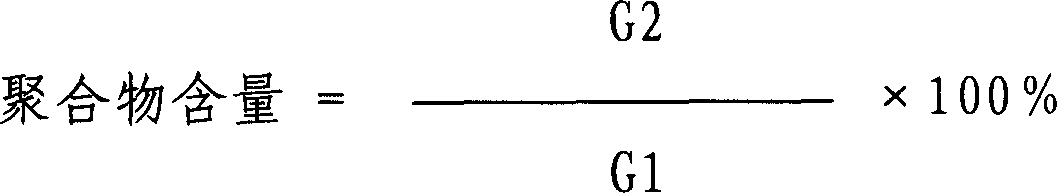Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
186 results about "Butylphthalide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
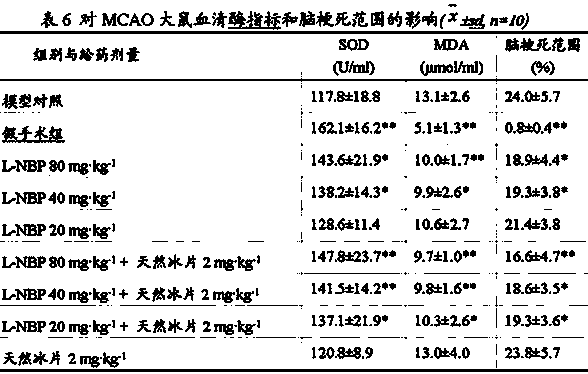
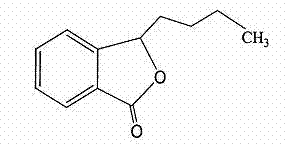
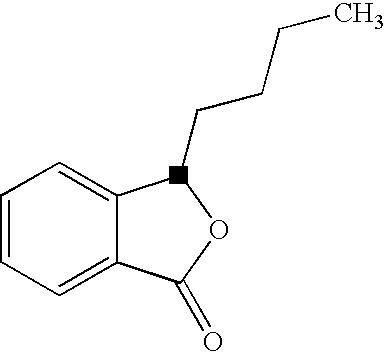
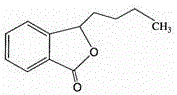
Butylphthalide (3-n-butylphthalide or NBP) is one of the chemical constituents in celery oil, along with sedanolide, which is primarily responsible for the aroma and taste of celery. Studies in animal models suggest that butylphthalide may be useful for the treatment of hypertension and may have neuroprotective effects. In 2002, NBP was approved in China for the treatment of cerebral ischemia.
Preparation method of butylphthalide
InactiveCN101962374AReaction raw materials are readily availableFew reaction stepsOrganic chemistryBenzoic acidGrignard reagent
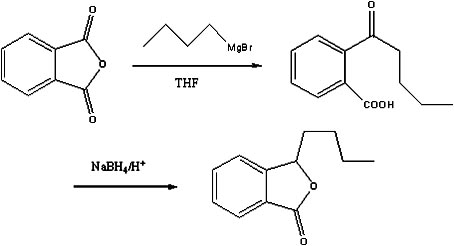
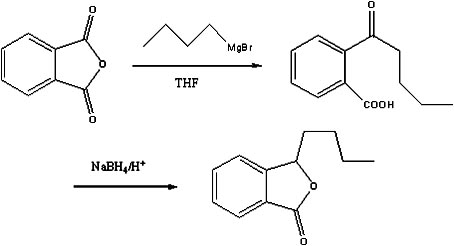
The invention discloses a preparation method of butylphthalide, which comprises the following steps: taking phthalic anhydride as a raw material; enabling the phthalic anhydride to carry out addition reaction with the Grignard reagent of butyl halide to obtain an intermediate of o-valeryl benzoic acid; and then, reducing by sodium borohydride, and carrying out acidic cyclization to obtain the butylphthalide. The phthalic anhydride and the butyl halide which are used as raw materials in the preparation method of the butylphthalide of the invention are commercial products, and the reaction raw materials can be obtained easily. Because the Grignard reaction, the sodium borohydride reduction and the acidic cyclization are classical reactions, the operation is simple, the industrialized production can be realized easily, the yield of the butylphthalide reaches 50-60%, and the purity of the butylphthalide reaches 97-98%.
Owner:SHANGHAI INST OF TECH
Application of butylphthalide and derivatives thereof in preparation of medicines for preventing and treating ALS
ActiveCN102397272ADelayed onset timeProlong lifeOrganic active ingredientsNervous disorderDegenerative changeButylphthalide
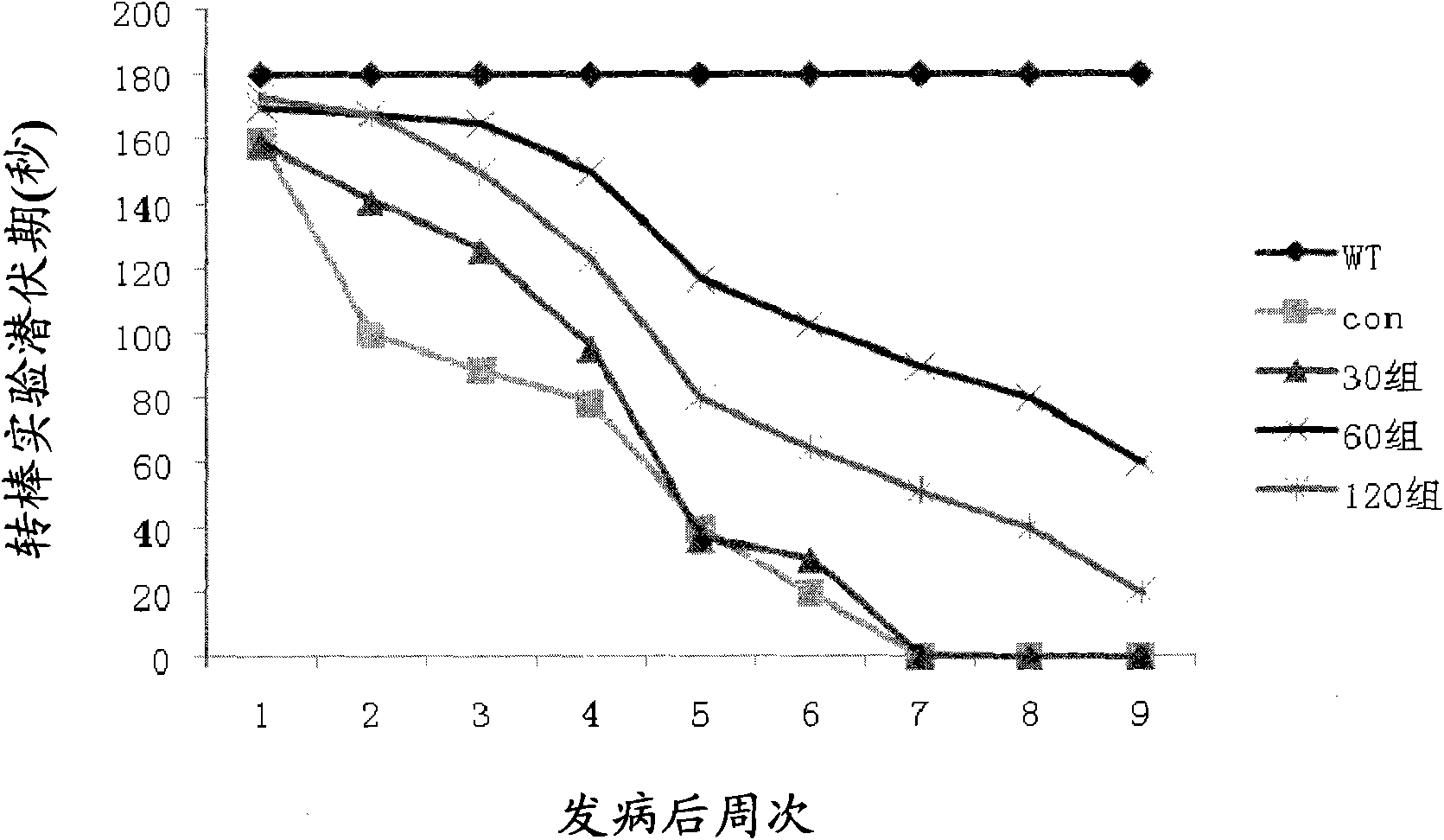
The invention which relates to the medicinal field discloses an application of butylphthalide and derivatives thereof in the preparation of medicines for preventing and treating ALS (amyotrophic lateral sclerosis). Butylphthalide and the derivatives, which can delay the disease time of an SOD1-G93A transgenic mouse, prolong the lifetime of the mouse, reduce the degenerative change of spinal motorneurons, obviously increase the survival number of spinal anterior horn motor neurons of the mouse, reduce the electrophysiological abnormality of the transgenic mouse, obviously improve the action potential amplitude and the motion unit number of compound muscles, substantially inhibit the activation of astrocytes and microglial cells in the spinal cord of the mouse, and obviously reduce the expression level of iNOS and NF-kappaBp65, have good application prospects in the prevention and the treatment of the ALS.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
3-(3'-hydroxyl)-butyl phthalide ester, and preparation thereof and uses
ActiveCN101289438AOrganic active ingredientsGroup 5/15 element organic compoundsOrganic acidSolubility
The invention relates to an ester obtained by the reaction of 3-(3'-hydroxyl)-butylphthalide and acids, wherein, the acids can be acceptable inorganic acids or organic acids in pharmacy. Proved by testing, the efficacy of the ester is increased significantly. Part of the ester can go on reacting with the acids or alkalis to form salts in order to increase water solubility and prepare injection preparations; being proved by testing, the injection preparations have no muscle and vascular stimulation.
Owner:BEIJING YILING BIOENG
Butylphthalide dripping pill and preparing method
ActiveCN1943571AFast oral onsetPromote absorptionOrganic active ingredientsDispersion deliveryButylphthalideHypromellose
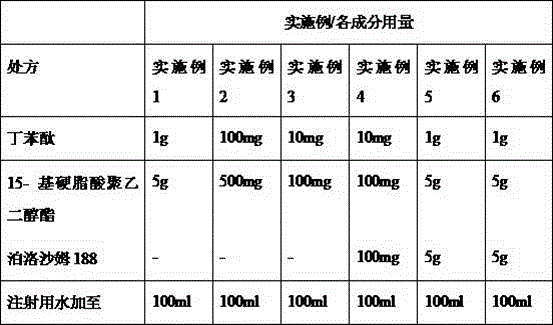
The invention relates to a drip pills of butylbenzene phthalein and its preparation method and it is characterized by comprising butylbenzene phthalein, base material, dispersing agent and coating materials. PEG4000, PEG6000, PEG20000 and poloxamer are selected as base materials. Dispersing agent is from lightweight aerosol and crospovidone. And coating materials are from hypromellose, hydroxypropyl cellulose, ethyl cellulose and Eudragit E30D.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
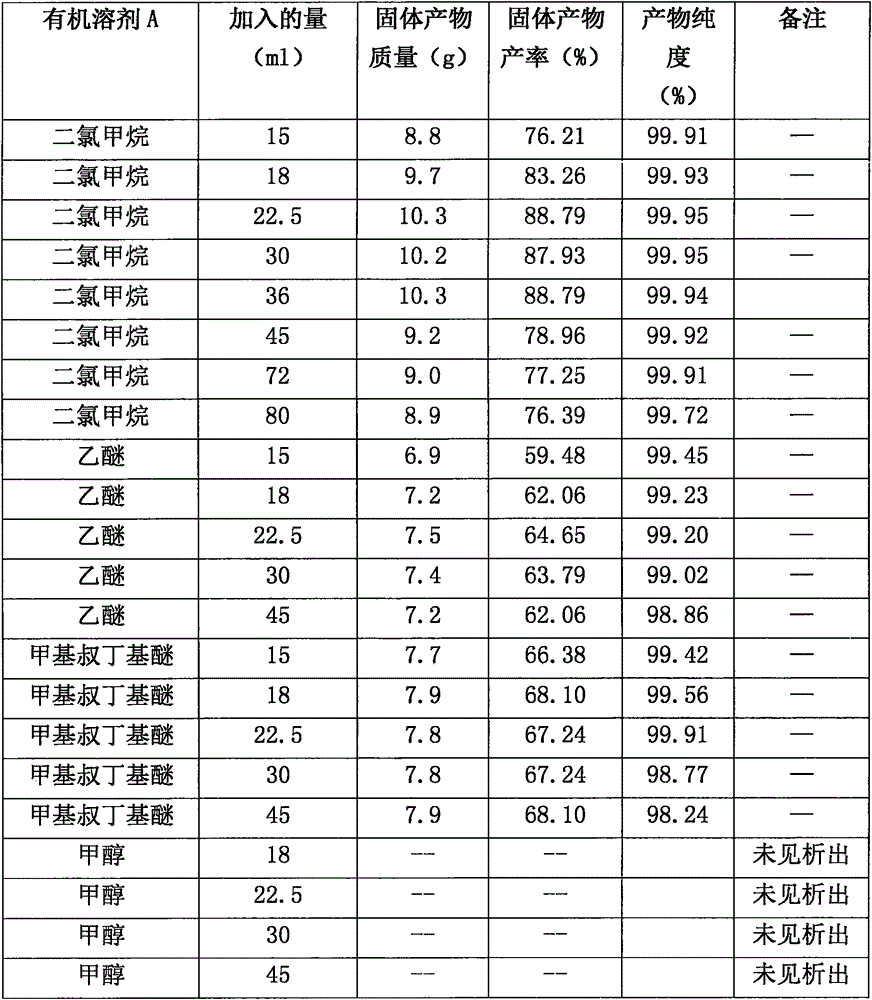
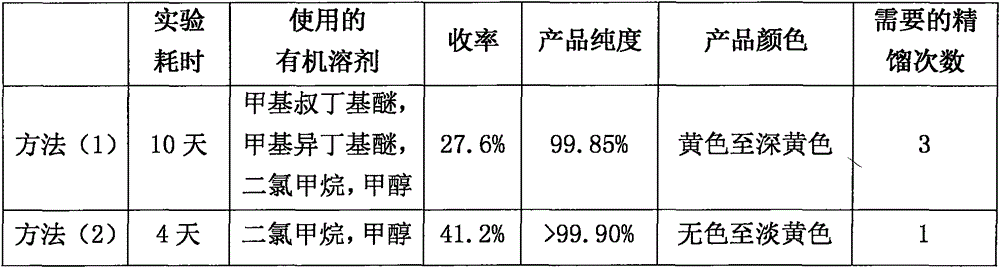
Butylphthalide synthesis method and purification technology
The invention relates to a butylphthalide synthesis method. The method comprises the steps that methyl 2-formyl benzoic acid is adopted as a starting material, THF is adopted as a solvent to react with an n-butyl magnesium chloride Grignard reagent, and acid regulation is performed to prepare a butylphthalide product. The invention further relates to a technology for preparing high-purity butylphthalide. The obtained crude butylphthalide product is subjected to hydrolysis treatment by an alkaline substance, acid regulation is performed to separate out solids, and filtering is performed to obtain a butylphthalide midbody; the acid regulation and alkali regulation processes are executed repeatedly, and finally ring closure and decompression desolvation are performed to obtain high-purity butylphthalide. According to the synthesis method, low-flash diethyl ether is prevented from being adopted as a solvent, the purification technology is easy to implement, the reagent can be purchased in bulk easily, column chromatography product purification and reduced pressure distillation under high temperature and high vacuum degree are not needed, and industrial enlarged production is easy.
Owner:福建省宝诺医药研发有限公司
Use of butyl phthalide and derivatives thereof in preparation of medicines for treating Parkinson disease
InactiveCN102125548ABlock or slow down the processNeuroprotectiveOrganic active ingredientsNervous disorderRotenonePhthalide
The invention relates to new use of 3-n-butylphthalide (abbreviated as butyl phthalide) and derivatives thereof, in particular to the use of the compounds in the preparation of the medicine for treating Parkinson disease. A mat model of hemiparkinsonian disease is built by using three-dimensional targeted injection of rotenone to perform a test, and the result of the test shows that the butyl phthalide and derivatives thereof can effectively improve symptoms aroused by Parkinson and have a nerve-protecting effect.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Butylphthalide medicine active composition and preparation method of butylphthalide medicine active composition
ActiveCN102716121AComply with medicinal requirementsQuality improvementOrganic active ingredientsOrganic chemistryButylphthalidePharmaceutical drug
The invention provides a butylphthalide medicine active composition, which comprises the following ingredients: first ingredients: the butylphthalide content is higher than or equal to 98.0 percent; second ingredients: the second ingredients are one kind of materials or several kinds of materials selected from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide, amylene phthalide, phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, in addition, the content of the second ingredients is higher than 0 but is lower than or equal to 2.0 percent, when the second ingredients comprise any one kind of materials from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide and amylene phthalide, the content of any one kind of included ingredients does not exceed 0.5 percent, and when the second ingredients comprise any one kind of materials from phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, the content of any one kind of included ingredients does not exceed 1.0 percent. The quality of the medicine active composition is stable, and the clinic curative effect and the medication safety of the butylphthalide preparation can be ensured.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
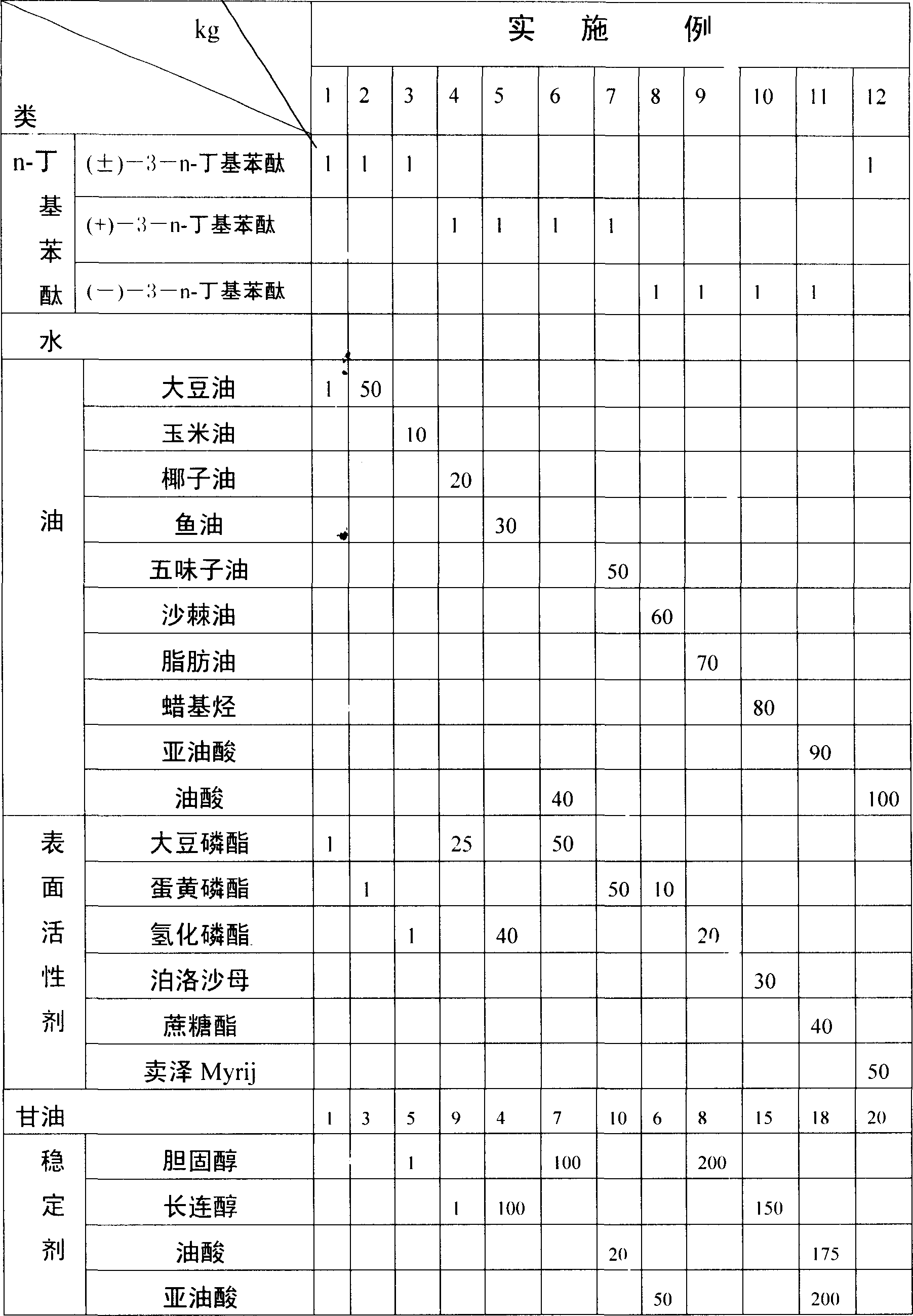
Emulsion medicine containing n-NBP for injection and its preparation method
InactiveCN1839824AImprove bioavailabilityUniform particle sizeOrganic active ingredientsEmulsion deliveryEmulsionCurative effect
The invention relates to a medicament for injection containing n-butylphthalide and its preparing process, wherein the constituents include (by weight parts) n-butylphthalide, oil, surface active agent, isotonic agent and water by the proportion of 1:1-100:1-50:1-20:50-70. The preparing process comprises charging the isotonic agent and water soluble surface active agent into water, thus obtaining aqueous phase mixture, then charging n-butylphthalide, surface active agent and stabilizing agent into oil, heating to 60-90 deg. C, high speed stirring and emulsifying.
Owner:高春平
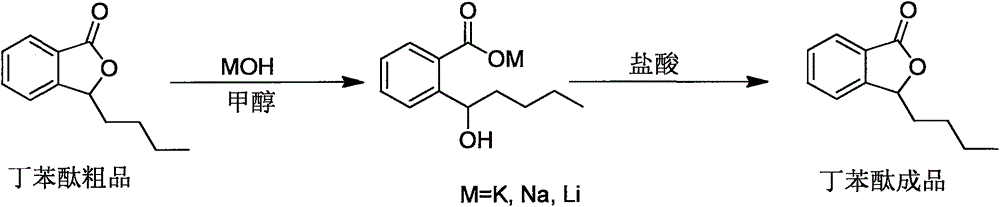
Preparation method of high-purity butylphthalide
The invention provides a preparation method of high-purity butylphthalide. The preparation method comprises the following steps: A. adding a crude product of rac-3-n-butylphthalide to a reaction flask and reducing the pressure until the system vacuum degree is 1-2mbar; raising the temperature and collecting a 130-140 DEG C fraction, thus obtaining a rac-3-n-butylphthalide fraction; B. adding the rac-3-n-butylphthalide fraction and methanol to the reaction flask, stirring to dissolve the materials, adding a methanol solution of inorganic base, heating till reflux reaction, carrying out filtration, removing methanol through concentration, adding dichloromethane, stirring the materials to precipitate solids, and carrying out filtration and drying, thus obtaining 1-hydroxypentyl-2-benzoate; and C. adding 1-hydroxypentyl-2-benzoate to water, stirring to dissolve 1-hydroxypentyl-2-benzoate, then adding the solution to a reaction flask with a hydrochloric acid solution, stirring to react at a temperature of 35-45 DEG C, standing for layering, washing an oil phase with a sodium bicarbonate solution and water in sequence, and carrying out drying and filtration, thus obtaining the pure product of rac-3-n-butylphthalide.
Owner:LIVZON PHARM GRP INC
Optically active butylphthalide open-ring derivative, preparation method and medical application
InactiveCN103193789AFuzzy structureReduce the numberNervous disorderOrganic chemistryDiseaseAntithrombotic Agent
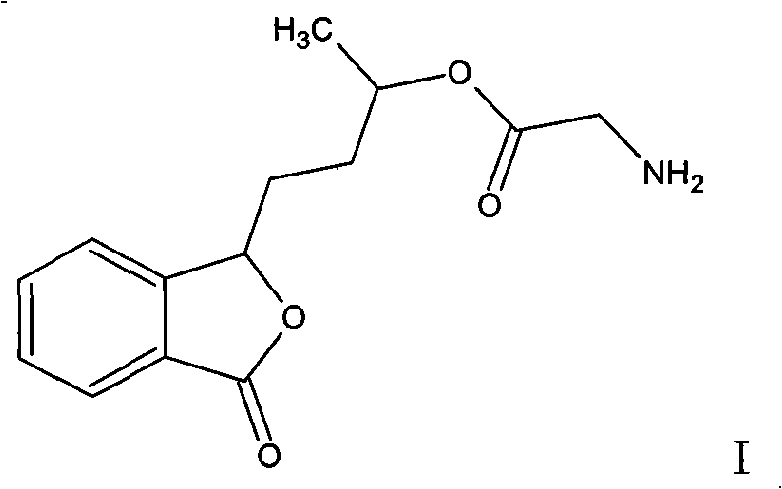
The present invention relates to the field of pharmaceutical chemistry and therapeutics, and particularly relates to an optically active butylphthalide open-ring derivative as shown in the general formula I or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier, preparation methods thereof, pharmaceutical compositions containing the compounds, and medical application thereof, especially application in medicines for prevention and treatment of cardiovascular and cerebrovascular and improvement of heart and brain circulatory disturbance, antiplatelet aggregation medicines, antithrombotic medicines, anti-ischemic medicines, anti-dementia medicines, anti-atherosclerotic medicines, and medicines for anti-diabetes and complications thereof. Pharmacological experimental results show that the compounds have good anti-platelet aggregation activity, anti-thrombotic activity, anti-ischemic activity and neuroprotective effects, and are clinically useful for the preparation of medicines for preventing or treating diseases associated with platelet aggregation.
Owner:CHINA PHARM UNIV
Penicillium vulpinum fungi strain and method for preparing levo 7-hydroxyl butylphthalide employing fungi strain
ActiveCN104726347AIncrease productionQuality improvementFungiMicroorganism based processesBiotechnologyMicroorganism
The invention discloses a penicillium vulpinum fungi strain and a method for preparing levo 7-hydroxyl butylphthalide ((S)-(-)-butyl-7-hydroxyl phthalide) employing the fungi strain. The fungi strain disclosed by the invention is a penicillium vulpinum fungi strain NCC3421 (penicillium vulpinum) and is preserved at the China General Microbiological Culture Collection Center (CGMCC for short); and the preservation number is CGMCC No.9094. Under the culture condition provided by the method, the fermentation unit of the levo 7-hydroxyl butylphthalide reaches over 1600mg / L; the fermented product is relatively single in components, and easy to separate and purify, and can be applied to industrialized production of the levo 7-hydroxyl butylphthalide.
Owner:NCPC NEW DRUG RES & DEV
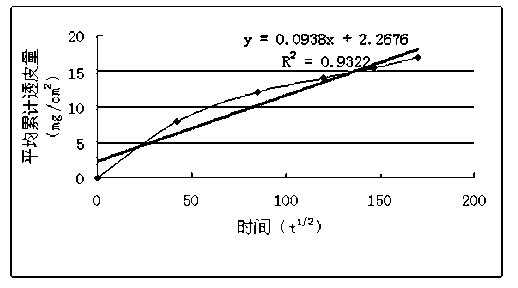
Microemulsion transdermal gel agent of butylphthalide or derivative thereof, and preparation method thereof
ActiveCN102178643APromote transdermal absorptionEasy to preparePharmaceutical delivery mechanismEster active ingredientsMedicineCentrifugation
The invention relates to a microemulsion transdermal gel agent of butylphthalide or a derivative thereof, and a preparation method thereof, and belongs to the technical field of medicaments. The technical scheme is that: the microemulsion transdermal gel agent is prepared mainly from the following ingredients in part by weight: 1 to 5 parts of oil phase, 1 to 5 parts of emulsifying agent, 0.1 to 1 part of auxiliary emulsifying agent, 1.5 to 15 parts of purified water and 0.003 to 0.5 part of transdermal accelerator. Compared with a comparison example, the microemulsion transdermal gel agent of the butylphthalide or the derivative thereof almost does not influence the microemulsion appearance and granular size under the conditions of centrifugation and high temperature, and is a dynamic and thermodynamic stable system. An in vitro transdermal performance test proves that the microemulsion transdermal gel agent of the butylphthalide or the derivative thereof has good transdermal absorbability. In conclusion, the microemulsion transdermal gel agent of the butylphthalide or the derivative thereof has the simple preparation method, does not need the procedures of high-speed cutting or homogenizing and the like, has small product granular size, and contributes to industrialization.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
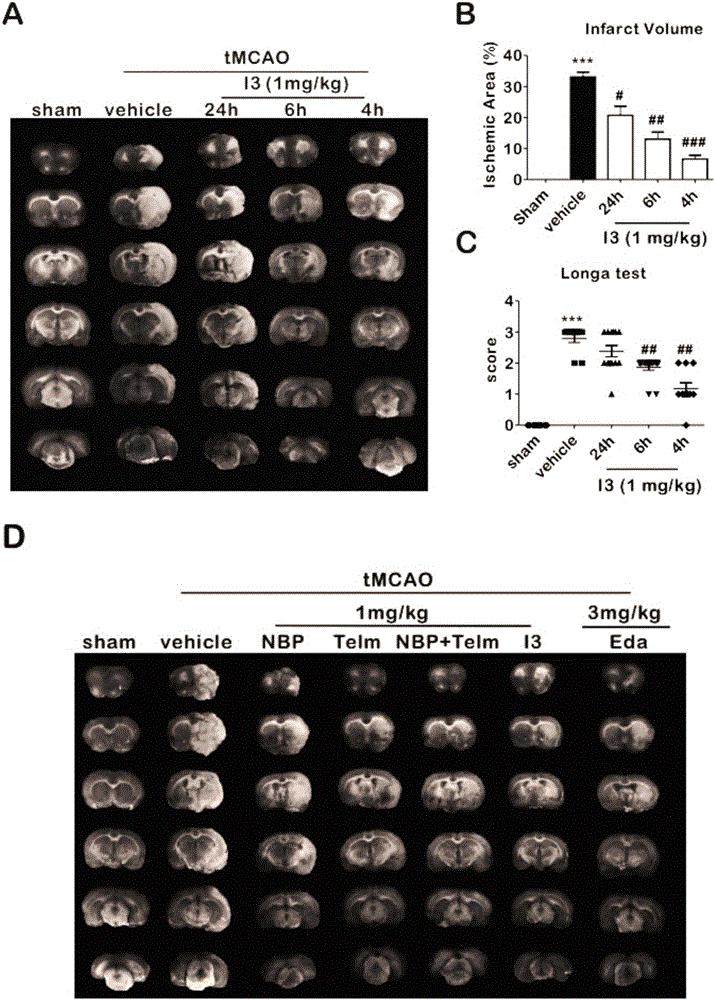
Butylphthalide-telmisartan heterocomplex and preparation method and application thereof
ActiveCN106800537AStrong anti-ischemic activityOrganic active ingredientsNervous disorderDiseaseMedicine
The invention relates to the fields of pharmaceutical chemistry and pharmaceutical therapeutics, and more particularly to an optically active ring-opening butylphthalide-telmisartan heterocomplex or a pharmaceutically acceptable salt or ester thereof, preparation methods thereof, pharmaceutical compositions containing the compounds, and pharmaceutical application of the pharmaceutical compositions, particularly application in prevention and treatment of neuroinflammation-related diseases, including ischemic stroke, alzheimer's disease, brain trauma, Parkinson's disease, multiple sclerosis and depression.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Compound air cleaning agent and preparing method and application thereof
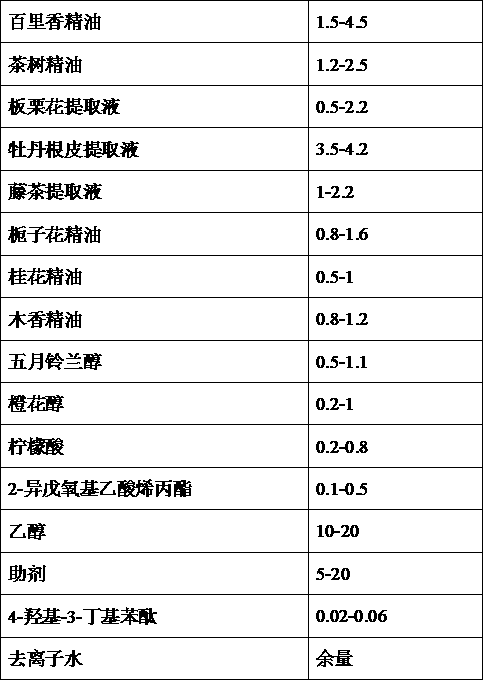
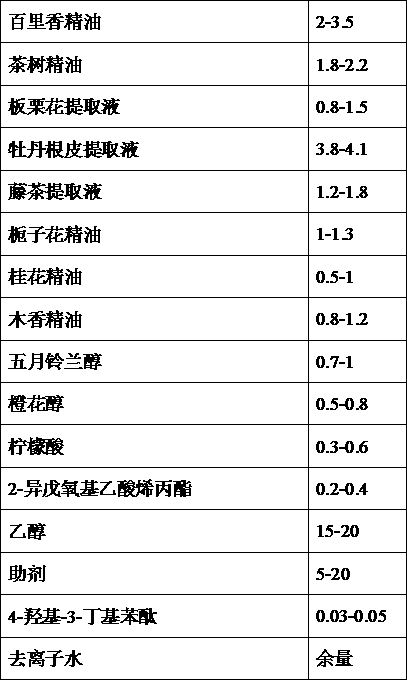
InactiveCN103239745ARich fragranceImprove mosquito repellent effectBiocidePest repellentsBiotechnologyAllyl acetate
The invention provides a compound air cleaning agent and a preparing method and application thereof. The compound air cleaning agent is formed by compounding multiple components of thyme essential oil, tea tree essential oil, castanea mollissima flower extracting solution, peony root and skin extracting solution, vine tea extracting solution, gardenia essential oil, osmanthus fragrans essential oil, costustoot essential oil, may lily of the valley alcohol, nerol, citric acid, 2-isopentyloxy allyl acetate, alcohol, assistant, 4-hydroxy-3-butylphthalide and deionized water. The air cleaning agent has the advantages of better sterilizing, mosquito repelling and aroma enhancing performances due to proper selecting of component and proportion and the coordinated action among the components, less using amount, long time effectiveness, and is more saved and fast in the aspect of life and use of people.
Owner:清远市立道精细化工有限公司
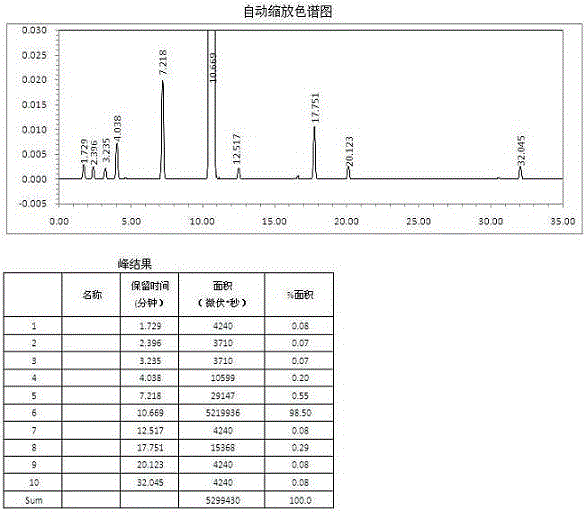
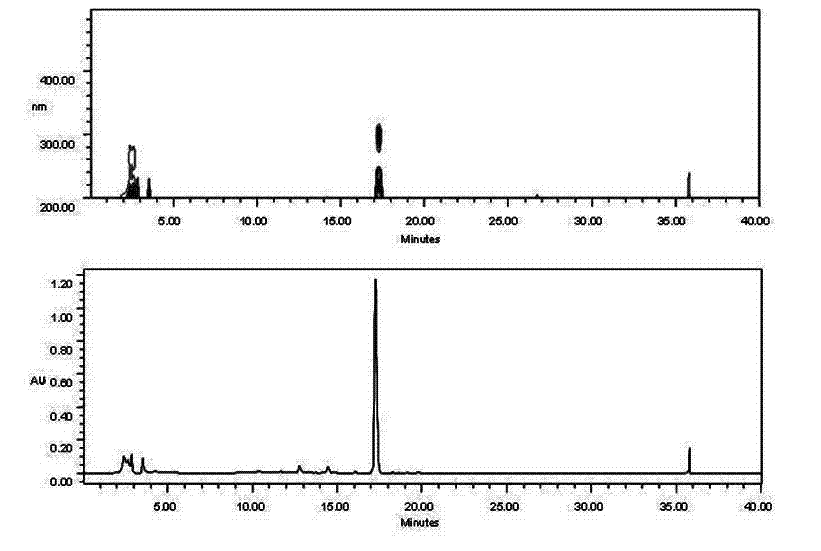
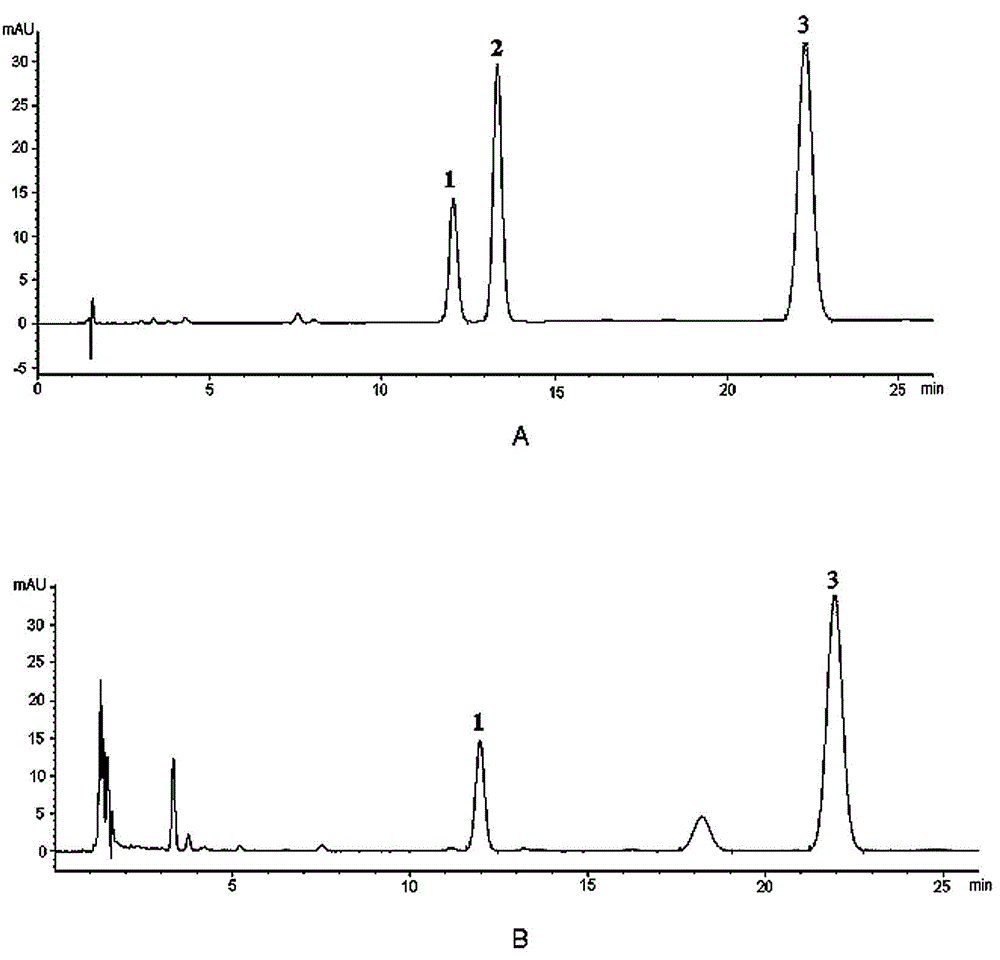
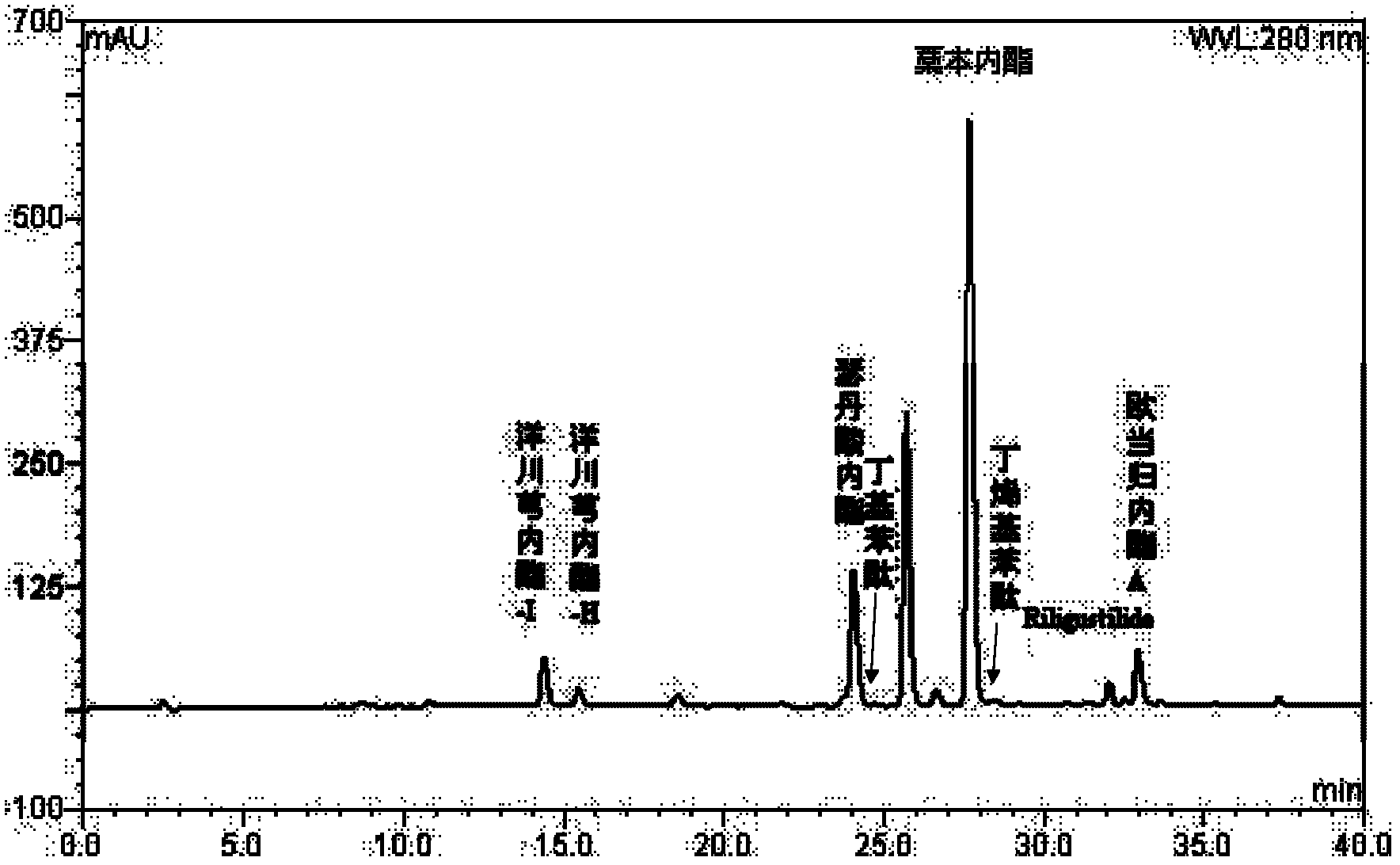
Method for simultaneously and quantitatively detecting ligustilide and senkyunolide A
The invention discloses a method for simultaneously and quantitatively detecting ligustilide and senkyunolide A. The method comprises the following steps: according to an HPLC (high performance liquid chromatography) method in which butylphthalide serves as a substitution reference substance and octadecyl silane bonded silica gel serves as a filling agent, respectively determining the peak area Ax and the peak area Ay of the ligustilide (X) and the senkyunolide A (Y) in a to-be-detected sample by using a single wavelength in a range of 275-284nm; and according to the concentration Cz and the peak area Az of the substitution reference substance, namely the butylphthalide (Z), calculating the concentration C of the detected components, namely the ligustilide and the senkyunolide A, according to a formula by using correction factors f, wherein a correction factor fx is within 0.20-0.25, and a correction factor fy is within 0.46-0.54. A detection result obtained by using the method provided by the invention is stable, and is in accordance with a detection result obtained by using a method in which the ligustilide and the senkyunolide A, freshly prepared, serve as reference substances, and the problems that the dual-wavelength detection needs to be simultaneously adopted, the requirements on HPLC equipment are high and the universality is poor in the prior art are solved.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Polymerization inhibitor for preventing self-polymerization or co-polymerization of C5 diolefins
InactiveCN1699311AGood inhibition effectHydrocarbon purification/separationHydrocarbonsButylphthalideComposition B
The invention relates to a polymerization inhibitor for preventing self-polymerization or co-polymerization of C5 diolefins, which comprises composition A: diethylhydroxylamine, composition B: tert-butyl pyrocatechol, and solvent, wherein the solvent is selected from benzene, toluene, dimethylbenzene or ethylbenzene, the weight ratio of the composition A and the composition B being (0.5-6):1, the total content of the composition A and the composition B in the polymerization inhibitor being 25-45wt%. Compared with the prior art, the invention can realize better polymerization inhibiting effect, and no extra separation process is needed in the current C5 distillate separation procedure.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
A pharmaceutical composition comprising butylphthalide and borneol and applications thereof
InactiveCN104224772AGood treatment effectReduce the effective doseHydroxy compound active ingredientsCardiovascular disorderDiseasePharmaceutical drug
A pharmaceutical composition comprising butylphthalide and borneol is disclosed. The pharmaceutical composition has synergistic effects on treating cerebrovascular diseases especially ischemic cerebrovascular diseases, and is capable of obviously enhancing treating effects of the butylphthalide, reducing the effective dose of the butylphthalide, and reducing the using amount. Untoward effects of the pharmaceutical composition during long-term application can be reduced.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Process for preventing self-polymerization or co-polymerization of C5 diolefins in separation process of petroleum C5 distillate
InactiveCN1699310AGood inhibition effectHydrocarbon purification/separationHydrocarbonsComposition BButylphthalide
The invention provides a process for preventing self-polymerization or co-polymerization of C5 diolefins in separation process of petroleum C5 distillate, which consists of charging polymerization inhibitor into C5 distillate material, and making the polymerization inhibitor maintain a finite amount in the C5 distillate material, the polymerization inhibitor comprises composition A: diethylhydroxylamine, and composition B: tert-butyl pyrocatechol, the weight ratio of the composition A and the composition B being (0.5-6):1, the quantity of polymerization inhibitor in the C5 distillate material is sustained at 30-600ppm calculated on the total amount of the composition A and the composition B. The invention can realize better polymerization inhibiting effect, and no extra separation process is needed in the current C5 distillate separation procedure.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD +1
Butylphthalide sublingual tablet and preparation method thereof
The invention provides a butylphthalide sublingual preparation. The butylphthalid sublingual preparation contains butylphthalide, lauric acid polyethylene glycol glyceride, an excipient, an adsorbent and a lubricating agent. A butylphthalide solid dispersion prepared by taking lauric acid polyethylene glycol glyceride as a carrier can be quickly dissolved in vitro, and a sample is prepared by adopting a hot melting extrusion method, so that drug loading capacity is high and sample stability is good.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Butylphthalide- and edaravone-containing compound injection and preparation method thereof
ActiveCN102526036ALow incidence of adverse reactionsGood curative effectOrganic active ingredientsNervous disorderAlcoholAntioxidant
The invention relates to a butylphthalide- and edaravone-containing compound injection and a preparation method thereof. The injection of every 100 ml contains 25-50 mg of active ingredient butylphthalide, 12.5-50 mg of edaravone, as well as absolute ethyl alcohol, hydroxypropyl-beta-cyclodextrin, an osmotic pressure regulator, an antioxidant and the like. With the adoption of the butylphthalide- and edaravone-containing compound injection, the administration dosage is greatly lowered, the burden of liver metabolism is reduced, and the incidence rate of liver adverse reactions is lowered. According to determination, with the adoption of the butylphthalide- and edaravone-containing compound injection, the administration dosage of the edaravone can be reduced by at least 20%, besides, the compound injection has a better curative effect, and the incidence rate of the liver adverse reactions is also remarkably lowered.
Owner:SHIJAZHUANG ZHONGSHUO PHARMA CO LTD
Butylphthalide Self-Emulsifying Drug Delivery System, Its Preparation Method and Application
ActiveUS20080319056A1Improve absorption rateReduce power consumptionBiocideNervous disorderAdditive ingredientButylphthalide
The present invention relates to a novel drug delivery and release system, i.e. Self-emulsifying Drug Delivery System (SEDDS), of butylphthalide, to a preparation process thereof, and to a use thereof in a pharmaceutical formulation. The drug delivery system comprises as essential ingredients 1% to 65% of butylphthalide and 10% to 65% of a emulsifying agent, together with various excipients as required depending on the desired dosage forms. The present invention significantly increases the contact area between butylphthalide and the mucous membrane of the gastrointestinal tract, and therefore improves the absorptivity of the drug.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Preparation containing ligustilide type component for treating cardio-cerebrovascular disease and preparation method thereof
InactiveCN102631387APrevent escapeImprove bioavailabilityCardiovascular disorderPlant ingredientsDiseaseWater vapor
The invention discloses a preparation containing a ligustilide type component and a preparation method thereof. The ligustilide type component comprises the following components by weight percent: ligustilide 14%-42%, sedanolide 3%-20%, butylidenephthalide 0.1%-5%, butylphthalide 0.1%-3%, senkyunolide-H 0.2%-3%, senkyunolide-I 0.4%-5%, levistilide A 0.5%-1.5%, and riligustilide 0.4%-1.2%. The preparation method prevents the ligustilide type component from being destroyed by the wet distillation method and the solvent extraction method, and the quality of the ligustilide type component is more ensured without solvent residue. The preparation containing the ligustilide type component is safe and controllable on the effective part. Drop pills and sublingual tablets containing the ligustilide type component can avoid the defects of the oral preparation after administration such as liver first pass effect and gastrointestinal reaction, and an injection containing the ligustilide type component can also avoid the possible situations during using the injections such as acute poisoning reaction and allergic reaction. The preparation containing the ligustilide type component is safer and more effective, and has good economic benefit and social benefit.
Owner:TIANJIN UNIV
Medical use of 7-hydroxy-butylphthalide
ActiveCN106214674AImprove protectionStrong medicinal effectOrganic active ingredientsAntinoxious agentsVascular diseaseButylphthalide
The invention belongs to the technical field of pharmaceutical chemistry and particularly relates to medical use of 7-hydroxy-butylphthalide in preparation of drugs for preventing and / or treating cardiac and cerebral vascular diseases.
Owner:NCPC NEW DRUG RES & DEV
Microencapsulated butylphthalide medicine composition, preparation method and applications
ActiveCN103417514APrevent volatile lossAvoid degradationOrganic active ingredientsNervous disorderSolubilityMedicine
The invention relates to a microencapsulated butylphthalide medicine composition, a preparation method and applications. Butylphthalide is microencapsulated to raise the solubility of butylphthalide, prevent volatilization, develop required solid preparations and liquid preparations clinically and give play to therapeutical effects of butylphthalide better. The composition contains butylphthalide effective component with a mass percent of 1-55%. Butylphthalide is coated with natural or synthesized high polymer materials as capsule wall materials or carrier materials to form microencapsules. The composition can be prepared into various preparations of powder, capsules, chewable tablets, buried tablets, buccal tablets, other oral compressed tablets, injection, liquid suspension, lotion, powder-injection, coating preparations, thin film preparations and the like.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
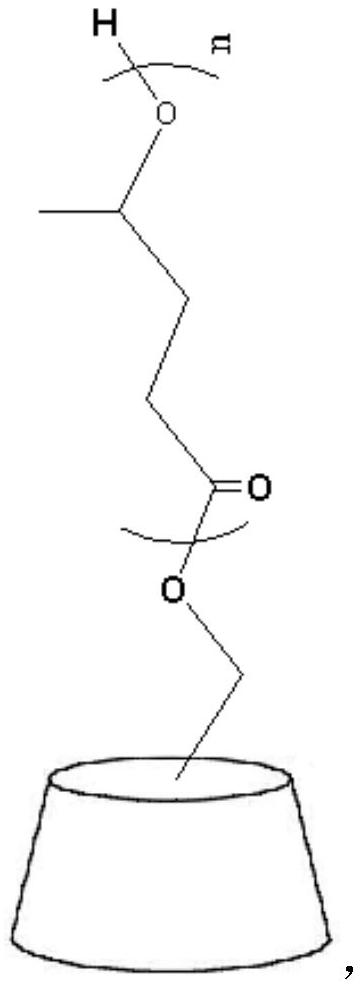
Stability-improved butylphthalide oral freeze-dried powder as well as preparation method and application thereof
ActiveCN113197872AImprove stabilityHigh purityOrganic active ingredientsPowder deliveryFreeze-dryingCyclodextrin
The invention relates to stability-improved butylphthalide oral freeze-dried powder which is characterized by being prepared from the following raw and auxiliary materials by a freeze-drying method: butylphthalide and 4-methylbutyrolactone modified sulfobutyl beta-cyclodextrin sodium, the 4-methylbutyrolactone modified sulfobutyl-beta-cyclodextrin sodium has the following simple structural formula: the butylphthalide oral freeze-dried powder provided by the invention overcomes the defects of capsule preparations and injections, and the freeze-dried powder is liquid after being redissolved, so that the freeze-dried powder is easier for patients to take. Besides, the plasma peak reaching time of butylphthalide capsules is 60-80 min, after the butylphthalide oral freeze-dried powder is redissolved, the peak reaching time of oral liquid is less than 30 min, and the butylphthalide oral freeze-dried powder can take effect quickly after being taken and has important significance for rescuing cerebral apoplexy patients. Meanwhile, the butylphthalide oral freeze-dried powder has good stability, and can keep good purity, solubility and bioavailability of butylphthalide after being stored for 10 days under the conditions of 25 DEG C and 40RH%.
Owner:奥信阳光(北京)药业科技有限公司
Butylphthalide controlled release preparation and preparation method thereof
ActiveCN103169685ASolve insolubleGood self-emulsifying performanceOrganic active ingredientsPharmaceutical delivery mechanismButylphthalidePharmaceutical Substances
The invention relates to a butylphthalide controlled release preparation and a preparation method thereof. Drugs can be uniformly released at a constant speed, and release of liquid drugs from the controlled release preparation in a liquid form is achieved.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Clathrates of butylphtualide with cyclodextrin or its derivatives, a process for their preparation and the use thereof
InactiveUS20060166931A1Less vascular irritationGood water solubilityBiocideOrganic active ingredientsTreatment effectOral medication
The present invention relates to the inclusion complexes of butylphthalide, which is D,L-mixed or levorotatory, with cyclodextrin or cyclodextrin derivatives, to a process for their preparation and the use thereof. In the invention, the butylphthalide is complexed with cyclodextrin or cyclodextrin derivatives, preferably with hydroxypropyl-β-cyclodextrin, in order to increase the water-solubility of butylphthalide, develop clinical solid or liquid formulations and improve the therapeutic effect of butylphthalide. The inclusion complex, in which the molar ratio of butylphthalide to cyclodextrin or cyclodextrin derivatives is in the range of 1:1-10, can be used to prepare infusion, injection, injectable powder, liquids for oral administration, syrup, tablets, granules, dispersible tablets and others.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Medicine composition comprising butylphthalide and cosolvent
InactiveCN105999279AReduce dosageImprove solubilityOrganic active ingredientsNervous disorderSolubilityAcupuncture
The invention relates to the field of medicine, particularly to a medicine composition comprising butylphthalide and cosolvent, and aims to adopt a novel cosolvent to improve the water solubility of butylphthalide, develop clinically required solid, semisolid or liquid preparations of butylphthalide, and better exert the therapeutical effect of butylphthalide. The composition can be used for preparing a plurality of dosage forms such as tablets, capsules, ointment, cream, gel, transfusion, aqueous acupuncture, powder for injection, oral liquid and the like. Compared with the prior art, the medicine composition realizes better safety and improvement on the water solubility of butylphthalide.
Owner:SICHUAN MANSAISI MEDICINE TECH CO LTD
Method for concurrently determining butylphthalide and relates substances thereof
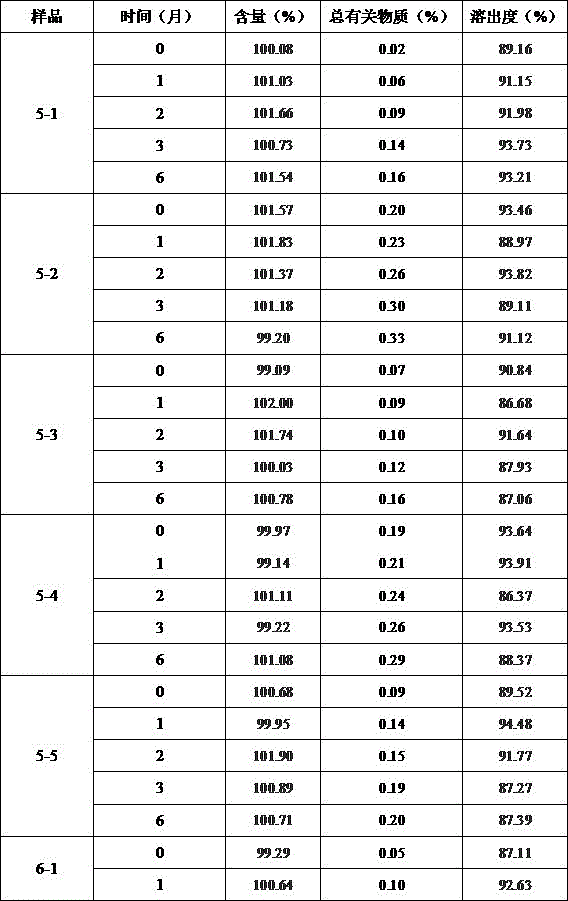
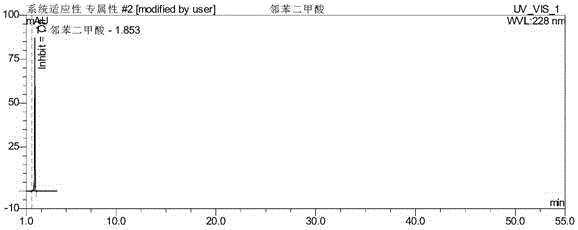
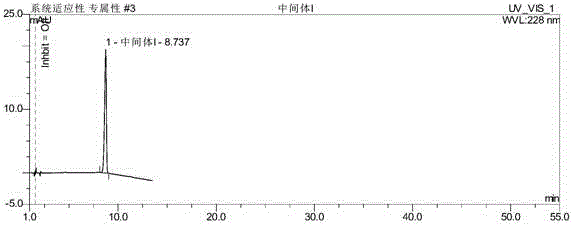
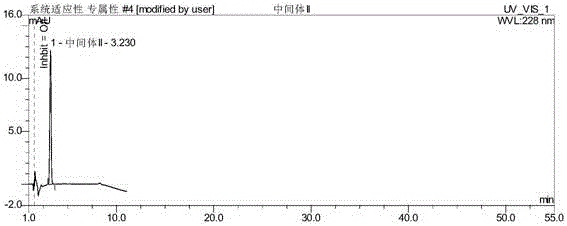
ActiveCN106093238AAvoid frequent replacementImprove work efficiencyComponent separationPhosphateButylphthalide
The present invention provides an analysis detection method for concurrently determining butylphthalide and relates substances thereof. According to the analysis detection method, through a high performance liquid chromatograph, an octadecylsilane bonded silica gel is adopted as a filler, a 0.2% phosphate buffer liquid and acetonitrile are adopted as a mobile phase to carry out gradient elution, and the detection wavelength is 226-230 nm. According to the present invention, the analysis method has characteristics of good specificity, high sensitivity, good accuracy and good durability, wherein the separation degrees of each absorption peak are more than or equal to 7.
Owner:NANJING YOKO PHARMA +2
Pharmaceutical composition containing butylphthalide and novel solubilizer
InactiveCN105688220AImprove securityLow hemolytic activityOrganic active ingredientsPowder deliverySolubilityButylphthalide
The invention relates to the field of medicine, in particular to a pharmaceutical composition containing butylphthalide and a novel solubilizer to improve the water solubility of butylphthalide by means of the novel solubilizer, clinically required solid form, or semisolid form or liquid form of butylphthalide is developed, so that the treatment effect of butylphthalide can be better realized. The composition can be used for preparing various drug forms such as tablets, capsules, particles, powder, ointment, cream, gel, infusion, squirt cut, powder filling and oral liquid. Compared with the prior art, the pharmaceutical composition is better in safety performance and water solubility of butylphthalide.
Owner:SICHUAN MANSAISI MEDICINE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com