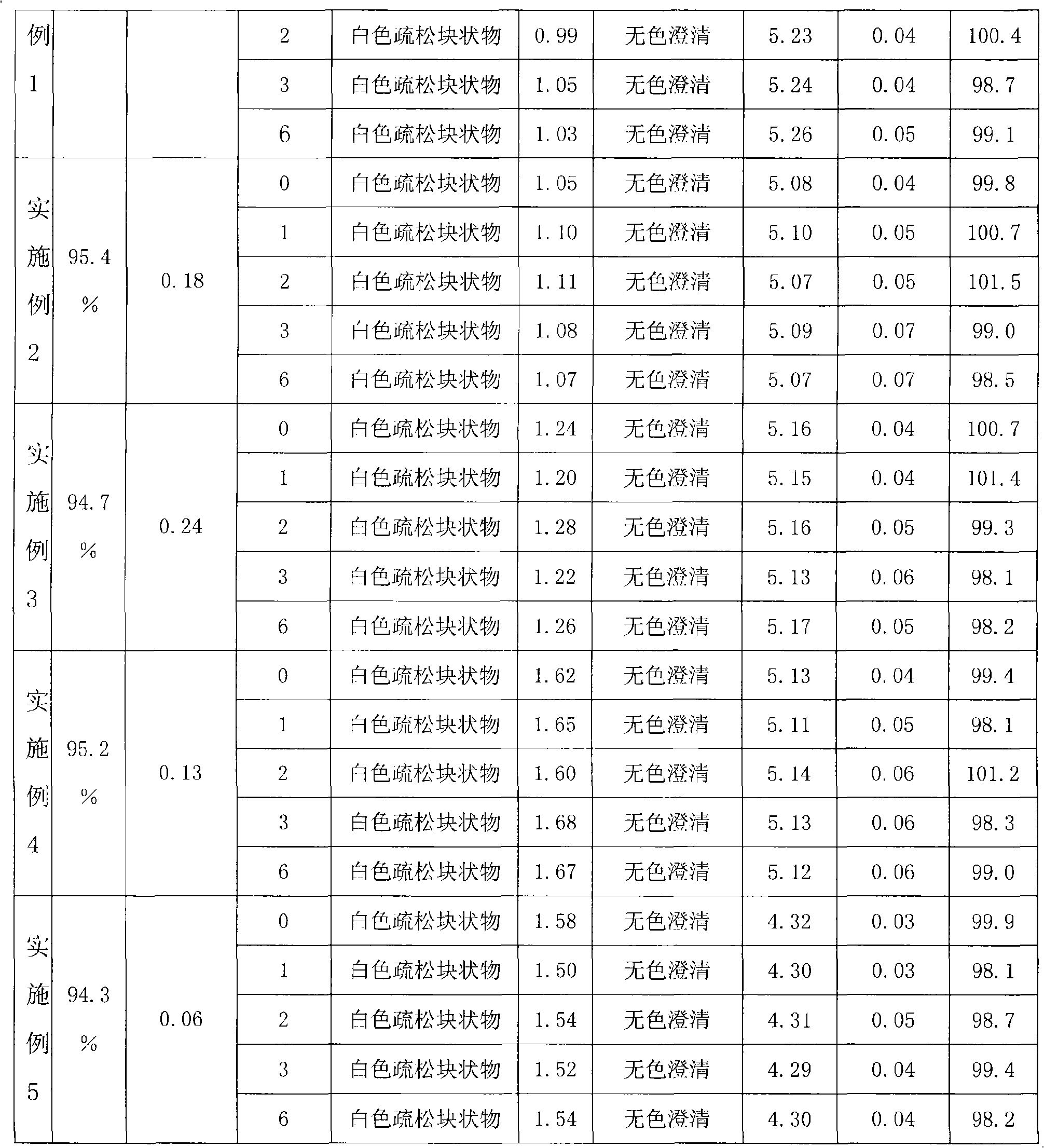Patents
Literature
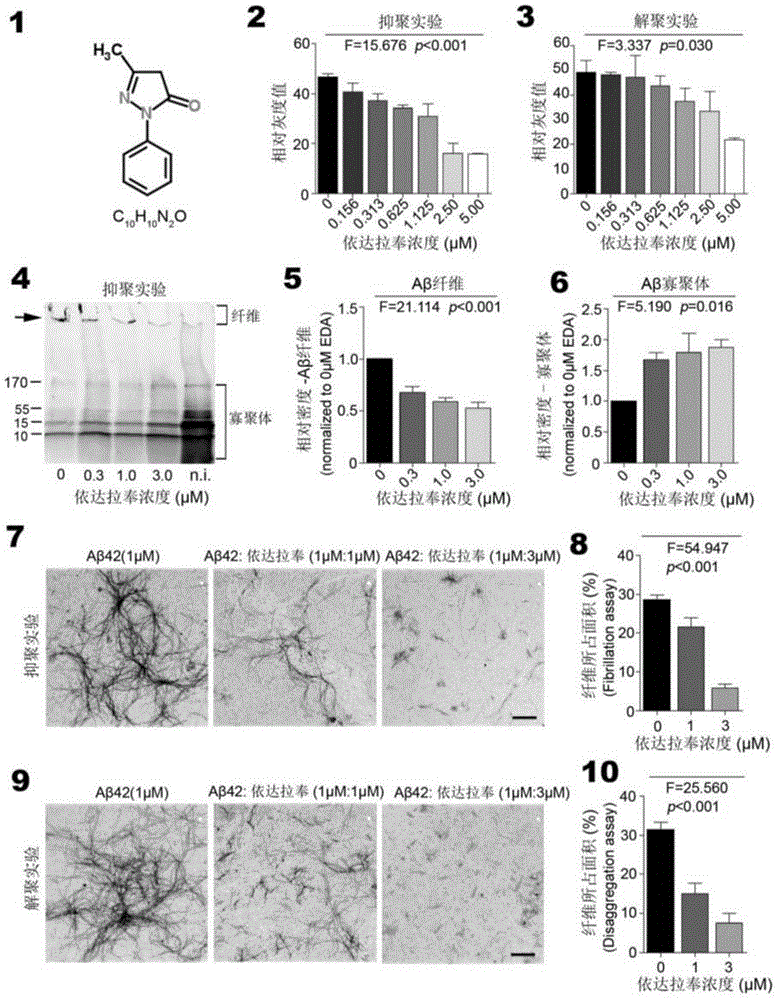
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
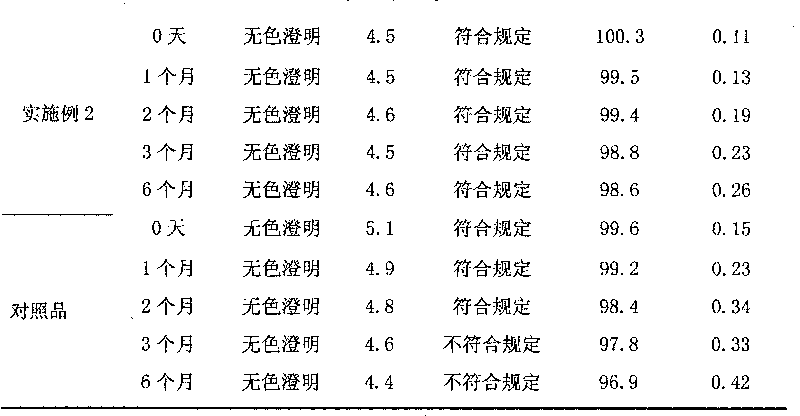
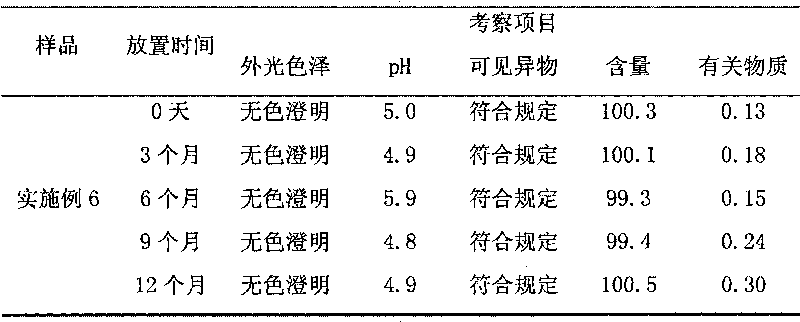
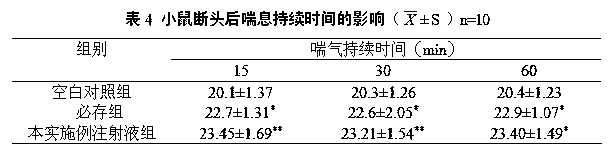
155 results about "Edaravone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
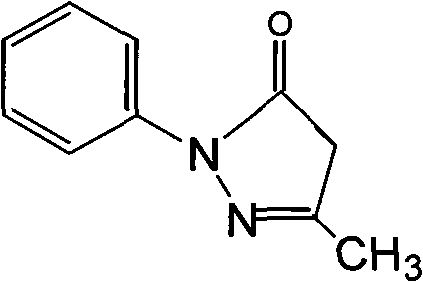
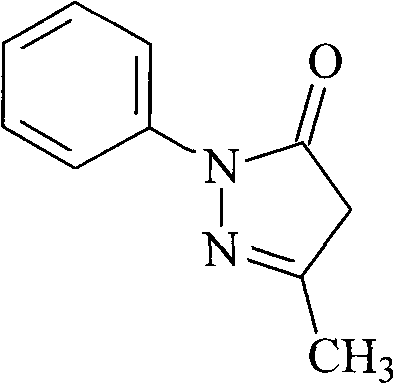
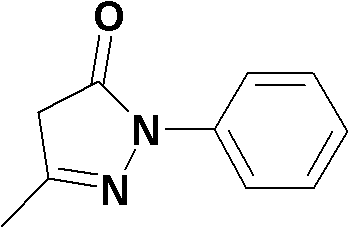
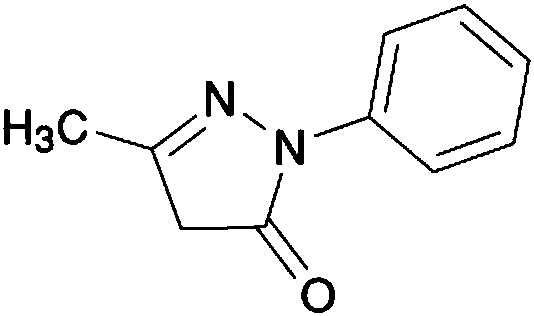
Edaravone is used to treat a certain type of nerve disease called amyotrophic lateral sclerosis (ALS, also commonly called Lou Gehrig's disease).
Edaravone injection for treating acute cerebral thrombus and its prepn
InactiveCN1440749AAdjust detection sensitivityOrganic active ingredientsSolution deliveryEdaravone InjectionEmulsion
The Edaravone injection of the present invention is a kind of free radical eliminating medicine to treat acute cerebral infarction. It is injection prepared with Edaravone, additive and solvent and in the form of sterilized solution, emulsion or suspension. It may be small capacity sterilized injection, great capacity sterilized injection, bacteria-free powder preparation for injection, freeze dried powder preparation for injection, sterilized suspension injection or sterilized emulsion injection.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Edaravone injection and preparation method thereof
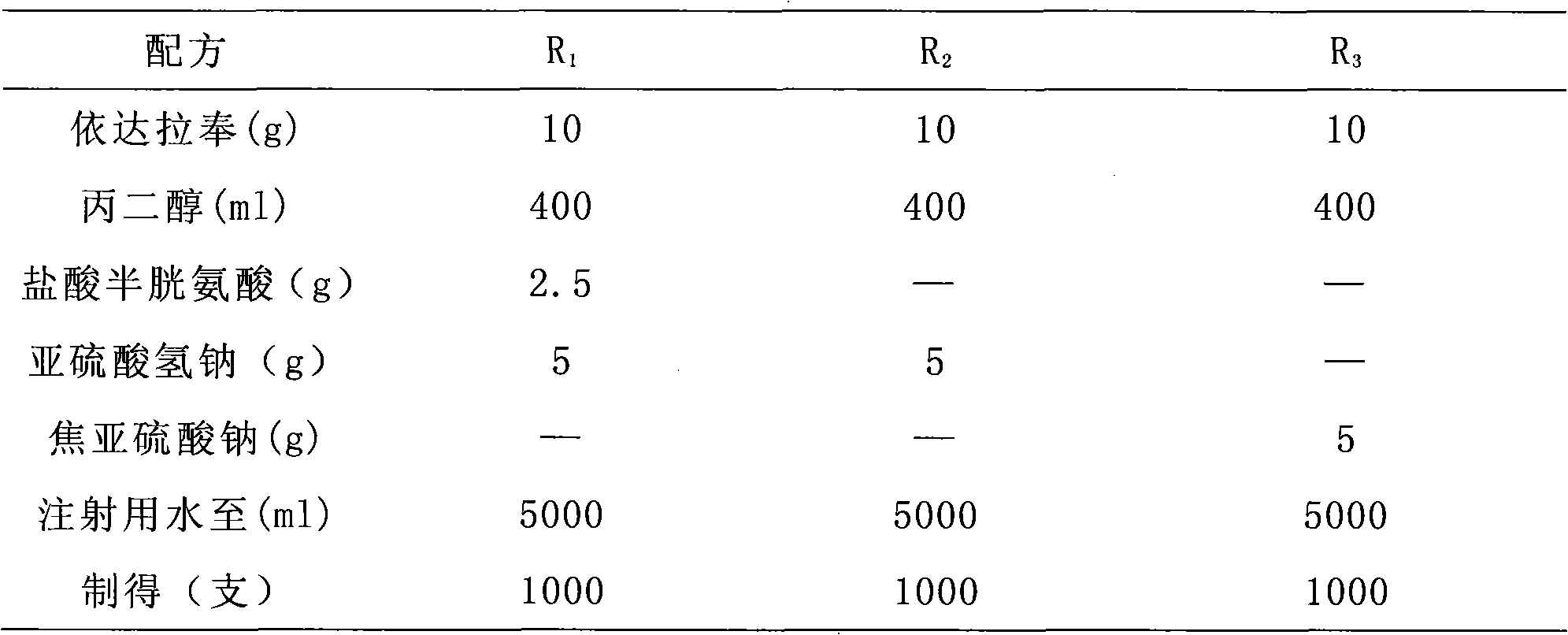
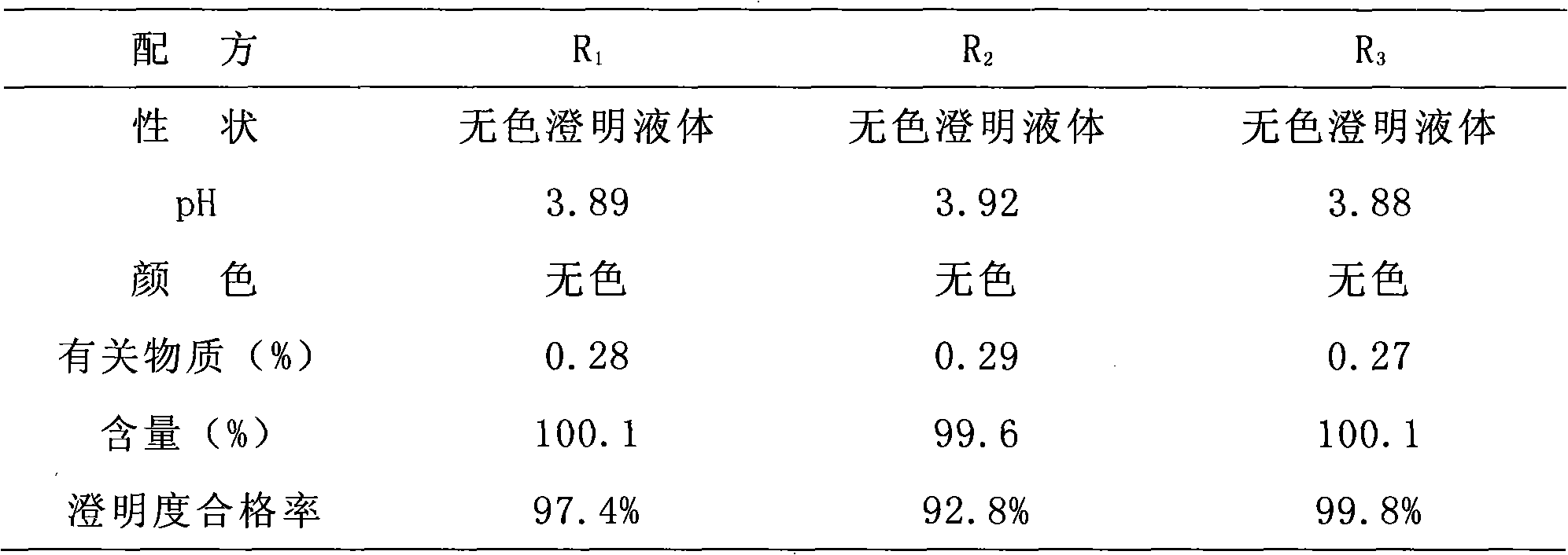
InactiveCN101933899AImprove stabilityLong validity periodOrganic active ingredientsNervous disorderEdaravone InjectionNitrogen
The invention discloses edaravone injection and a preparation method thereof. The injection contains edaravone serving as an active material, sodium pyrosulfite serving as antioxygen and propylene glycol serving as cosolvent. In the invention, in an edaravone injection production process, the sodium pyrosulfite antioxygen is added and high-purity nitrogen is charged into an ampoule bottle before and after liquid medicine canning and sealing to ensure the residual oxygen in the air above the liquid medicine in the ampoule bottle is less than 3 percent, so the edaravone is kept in a low-oxygen environment all the time. In the invention, the stability of the edaravone injection is improved, the period of validity of the edaravone injection is prolonged, and the safety and effectiveness of the edaravone injection in clinical use are ensured.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD
Edaravone compound synthesized by new method
InactiveCN101830852AHigh purityReduce manufacturing costOrganic chemistryAcetic acidPhenylhydrazine hydrochloride
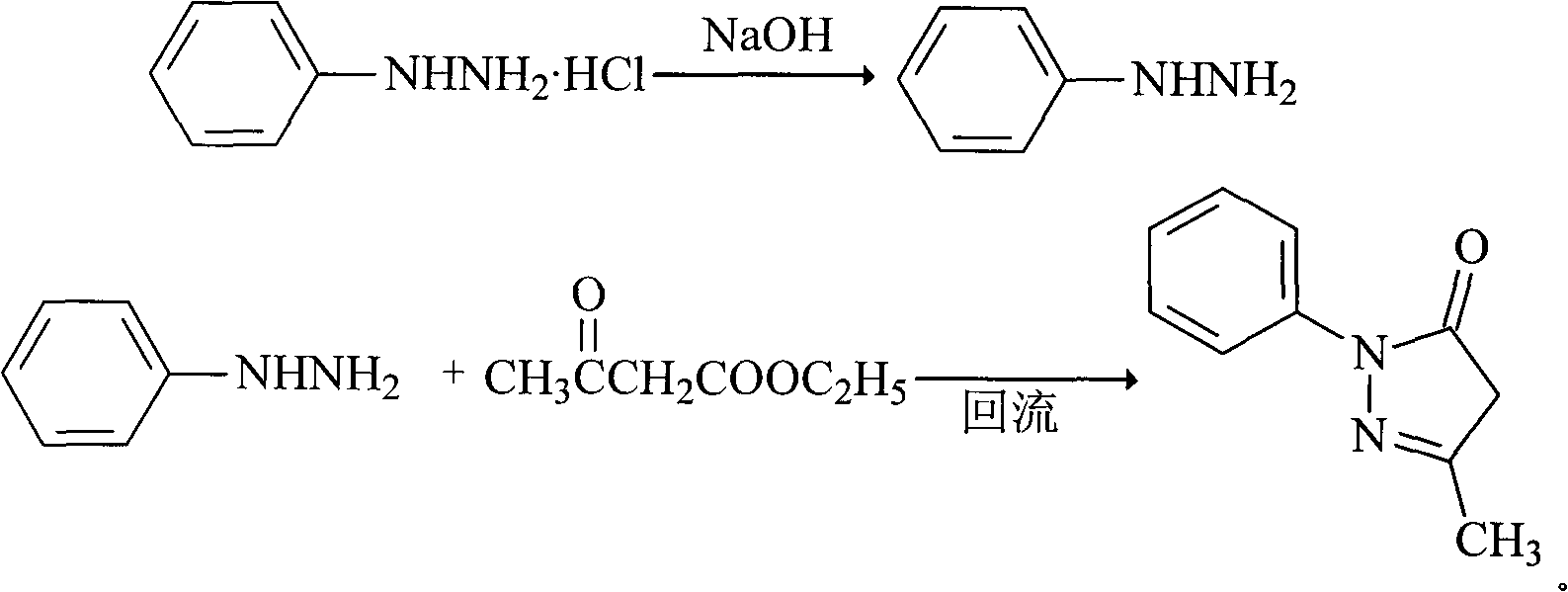
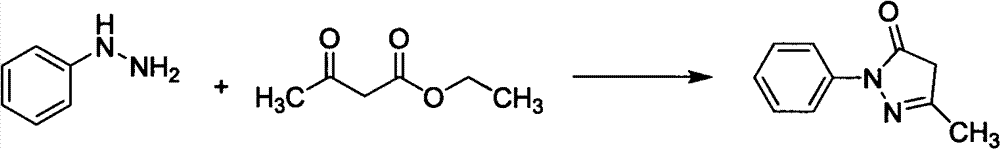
The invention relates to Edaravone synthesized by a new method. The method comprises the following steps of: reacting phenylhydrazine hydrochloride used as initial raw material with sodium hydroxide to generate phenylhydrazine; mixing the phenylhydrazine and ethyl acetoacetate to generate reflux reaction to prepare crude Edaravone; dissolving the crude Edaravone into an isopropanol water solution, adding active carbon for adsorbing and filtering to prepare fine white crystalloid powder. The invention has the advantages of low cost, high product yield and high purity.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method for edaravone
ActiveCN102180834ASimple processThe reaction conditions are mild and easy to controlOrganic chemistryAcetic acidAlcohol
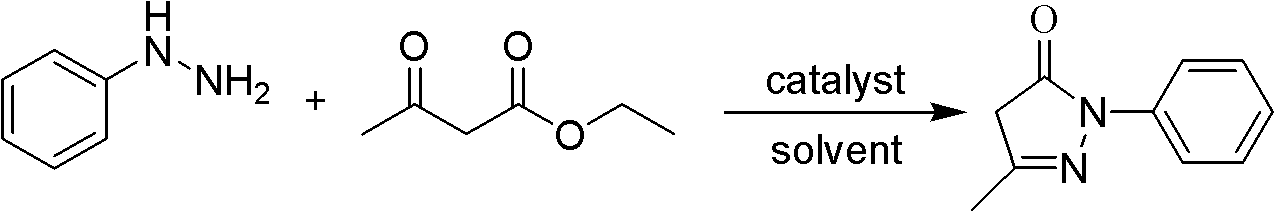
The invention discloses a preparation method for edaravone. The preparation method comprises the following steps of: reacting phenylhydrazine with ethyl acetoacetate in an alcohol solvent under the action of a catalyst to prepare the edaravone; and after the reaction, adding a non-alcohol solvent to cool and crystallize to obtain the edaravone crude product. The high-yield and high-purity edaravone is prepared from the phenylhydrazine and the ethyl acetoacetate which serve as the raw materials in the presence of acid serving as the catalyst. The quality of the edaravone product is high; the process is simple; the reaction condition is mild and easy to control; the aftertreatment is simple; the problem of three wastes is avoided; cost is low; and the preparation method is suitable for industrialized production.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
Edaravone lyophilized preparation and preparation technique thereof
ActiveCN101288650ASimple preparation processSuitable for industrial productionPowder deliveryOrganic active ingredientsFreeze-dryingFreeze dry
The invention relates to a stable edaravone freeze-dried preparation, a preparation process and a using method. The preparation is composed of edaravone and a freeze-dried stabilizer. The preparation process is that the edaravone and the freeze-dried stabilizer are dissolved in a mixed solvent system with tertiary butyl alcohol / water, and the freeze-drying process is carried out for freeze-drying. The preparation has stable quality and the preparation process is applicable to the industrial production.
Owner:JIANGSU SIMCERE PHARMA +1
Edaravone-containing injection
InactiveCN101732247AReduce the amount of infusionSuitable for useOrganic active ingredientsInorganic non-active ingredientsHigh concentrationEdaravone Injection
The invention relates to an edaravone-containing injection, which belongs to the field of pharmaceutical preparation. The edaravone-containing high-concentration injection consists of 1 to 5 percent (w / v) of edaravone, 5 to 25 percent (v / v) of 1,2-propanediol, 5 to 15 percent (v / v) of ethanol and water for injection, and has a pH of 4.5 to 5.5. The edaravone-containing injection in clinical use is added to sodium chloride infusion solution for the intravenous drip of patients, increases the population of applicable patients, and is applicable to the patients with acute cerebral infarction.
Owner:湖南万健康品生物科技有限公司
Preparation method and detection method for edaravone dimer and tautomer thereof
ActiveCN102180833ASimple and fast operationStarting materials are readily availableOrganic chemistryComponent separationSolventHigh-performance liquid chromatography
The invention belongs to the pharmaceutical field, and specifically relates to a preparation method and a detection method for edaravone dimers and tautomers thereof. The preparation method is characterized in that edaravone is dissolved in a solvent; a catalyst is added; the mixture is heated and filtered to obtain an insoluble substance; the substance is washed and dried to obtain a product. The detection method adopts a high performance liquid chromatography method. The preparation method of the invention has convenient operation and easily available initial raw materials, and the detection method is applicable to the detection of edaravone dimers in edaravone raw materials and preparation samples.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
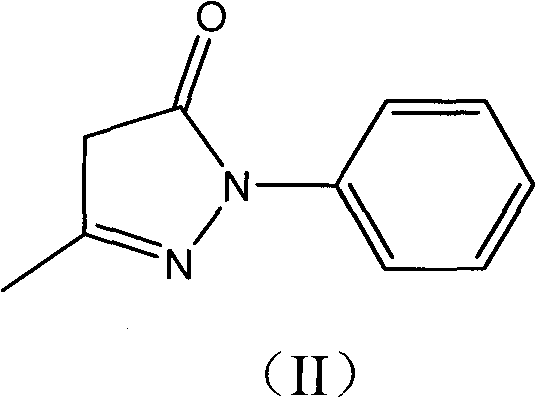
New application of 3-methyl-1-phenyl-2-pyrazoline-5-ketone and 2-borneol composition
ActiveCN102579432AProtectiveLow therapeutic useHydroxy compound active ingredientsCardiovascular disorderReperfusion injuryCardiac muscle
The invention relates to application of an edaravone and borneol medicinal composition to preparation of a medicament for preventing and / or treating myocardial ischemia-reperfusion injury.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD +1
Methods for treating acute acoustic trauma
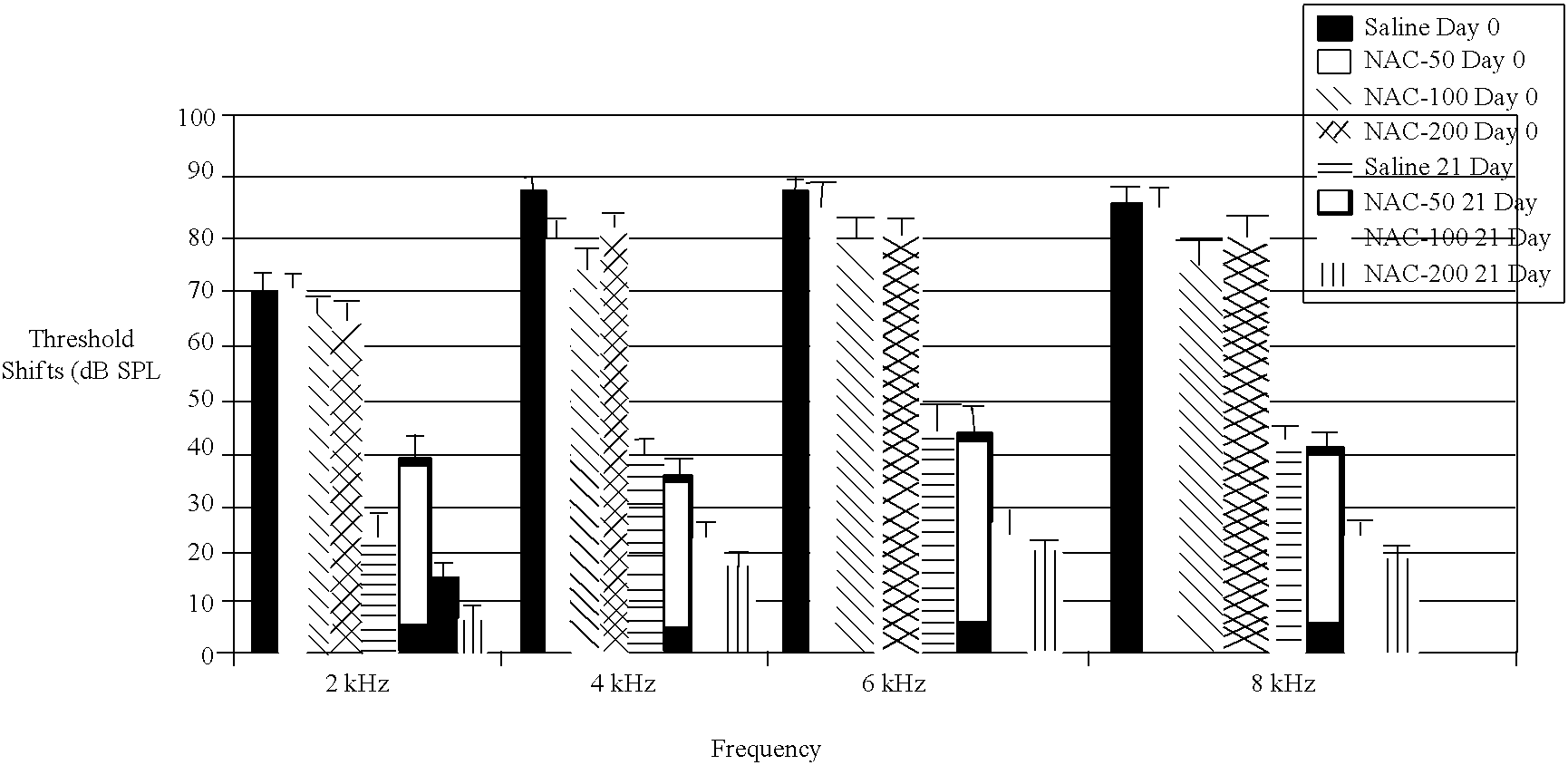
The current invention provides methods and compositions for treating sensorineural hearing loss including but not limited to acute acoustic trauma (AAT). The composition includes compounds which function as free radical traps such as phenyl butyl nitrone (PBN), free radical scavengers, such as edaravone, resveratrol, ebselen and iron chelator and compounds from the family of antioxidant compounds including, but not limited to, N-acetylcysteine (NAC), Acetyl-L-Carnitine (ALCAR), glutathione monoethylester, ebselen, D-methionine and carbamathione. The compositions of the current invention may be delivered by injections or orally.
Owner:OKLAHOMA MEDICAL RES FOUND
Edaravone lipid microsphere formulation and preparation method
InactiveCN101536979ASafety proofImprove stabilityOrganic active ingredientsNervous disorderLipid formationEdaravone Injection
The invention relates to an edaravone lipid microsphere formulation and a preparation method thereof. The edaravone lipid microsphere formulation is prepared by taking the mixed emulsifier of granulesten and poloxamer188 with specific rate as an emulsifier and injection soybean oil as oil phase solvent, and also contains pharmaceutically regular antioxidant. Not only does the prepared edaravone lipid microsphere injection have simple process, low cost and easy industrial production, but also obviously improved stability compared with the existing edaravone injection.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method for edaravone
The invention discloses a preparation method for edaravone. The preparation method comprises a step of subjecting hydrazinobenzene and ethyl acetoacetate to a cyclization reaction under a solvent-free condition and action of an acid so as to prepare edaravone, wherein the usage amount of the acid is 0.02 to 10 equivalent of the molar weight of hydrazinobenzene. The preparation method provided by the invention has the advantages of mild conditions, fast reaction, safety, reliability, easy and convenient operation, low cost, almost quantitative completion of the reaction, capacity of obtaining a high purity (greater than 98%) crude product which accords with medicinal standards after simple recrystallization, and suitability for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Stable and safe edaravone injecta
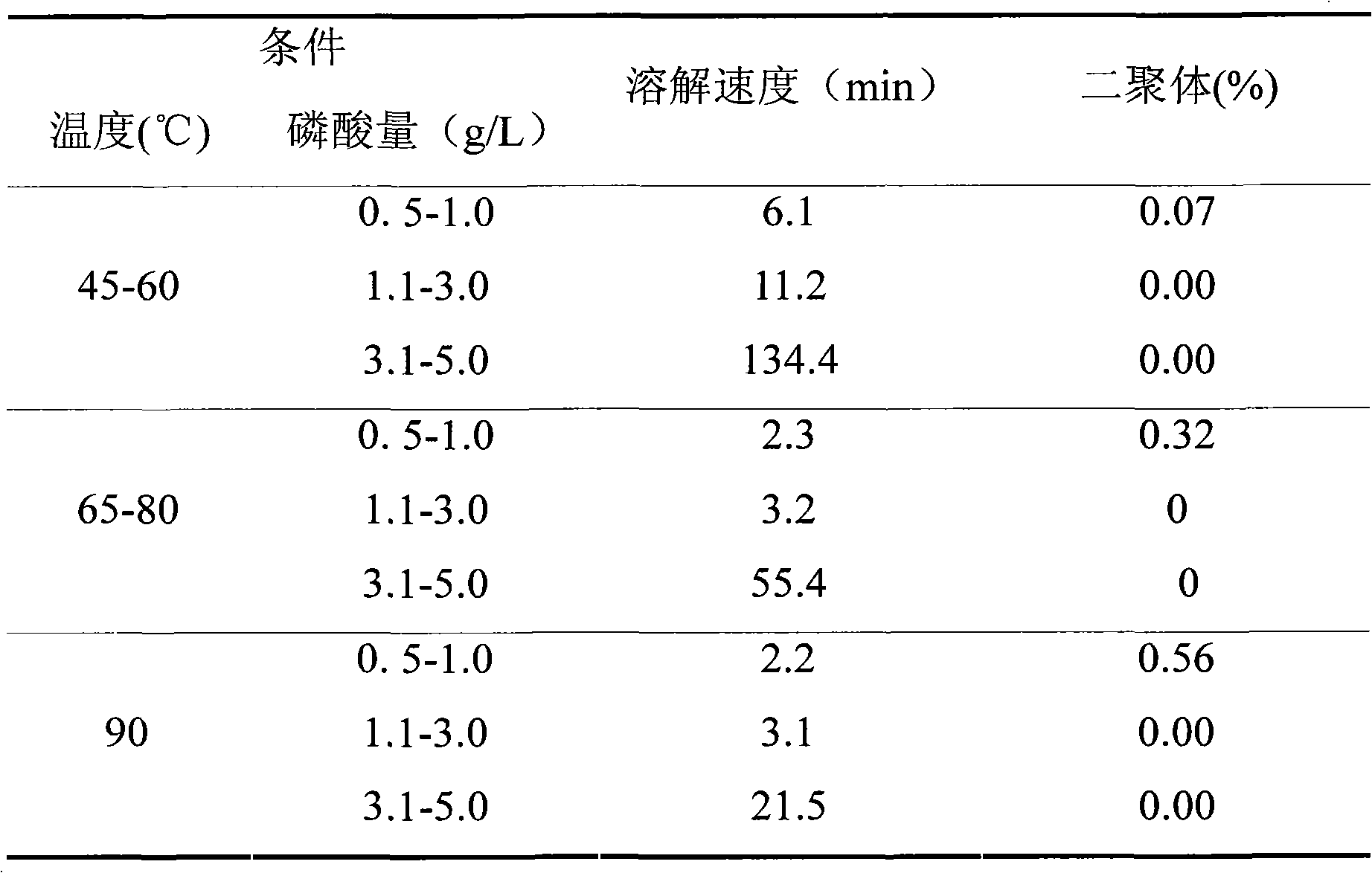
ActiveCN102144964AImprove stabilityQuality improvementOrganic active ingredientsInorganic non-active ingredientsEdaravone InjectionPhosphate
The invention belongs to the field of pharmaceutical preparations, in particular to an edaravone injecta and a preparation process thereof. The edaravone injecta contains edaravone and pharmaceutically acceptable auxiliary materials and is characterized in that the injecta contains an antioxidant and a pH regulator, wherein the antioxidant contains phosphate, L-cysteine hydrochloride and sodium bisulfite. A whole-process nitrogen-filing and oxide-emitting method and a post-sterilization rapid cooling method are adopted in the preparing and filling process of the edaravone injecta to improve the stability of the edaravone injecta, remarkably reduce the content of a related substance, i.e., dimmer and provide the stable and safe edaravone injecta for clinical administration.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
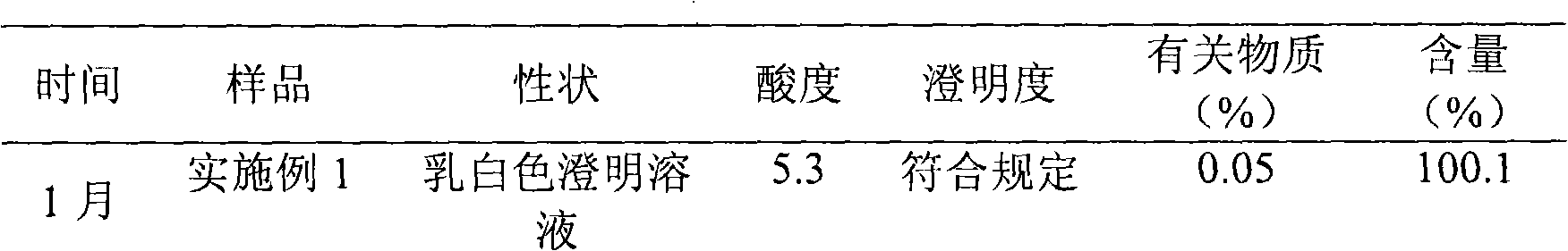
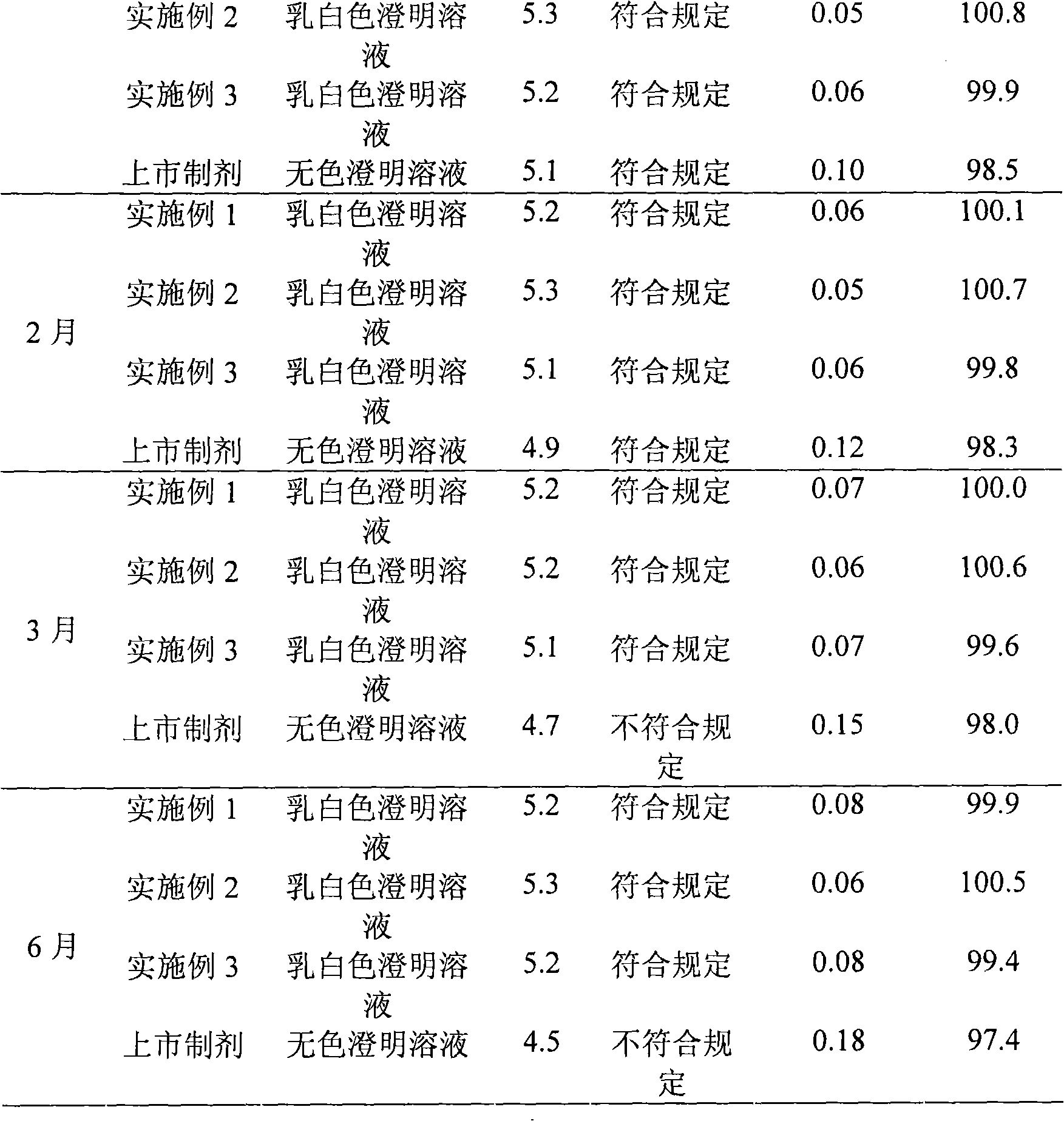
Edaravone compound with stable crystal form
The invention belongs to the technical field of medicines and provides an edaravone compound with a stable crystal form and a preparation method thereof. The method comprises the following steps of: stirring to dissolve a crude edaravone product in a pure aqueous solution of ethanol, regulating the pH value to be 3 to 5 by using hydrochloric acid, adding a mixed solution in which the volume ratio of ethers to ketones is 1:(1-4), and stirring to precipitate crystals; and filtering, collecting, washing, and performing vacuum drying to obtain the edaravone compound. The yield is 90 percent, and the purity is 99.6 percent.
Owner:TIANJIN SONGRUI MEDICAL TECH
Edaravone liposome injection and new application thereof
InactiveCN101601656AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolThrombus
The invention discloses an edaravone liposome preparation form. The edaravone liposome injection is prepared from excipients, such as edaravone, phospholipid, cholesterol, polysorbate 80, and the like. The invention further discloses new application of the edaravone liposome injection in treating thrombus type phlebitis.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
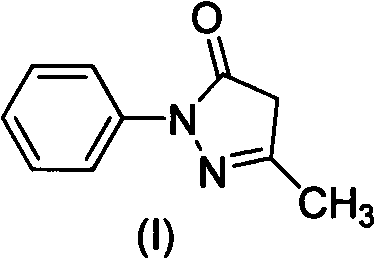
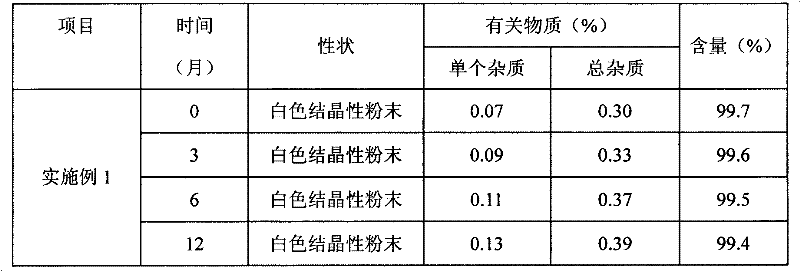
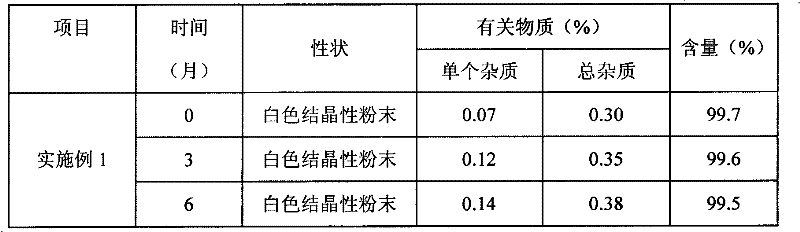
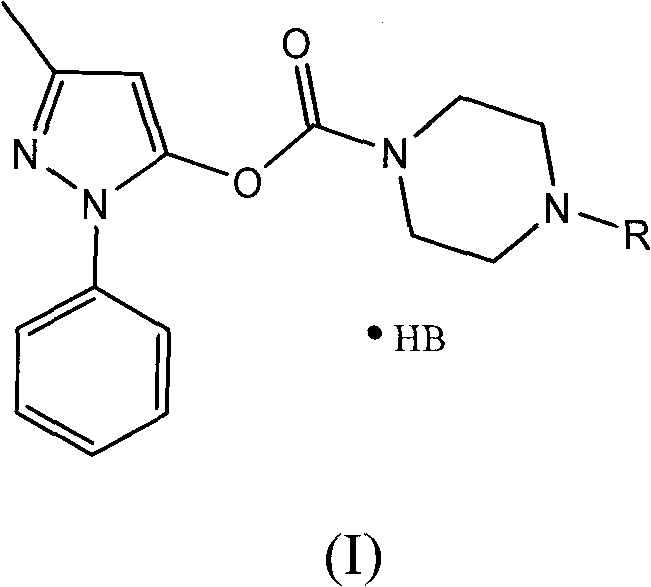
Water-soluble derivatives of edaravone, preparation method and application thereof
ActiveCN102190622ANo significant difference in efficacyAvoid side effectsOrganic active ingredientsNervous disorderSolubilityOrganic group
The invention discloses a series of amino carbonic ether derivatives of edaravone capable of reacting with acid salt to form water-soluble derivatives, and a preparation method thereof. The water-soluble derivatives are represented by the general formula (I), wherein R represents H, methyl, ethyl, propyl, isopropyl, butyl or benzyl, and HB represents the acid capable of reacting with organic groups containing nitrogen to form salt. The compound disclosed in the invention has favorable water-solubility and has the same cerebral protective effect in animals with edaravone.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
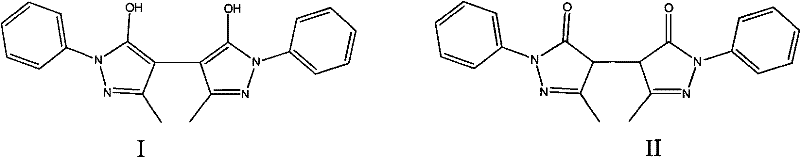
Pyrazolines compound as well as application and preparation method thereof
The invention relates to a compound having a structure as shown in a specification as well as a preparation method and application thereof. The preparation method comprises the steps of: reacting edaravone, sodium pyrosulfite and 1,2-propylene glycol; placing the solution of the reaction under an environment with the temperature of 35-55 DEG C for 96-168 hours and filtering for later use; and separating the edaravone solution by using a reversed phase high performance liquid chromatography method for preparing 4-(1-hydroxy-2-sulfo-propane-2-yl)-3-methyl-1-phenyl-2-pyrazoline-5-ketone. The compound can be used a comparison product of edaravone impurities and is convenient for controlling edaravone and the content of the edaravone in relevant preparations.
Owner:JIANGSU SIMCERE PHARMA +2
Use of edaravone in preparation of drug for preventing and treating cerebral amyloid angiopathy (CAA)
ActiveCN105616405AEliminate depositsAvoid depositionOrganic active ingredientsNervous disorderAmyloid angiopathyProtein C
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Edaravone injection solution and preparation method thereof
ActiveCN103349776AGood antioxidant propertiesGood treatment effectOrganic active ingredientsNervous disorderVitamin CEdaravone Injection
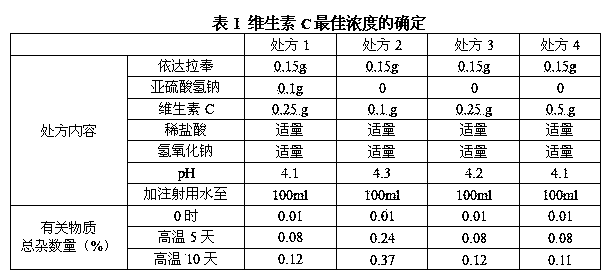
The invention relates to an edaravone injection solution and a preparation method thereof. The edaravone injection solution contains vitamin C and glutathione, wherein the concentration of edaravone is 0.1%-0.2% (W / V), the concentration of the vitamin C is 0.1%-0.5% (W / V), and the concentration of glutathione is 1.0%-10.0% (W / V). Compared with the prior art, the edaravone injection solution has the beneficial effects that the edaravone, the vitamin C and the glutathione are reasonably matched to enable the three ingredients to exert a synergistic effect, so that the excellent oxidation property is given full play, the cerebral injury and the complications are well treated, and the stability is high.
Owner:北京普瑞博思生物科技有限公司
Edaravone injection without antioxidant and preparation method thereof
ActiveCN103083232AResidual oxygen guaranteeOrganic active ingredientsNervous disorderEdaravone InjectionAntioxidant
The invention belongs to the field of medicine preparation, and particularly relates to edaravone injection without an antioxidant and a preparation method thereof. The invention aims at providing the edaravone injection which contains few impurities, is short in preparation time and is free of addition of the antioxidant. The edaravone injection comprises edaravone, a pH value conditioning agent and water for injection; and the preparation method comprises the steps of: A. preparing liquid; B filtering; C. filling; and D. sterilizing, wherein nitrogen is fed for protection in the previous three steps, and the remaining oxygen in the water and the impurity content of the product are strictly controlled so as to ensure the safety and effectiveness of the edaravone injection in clinical application.
Owner:CHENGDU BAIYU PHARMA CO LTD
Edaravone and (+)-2-camphol sublingual medicine composition
InactiveCN107773545AHydroxy compound active ingredientsPill deliveryPharmaceutical drugSublingual administration
The invention discloses an edaravone and (+)-2-camphol composition sublingual medication preparation and a preparation method thereof, wherein the sublingual medication preparation is prepared from edaravone, (+)-2-camphol, excipients, filling agents, bonding agents, disintegrating agents, lubricating agents and the like. The sublingual medication preparation has the advantages that the first passeffect of the liver can be avoided; the sample stability is high; the transportation is convenient; the use is convenient, and the like.
Owner:YANTAI YENEPHARMA BIOMEDICAL
Method for detecting impurity phenylhydrazine in edaravone
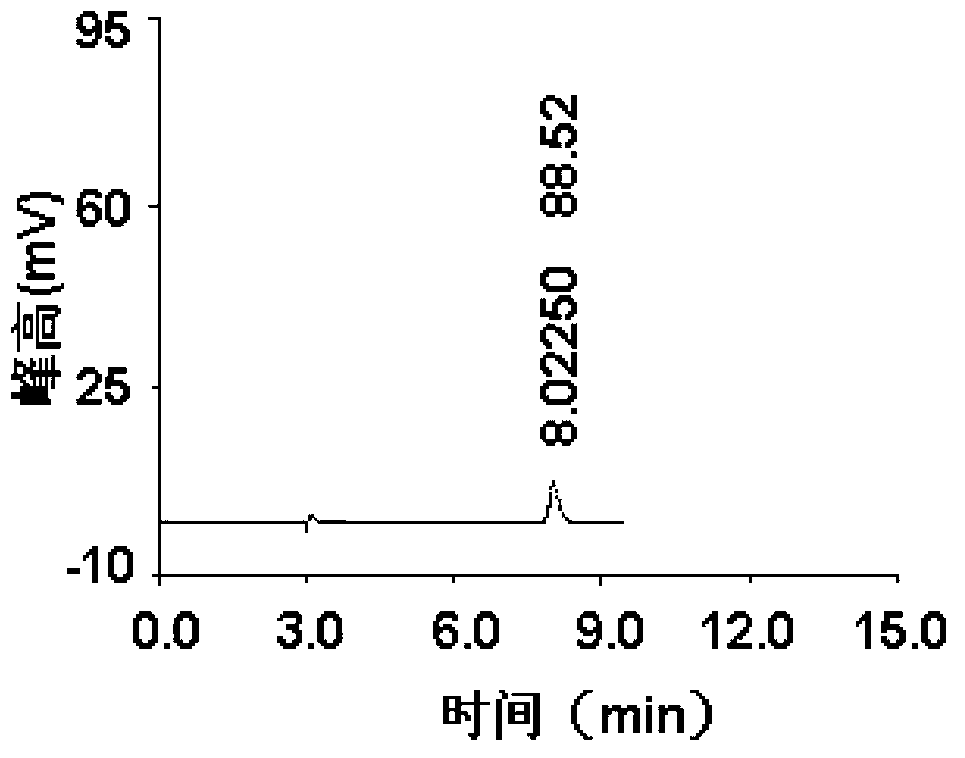
InactiveCN102841170ASharp peakSymmetrical peak shapeComponent separationRetention timeColumn temperature
Owner:CHENGDU BAIYU PHARMA CO LTD
Quality control method for edaravone and edaravone-containing preparation
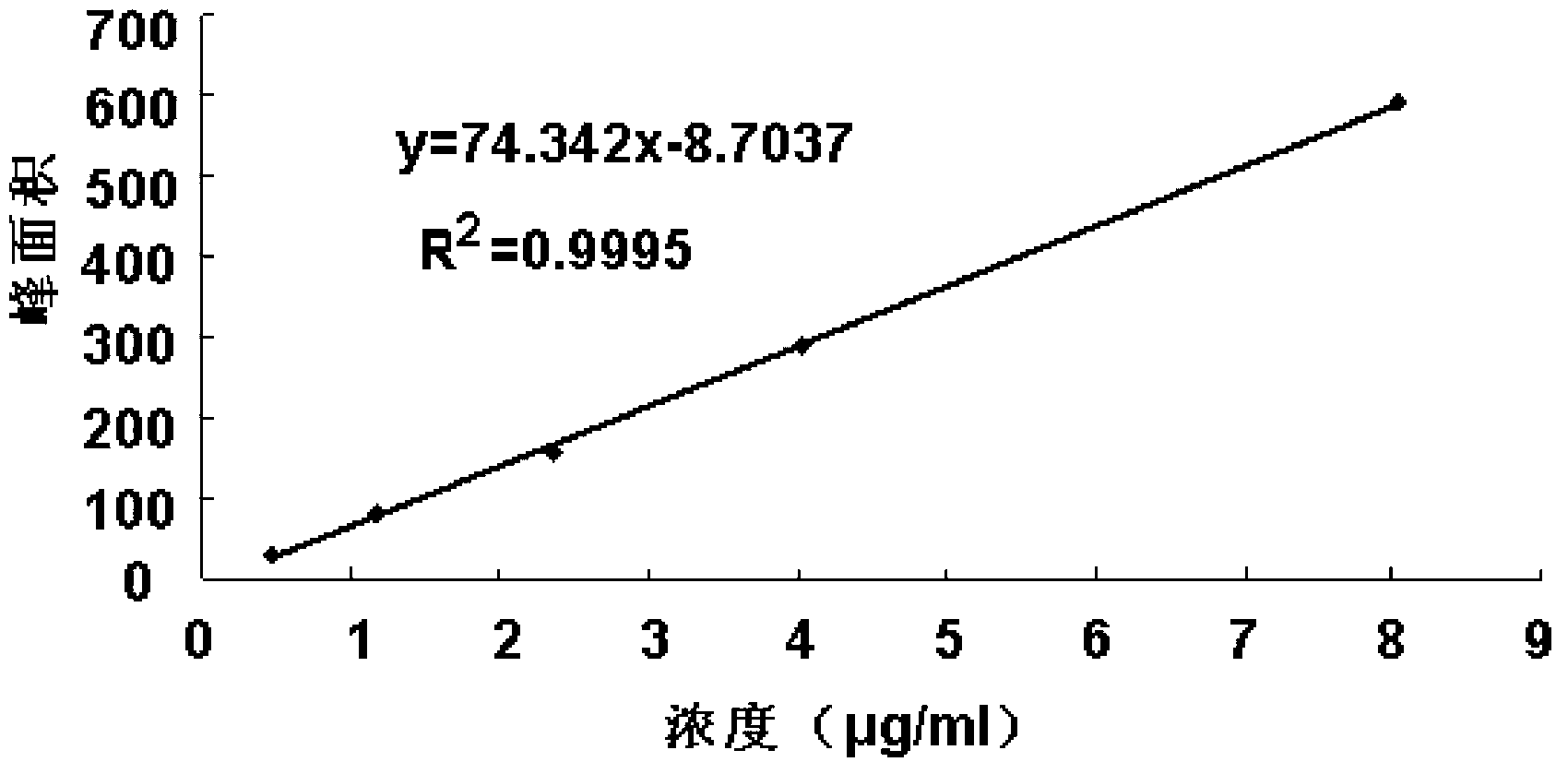
InactiveCN101968467AReliable evaluationAccurate evaluationComponent separationDiketoneQuality control
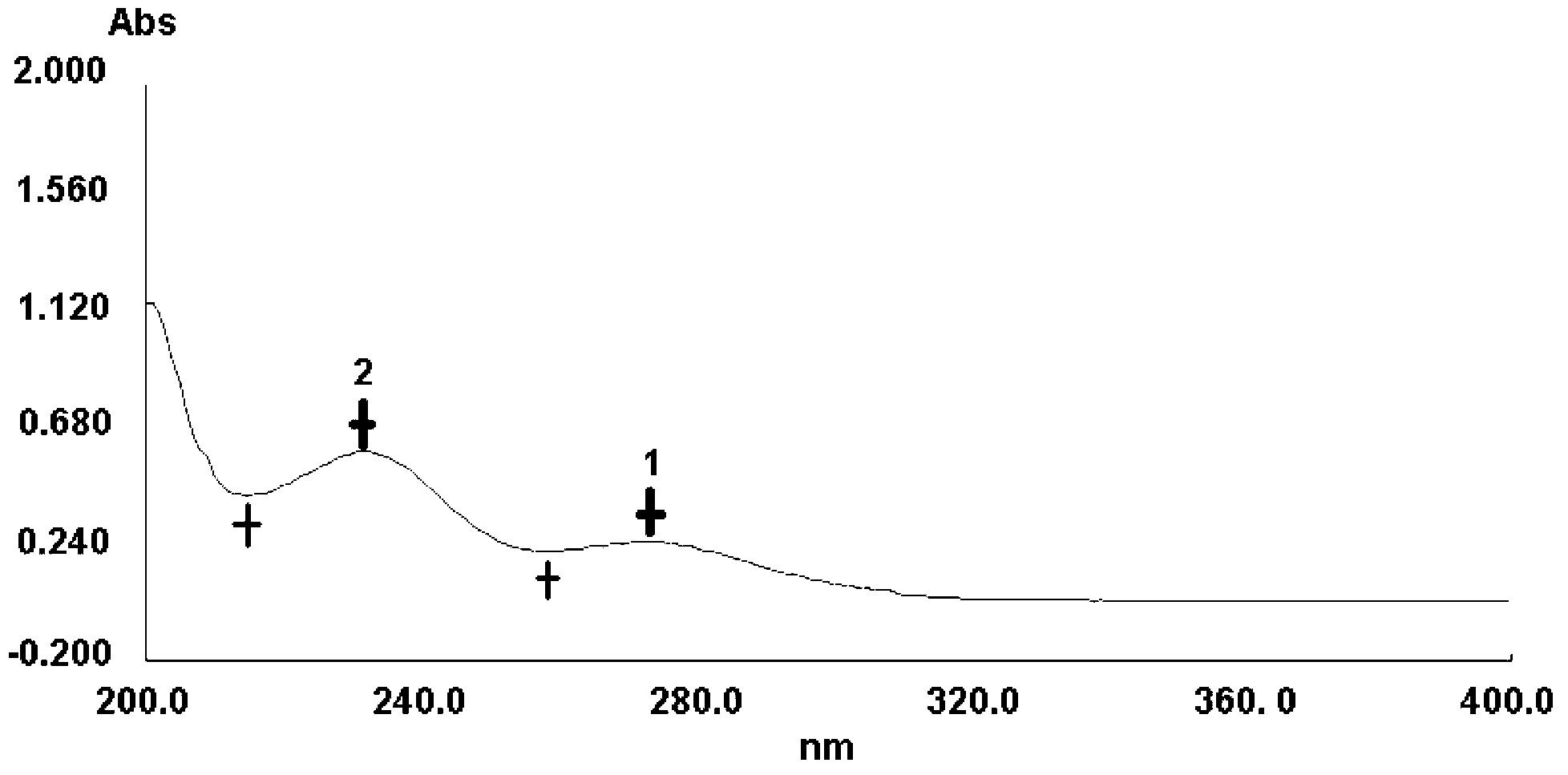
The invention discloses a quality control method for edaravone and an edaravone-containing preparation. In the method, an impurity, namely, 2,2',4,4'-tetrahydrophthalic anhydride-5,5'-dimethyl-2,2'-diphenyl-[4,4'-di-3H-parazole]-3,3'-dione in the edaravone and an edaravone preparation is determined quantitatively by photoelectric technology. By the technical scheme of the invention, the qualities of the edaravone and an edaravone-containing medicament preparation can be evaluated and controlled more reliably and accurately.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +2
Stable large volume parenteral edaravone injection and preparation process thereof
InactiveCN108324683AImprove stabilityOrganic active ingredientsInorganic non-active ingredientsFiltrationPhosphate
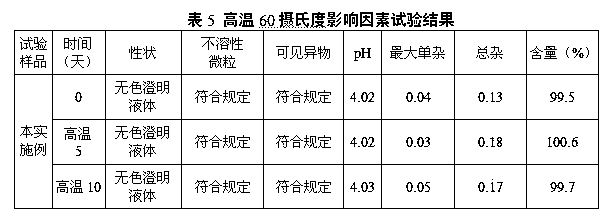
The invention discloses a stable large volume parenteral edaravone injection and a preparation process thereof, and belongs to the field of medicine preparation. The edaravone injection includes a therapy active component raw material edaravone, an osmotic pressure regulator NaCl, and a pH regulator being a phosphate buffer solution composed of phosphoric acid and NaOH, in which the NaOH also serves as a solution additive, and an antioxidant L-cysteine hydrochloride and sodium hydrogen sulfite. The specification of the large volume parenteral edaravone injection is 100 ml, containing 0.03 g ofthe edaravone and 0.855 g of NaCl. The preparation method includes the steps of: 1) liquid preparation, 2) filtration, 3) fill packaging, and 4) sterilization, wherein in the steps 1-3, a system is filled with high-purity nitrogen gas for protection, water-for-injection is boiled for removing oxygen, and residual oxygen content in medicine liquids and content of related substances in the productare strictly controlled in order to guarantee the safety and efficacy of the product. Influence factor experiments prove that generation of related substances is relative low. The production has stable quality and is safe.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
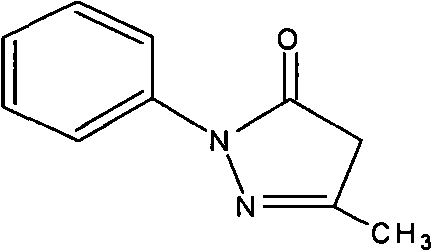
Edaravone A-type crystal and preparation method thereof
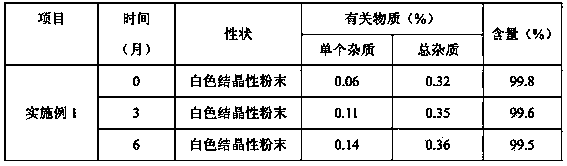
The invention discloses an edaravone A-type crystal and a preparation method thereof. The X-ray diffraction pattern of the crystal is basically shown in the figure 1. According to the crystal and the method thereof, related substance of the product can be effectively reduced, the purity is improved, and the reaction yield is increased. The crystal has strengthened stability, and is suitable for storing particularly in high-temperature high-humidity conditions. The crystal has excellent solubility due to its better chemical property and stable crystal distribution. When medicine is prepared, satisfactory dosage accuracy can be achieved, so as to improve the safety of the product and reduce the patient's risk. Therefore, the crystal provided by the invention is suitable for preparing stable medicinal preparation.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
A kind of optimized Edaravone synthetic method
ActiveCN102285920ALow costTroubleshoot problems during synthesisOrganic chemistrySynthesis methodsFiltration
The invention belongs to the technical field of medical chemistry, and particularly relates to an optimal edaravone synthesis method. The method comprises the following steps in sequence: (1) adding phenyl hydrazine or phenylhydrazine hydrochloride serving as an initial raw material into a container in which water is held under a stirring condition and adjusting the pH value to be 6.0 by taking a proper amount of concentrated hydrochloric acid or ammonia water; (2) slowly dropwise adding ethyl acetoacetate into the solution obtained in the step (1), reacting and discharging heat, adding sodium hyposulfate after the solution is cooled to room temperature, heating the solution, performing reflux reaction for 2-5 hours, stopping heating, stirring, cooling, and performing vacuum filtration to obtain a light yellow granular edaravone rough product; and (3) recrystallizing the edaravone rough product obtained in step (2) by using absolute ethanol, performing vacuum filtration and drying to obtain white crystalline powder solid, namely an edaravone finished product. The method has the advantages of low cost, high yield and high purity; and the problems existing in the conventional edaravone synthesis process are solved.
Owner:JILIN BODA PHARMA
Compound injection preparation for treating cardiovascular and cerebrovascular diseases and preparation method thereof
InactiveCN102526065AEffective treatmentOvercome the disadvantages of usingOrganic active ingredientsPowder deliveryArterial Occlusive DiseasesAdditive ingredient
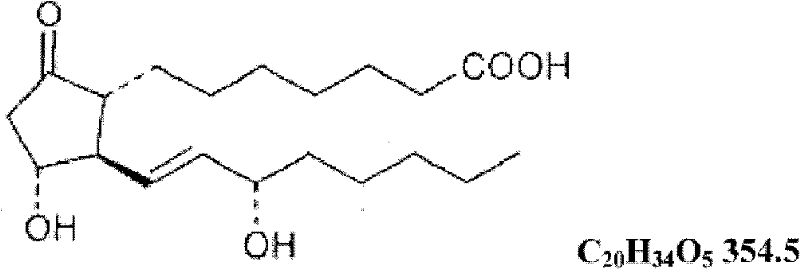
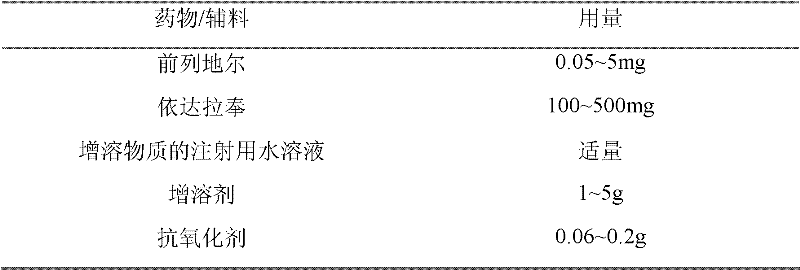
The invention discloses a compound injection preparation for treating cardiovascular and cerebrovascular diseases and a preparation method thereof. The preparation method comprises the following steps of: taking alprostadil and edaravone as active pharmaceutical ingredients, and compounding the alprostadil and the edaravone according to a certain ratio to form a certain injection preparation. With the adoption of the compound injection preparation, heart vessels or brain vessels can be effectively dilated, the chronic arterial occlusive diseases, caused by thromboangiitis obliterans, diabetes and the like, can be effectively treated, and meanwhile, the pain generated in the conventional injection of an alprostadil preparation can be remarkably abated due to the adoption of the compound preparation of the alprostadil and the edaravone.
Owner:XIAN LIBANG ZHAOXIN BIOTECH CO LTD
Synthesis process of high-purity edaravone
ActiveCN106117144AReduce build timeShort reaction timeOrganic chemistryAcetic acidTemperature control
The invention discloses a synthesis process of high-purity edaravone. The synthesis process comprises the steps of dissolving ethyl acetoacetate into lower alcohol, adding phenylhydrazine drop by drop under the condition of temperature control, adding a catalytic amount of alkali, carrying out heating reflux cyclization, crystallizing, filtering, washing and refining to obtain the edaravone, wherein the purity of the obtained edaravone is higher than 99.97%. The synthesis process is simple in process, high in yield and pure in product, thus being suitable for industrial production.
Owner:HEFEI JIUNUO MEDICAL TECH
Preparation method of fat emulsion of cerebral protection therapeutic drug
ActiveCN102058531AImprove solubilityImprove stabilityOrganic active ingredientsAntinoxious agentsYolkHemolysis
The invention relates to an Edaravone fat emulsion injection and a preparation method thereof. The method comprises the following steps of: preparing Edaravone into a fat emulsion injection; and preparing a fat emulsion injection capable of being used for intravenous injection through homogenizing under high pressure by utilizing injection oil as a solvent and high-purity yolk lecithin with good biocompatibility as an emulsifier. Compared with the preparations clinically applied at present, the preparation is prepared without using organic solvents such as alcohol, propylene glycol, and the like and adding cosolvents such as poloxamer, and the like, thereby improving the medication safety, meanwhile, proved by animal experiments, the preparation has stronger antioxidative cerebral protection effect, and shown as relevant safety test results, the new preparation does not have the phenomena of hemolysis and blood vessel stimulation.
Owner:XIAN LIBANG PHARMA
Butylphthalide- and edaravone-containing compound injection and preparation method thereof
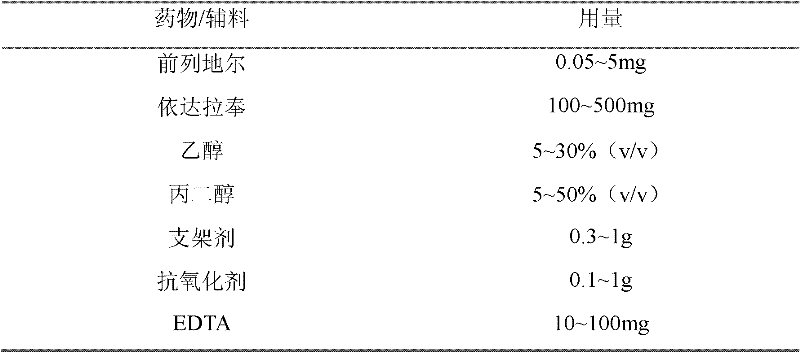
ActiveCN102526036ALow incidence of adverse reactionsGood curative effectOrganic active ingredientsNervous disorderAlcoholAntioxidant
The invention relates to a butylphthalide- and edaravone-containing compound injection and a preparation method thereof. The injection of every 100 ml contains 25-50 mg of active ingredient butylphthalide, 12.5-50 mg of edaravone, as well as absolute ethyl alcohol, hydroxypropyl-beta-cyclodextrin, an osmotic pressure regulator, an antioxidant and the like. With the adoption of the butylphthalide- and edaravone-containing compound injection, the administration dosage is greatly lowered, the burden of liver metabolism is reduced, and the incidence rate of liver adverse reactions is lowered. According to determination, with the adoption of the butylphthalide- and edaravone-containing compound injection, the administration dosage of the edaravone can be reduced by at least 20%, besides, the compound injection has a better curative effect, and the incidence rate of the liver adverse reactions is also remarkably lowered.
Owner:SHIJAZHUANG ZHONGSHUO PHARMA CO LTD
Stable edaravone trihydrate and pharmaceutical compositions thereof
InactiveCN103483262ASolve insolubleHigh yieldOrganic active ingredientsOrganic chemistryAlcoholEther
The invention belongs to the technical field of medicine, and provides stable edaravone trihydrate, edaravone trihydrate pharmaceutical compositions for injection, a method for preparing the edaravone trihydrate and a method for preparing the edaravone trihydrate pharmaceutical compositions. Crude edaravone trihydrate is stirred and dissolved in an ethyl alcohol-pure water solution, the pH value is adjusted through hydrochloric acid to range from 3 to 5, and then liquor formed by mixing ethers and ketone according to the volume ratio of 1:(1-4) is added to the solution, and stirring and crystallization are carried out; filtering, collecting, washing and vacuum drying are carried out to obtain the edaravone trihydrate which is high in yield and purity and stable in quality. The edaravone trihydrate pharmaceutical compositions, for injection, prepared according to the preparation formulation and the preparation technology are stable in quality and can be easily employed in an industrialized mode.
Owner:TIANJIN SONGRUI MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com