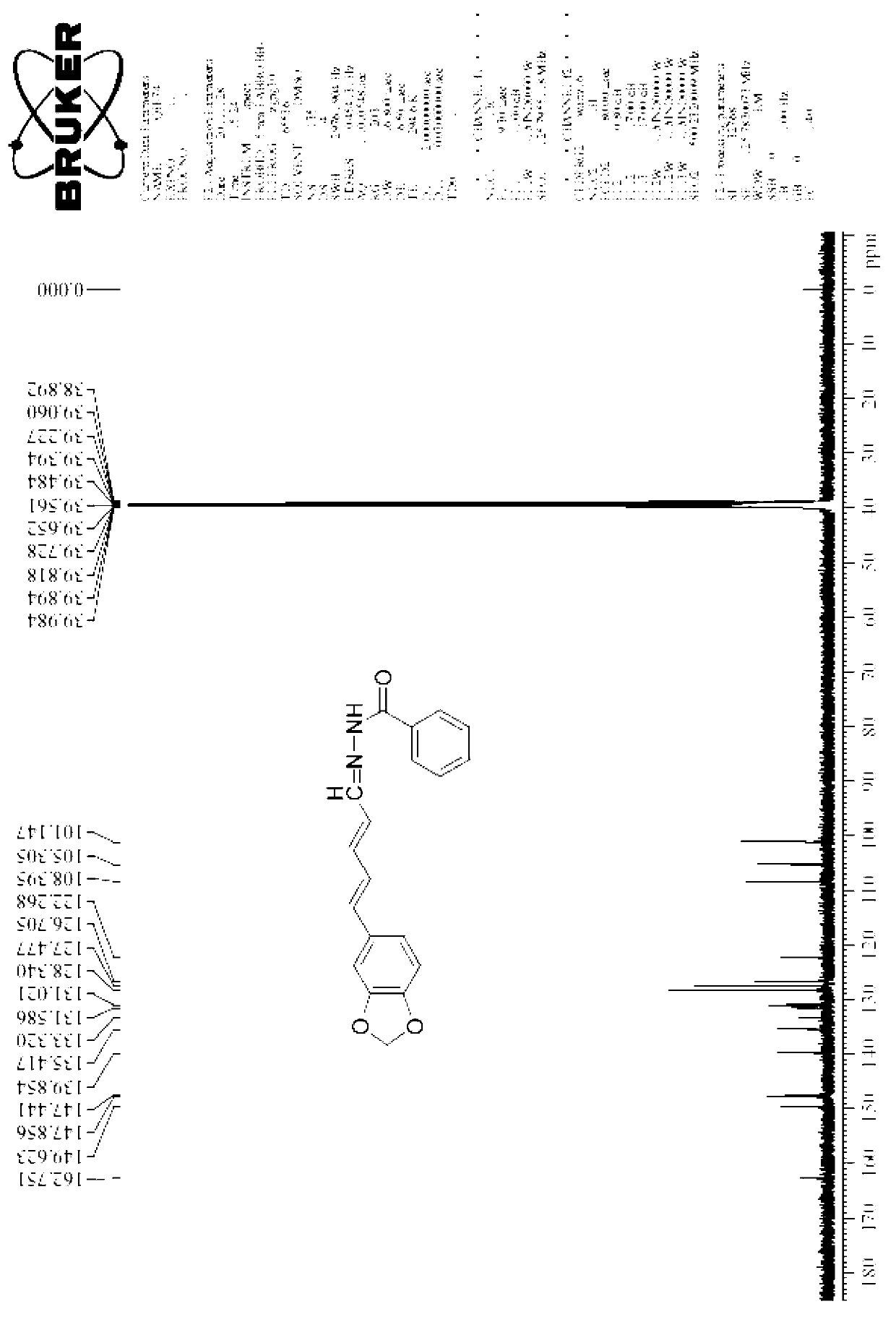
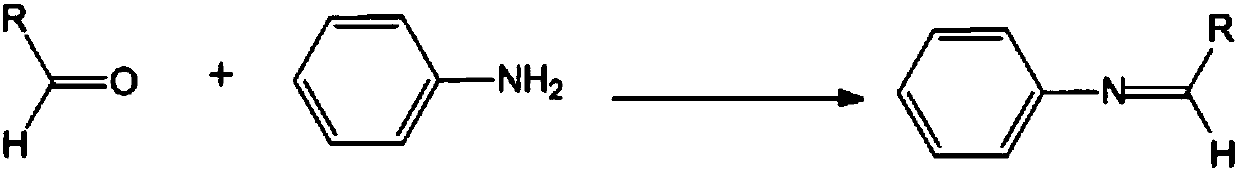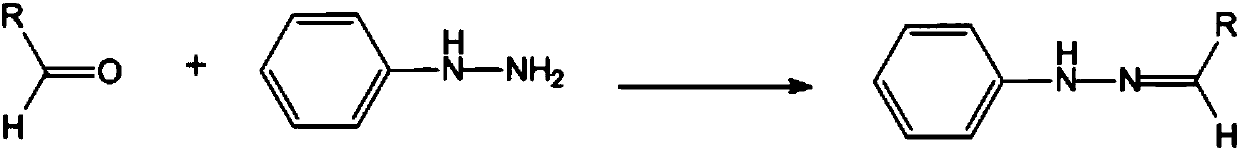Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
266 results about "Phenylhydrazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
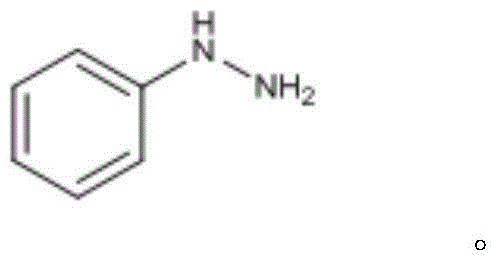
Phenylhydrazine is the chemical compound with the formula C₆H₅NHNH₂. It is often abbreviated as PhNHNH₂.
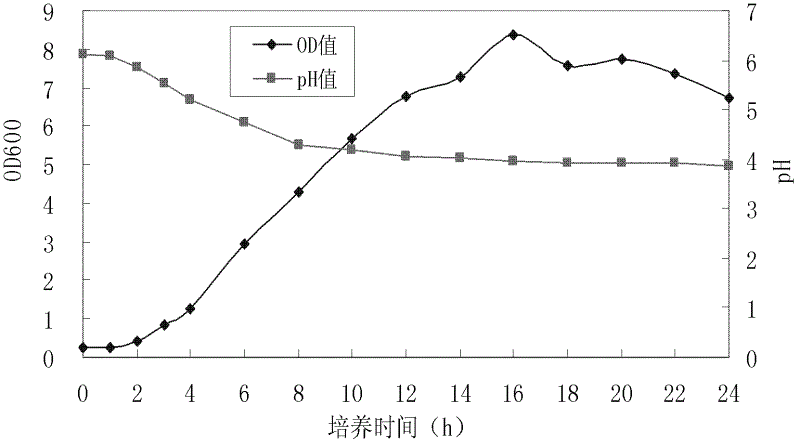
Lactobacillus rhamnosus capable of relieving chronic alcohol liver injury and application thereof
The invention relates to lactobacillus rhamnosus CCFM1107 capable of resisting oxidation and relieving chronic alcohol liver injury and also relates to application of the lactobacillus rhamnosus CCFM1107 to preparation of dairy products by using a working fermentation agent of the lactobacillus rhamnosus CCFM1107. The dairy product is milk, milk power, milk capsule products or fermented milk containing the lactobacillus rhamnosus CCFM1107. The lactobacillus rhamnosus CCFM1107 disclosed by the invention has high oxidation resistance, the capability of removing diphenyl picrylhydrazyl (DPPH) free radical and hydroxyl free radical, the capability of inhibiting lipid peroxidation and the capabilities of tolerating cholate, sodium chloride and pH. According to the lactobacillus rhamnosus CCFM1107 disclosed by the invention, the liver functions of a mouse suffered from the chronic alcohol liver injury and the antioxidant indexes can be improved; the serum endotoxin level is reduced; the distribution of intestinal flora is adjusted; and the chronic alcohol liver injury can be effectively relieved.
Owner:JIANGNAN UNIV
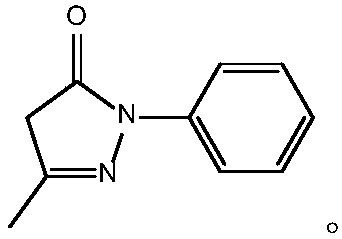
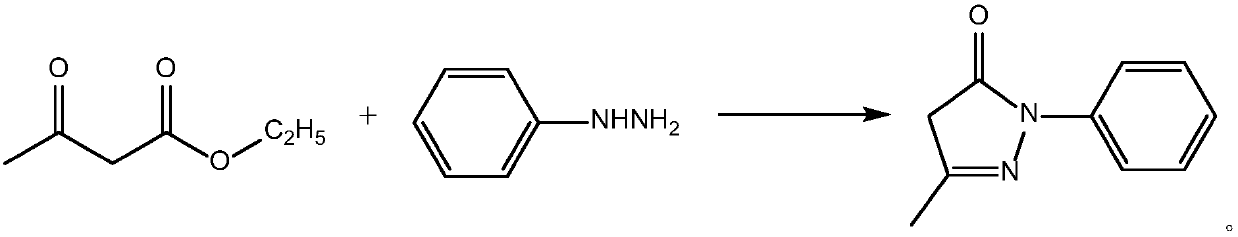
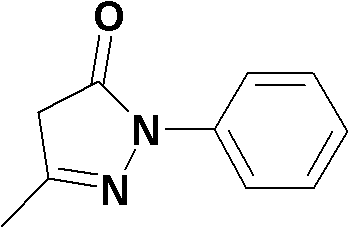
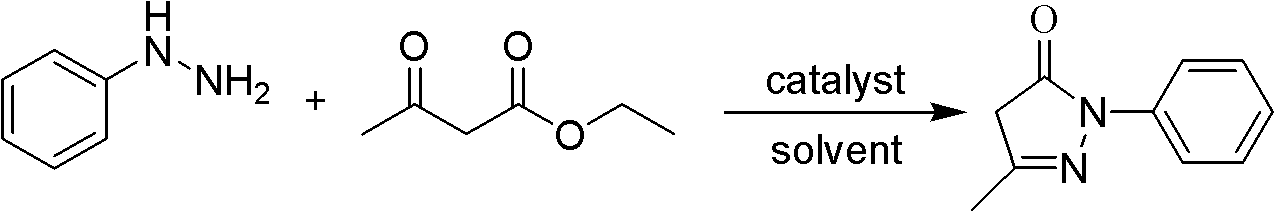
Preparation method for edaravone
ActiveCN102180834ASimple processThe reaction conditions are mild and easy to controlOrganic chemistryAcetic acidAlcohol
The invention discloses a preparation method for edaravone. The preparation method comprises the following steps of: reacting phenylhydrazine with ethyl acetoacetate in an alcohol solvent under the action of a catalyst to prepare the edaravone; and after the reaction, adding a non-alcohol solvent to cool and crystallize to obtain the edaravone crude product. The high-yield and high-purity edaravone is prepared from the phenylhydrazine and the ethyl acetoacetate which serve as the raw materials in the presence of acid serving as the catalyst. The quality of the edaravone product is high; the process is simple; the reaction condition is mild and easy to control; the aftertreatment is simple; the problem of three wastes is avoided; cost is low; and the preparation method is suitable for industrialized production.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
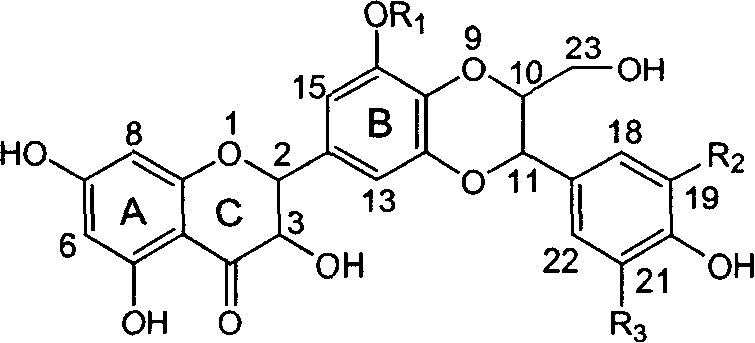
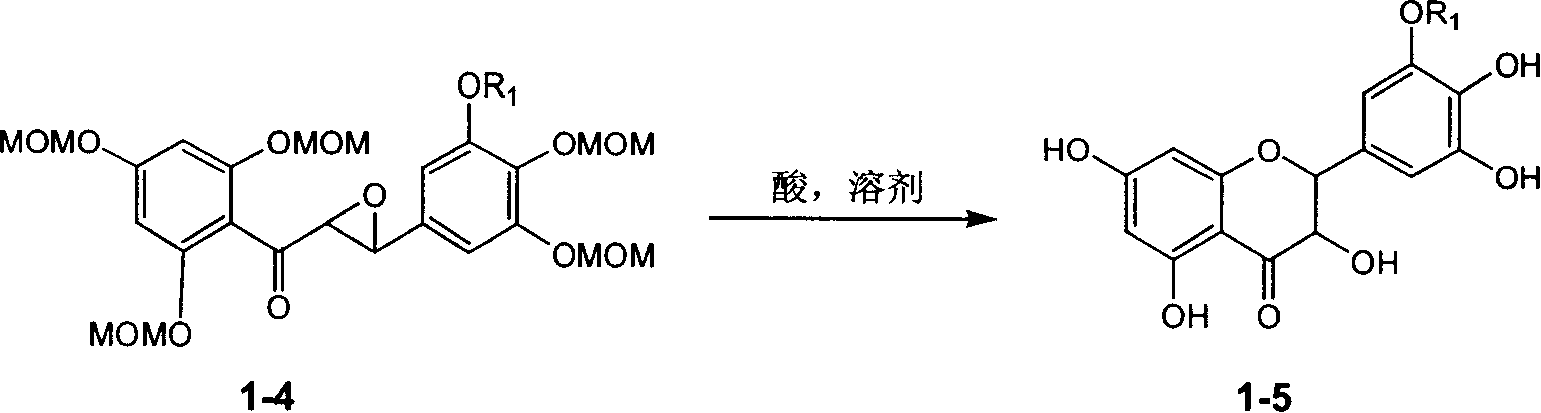
Silybin flavonolignan and their production method and use
The invention relates to a silibinin flavonolignan and its medicine salt or solvates. The invention also relates to the method preparing its important mediate and its drug combination and medical application. The compound can protect primary hepatocyte from oxidative damage for SD newly born rat hepatitis virus, so it is expected to be used as drug preventing liver damage. The compound can remove superoxide anion free radical and diphenyl picryl phenylhydrazine free radical, inhibit free radical from inducing generation of fat oxidatant, protect PC 12 cell from being damaged by free radical, so it is expected to be used as drug treating diseases caused by free radical.
Owner:ZHEJIANG HISUN PHARMA CO LTD
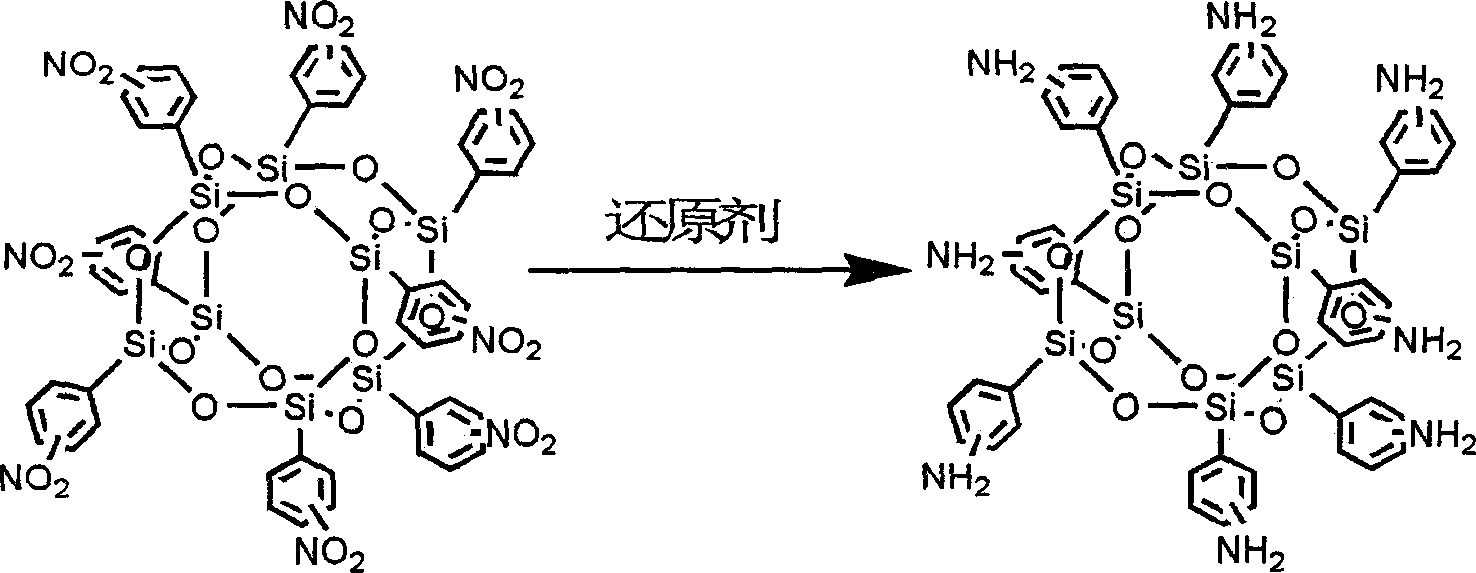
Process for preparing amino phenyl silsesquioxane
InactiveCN1844126AReduce manufacturing costRaw materials are easy to getSilicon organic compoundsHydrazine compoundNitrogen gas
This invention belonging to nano material technology is the synthesis of eight-amino-benzene cage model sesquialter siloxane. The previous method adopts aminic acid as hydrogen donor and Pd / C catalysis system. Our method is as followings:mix the eight-nitrobenzene cage model sesquialter siloxane, solvent and Fe / C catalysis in the weight propotion of 10:50-200:2-10,stiring in the air or nitrogen at 50- 100deg C; add 2-4 weight folds hydrazine hydrate or phenylhydrazine into eight-nitrobenzene cage model sesquialter siloxane, react for 2-12 hours; cool to ambience temperature, filter and add acetic ester for extraction, wait to layers; Get organic facies and precipitate in ligarine,obtain white sediment after separation and dry. This invention is advantageous in cheap, stable and long-term active Fe / C catalysis system, is advantageous in production efficiency.
Owner:BEIJING UNIV OF CHEM TECH
Celecoxib preparation process
InactiveCN102746231AMild reaction conditionsEasy to operateOrganic chemistryTrifluoromethylCyclooxygenase
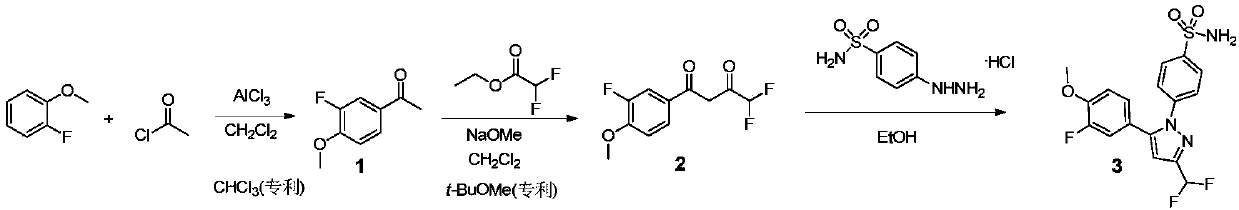
The invention provides a process for simply and efficiently preparing a selective cyclooxygenase-2 (COX-2) inhibitor 4-[3-trifluoromethyl-5-(4-methylphenyl)-1H-pyrazolyl]benzenesulfonamide (celecoxib, I). The process comprises the following steps: reacting an alkyl trifluoroacetate and 4-methylacetophenone which are initial raw materials under the action of an organic alkali solution, directly adding a dilute acid, an organic solvent and 4-aminosulfonylhydrazinobenzene or its acid salt to the resulting system, and heating to prepare the celecoxib. Above reaction adopts one kettle way, so the process has the advantages of mild condition, simple operation, high product yield and high product purity, and is suitable for industrialized production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
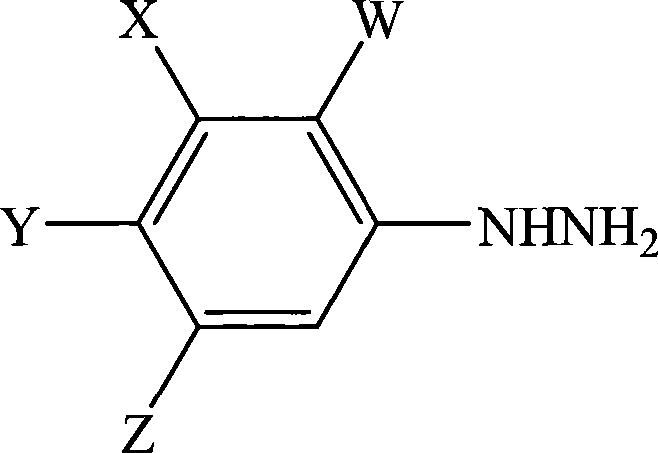
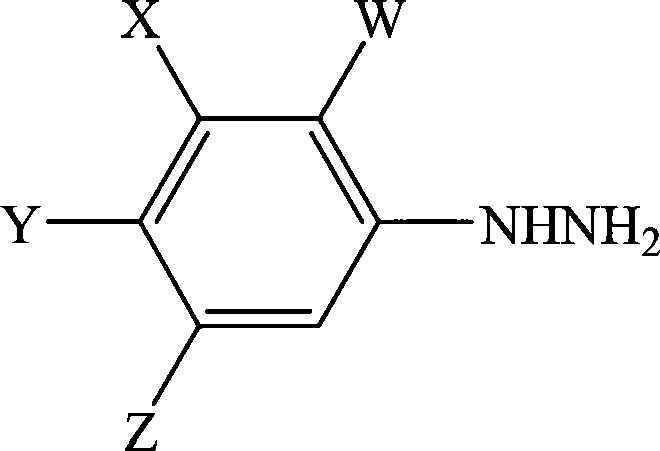
Process for the preparation of phenylhydrazines
InactiveUS6852890B1Efficient preparationHydrazine preparationOrganic compound preparationOxygenHydrolysis
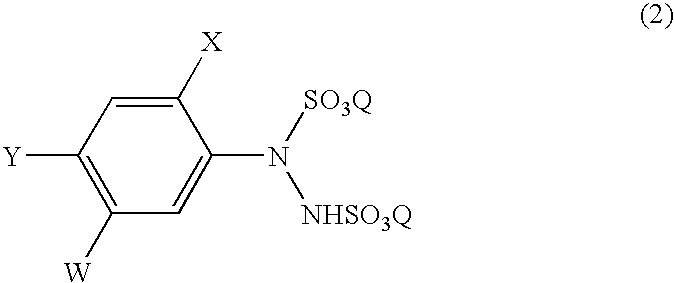
A process for the preparation of a phenylhydrazine or an inorganic acid salt thereof of the formula (1): wherein X is a hydrogen or halogen atom; Y is a halogen atom; and W is a hydrogen atom or —ZR in which Z is an oxygen or sulfur atom, and R is a hydrogen atom, an alkyl group, a haloalkyl group, and so on, by the hydrolysis of a phenylhydrazine derivative of the formula (2): where X, Y and W are the same as defined above, and the Q groups are a hydrogen atom, an ammonium group or an alkali metal atom in the presence of water and an inorganic acid, in which the concentration of the inorganic acid is at least 6 moles per 1 kg of water in a reaction system.
Owner:SUMITOMO CHEM CO LTD
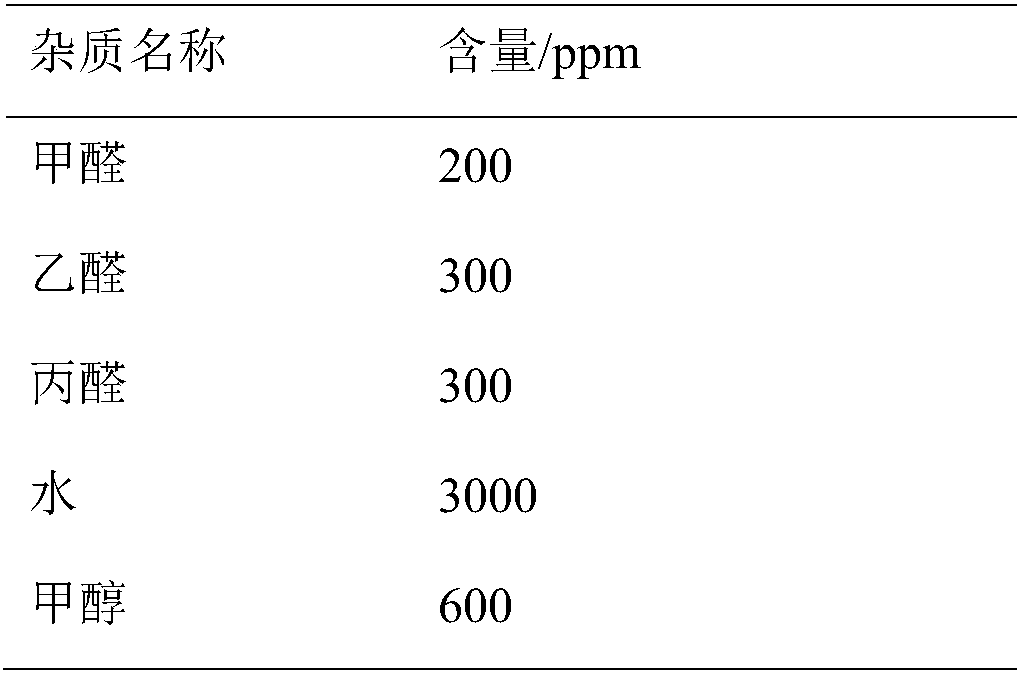
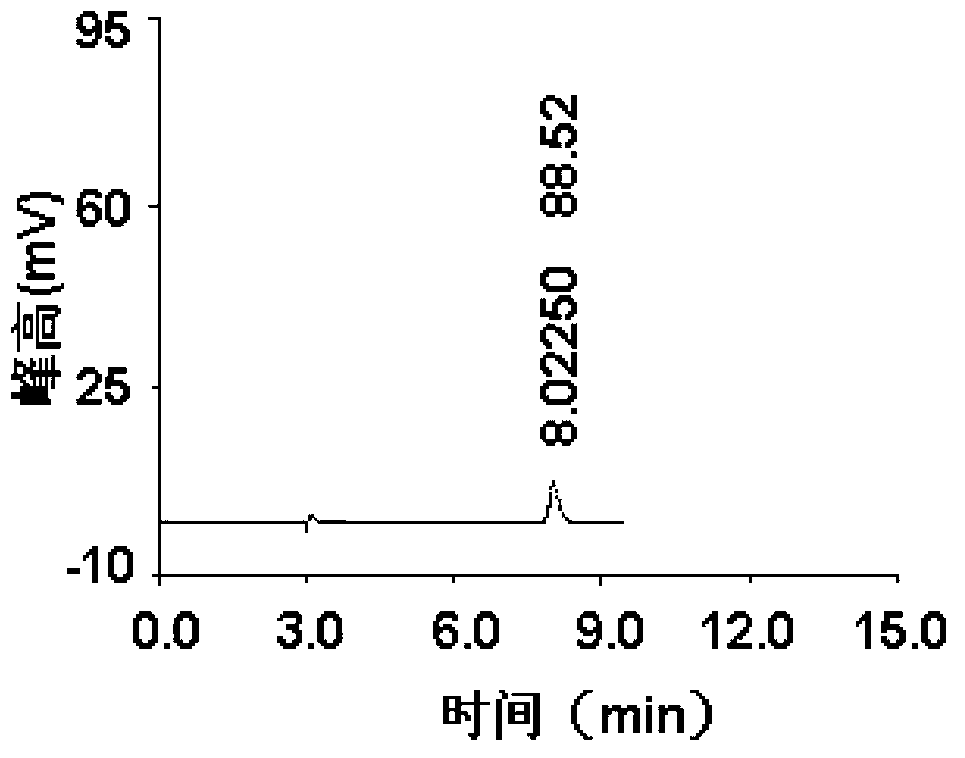
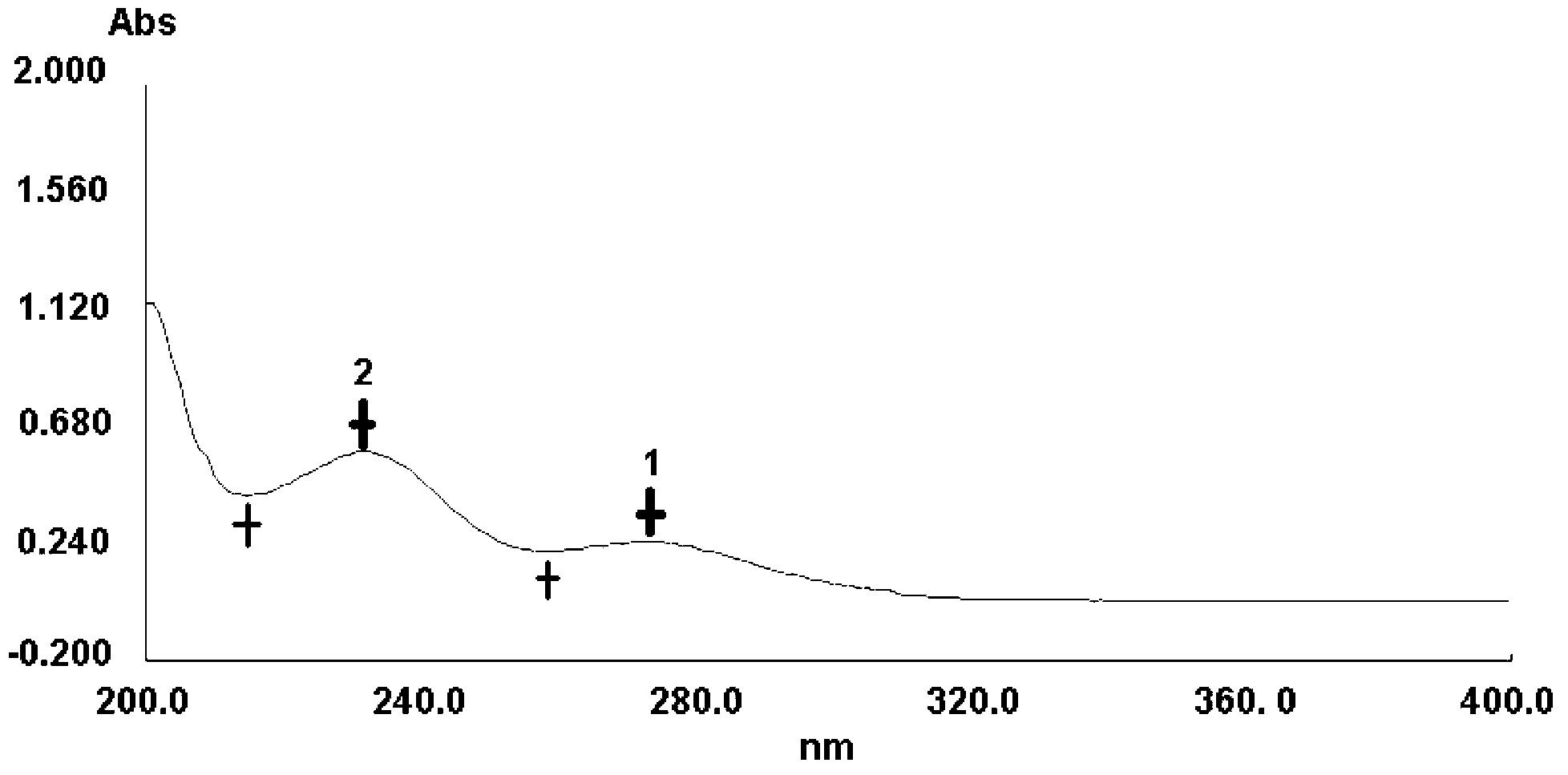
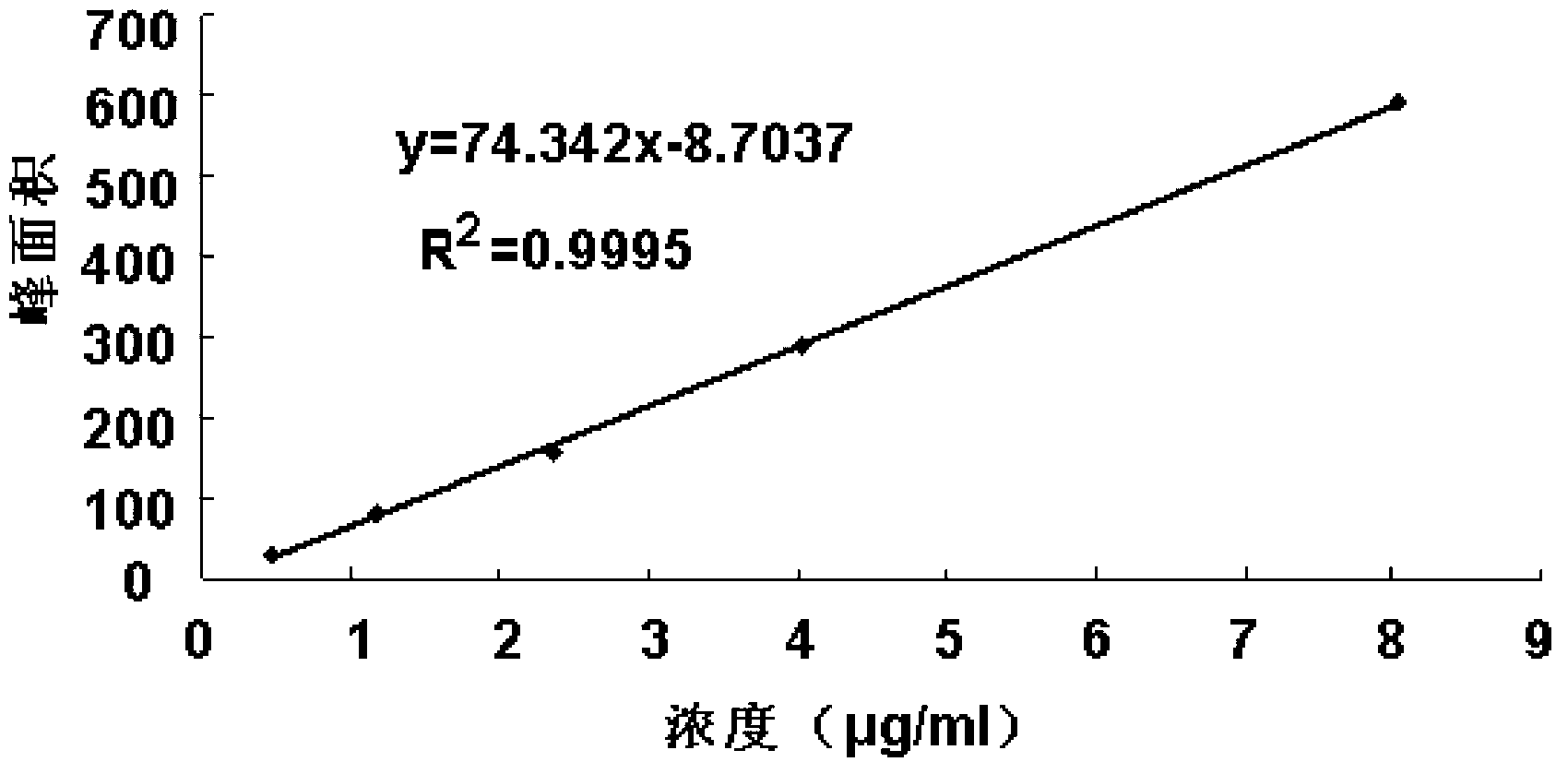
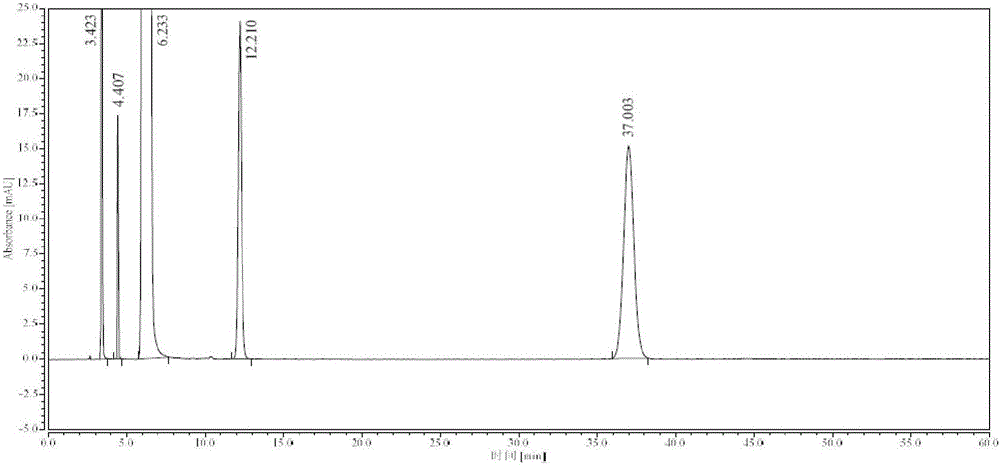
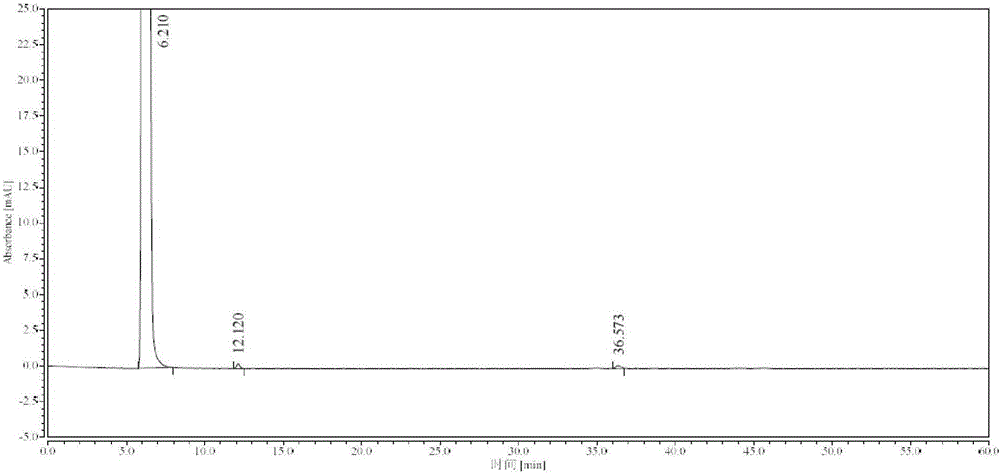
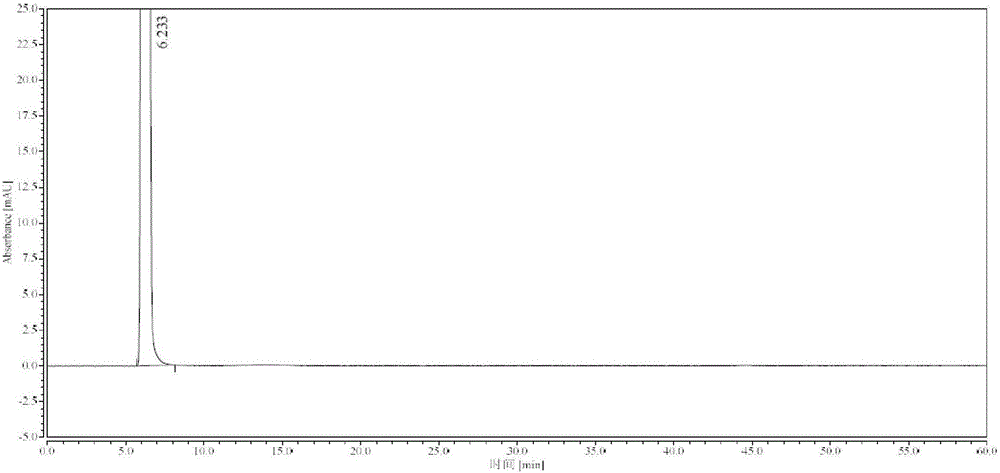
Method for detecting impurity phenylhydrazine in edaravone
InactiveCN102841170ASharp peakSymmetrical peak shapeComponent separationRetention timeColumn temperature
Owner:CHENGDU BAIYU PHARMA CO LTD
Synthesis process of high-purity edaravone
ActiveCN106117144AReduce build timeShort reaction timeOrganic chemistryAcetic acidTemperature control
The invention discloses a synthesis process of high-purity edaravone. The synthesis process comprises the steps of dissolving ethyl acetoacetate into lower alcohol, adding phenylhydrazine drop by drop under the condition of temperature control, adding a catalytic amount of alkali, carrying out heating reflux cyclization, crystallizing, filtering, washing and refining to obtain the edaravone, wherein the purity of the obtained edaravone is higher than 99.97%. The synthesis process is simple in process, high in yield and pure in product, thus being suitable for industrial production.
Owner:HEFEI JIUNUO MEDICAL TECH
Method for preparing phenylhydrazine derivant
InactiveCN101134734AReduce manufacturing costShort reaction timeHydrazine preparationAromatic amineReducing agent
The present invention relates to phenyl hydrazine derivative preparing process. By using aromatic amine as the initial material, and through diazo reaction, reduction reaction with sodium pyrosulfite as reductant at 10-35 deg.c and in pH 7-9 condition, purifying and drying, phenyl hydrazine derivative product of high quality is produced in low production cost.
Owner:太仓市华联化工实业有限公司
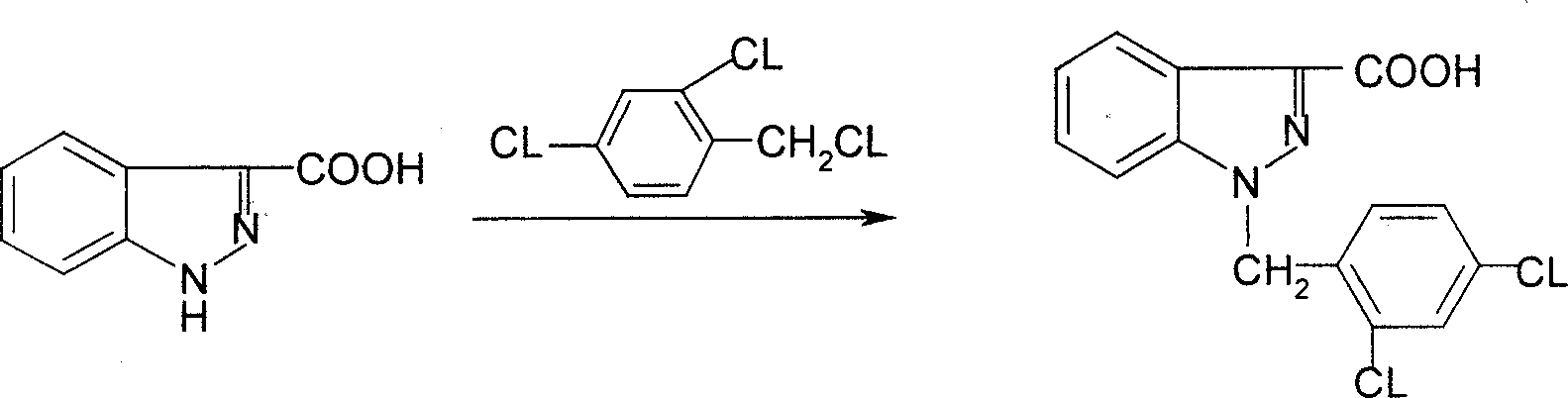
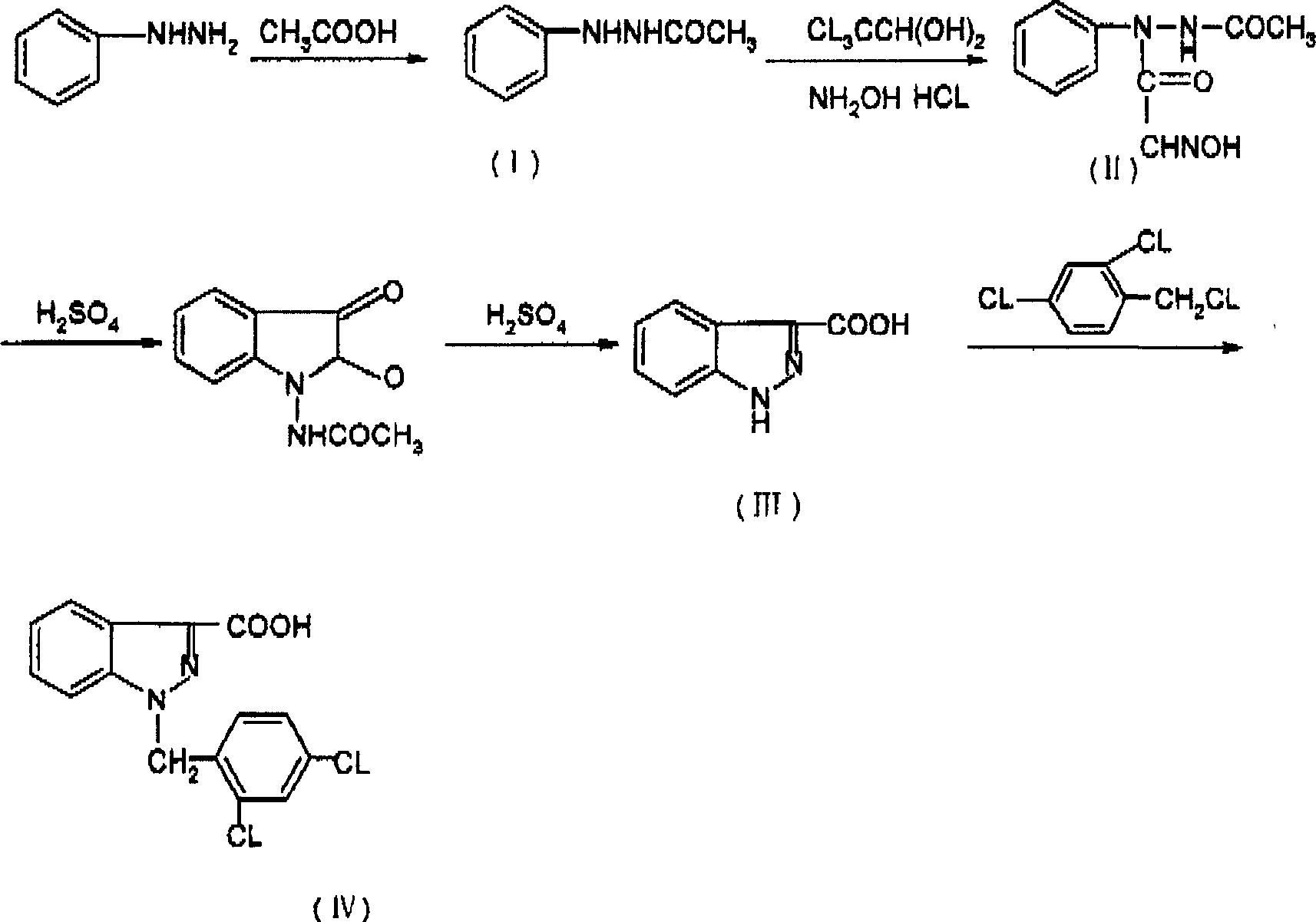
Process for synthesis of lonidamine
ActiveCN1594297AReduce pollutionLow costOrganic chemistryAntineoplastic agentsSynthesis methodsCarboxylic acid
The invention relates to a process for synthesizing 1-[(2,4-dichlorobenzene) methyl]-1H-indazole-3-carboxyl acid which consists of, using phenylhydrazine as starting raw material, producing beta-acetylphenylhydrazine through reaction with glacial acetic acid, reacting with hydrated chloral and hydroxylamine hydrochloride, obtaining N-acetamido-isonitro-acetanilide, preparing 1H-indazole-3-carboxylic acid under the condition of concentrated sulfuric acid, finally subjecting 1H-indazole-3-carboxylic acid with 2,4-dichlorin benzyl chloride.
Owner:SHANGHAI ZHAOHUI PHARMA +1
Synthesis method of parachlorophenylhydrazine hydrochloride
InactiveCN105837466AHigh yieldThe synthesis process is simpleHydrazine preparationAfter treatmentSynthesis methods
The invention provides a synthesis method of parachlorophenylhydrazine hydrochloride. The synthesis method comprises the following steps: 1. diazotization of parachloroaniline; 2. reduction; 3. acidification; and 4. after-treatment. The synthesis method has the advantages of environment-friendly raw materials, simple synthesis technique, environment friendliness, low cost, high product yield, energy saving and consumption reduction, effectively lowers the production cost, and can enhance the economic benefit of the enterprise.
Owner:JIANGSU TUOQIU AGRI CHEM CO LTD
Synthetic method of biphenyl compounds
InactiveCN103553856ASimple and fast operationImprove reaction efficiencyOrganic compound preparationOrganic substitutionSulfohydrazideHalogen
The invention relates to a synthetic method of biphenyl compounds. Based on novel coupling reaction of phenylhydrazine or benzenesulfonyl hydrazide, the phenylhydrazine or benzenesulfonyl hydrazide is used as a substrate, and coupled after being acted by transition metal in a catalytic manner, so that the biphenyl compounds can be conveniently and effectively combined. Compared with the existing method, the synthetic method disclosed by the invention is extensive in applicable substrate range, mild in reaction condition, simple and convenient to operate and high in reaction efficiency, and is a method with important application value. The method disclosed by the invention is further used for synthesizing biphenyl compounds containing halogens. The invention provides a simple, novel and efficient method.
Owner:TONGJI UNIV
Industrial synthesis method for 4-trifluoromethylphenylhydrazine Hydrochloride
InactiveCN101209980ALower requirementReasonable choice of preparation processHydrazine preparationHydrazine compoundFiltration
The invention relates to a preparation method for composing aromatic hydrazine hydrochloride, in particular to an industrial preparation method of 4-trifluoromethyl phenylhydrazine hydrochlorid. The invention mainly aims at solving the technical problems that low yield of the existing synthetic methods has low yield and industrial production can not be realized. The invention takes 4-trifluoromethyl aniline as raw material, successive reactions 'in one pot' happen, diazotization and reduction are carried out successively, diazonium hydrochloride is prepared after diazo reaction of sodium nitrite in aqueous hydrochloric acid solution, then hydrochloric acid solution of reducer stannous chloride is used for achieving reduction reaction, finally, filtration and washing are carried out and the 4-trifluoromethyl phenylhydrazine hydrochlorid is prepared. The invention can be used for the industrial production of the 4-trifluoromethyl phenylhydrazine hydrochlorid, and the yield can be up to more than 75 percent.
Owner:上海药明康德新药开发有限公司
Synthetic method of phenylhydrazine
ActiveCN104610093AReduce water consumptionHigh recycling valueHydrazine preparationDistillationAniline
The invention discloses a synthetic method of phenylhydrazine. The synthetic method comprises the following steps: adding dilute sulfuric acid into aniline, dropwise adding ammonium nitrite at 0-5 DEG C, and then performing thermal reaction for 4-6 minutes; then cooling to a temperature between -5 and -10 DEG C, adding a solid reducing agent, and stirring violently; when the temperature of the reaction system naturally rises to be more than 40 DEG C, adding dilute sulfuric acid to adjust the pH value to be 2-4, and heating to 70-100 DEG C to react for 4-6 hours; and separating the obtained reaction product to obtain phenylhydrazine. According to the synthetic process of phenylhydrazine, disclosed by the invention, aniline is used as a raw material, dilute sulfuric acid is used as salt forming and acidifying reagents, ammonium nitrite is used as a diazotization reagent, ammonium sulfite and / or ammonium hydrogen sulfite is used as the reducing agent, ammonia gas is used for neutralizing a reaction solution, phenylhydrazine is finally obtained by virtue of extraction and distillation, and a byproduct salt is single ammonium sulfate.
Owner:江苏艾科维科技有限公司
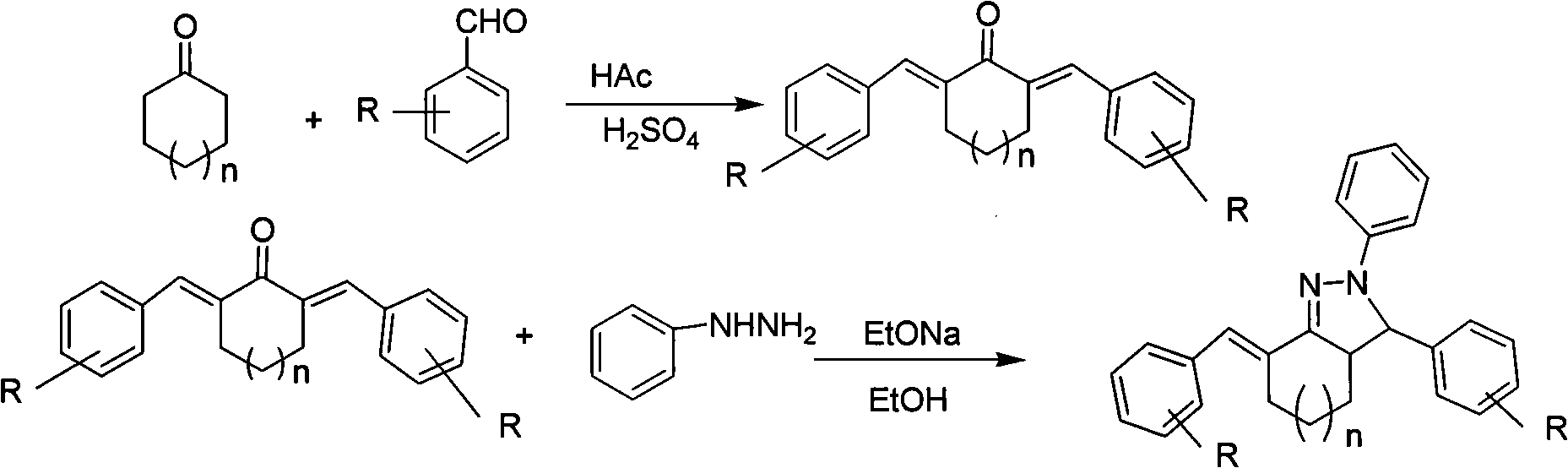
2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof
The invention belongs to the field of organic synthesis and relates to a 2H-pyrazolo [3, 4-d] pyrimidine derivative (i.e., 2-(4-substituted phenyl)-4-chloro-2H-pyrazolo [3, 4-d] pyrimidine) and a synthetic method thereof. In the general structural formula of 2-(4-substituted phenyl)-4-chloro-2H-pyrazolo [3, 4-d] pyrimidine, R refers to hydrogen, alkyl, alkoxyl, halogen, cyan, nitro, carboxyl, ester group and the like; and tetrahydrofuran is adopts as solvent, 4-substituted phenylhydrazine reacts with 4, 6-(dichloro-pyrimidine)-5-methanal under the action of triethylamine, most solvent is vaporized after reaction completion, a crude product precipitates out as a solid by adding water, the crude product filtered out is recrystallized with absolute methanol, and then isomer is removed by a column separation method to obtain corresponding 2-(4-substituted phenyl)-4-chloro-2H-pyrazolo [3, 4-d] pyrimidine. The 2H-pyrazolo [3, 4-d] pyrimidine derivative is synthesized for the first time by selecting reasonable reaction conditions.
Owner:CHANGZHOU UNIV
Method for preparing 4-chlorine phenylhydrazine
InactiveCN101157634AShort reaction timeReduce manufacturing costHydrazine preparationSodium metabisulfiteHydrolysis
The invention relates to a preparative method of 4-chlorophenyl hydrazi. By using 4-chloraniline as the initial raw material, the preparative method is capable of producing the 4-bromophenylhydrazine after the diazotization reaction, the reduction reaction and hydrolysis, wherein, the reduction reaction which utilizes sodium metabisulfite as the reduction agent is carried out under the conditions that the temperature is 10 to 35 DEG C and the PH is 7 to 9. The 4- chlorophenyl hydrazi produced by the preparative method of the invention is characterized in high product purity and low production cost.
Owner:太仓市华联化工实业有限公司
Method for synthesizing caulerpin
InactiveCN104016989ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryPtru catalystBiochemical engineering
The invention discloses a method for synthesizing caulerpin. In the method, the caulerpin is prepared from phenylhydrazine and 3-oxo-1,5-dimethyl glutarate by the following synthesis route. The method comprises the following steps: 1. carrying out Fischer indole reaction on phenylhydrazine 2 and 3-oxo-1,5-dimethyl glutarate 3 by using an acid catalyst to obtain methyl 2-(3'-methoxycarbonyl)indolyl-acetate 4; 2. carrying out Claisen ester condensation on the methyl 2-(3'-methoxycarbonyl)indolyl-acetate 4 by using an alkali catalyst to prepare an intermediate 5; and 3. carrying out catalytic hydrogenation or NaBH4 reduction on the intermediate 5, and dehydrating to obtain the caulerpin 1.Compared with the existing caulerpin synthesis route, the synthesis method disclosed by the invention has the advantage of cheap and accessible raw materials; the reaction conditions are mild, and every step can be performed at normal temperature; and the method is simple and convenient to operate, is easy for mass synthesis, and is applicable to industrial production.
Owner:SHANGHAI MARITIME UNIVERSITY
Preparing method of edaravone
The invention relates to a preparing method of edaravone, and belongs to the technical field of preparation of heterocyclic compounds. The preparing method of edaravone comprises the steps of carryingout a reaction between phenylhydrazine and ethyl acetoacetate at 25-30 DEG C for 1-4 h in an organic solvent, and then removing the solvent to obtain an oily substance, wherein the mole ratio of phenylhydrazine to ethyl acetoacetate is 1:(1.019-1.080); uniformly mixing the obtained oily substance with acetic acid, carrying out a reflux reaction at 105-115 DEG C for 6-10 h, then removing unreactedacetic acid, then adding an alcohol solvent for uniform mixing, and conducting soil-liquid separation to obtain edaravone. According to the preparing method of edaravone, by controlling the reactioncondition, stepwise reactions are carried out, the reaction process is more easily controlled, the content of impurities in the product is also greatly reduced, particularly, the contents of residualphenylhydrazine and derivative impurities thereof can be reduced, the genotoxicity of the product is greatly lowered, and the safety of using edaravone is improved.
Owner:HENAN RUNHONG PHARMA
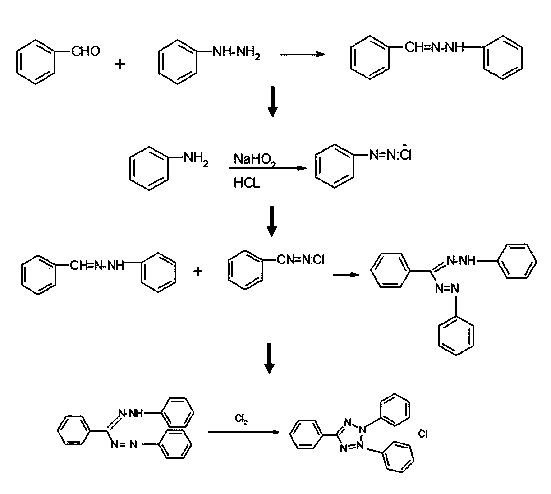
Synthesis method of chloridized-2,3,5-triphenyl tetrazolium chloride
InactiveCN103980216AThe source is easy to getThe synthesis method is simpleOrganic chemistryBenzaldehydePhenyl group
Owner:湖北百诺捷生物科技有限公司
Piperine hydrazone or acylhydrazone or sulfonyl hydrazone derivative substances and application for preparing a botanical insecticide
InactiveCN103130766AHigh insecticidal activityLow toxicity insecticidal activityBiocideOrganic chemistryHydrazoneSulfohydrazide
The invention relates to piperine hydrazone, acylhydrazone or sulfonyl hydrazone derivative substances and application for preparing a botanical insecticide. The piperine hydrazone, acylhydrazone or sulfonyl hydrazone derivative substance takes the piperine hydrazone as raw materials, piperine aldehyde is obtained by means of hydrolysis, esterification, reduction and oxidation, and the piperine aldehyde is respectively reacted with replaced phenylhydrazine, the acylhydrazone and sulfonyl hydrazide. The structural formula is shown as follows. The experiment shows that the piperine hydrazone, or the acylhydrazone or the sulfonyl hydrazone derivative substances has the advantages of having good antifeedant and insecticidal activity, partly exceeds the parent piperine, wherein the insecticidal activity of some components is higher than the commercialized botanical pesticide toosendanin, and therefore the piperine hydrazone, or the acylhydrazone or the sulfonyl hydrazone derivative substances is expected to be used for preparing high-efficient, environment-friendly and low-toxic botanical insecticides.
Owner:NORTHWEST A & F UNIV
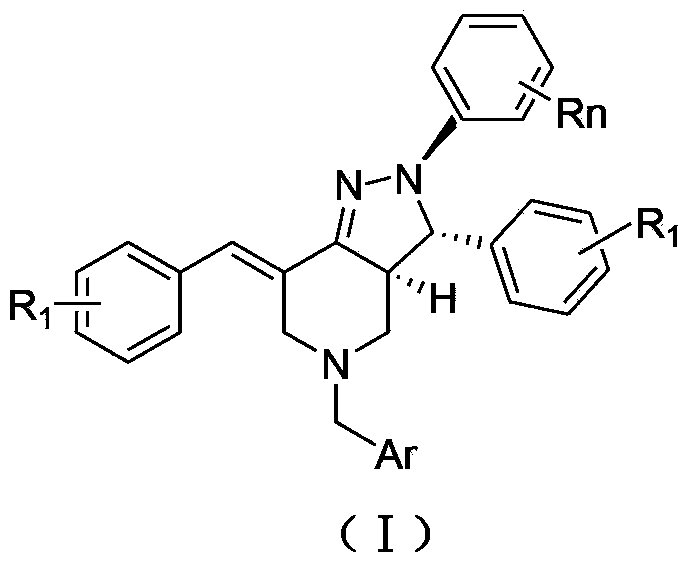
2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and preparation method and application thereof
The invention provides 2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and a preparation method and application thereof. The derivative is 2, 3-bis(substituted phenyl)-5-subsituted arylmethyl-7-substituted benzylidene dihydro-pyrazolo piperidine derivative, having the following formula (I). The preparation method includes using substituted arylmethyl amine and methyl acrylate as raw materials; subjecting the materials to Michael addition, Dieckmann condensation and hydrolysis-decarboxylation sequentially; allowing for Aldol reaction with substituted benzaldehyde to obtain intermediate N-substituted arylmethyl-3, 5-bis(substituted benzylidene)-4-piperidone; allowing for condensation with substituted phenylhydrazine to obtain a compound according to the formula (I). The derivative is efficient in inhibiting multiplication of various carcinoma cell lines such as leukemia, esophagus cancer, ovarian cancer and breast cancer in human, is well stably metabolic in liver microsomes of human and rat, is free of direct and competitive inhibition on five enzymes of liver microsomes, such as CYP3A4, CYP2D6, CYP2C9, CYP1A2 and CYP2C19, is highly bioavailable, is low in toxicity to normal cells, and is available for the preparation of drugs for the cancers.
Owner:SHANGHAI NORMAL UNIVERSITY
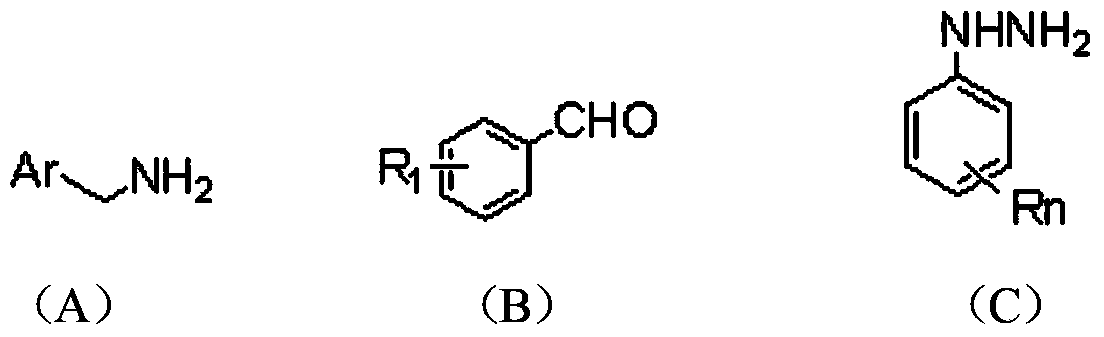
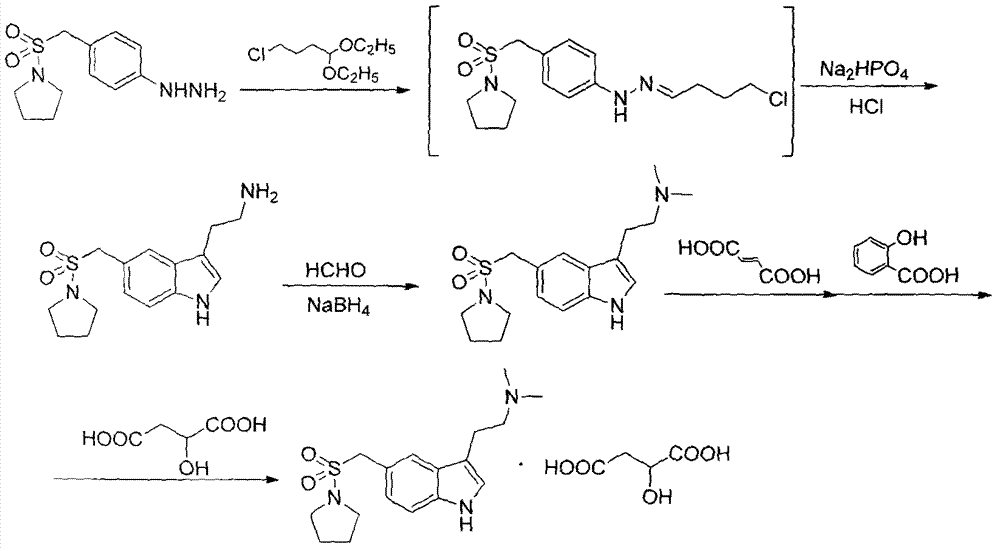
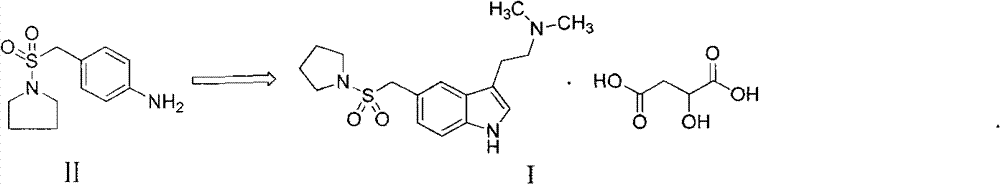
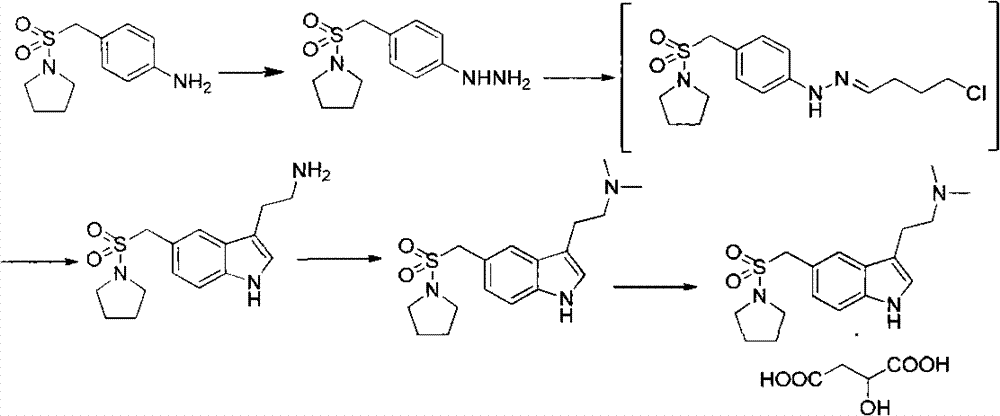
Method for preparing almotriptan malate
InactiveCN102827062AQuality improvementHigh yieldCarboxylic acid salt preparationSalicylic acidImpurity
The invention provides a method for preparing almotriptan malate, which comprises that (1) 4-(1-pyrrolidinylsulforylmenthyl)phenylhydrazine and 4-chlorobutyraldehyde are reacted with each other to obtain the corresponding phenylhydrazone, and 3-[2-(dimethylamino)ethyl]-5-(1-pyrrolidinylsulforylmenthyl)-1H-benzazole (ATP-2) is formed through cyclization under the acid condition; (2) ATP-2 is methylated under the action of formaldehyde and sodium borohydride to obtain crude almotriptan alkali (ATP-3); (3) the crude almotriptan alkali (ATP-3) is respectively salified and crystallized in fumaric acid and salicylic acid ethanol solution; and (4) the refined ATP-3 and DL-malic acid are reacted with each other in carbine to obtain the almotriptan malate. The almotriptan malate with high purity can be prepared through adopting the method; and the method has a simple synthetic technology, easily obtained raw materials, high product yield and low impurity content, and can satisfy the requirement of industrial large-scale production.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
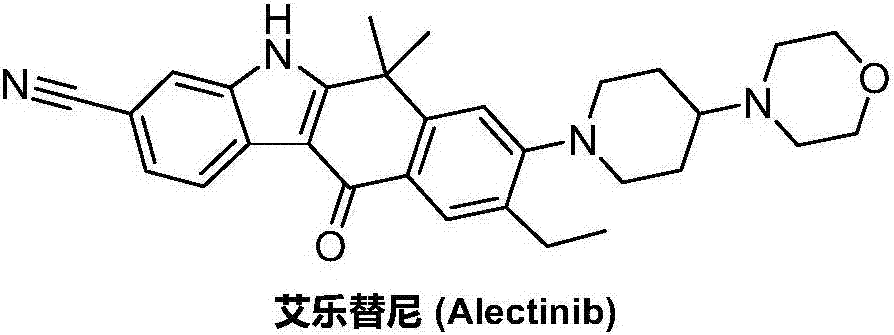
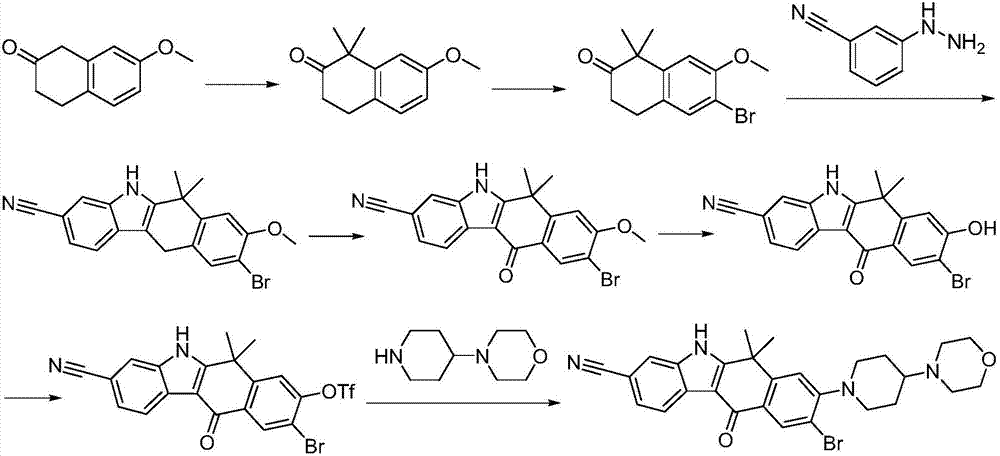
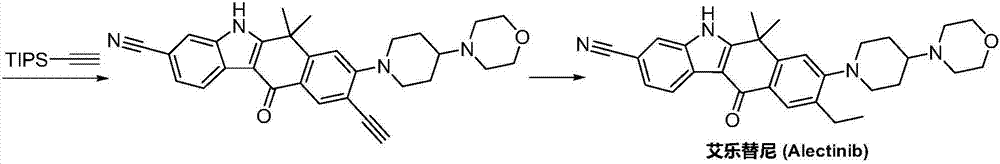
Preparation method of alectinib
InactiveCN106995433AMeet the needs of useApplicable generationOrganic chemistryFischer indole synthesisMorpholine
The invention discloses a preparation method of alectinib. The preparation method comprises the following steps of using 2-(4-bromo-3-hydroxyphenyl)ethyl acetate as a raw material; performing trifluoromethanesulfonic acid etherification with trifluoromethyl sulfonic anhydride, so as to obtain a trifluoromethanesulfonic acid etherification compound; performing substitution reaction with the other raw material, namely 4-(4-piperidyl)morpholine, so as to obtain 2-{4-bromo-3-[4-morpholine-4-yl]piperidine-1-yl]phenyl}ethyl acetate, then performing dimethylation reaction and hydrolysis reaction to obtain 2-{4-bromo-3-[4-morpholine-4-yl]piperidine-1-yl]phenyl}-2-methyl propionate to be subjected to condensation reaction with malonic acid mono-tert-butyl ester, so as to obtain the 4-{4-bromo-3-[4-morpholine-4-yl]piperidine-1-yl]phenyl}-4-methyl-3-oxopentanoate tert-butyl; utilizing a typical Fischer indole synthesis method, enabling carbonyl and phenylhydrazine to cyclize under the acid catalyzing action to form indole nuclear parent; finally, performing cyclizing reaction, boric acidifying and catalytic coupling reaction, so as to prepare the alectinib. The preparation method has the advantages that the design of route method is reasonable, the price of raw material is low, the obtaining is easy, and the reaction condition is easily and effectively controlled.
Owner:HUNAN BOAODE BIOPHARML TECH DEV
Method for preparing chenopodium vulvaria volatile oil and application of chenopodium vulvaria volatile oil
InactiveCN102618386ABroad-spectrum bactericidalBroad-spectrum antibacterialAntibacterial agentsCosmetic preparationsBiotechnologyEscherichia coli
The invention relates to application of chenopodium vulvaria volatile oil to bacteriostatic / bactericidal and antioxidative products. The chenopodium vulvaria volatile oil can be obtained by a water steam distillation or CO2 supercritical extraction method, and main ingredients of the chenopodium vulvaria volatile oil are identified by gas chromatograph / mass spectrum. The chenopodium vulvaria volatile oil has an obvious effect of killing or inhibiting bacteria such as staphylococcus, streptococcus, enterobacter, salmonella, shigella, pseudomonas, bacillus, escherichia coli and the like and fungi such as candida, cryptococcus, penicillin, aspergillus, mucor, microsporon, trichophyta, epidermophyton and the like. A diphenyl picryl phenylhydrazine (DPPH) method proves that the chenopodium vulvaria volatile oil has the high antioxidant activity, can be widely applied to industry of food, medicines, cosmetics, health-care products and the like, and is used for preparing the bacteriostatic / bactericidal and antioxidative products.
Owner:SOUTHEAST UNIV
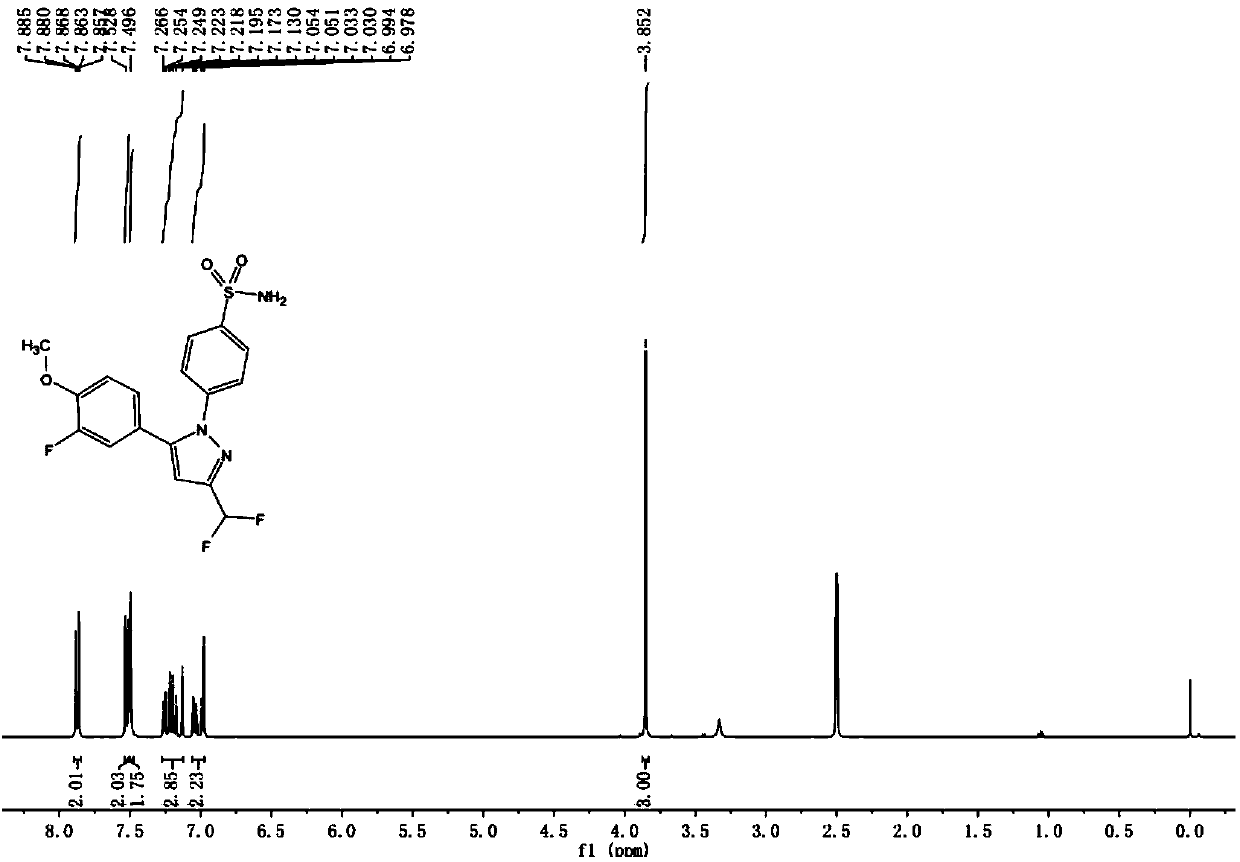
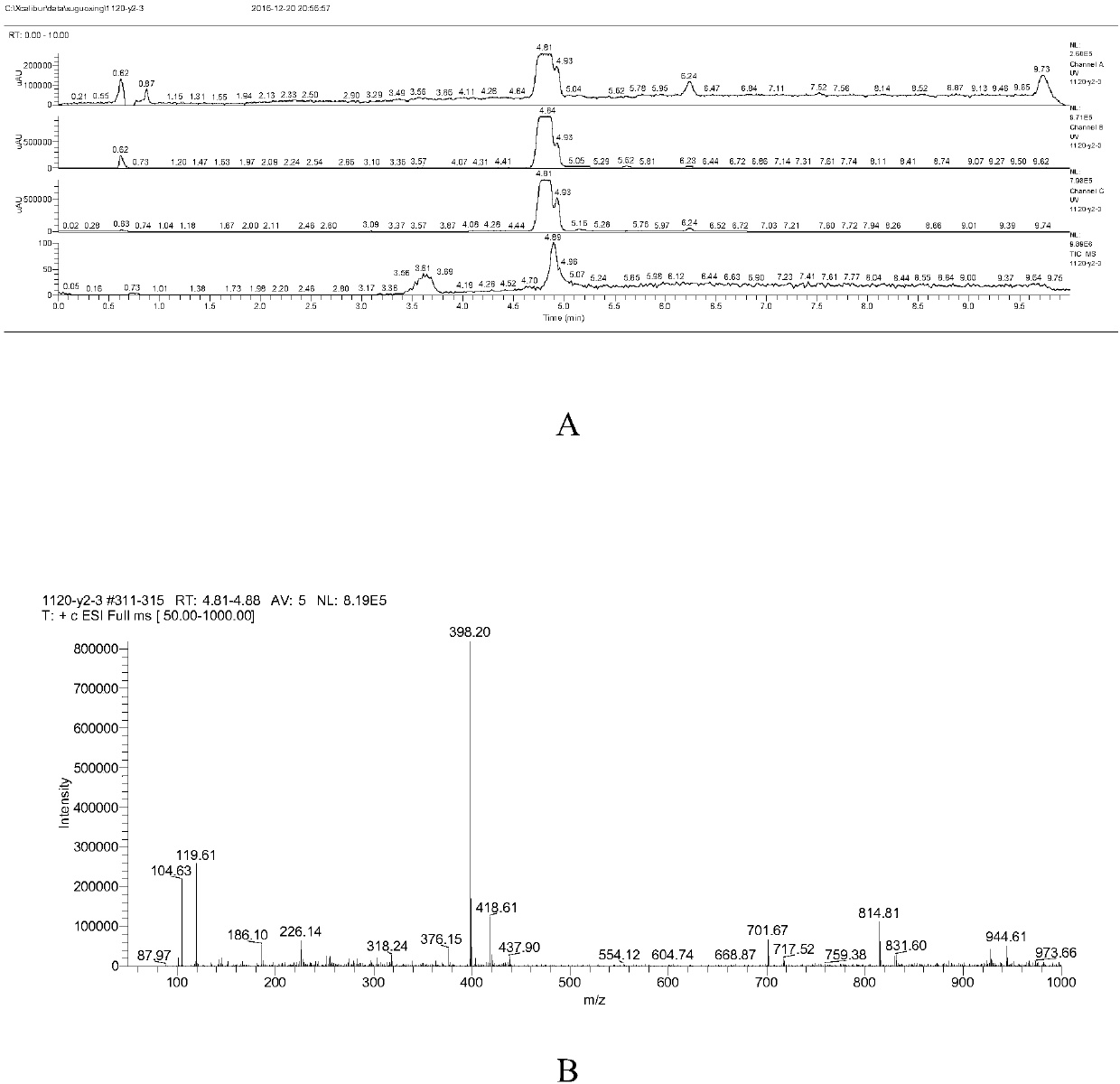
Preparation method of Deracoxib
The invention discloses a preparation method of Deracoxib, and belongs to the field of Deracoxib preparation. The preparation method of Deracoxib disclosed by the invention comprises the following steps: (1) taking methane chloride as a reaction solvent, and reacting 2-fluoroanisole with acetylchloride under the effect of acid so as to obtain 3-fluoro-4-methoxyacetophenone; (2) taking methane chloride as a reaction solvent, and reacting 3-fluoro-4-methoxyacetophenone with ethyl difluoroacetate under the effect of alkaline so as to obtain 4,4-difluoro-1-(3-fluoro-4-methoxyphenyl)-1,3-butanedione; and (3) under the existence of an ethyl alcohol solvent, reacting 4,4-difluoro-1-(3-fluoro-4-methoxyphenyl)-1,3-butanedione with para-sulfamine phenylhydrazine or salt thereof so as to obtain Deracoxib. According to the preparation method of Deracoxib disclosed by the invention, dichloromethane is used as a solvent, the toxicity of the solvent is low, the cost of the solvent is lower than thatof methyl tertiary butyl ether, and the production cost is obviously reduced on the premise of guaranteeing the yield.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
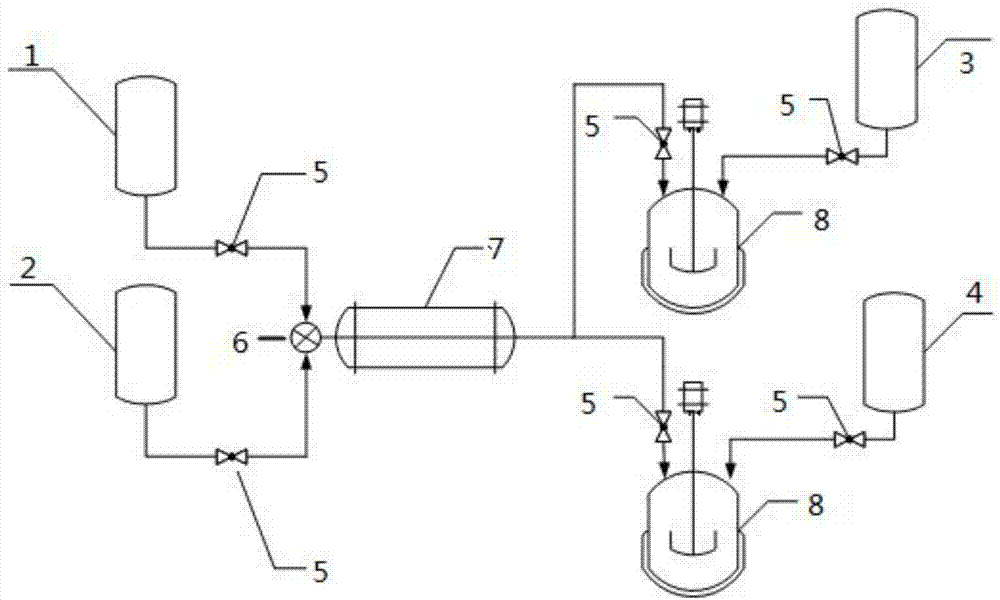
Continuous production method and device of 4-chlorophenylhydrazine hydrochloride
ActiveCN107573256AWell mixedHigh purityHydrazine preparationChemical/physical/physico-chemical processesOrganic synthesisSide reaction
The invention relates to the field of organic synthesis and in particular relates to a continuous production method and device of 4-chlorophenylhydrazine hydrochloride. A continuous production mannercombining tubular reaction and kettle type reaction is adopted; diazotization reaction is carried out in a tubular reactor and raw materials are uniformly mixed; the reaction is stably carried out, the safety of the reaction is improved, side reaction is reduced and the purity and the yield of a final product are improved; reduction reaction and acidification reaction are carried out in a reduction reaction kettle so that the production continuity is improved, the total reaction time is reduced, the production efficiency is improved, the energy consumption is saved and the production cost is reduced; the yield of the prepared 4-chlorophenylhydrazine hydrochloride is greater than 90 percent and the purity is greater than 96 percent; the continuous production method and device meet industrial requirements and are suitable for industrial continuous production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Method for preparing antiseptic triaryl-2-pyrazoline derivative by microwave
InactiveCN101580492AHigh purityEasy post-processingBiocideOrganic chemistryCyclohexanoneProduction rate
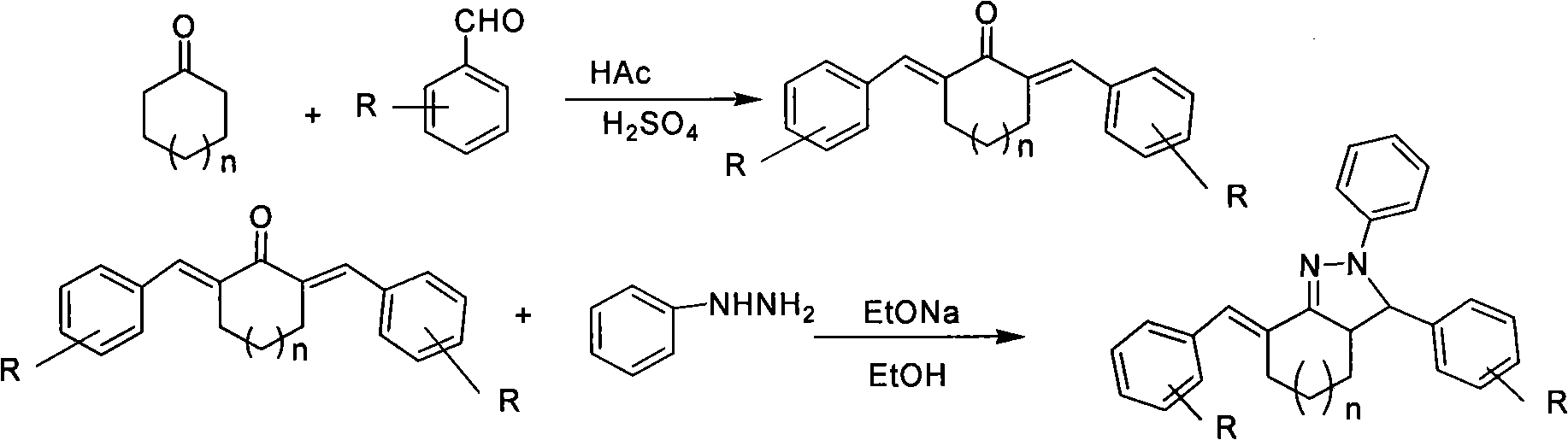
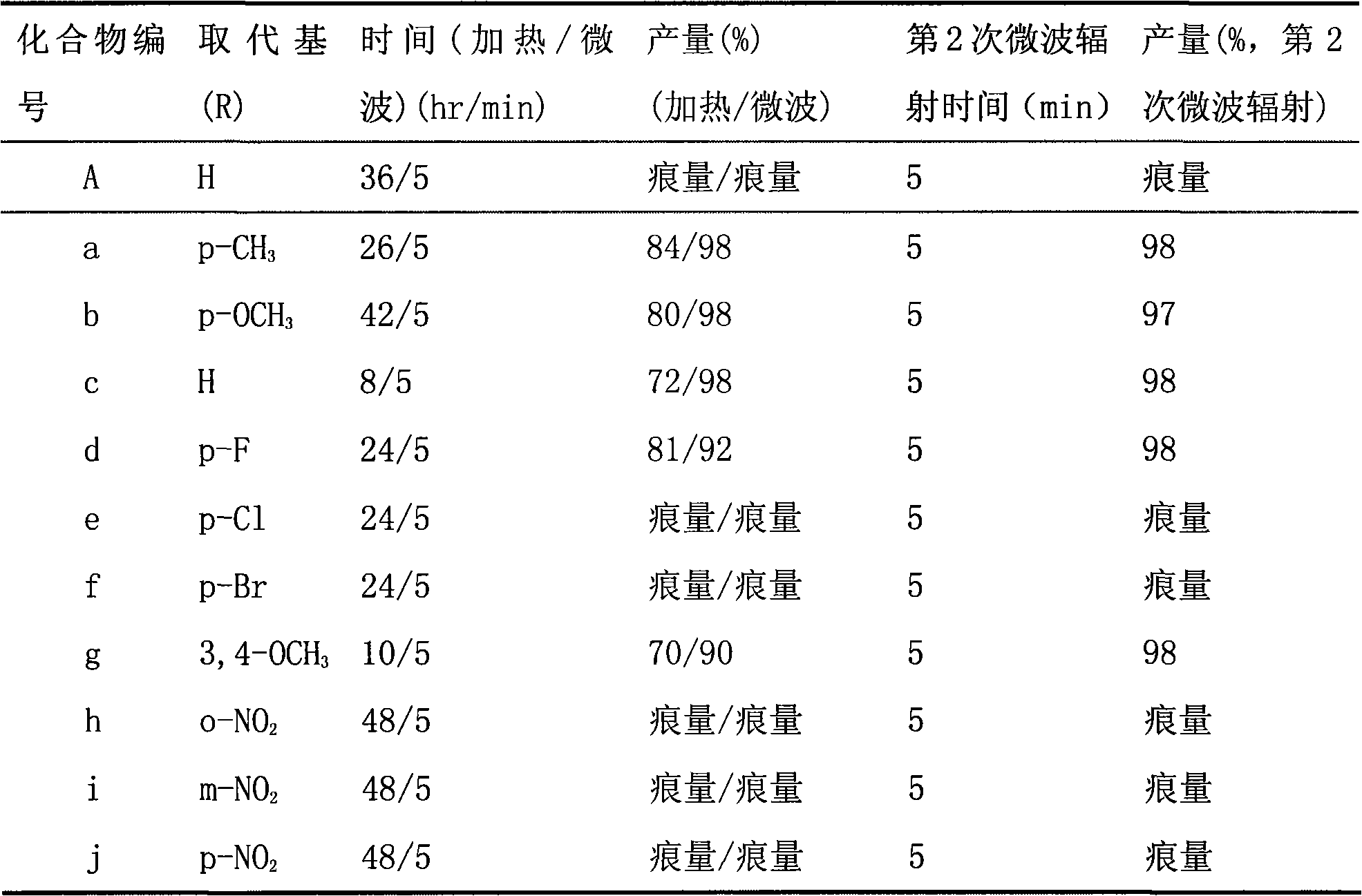
The invention relates to a method for preparing antiseptic triaryl-2-pyrazoline derivative by microwave, which is characterized by taking alpha, alpha'-dibenzyl cyclohexanone derivative having alpha, beta-unsaturated carboxide structure and phenyl hydrazine derivative as main raw materials; the raw materials react for 5-10min in sodium alcoholate and alcohol solution under the condition that the microwave power is 200-500W to be directly cyclized and synthesized to be the antiseptic triaryl-2-pyrazoline derivative which maintains one double-bond and has alpha-benzal cyclohexyl and pyrazoline ring structure. Therefore, the method has the advantages of high reaction selection, production rate and purity of the product, easy purification, etc.
Owner:ZHEJIANG UNIV
Prepn process of 7-ethyl tryptophol
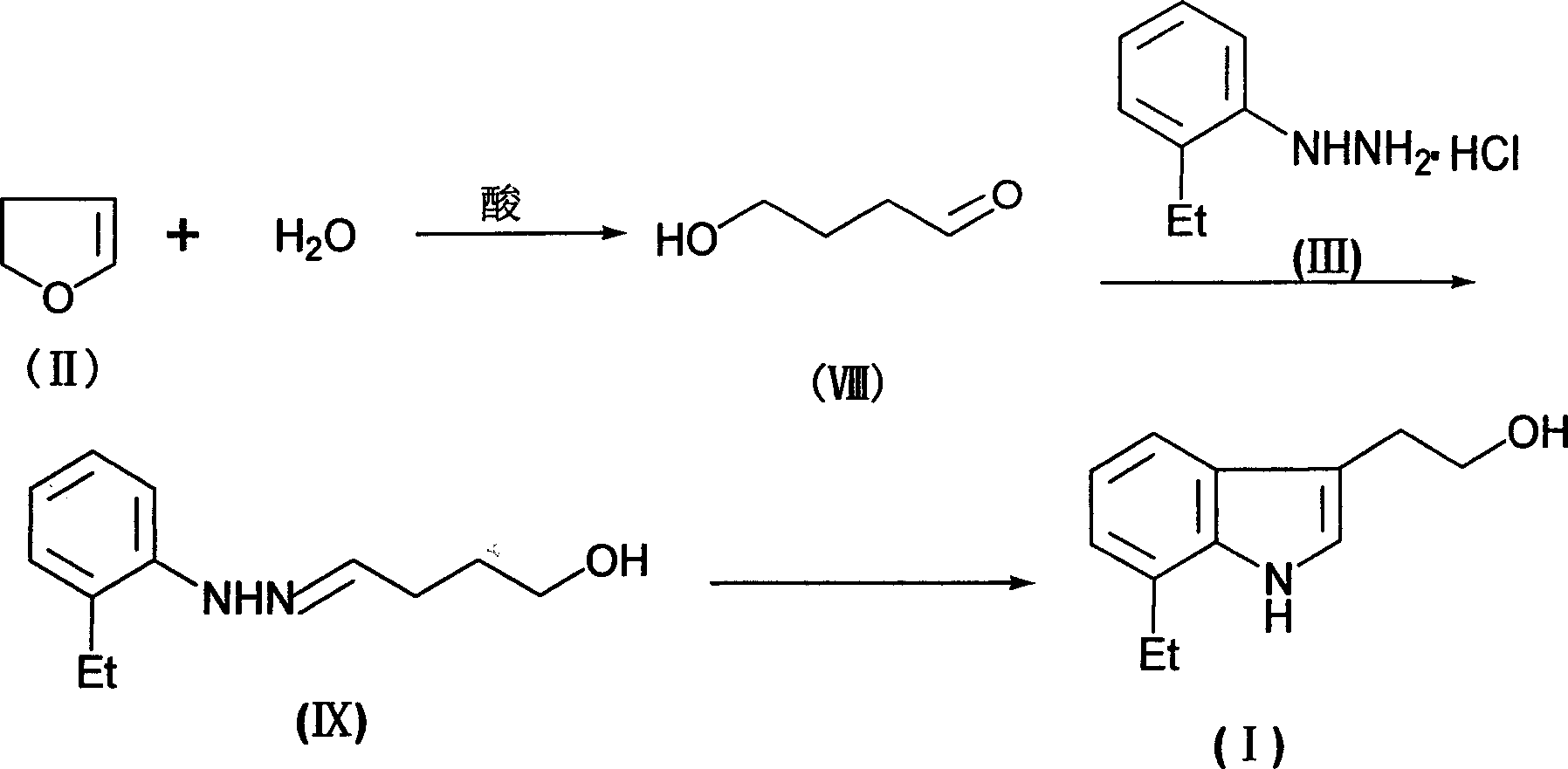
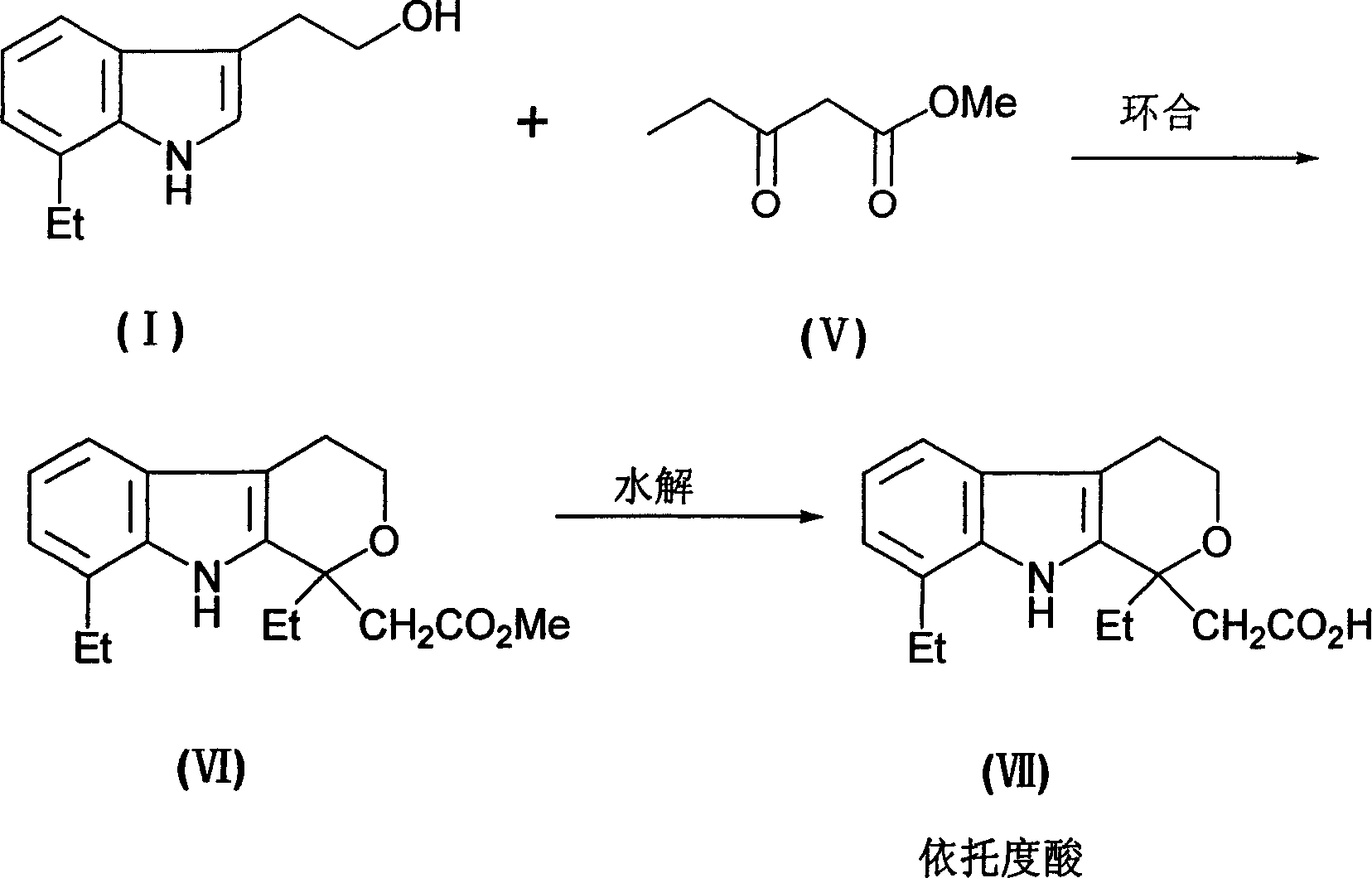
The preparation process of 7-ethyl tryptophol includes the following steps: hydrolyzing 2, 3-dihydrofuran inside glycol-ether solvent to obtain 4-hydroxy butyraldehyde; reacting 4-hydroxy butyraldehyde and o-ethyl phenylhydrazine hydrohcloride inside glycol-ether solvent in the temperature from ¿C20 deg.c to solvent boiling point for 1-24 hr; and post-treatment. The 7-ethyl tryptophol preparing process of the present invention has high yield, convenient operation and high product purity, and is suitable for industrial production. The product is used in further production of etodolac.
Owner:杭州科本药业有限公司
Preparation method of cefodizime acid
The invention belongs to the technical field of medicament synthesis, and particularly relates to a preparation method of cefodizime acid. The preparation method is characterized by comprising the following steps of: adding 7-aminocephalosporanic acid, 2-Mercapto-4-methyl-1,3-thiazol-5-yl-acetic acid(MMTA) and water, dropwise adding an alkaline solution, adjusting pH, adding an organic solvent, stirring and mixing, and adjusting pH by using glacial acetic acid; leaching, rinsing by the organic solution, and drying; directly adding the dried substance obtained from the former step into a dichloromethane solution after rinsing, adding 2-methoxy imino-2-(2-azyl-4-thiazolyl)-(z)-thioacetic acid phenylhydrazine thiazole ester, and dropwise adding a 7-aminocephalosporanic acid basic catalyst for reaction; and extracting with water, and adjusting pH with glacial acetic acid to obtain cefodizime acid. After the special alkaline solution is used, the reaction yield of 7-aminocephalosporanic acid and MMTA can be improved. After the amide basic catalysts are used, the product quality of synthesized cefodizime acid can be guaranteed.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD +1
Method of rectifying and refining epoxypropane through reaction
InactiveCN107686469AImprove removal efficiencyHigh process integrationOrganic chemistryChemical industryHigh energyReactive distillation
The invention discloses a method of rectifying and refining epoxypropane through a reaction. The method comprises the following steps: performing reactive distillation of to-be-refined crude epoxypropane containing aldehyde impurities and a reaction entrainer, wherein the reaction entrainer is one or more of phenylamine, phenylhydrazine and semi carbazide, preferably phenylhydrazine and semi carbazide, and most preferably phenylhydrazine. The method disclosed by the invention has relatively high aldehyde removal efficiency, can solve the problem of high energy consumption caused by rectifyingand recycling an extraction agent in traditional extraction and rectification for aldehyde removal, and is a novel efficient epoxypropane refining technology.
Owner:WANHUA CHEM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

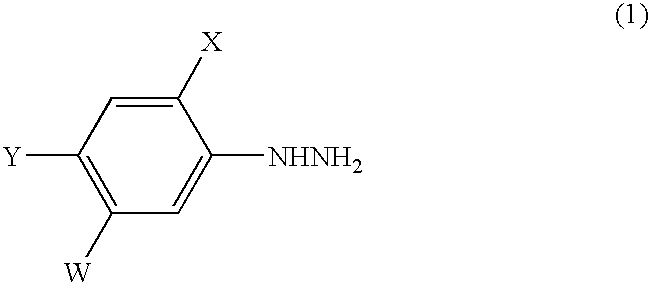
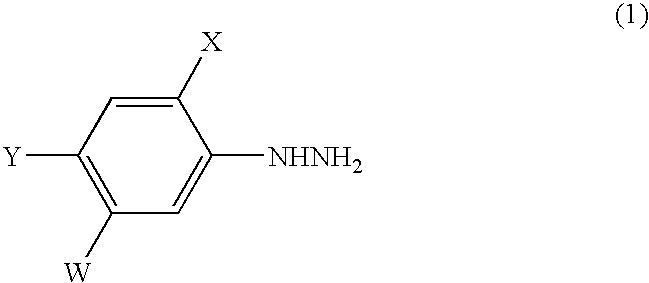

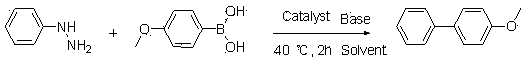
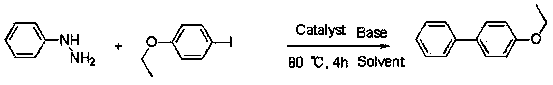
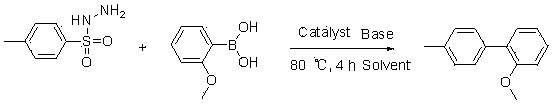
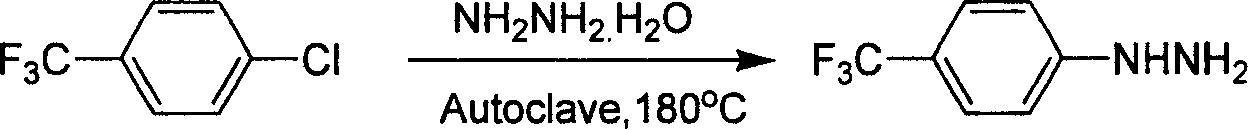
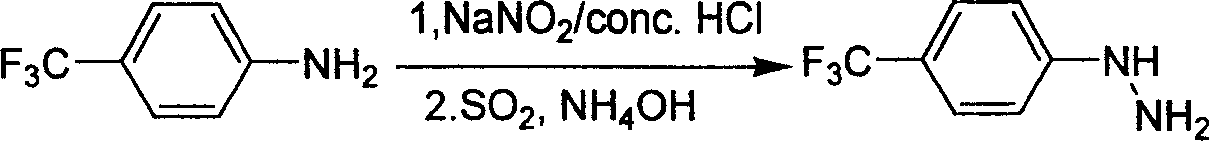
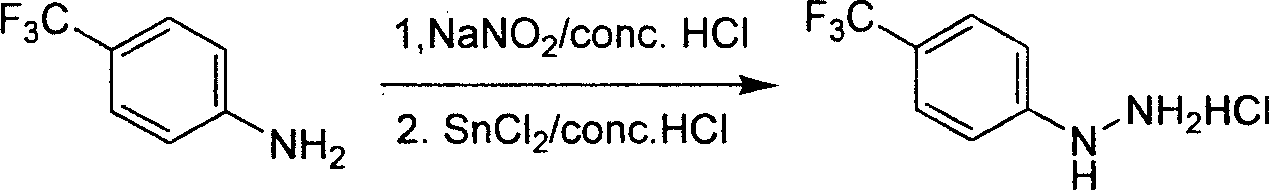
![2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof 2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d119ea34-30ed-469a-9e03-c87617ab205d/31445DEST_PATH_IMAGE008.png)
![2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof 2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d119ea34-30ed-469a-9e03-c87617ab205d/96670DEST_PATH_IMAGE006.png)
![2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof 2H-pyrazolo [3, 4-d] pyrimidine derivative and synthetic method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d119ea34-30ed-469a-9e03-c87617ab205d/361199DEST_PATH_IMAGE010.png)