Flavonoid glycoside compound in flos elaeagni angustifoliae, and preparation method thereof
A technology of middle flavonoid glycosides and anthocyanidin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of seldom research on monomer components of jujube flower.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
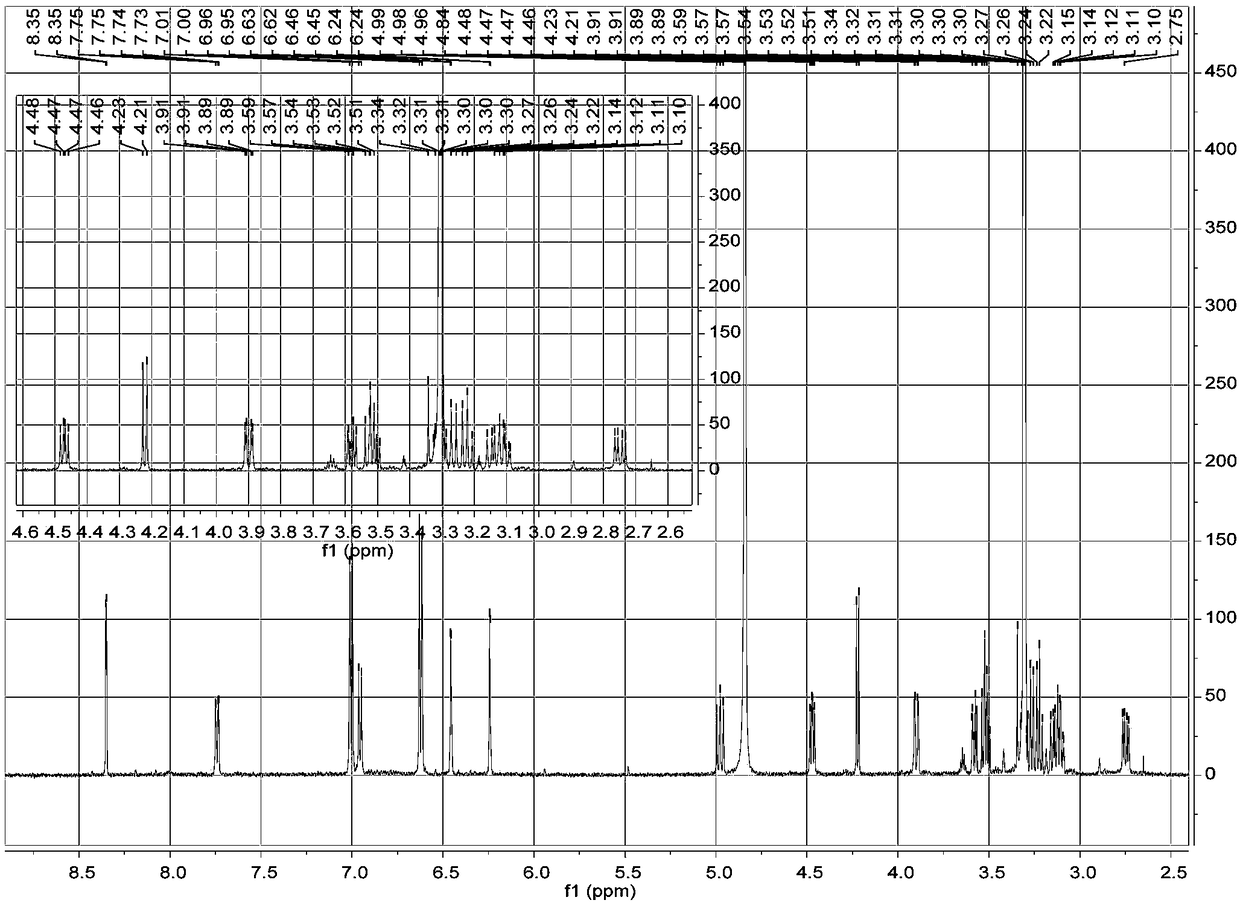
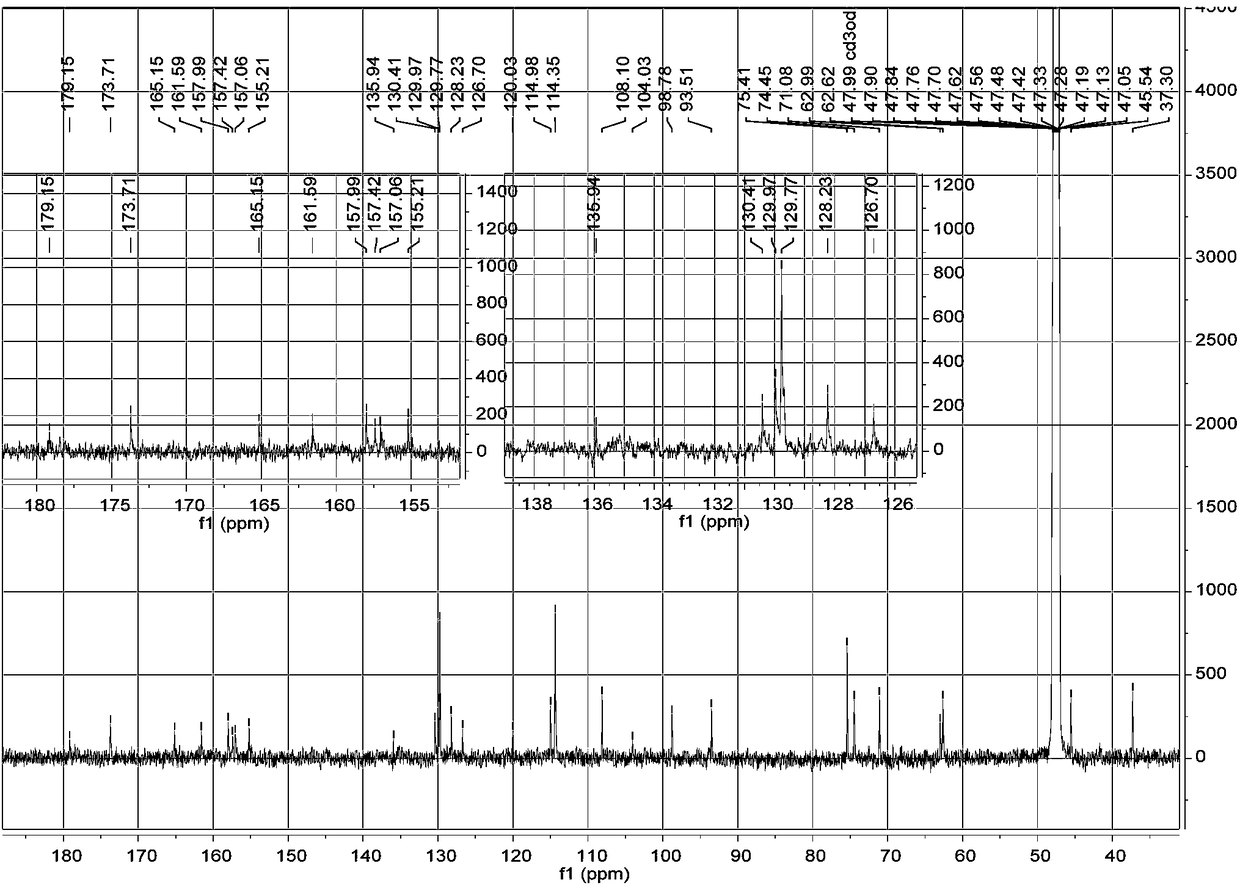
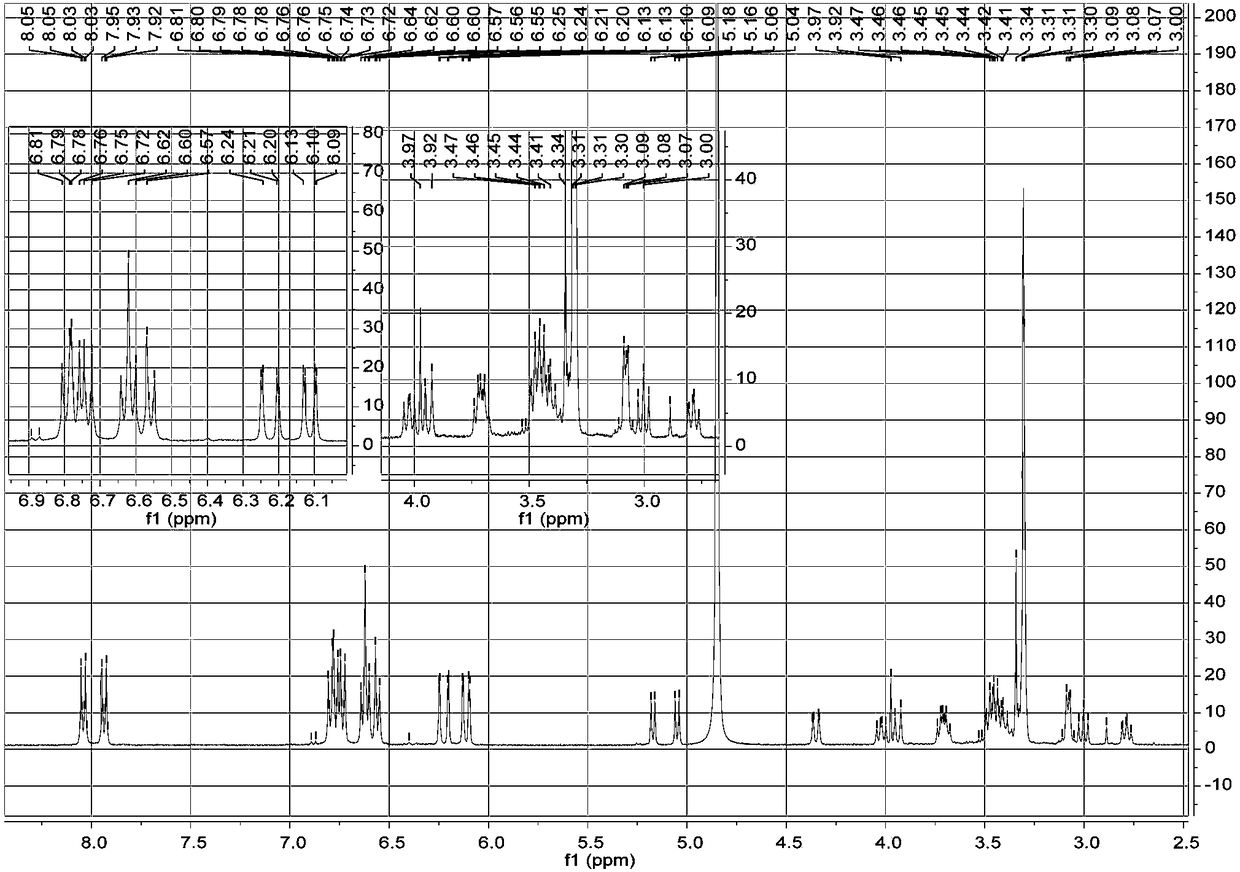
[0023] a. Take 1 kg of dried whole flowers of Elaeagnus eraeae, after pulverization, use 5 times the volume concentration of 95% methanol aqueous solution to extract once at room temperature, combine the filtrates, evaporate to dryness, and obtain the extract;
[0024] b. Disperse the extract obtained in step a with water, extract 3 times with petroleum ether, collect the extract, evaporate the solvent in vacuo to obtain petroleum ether extract extract; use ethyl acetate to extract the remaining water layer Extract 3 times, collect the ethyl acetate extract, combine and dry to obtain the ethyl acetate extract extract; extract the remaining water layer with n-butanol for 3 times, collect the n-butanol extract, combine and dry , to obtain n-butanol extract extract;
[0025] c. The n-butanol extract extract obtained in step b is separated by MCI column chromatography, and gradient elution is carried out with methanol aqueous solution with a volume ratio of 10%-100%, and the volum...
Embodiment 2
[0027] a. Take 1 kg of dried whole flowers of Elaeagnus eraeae, after pulverization, use 5 times the volume concentration of 95% ethanol aqueous solution to extract twice at room temperature, combine the filtrates, evaporate to dryness, and obtain the extract;
[0028] b. Disperse the extract obtained in step a with water, extract 4 times with petroleum ether, collect the extract, evaporate the solvent in vacuo to obtain petroleum ether extract extract; use ethyl acetate to extract the remaining water layer Extract 4 times, collect the ethyl acetate extract, combine and dry to obtain the ethyl acetate extract extract; extract the remaining water layer with n-butanol for 4 times, collect the n-butanol extract, combine and dry , to obtain n-butanol extract extract;
[0029] c. The n-butanol extract extract obtained in step b is separated by MCI column chromatography, and gradient elution is carried out with methanol aqueous solution with a volume ratio of 10%-100%, and the volum...
Embodiment 3
[0031] a. Take 1 kg of dried whole flowers of Elaeagnus eraeae, after pulverization, use 10 times volume concentration of 95% methanol aqueous solution at a temperature of 70°C to extract by heating and refluxing for 3 times, and evaporate the extract to dryness to obtain an extract;
[0032] b. Disperse the extract obtained in step a with water, extract 5 times with petroleum ether, collect the extract, evaporate the solvent in vacuo to obtain petroleum ether extract extract; use ethyl acetate to extract the remaining water layer Extract 5 times, collect the ethyl acetate extract, combine and dry to obtain the ethyl acetate extract extract; extract the remaining water layer with n-butanol for 5 times, collect the n-butanol extract, combine and dry , to obtain n-butanol extract extract;
[0033] c. The n-butanol extract extract obtained in step b is separated by MCI column chromatography, and gradient elution is carried out with methanol aqueous solution with a volume ratio of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com