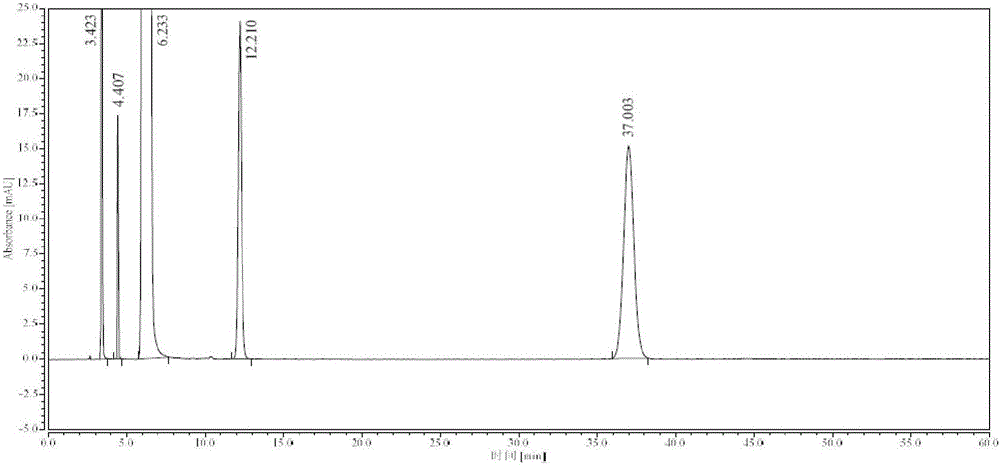
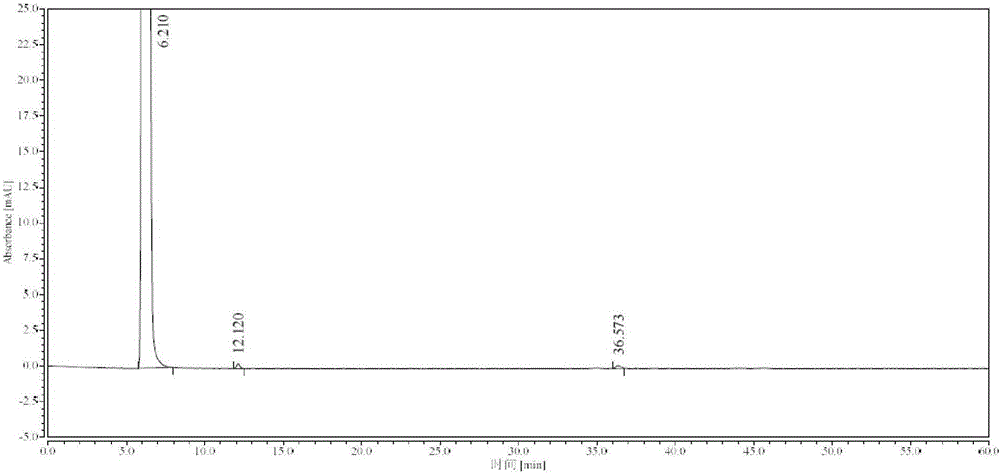
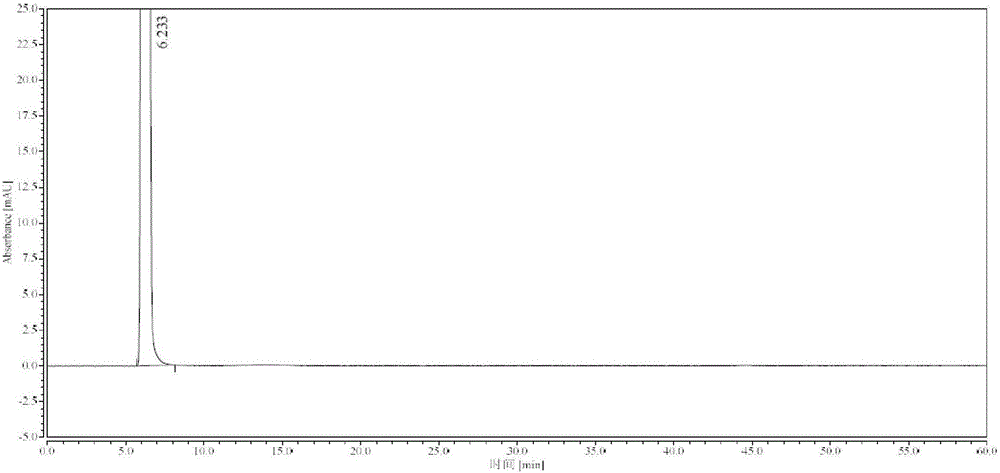
Synthesis process of high-purity edaravone
A synthetic process, edaravone technology, applied in the field of medicine, can solve the problems of dark color, many impurities, high cost, etc., and achieve the effects of improving product purity, shortening reaction time, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Take 18kg (138.31mol) of ethyl acetoacetate, add 21L of absolute ethanol to dissolve, control the temperature at 15-25°C, add 14.9kg (137.78mol) of phenylhydrazine dropwise, and keep stirring at 15-25°C for 10min, add hydrogen Sodium oxide 44g (1.1mol), reflux at 80°C for 1h, stir to cool down to 0-10°C, stir and crystallize for 1h, filter, wash the filter cake with ethyl acetate until the filtrate is colorless, and obtain 23kg of wet product of Edaravone, Dissolve the filter cake with 57L of absolute ethanol under reflux, filter, stir the filtrate to cool down to 0-10°C, stir and crystallize for 3h, filter, wash the filter cake with 4L of cold absolute ethanol, and vacuum dry at 60-70°C (≥0.09MPa) for 6h 17.1 kg of white crystalline Edaravone was obtained, the total yield was 71.2%, and the purity was greater than 99.97% (HPLC method).
Embodiment 2
[0044] Take 18kg (138.31mol) of ethyl acetoacetate, add 21L of isopropanol to dissolve, control the temperature at 15-25°C, add 14.9kg (137.78mol) of phenylhydrazine dropwise, keep stirring at 15-25°C for 10min, add hydrogen Sodium oxide 44g (1.1mol), reflux at 85°C for 45min, stir to cool down to 0-10°C, stir and crystallize for 1h, filter, wash the filter cake with ethyl acetate until the filtrate is colorless, and obtain 23.5kg of wet product of Edaravone , the filter cake was dissolved with 58L of absolute ethanol under reflux, filtered, the filtrate was stirred and cooled to 0-10°C, stirred and crystallized for 3h, filtered, the filter cake was washed with 4L of cold absolute ethanol, and vacuum-dried at 60-70°C (≥0.09MPa) After 6 hours, 16.8 kg of white crystalline Edaravone was obtained, with a total yield of 70.0% and a purity greater than 99.97% (HPLC method).
Embodiment 3
[0046] Take 18kg (138.31mol) of ethyl acetoacetate, add 21L of absolute ethanol to dissolve, control the temperature at 15-25°C, add 14.9kg (137.78mol) of phenylhydrazine dropwise, and keep stirring at 15-25°C for 10min, add hydrogen Potassium oxide 62g (1.1mol), reflux at 76°C for 1.5h, stir and cool down to 0-10°C, stir and crystallize for 1h, filter, wash the filter cake with ethyl acetate until the filtrate is colorless, and obtain the wet product of Edaravone 23.3 kg, the filter cake was dissolved with 58L of absolute ethanol under reflux, filtered, the filtrate was stirred and cooled to 0-10°C, stirred and crystallized for 3h, filtered, the filter cake was washed with 4L of cold absolute ethanol, and vacuum-dried at 60-70°C (≥0.09MPa ) 6h, 17.0kg of white crystalline Edaravone was obtained, with a total yield of 70.8% and a purity greater than 99.97% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com