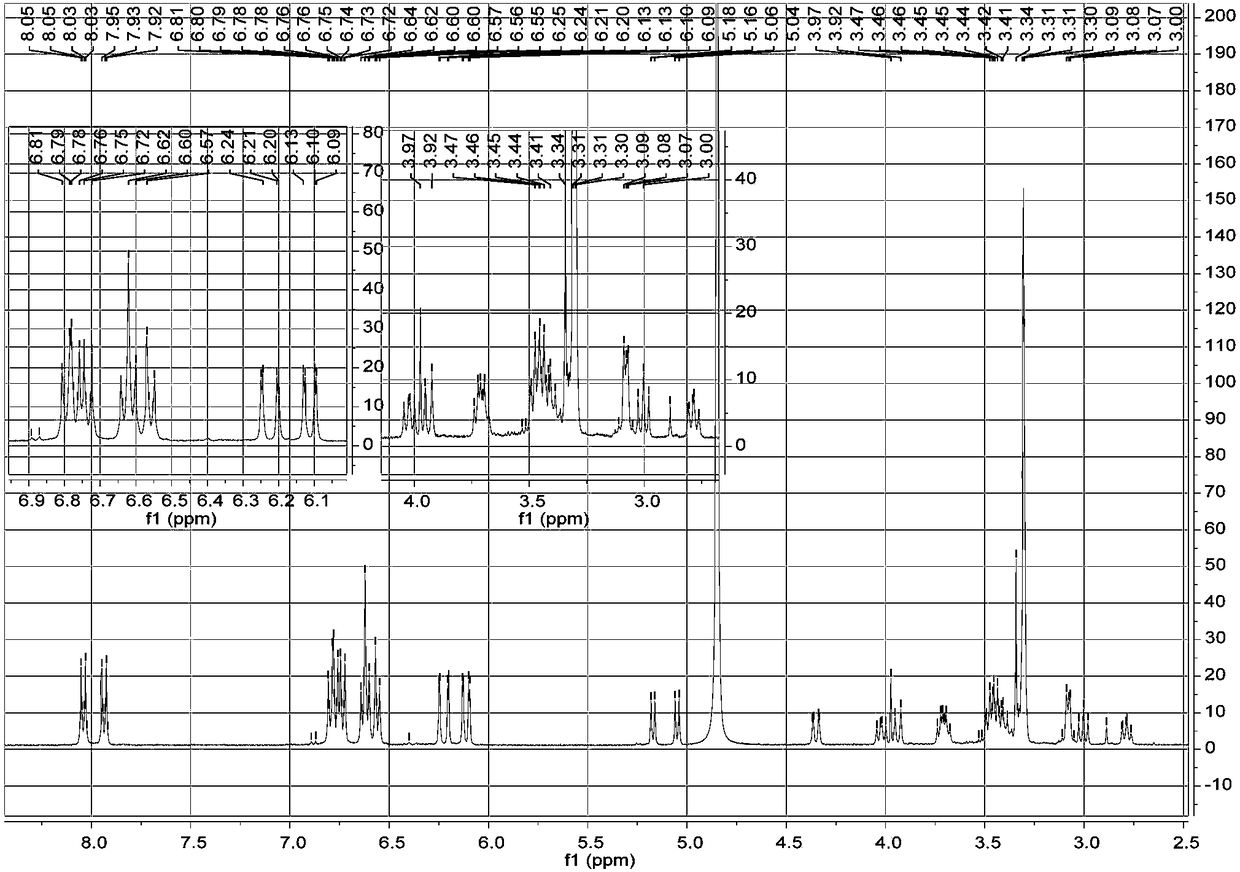
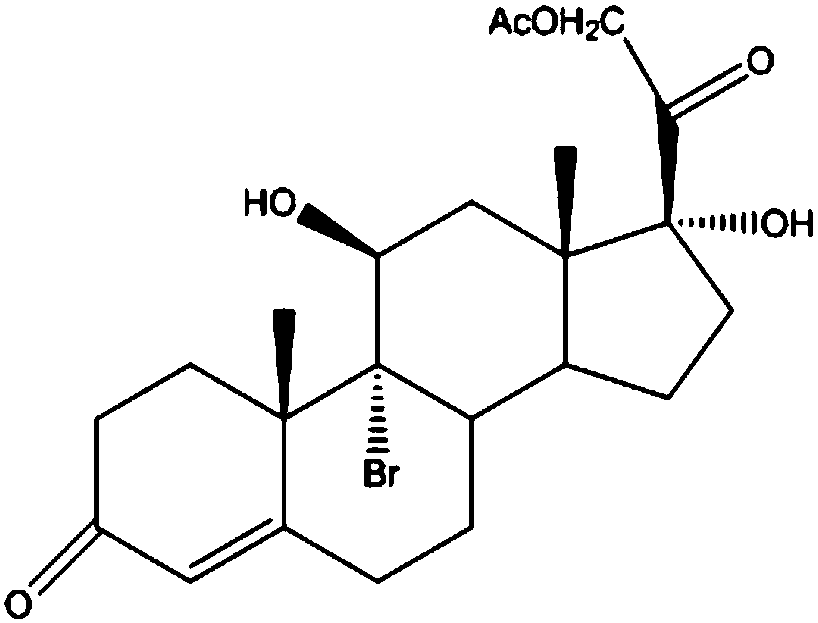
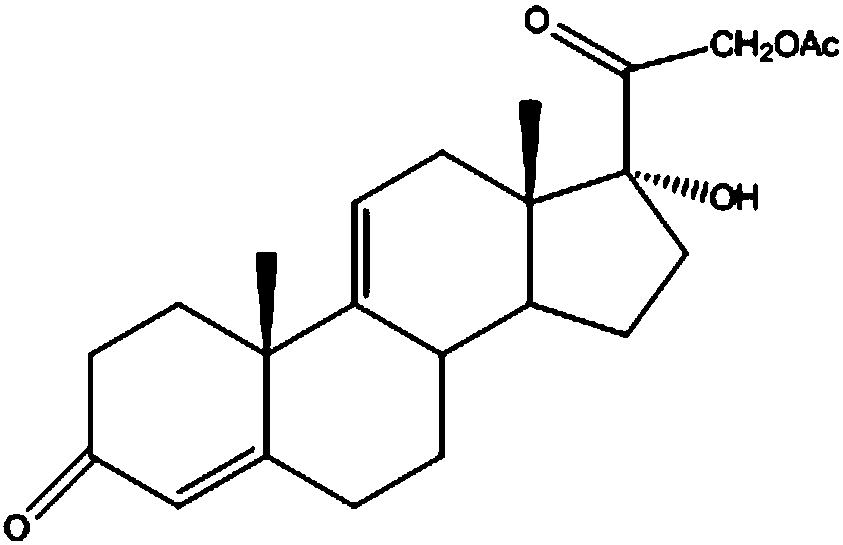
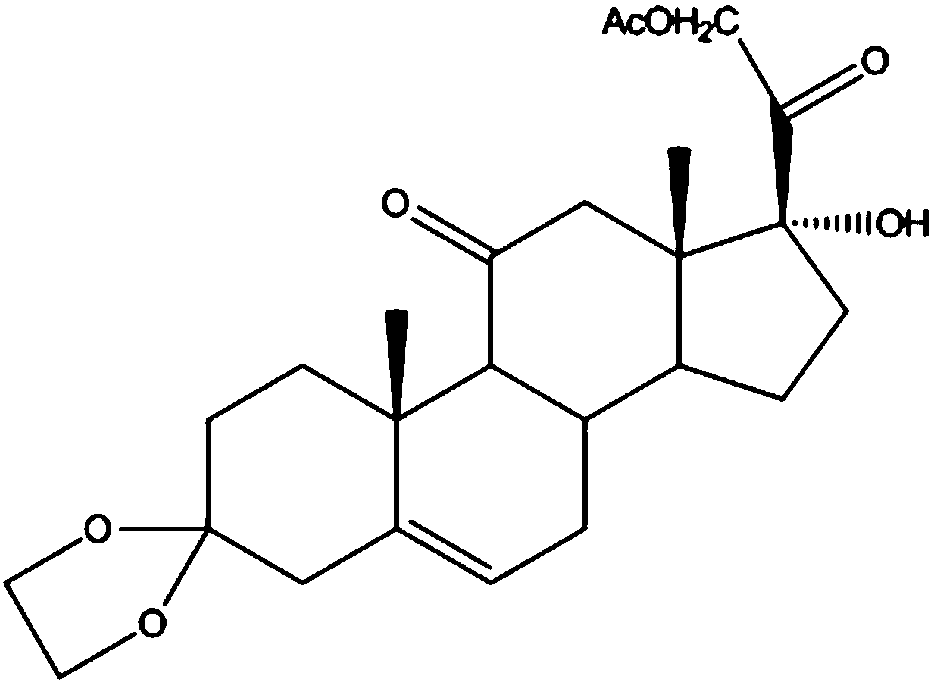
Preparation method of steroid intermediate
The invention discloses a preparation method of a steroid intermediate. The preparation method comprises the following steps: adding a first solvent, a first acid solution, water and 17-alpha-hydroxypregnenolone steroid-4,9-diene-3,20-diketone acetate into a first reaction tank, and uniformly stirring; adding a bromination agent in four times at 2-4 DEG C, maintaining the temperature for reactingfor 2h, and carrying out thin-layer chromatography analysis until the reaction of the raw materials is finished; and regulating the pH value to be more than 7, regulating the pH value back to 6, carrying out reduced pressure concentration until the first solvent is completely concentrated, adding water, cooling to 5 DEG C, and carrying out centrifugation, cleaning, spin-drying and drying, so as toobtain the steroid intermediate, namely cortisone. According to the preparation method, an oxidant and a catalyst which contain heavy metal ions are not used, the used solvent can be recycled and reused, wastewater does not contain heavy metal ions in the production process, the method belongs to an environment-friendly process and has very good process prospects, and the yield and purity of theproduct are high.
Owner:JIANGSU YUANDA XIANLE PHARMA
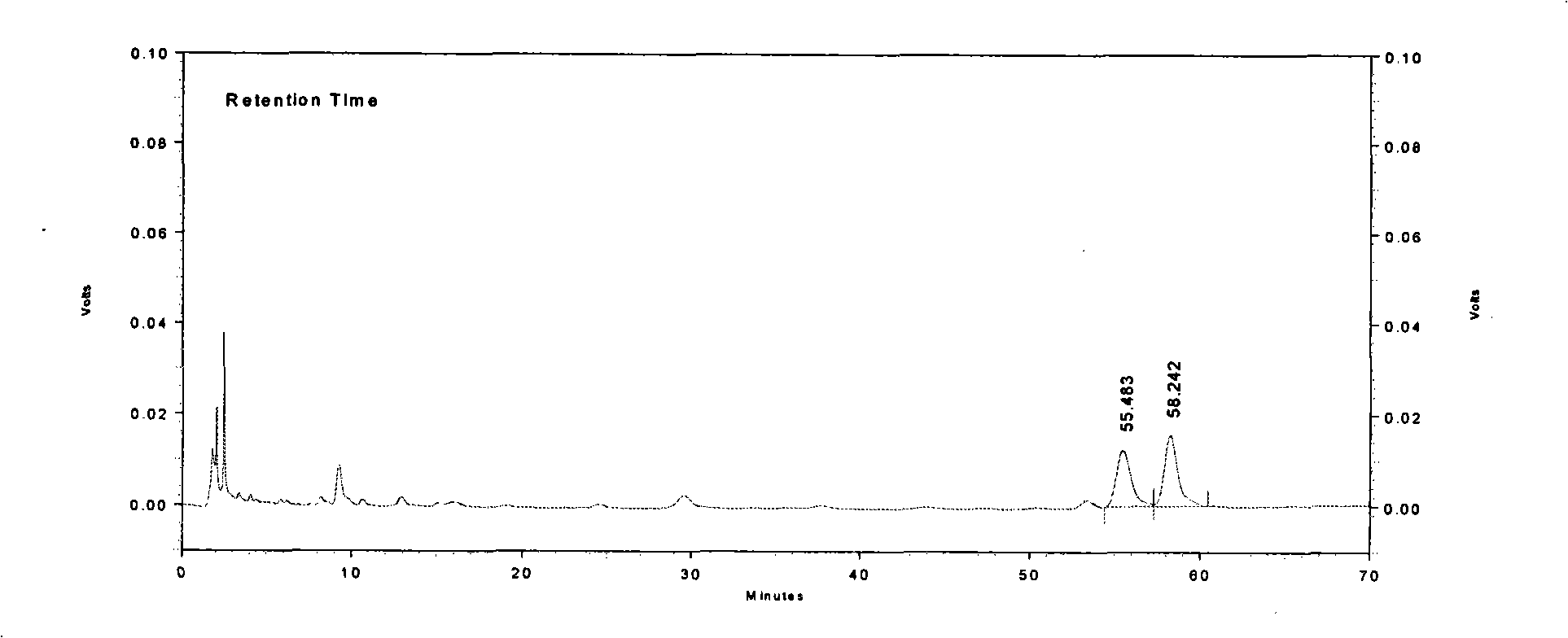
Detection method of Wuweiganlu preparation
ActiveCN102645493AQuality improvementStable quality detection methodComponent separationPreparing sample for investigationPseudoephedrine HydrochlorideJuniperus formosana
The invention provides a detection method of a Wuweiganlu preparation. The method comprises the following steps of: performing microscopic identification of the microscopic characteristics of juniperus formosana and myricaria in the Wuweiganlu preparation; performing thin-layer chromatography identification of rhododendron anthopogonoide, juniperus formosana and artemisia sieversiana in the Wuweiganlu preparation; and measuring the content of ephedrine hydrochloride and pseudoephedrine hydrochloride in ephedra and the content of artemisetin in artemisia sieversiana in the preparation by a high performance liquid chromatography. The detection method provided by the invention has the advantages of good reproducibility and stability, high precision, strong specificity, clear spot color, high separation degree, accurate content and the like, and is simple to operate; and by creating a reliable quality detection method with strong specificity, the quality of the Wuweiganlu preparation can be effectively controlled so that the quality of the Wuweiganlu preparation is stable, safe and controllable.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Preparation for treating hysteritis and its quality control method
A Chinese medicine Gongyanping for treating gynecological inflammations is prepared from 5 Chinese-medicinal materials including shing bugleweed herb, zanthoxylum nitidum, Chinese angelica root, hispid fig root, etc. Its quality control method features that the efficient liquid-phase chromatographic analysis method or UV spectrophotometric method is used for measuring the content of tannin or gallic acid, and the thin-layer method is used to measure the contents of the one or more active components contained in its raw materials.
Owner:GUANGDONG LUOFUSHAN SINOPHARM
Quality control method for liuwei Dihuang soft extract
The present invention is the new quality control method for Rehmanniae ointment of six ingredients. The medicine Rehmanniae ointment of six ingredients is prepared with six kinds of Chinese medicinal materials, including prepared rhizome of rehmannia, dogwood, Chinese yam, tree peony bark, tuckahoe and oriental water plantain. The quality control method includes the high efficiency liquid phase chromatographic process to measure the content of paeoniflorin and the thin-layer chromatographic process to identify dogwood, and the method is accurate and advanced.
Owner:JIANGZHONG PHARMA
Preparation method and use of hemp seed reference extract product
InactiveCN104398580AReduced stabilityImprove stabilityComponent separationPlant ingredientsWater bathsFiltration
preparation method of a hemp seed reference extract product comprises the following steps: 1, carrying out ether degreasing: taking 200g of hemp seed medicinal material powder, sieving by a No.2 sieve, adding 1500ml of ether, carrying out water bath heating refluxing for 1h at a water bath temperature of 50DEG C, carrying out reduced pressure filtration, adding 500ml to medicinal residues, washing, and removing the obtained ether liquid; 2, extracting with methanol to obtain an extract: adding 1500ml to the obtained medicinal residues, carrying out water bath heating refluxing at 70DEG C for 1h for extraction, carrying out reduced pressure filtration, and evaporating to remove methanol to obtain an extract, and weighing; and 3, carrying out silicon gel mixing and reduced pressure drying: re-dissolving the extract obtained in step 2 in 100ml of methanol, adding column chromatography silica gel having a same amount with the extract, uniformly mixing, carrying out reduced pressure evaporation to remove methanol, and sieving the obtained substance by a No.9 sieve in order to obtain the hemp seed reference extract product. The invention also provides a use of the hemp seed reference extract product. The hemp seed reference extract product substitutes a hemp seed reference medicinal material to be used as a reference for thin layer chromatogram discrimination.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com